Recent Developments in Automated Reactors for Plasmonic Nanoparticles
Abstract
1. Introduction
2. Batch Platforms
3. Continuous Flow Platforms
4. Challenges and Future Outlook
5. Conclusions
Funding
Conflicts of Interest
References
- Ahrberg, C.D.; Wook Choi, J.; Geun Chung, B. Automated droplet reactor for the synthesis of iron oxide/gold core-shell nanoparticles. Sci. Rep. 2020, 10, 1737. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.D.; Pyzer-Knapp, E.O.; Purdie, M.; Jones, M.F.; Barthelme, A.; Pavey, J.; Kapur, N.; Chamberlain, T.W.; Blacker, A.J.; Bourne, R.A. Bayesian Self-Optimization for Telescoped Continuous Flow Synthesis. Angew. Chem. 2023, 135, e202214511. [Google Scholar] [CrossRef]
- Clayton, A.D. Recent Developments in Reactor Automation for Multistep Chemical Synthesis. Chem. Methods 2023, 3, e202300021. [Google Scholar] [CrossRef]
- Taylor, C.J.; Pomberger, A.; Felton, K.C.; Grainger, R.; Barecka, M.; Chamberlain, T.W.; Bourne, R.A.; Johnson, C.N.; Lapkin, A.A. A Brief Introduction to Chemical Reaction Optimization. Chem. Rev. 2023, 123, 3089–3126. [Google Scholar] [CrossRef]
- Kioumourtzoglou, S.; Hof, S.; Kalk, C.; Toth, V.; Görlin, M.; Nováková, J.; Sá, J. Nanomaterials as a Service (NaaS) concept: On-demand protocols for volume synthesis of nanomaterials. Nanoscale Horiz. 2024, 9, 1364–1371. [Google Scholar] [CrossRef]
- Liu, J.; Luo, H.; Li, Z.; Peng, Y.; Zhang, J.; Khaniyev, B.; Mukhametkarimov, Y.; Ibraimov, M.; Xu, Y. Designing Hybrid Plasmonic Superlattices with Spatially Confined Responsive Heterostructural Units. Nano Lett. 2025, 25, 754–761. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef]
- Seifert, J.S.; Nees, N.; Khan, H.; Traoré, N.E.; Drobek, D.; Peukert, W.; Apeleo Zubiri, B.; Spiecker, E.; Stingl, M.; Pflug, L.; et al. Continuous flow synthesis and simulation-supported investigation of tunable plasmonic gold patchy nanoparticles. Nanoscale 2024, 16, 19284–19297. [Google Scholar] [CrossRef]
