Facile Immunoassay Constructed by Gold Nanostar-Labeled Rabbit-AFP Antibody and Gold Nanoparticle-Conjugated Goat Anti-Rabbit IgG
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagent and Apparatus
2.2. GNSs’ Synthesis [23]
2.3. Gold Nanoparticles’ Synthesis
2.4. Labeling of Antibodies
2.5. Combination of Antibody and Antigen
2.6. Sensitivity, Reproducibility, Accuracy and Specificity
2.7. Real Serum Application and Comparison of Methodology
3. Results and Discussions
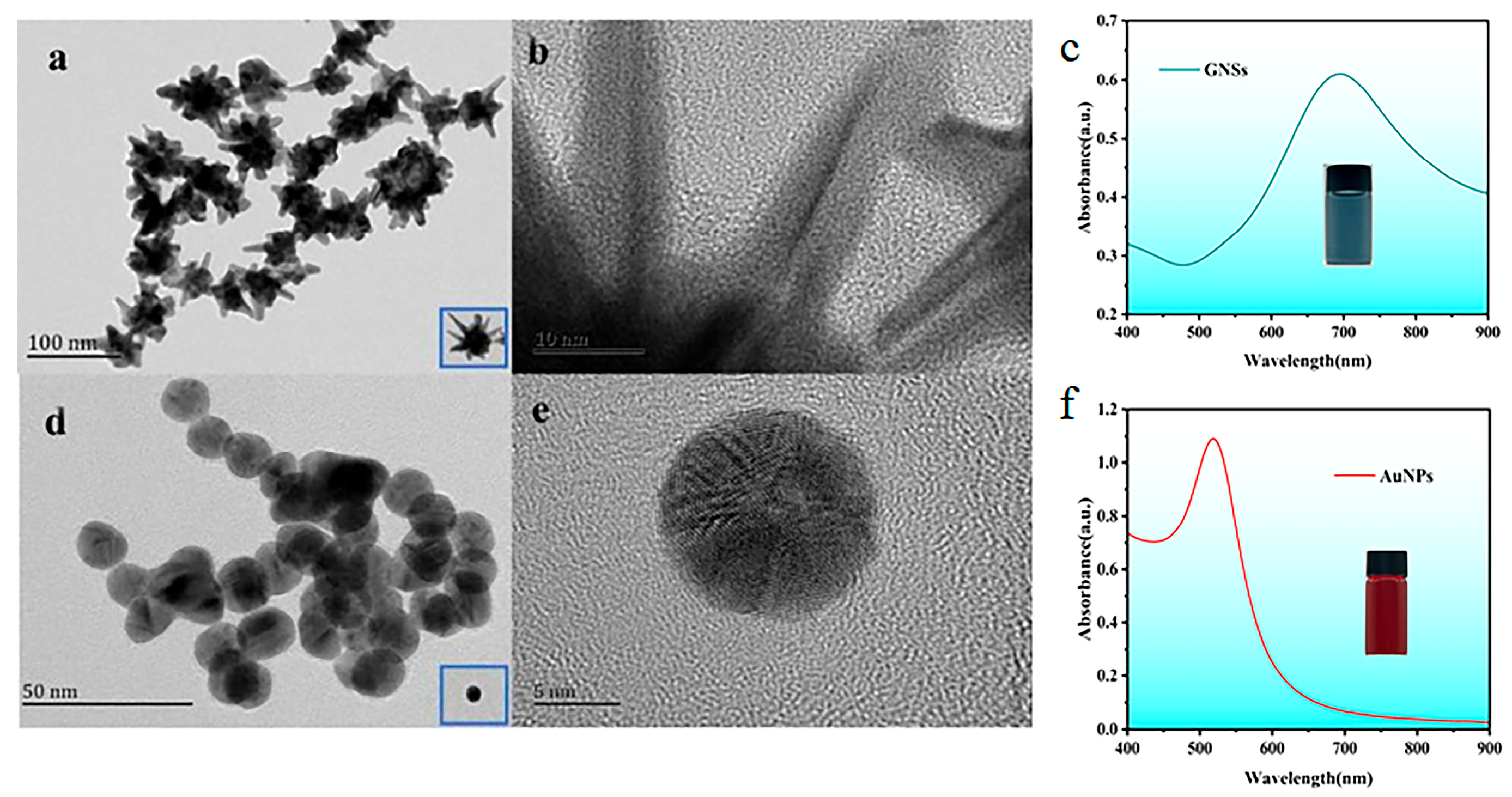
3.1. Synthesis and Characterization of Nanomaterials
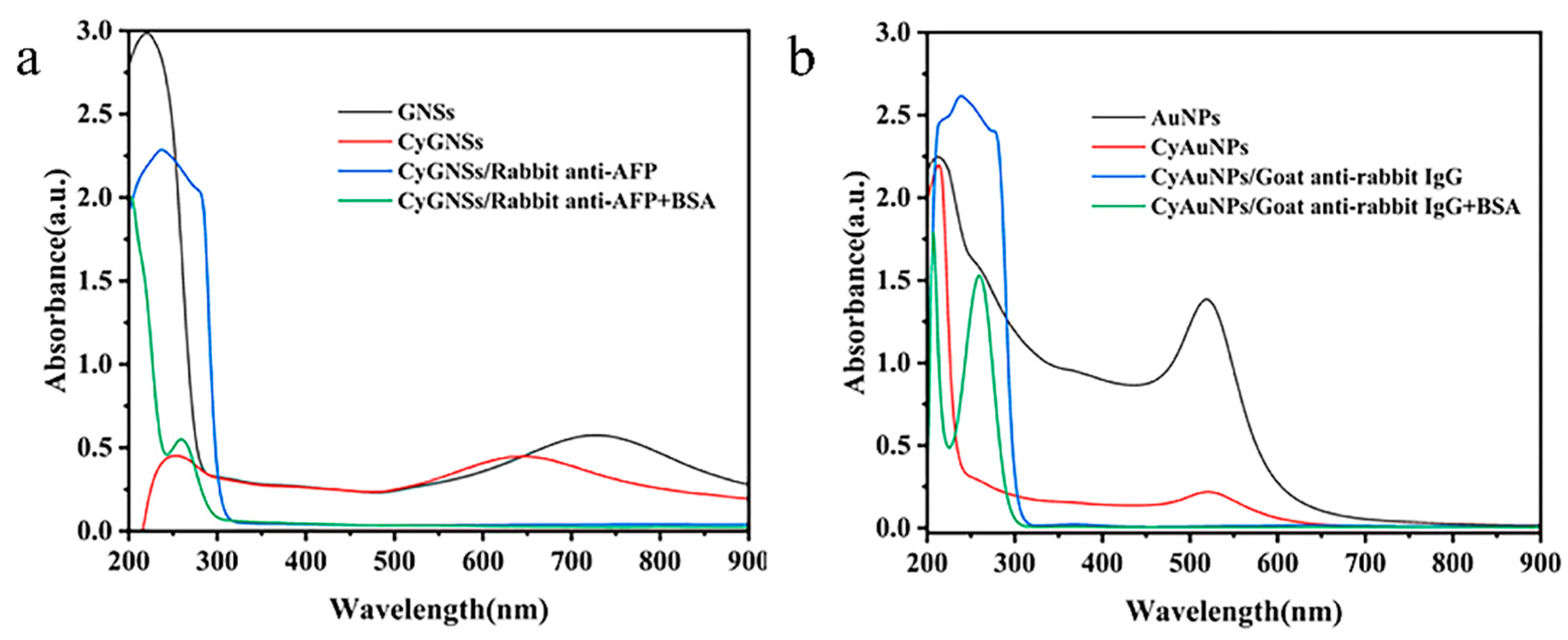