- Patrizia Di, P.; Gaetano, S.; Lidia, Z.; Cristina, S. Gold and Silver Nanoparticles for Applications in Theranostics. Curr. Top. Med. Chem. 2016, 16, 3069–3102. [Google Scholar] [CrossRef]
- Jellicoe, M.; Yang, L.; He, S.; Lobel, B.; Young, D.; Harvie, A.; Bourne, R.; Elbourne, A.; Chamberlain, T. Flowing into the Future: The Promise and Challenges of Continuous Inorganic Nanoparticle Synthesis and Application. ChemRxiv 2025. [Google Scholar] [CrossRef]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef] [PubMed]
- Pokrajac, L.; Abbas, A.; Chrzanowski, W.; Dias, G.M.; Eggleton, B.J.; Maguire, S.; Maine, E.; Malloy, T.; Nathwani, J.; Nazar, L.; et al. Nanotechnology for a Sustainable Future: Addressing Global Challenges with the International Network4Sustainable Nanotechnology. ACS Nano 2021, 15, 18608–18623. [Google Scholar] [CrossRef] [PubMed]
- Truong, T.T.; Mondal, S.; Doan, V.H.M.; Tak, S.; Choi, J.; Oh, H.; Nguyen, T.D.; Misra, M.; Lee, B.; Oh, J. Precision-engineered metal and metal-oxide nanoparticles for biomedical imaging and healthcare applications. Adv. Colloid Interface Sci. 2024, 332, 103263. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Salley, D.; Sharma, A.; Keenan, G.; Mullin, M.; Cronin, L. An artificial intelligence enabled chemical synthesis robot for exploration and optimization of nanomaterials. Sci. Adv. 2022, 8, eabo2626. [Google Scholar] [CrossRef]
- Kardamaki, A.; Nikolakopoulos, A.; Kavousanakis, M.; Doganis, P.; Jellicoe, M.; Stokes, W.; Hongisto, V.; Simmons, M.D.; Chamberlain, T.W.; Kapur, N.; et al. The Sabydoma Safety by Process Control Framework for the Production of Functional, Safe and Sustainable Nanomaterials. Comput. Chem. Eng. 2025. [Google Scholar] [CrossRef]
- Khositanon, C.; Adpakpang, K.; Bureekaew, S.; Weeranoppanant, N. Continuous-flow purification of silver nanoparticles and its integration with flow synthesis. J. Flow Chem. 2020, 10, 353–362. [Google Scholar] [CrossRef]
- Jellicoe, M.; Igder, A.; Chuah, C.; Jones, D.B.; Luo, X.; Stubbs, K.A.; Crawley, E.M.; Pye, S.J.; Joseph, N.; Vimalananthan, K.; et al. Vortex fluidic induced mass transfer across immiscible phases. Chem Sci. 2022, 13, 3375–3385. [Google Scholar] [CrossRef]
- Jellicoe, M.; Yang, Y.; Stokes, W.; Simmons, M.; Yang, L.; Foster, S.; Aslam, Z.; Cohen, J.; Rashid, A.; Nelson, A.L.; et al. Continuous Flow Synthesis of Copper Oxide Nanoparticles Enabling Rapid Screening of Synthesis-Structure-Property Relationships. Small 2025, 21, 2403529. [Google Scholar] [CrossRef]
- Alharbi, T.M.D.; Jellicoe, M.; Luo, X.; Vimalanathan, K.; Alsulami, I.K.; Al Harbi, B.S.; Igder, A.; Alrashaidi, F.A.J.; Chen, X.; Stubbs, K.A.; et al. Sub-micron moulding topological mass transport regimes in angled vortex fluidic flow. Nanoscale Adv. 2021, 3, 3064–3075. [Google Scholar] [CrossRef]
- Jackson, C.; Robertson, K.; Sechenyh, V.; Chamberlain, T.W.; Bourne, R.A.; Lester, E. Self-optimising continuous-flow hydrothermal reactor for nanoparticle synthesis. React. Chem. Eng. 2025, 10, 511–514. [Google Scholar] [CrossRef]
- Karthik, V.; Karuna, B.; Kumar, P.S.; Saravanan, A.; Hemavathy, R.V. Development of lab-on-chip biosensor for the detection of toxic heavy metals: A review. Chemosphere 2022, 299, 134427. [Google Scholar] [CrossRef] [PubMed]
- Schweidtmann, A.M.; Clayton, A.D.; Holmes, N.; Bradford, E.; Bourne, R.A.; Lapkin, A.A. Machine learning meets continuous flow chemistry: Automated optimization towards the Pareto front of multiple objectives. Chem. Eng. J. 2018, 352, 277–282. [Google Scholar] [CrossRef]
- Müller, P.; Clayton, A.D.; Manson, J.; Riley, S.; May, O.S.; Govan, N.; Notman, S.; Ley, S.V.; Chamberlain, T.W.; Bourne, R.A. Automated multi-objective reaction optimisation: Which algorithm should I use? React. Chem. Eng. 2022, 7, 987–993. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, G.; Hui, Y.; Ranaweera, S.; Zhao, C.-X. Microfluidic Nanoparticles for Drug Delivery. Small 2022, 18, 2106580. [Google Scholar] [CrossRef]
- Manson, J.A.; Chamberlain, T.W.; Bourne, R.A. MVMOO: Mixed variable multi-objective optimisation. J. Glob. Optim. 2021, 80, 865–886. [Google Scholar] [CrossRef]
- Salley, D.; Keenan, G.; Grizou, J.; Sharma, A.; Martín, S.; Cronin, L. A nanomaterials discovery robot for the Darwinian evolution of shape programmable gold nanoparticles. Nat. Commun. 2020, 11, 2771. [Google Scholar] [CrossRef]
- Wolf, J.B.; Stawski, T.M.; Smales, G.J.; Thünemann, A.F.; Emmerling, F. Towards automation of the polyol process for the synthesis of silver nanoparticles. Sci. Rep. 2022, 12, 5769. [Google Scholar] [CrossRef]
- Yoo, H.J.; Kim, N.; Lee, H.; Kim, D.; Ow, L.T.C.; Nam, H.; Kim, C.; Lee, S.Y.; Lee, K.-Y.; Kim, D.; et al. Bespoke Metal Nanoparticle Synthesis at Room Temperature and Discovery of Chemical Knowledge on Nanoparticle Growth via Autonomous Experimentations. Adv. Funct. Mater. 2024, 34, 2312561. [Google Scholar] [CrossRef]
- Pinho, B.; Torrente-Murciano, L. Dial-A-Particle: Precise Manufacturing of Plasmonic Nanoparticles Based on Early Growth Information—Redefining Automation for Slow Material Synthesis. Adv. Energy Mater. 2021, 11, 2100918. [Google Scholar] [CrossRef]
- Mekki-Berrada, F.; Ren, Z.; Huang, T.; Wong, W.K.; Zheng, F.; Xie, J.; Tian, I.P.S.; Jayavelu, S.; Mahfoud, Z.; Bash, D.; et al. Two-step machine learning enables optimised nanoparticle synthesis. NPJ Comput. Mater. 2021, 7, 55. [Google Scholar] [CrossRef]
- Hall, B.L.; Taylor, C.J.; Labes, R.; Massey, A.F.; Menzel, R.; Bourne, R.A.; Chamberlain, T.W. Autonomous optimisation of a nanoparticle catalysed reduction reaction in continuous flow. Chem. Commun. 2021, 57, 4926–4929. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Kheiri, S.; Hickman, R.J.; Tao, H.; Wu, T.C.; Yang, Z.-B.; Ge, X.; Zhang, W.; Abolhasani, M.; Liu, K.; et al. Self-driving lab for the photochemical synthesis of plasmonic nanoparticles with targeted structural and optical properties. Nat. Commun. 2025, 16, 1473. [Google Scholar] [CrossRef] [PubMed]
- Tao, H.; Wu, T.; Kheiri, S.; Aldeghi, M.; Aspuru-Guzik, A.; Kumacheva, E. Self-Driving Platform for Metal Nanoparticle Synthesis: Combining Microfluidics and Machine Learning. Adv. Funct. Mater. 2021, 31, 2106725. [Google Scholar] [CrossRef]
- Bui, H.K.; Nguyen, T.T.H.; Dao, T.D.; Seo, T.S. A Proportional–Integral Feedback Controlled Automatic Flow Chemistry System to Produce On-Demand AgAu Alloy Nanoboxes. Small Struct. 2024, 5, 2300397. [Google Scholar] [CrossRef]
- Joseph, N.; Mirzamani, M.; Abudiyah, T.; Al-Antaki, A.H.M.; Jellicoe, M.; Harvey, D.P.; Crawley, E.; Chuah, C.; Whitten, A.E.; Gilbert, E.P.; et al. Vortex fluidic regulated phospholipid equilibria involving liposomes down to sub-micelle size assemblies. Nanoscale Adv. 2024, 6, 1202–1212. [Google Scholar] [CrossRef]
- Adamo, A.; Beingessner, R.L.; Behnam, M.; Chen, J.; Jamison, T.F.; Jensen, K.F.; Monbaliu, J.-C.M.; Myerson, A.S.; Revalor, E.M.; Snead, D.R.; et al. On-demand continuous-flow production of pharmaceuticals in a compact, reconfigurable system. Science 2016, 352, 61–67. [Google Scholar] [CrossRef]
- Jellicoe, M.; Gibson, C.T.; Quinton, J.S.; Raston, C.L. Coiling of Single-Walled Carbon Nanotubes via Selective Topological Fluid Flow: Implications for Sensors. ACS Appl. Nano Mater. 2022, 5, 11586–11594. [Google Scholar] [CrossRef]
- Britton, J.; Stubbs, K.A.; Weiss, G.A.; Raston, C.L. Vortex Fluidic Chemical Transformations. Chem. Eur. J. 2017, 23, 13270–13278. [Google Scholar] [CrossRef]
- Britton, J.; Raston, C.L. Multi-step continuous-flow synthesis. Chem. Soc. Rev. 2017, 46, 1250–1271. [Google Scholar] [CrossRef]
- Pu, S.; Gong, C.; Robertson, A.W. Liquid cell transmission electron microscopy and its applications. R. Soc. Open Sci. 2020, 7, 191204. [Google Scholar] [CrossRef]


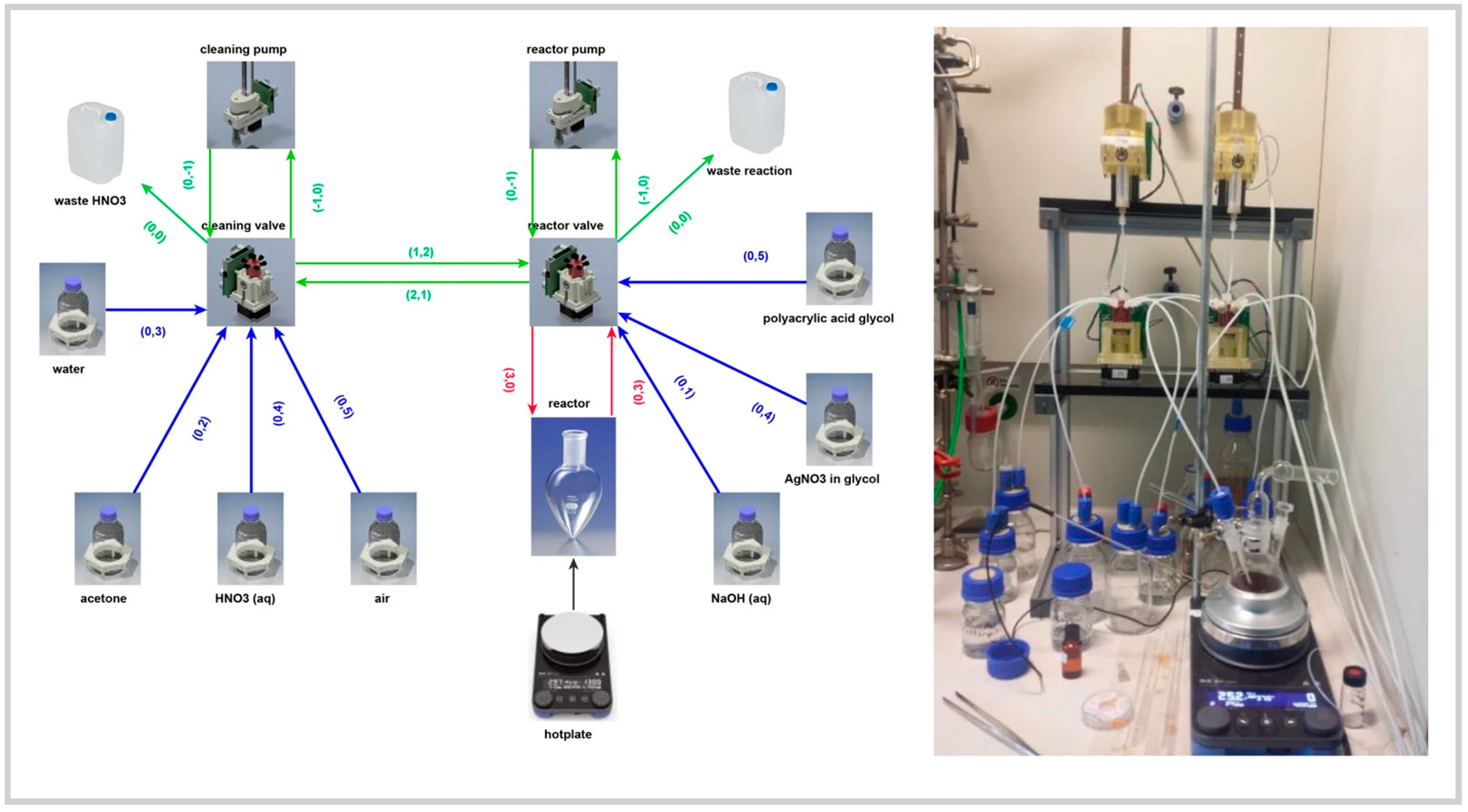
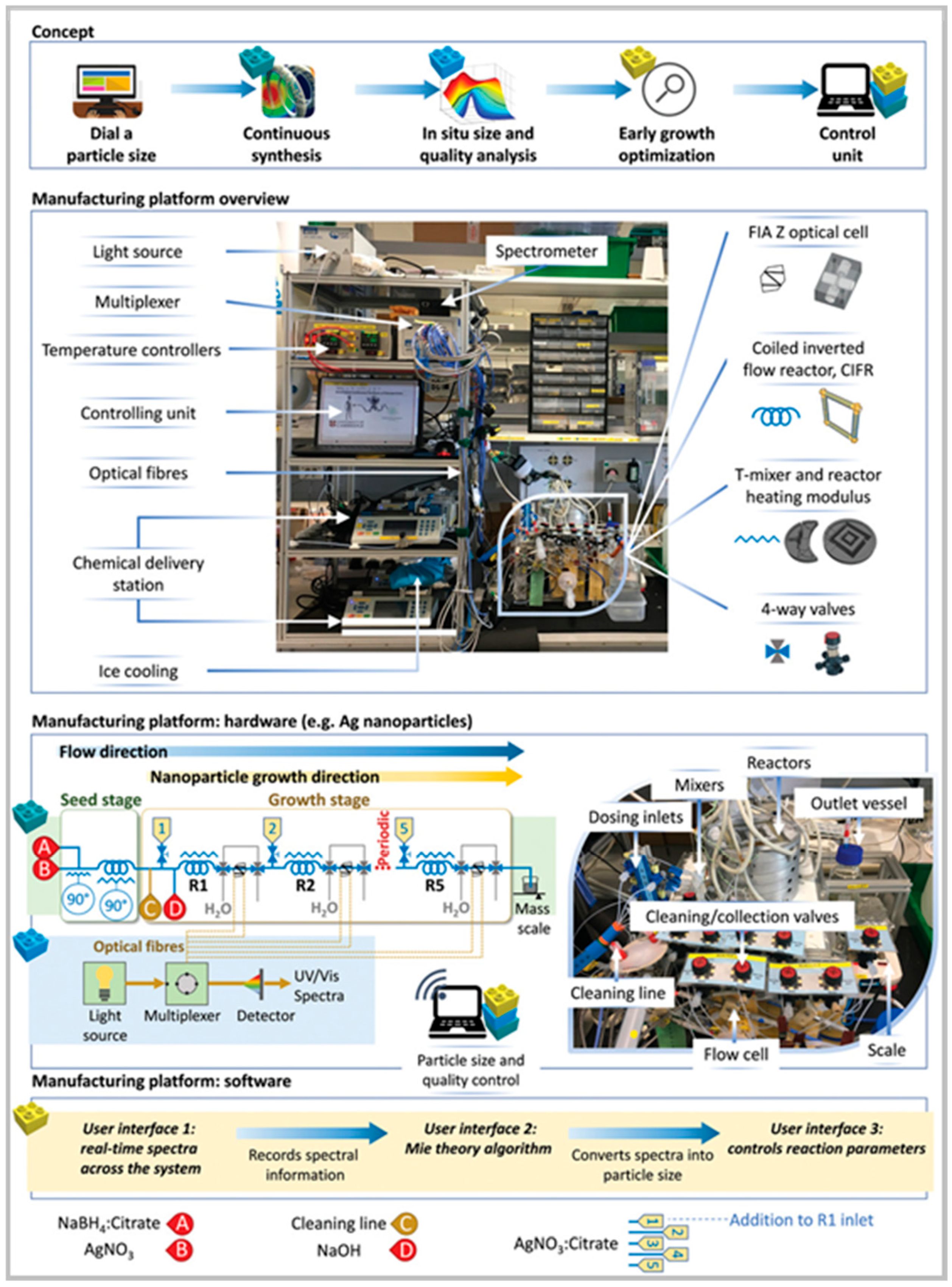

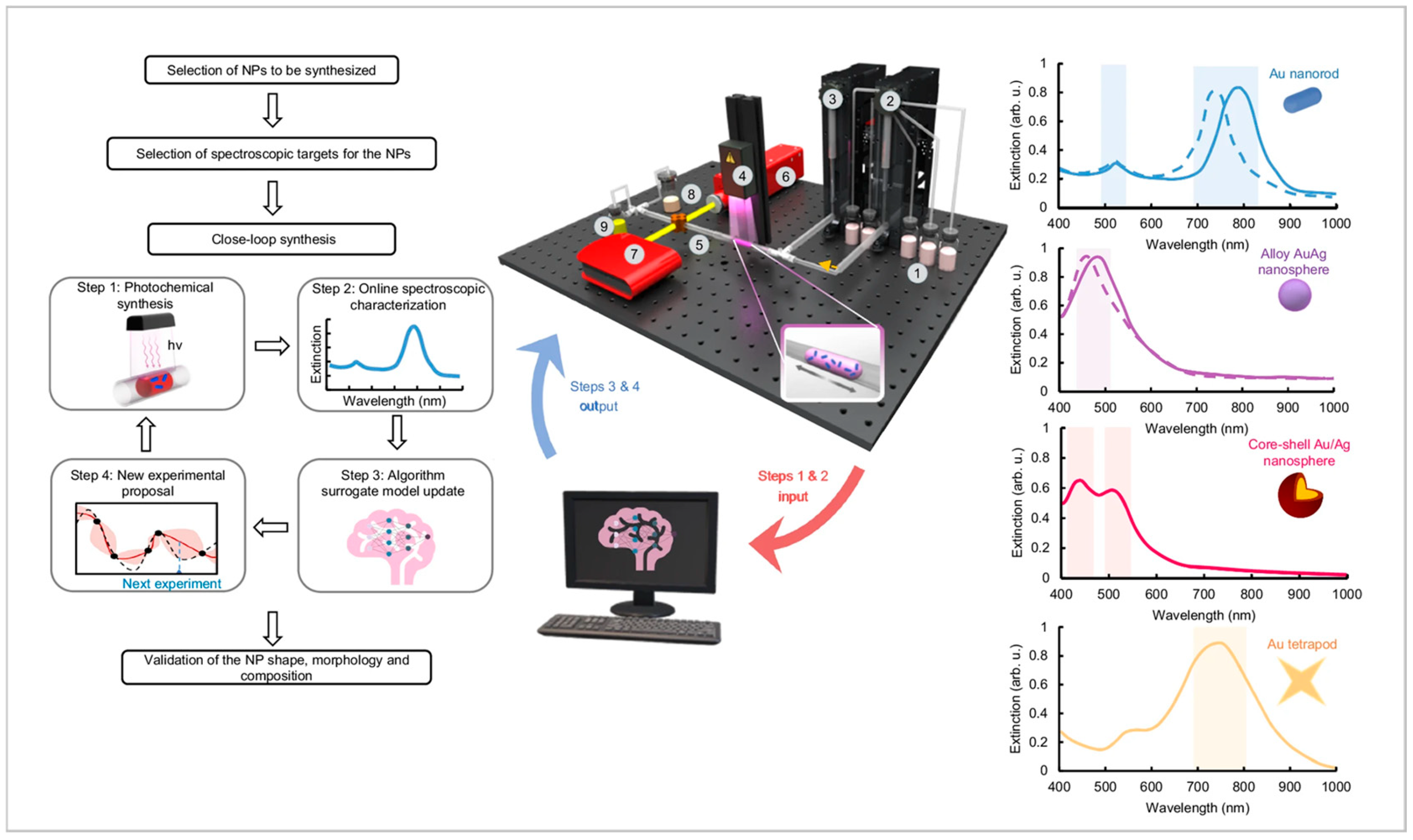
| Reactor Type | Nanoparticles (NPs) | Morphology (Size and Shape) | Analysis (Online/Offline) | Advantages | Disadvantages | Reference |
|---|---|---|---|---|---|---|
| Batch Platforms | ||||||
| Chemputer-Based Batch Reactor | Silver | 3–5 nm, Spherical | Offline (Small-angle X-ray scattering, Dynamic Light Scattering) | High precision, integration of Chemputer for automation | Relies on predefined reaction conditions, limited adaptability | Wolf et al., 2022 [27] |
| AI-Guided Batch Reactor | Silver | 18–32 nm, Spherical and Prisms | Online (UV-Vis Spectroscopy) | AI-guided self-optimization, rapid adaptation to conditions | Limited by reliance on a single analytical technique | Yoo et al., 2024 [28] |
| Genetic Algorithm Batch Reactor | Gold | 10–80 nm, Rods, octahedral structures | Online (UV-Vis Spectroscopy) | Genetic algorithm-driven self-optimization, diverse morphologies | Requires extensive experimental iterations for optimization | Salley et al., 2020 [26] |
| Machine Learning-Assisted Batch Reactor | Gold | 2–100 nm, Multiple shapes | Online (Real-time spectroscopic feedback) | High reproducibility, AI-assisted optimization | Need for additional offline validation (e.g., electron microscopy) | Jiang et al., 2022 [14] |
| Continuous Flow Platforms | ||||||
| Microfluidic Continuous Flow Reactor | Gold, Silver | 4–100 nm, Spherical | Inline (UV-Vis Spectroscopy) | Modular plug-and-play system, rapid parameter adjustments | Limited morphological insights, lacks high-resolution characterization | Pinho and Torrente-Murciano, 2021 [29] |
| Bayesian Optimization Continuous Flow Reactor | Silver | 20–70 nm, Prisms | Inline (UV-Vis Spectroscopy) | Fast, high-throughput, data-driven synthesis | Requires extensive data collection for training | Mekki-Berrada et al., 2021 [30] |
| Catalytic Continuous Flow Reactor | Gold | 11–22 nm, Spherical | Inline (UV-Vis Spectroscopy) | AI-driven optimization of catalytic efficiency, real-time feedback | Limited inline techniques for broader characterization | Hall et al., 2021 [31] |
| Self-Driving Photochemical Flow Reactor | Gold, Silver | 6–92 nm, Multiple shapes | Inline (UV-Vis Spectroscopy excitation) | Photochemical synthesis integration, AI-driven optimization | Requires sophisticated inline analytical tools | Wu et al., 2025 [32] |
| Oscillatory Microfluidics Flow Reactor | Metal Nanoparticles | 12–60 nm, Spherical | Online (Machine learning + Spectroscopy) | Fully autonomous parameter adjustment, rapid material discovery | High computational demand, complex instrumentation | Tao et al., 2021 [33] |
| Proportional–Integral Feedback Flow Reactor | Ag/Au Alloy | 40–60 nm, Cubes | Online (Proportional–Integral feedback, UV-Vis Spectroscopy) | High reproducibility, precise optical tuning | Performance depends on real-time analytical accuracy | Bui et al., 2024 [34] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Luo, T.; Chen, X.; Young, D.J.; Jellicoe, M. Recent Developments in Automated Reactors for Plasmonic Nanoparticles. Nanomaterials 2025, 15, 607. https://doi.org/10.3390/nano15080607
He S, Luo T, Chen X, Young DJ, Jellicoe M. Recent Developments in Automated Reactors for Plasmonic Nanoparticles. Nanomaterials. 2025; 15(8):607. https://doi.org/10.3390/nano15080607
Chicago/Turabian StyleHe, Shan, Tong Luo, Xiao’e Chen, David James Young, and Matt Jellicoe. 2025. "Recent Developments in Automated Reactors for Plasmonic Nanoparticles" Nanomaterials 15, no. 8: 607. https://doi.org/10.3390/nano15080607
APA StyleHe, S., Luo, T., Chen, X., Young, D. J., & Jellicoe, M. (2025). Recent Developments in Automated Reactors for Plasmonic Nanoparticles. Nanomaterials, 15(8), 607. https://doi.org/10.3390/nano15080607







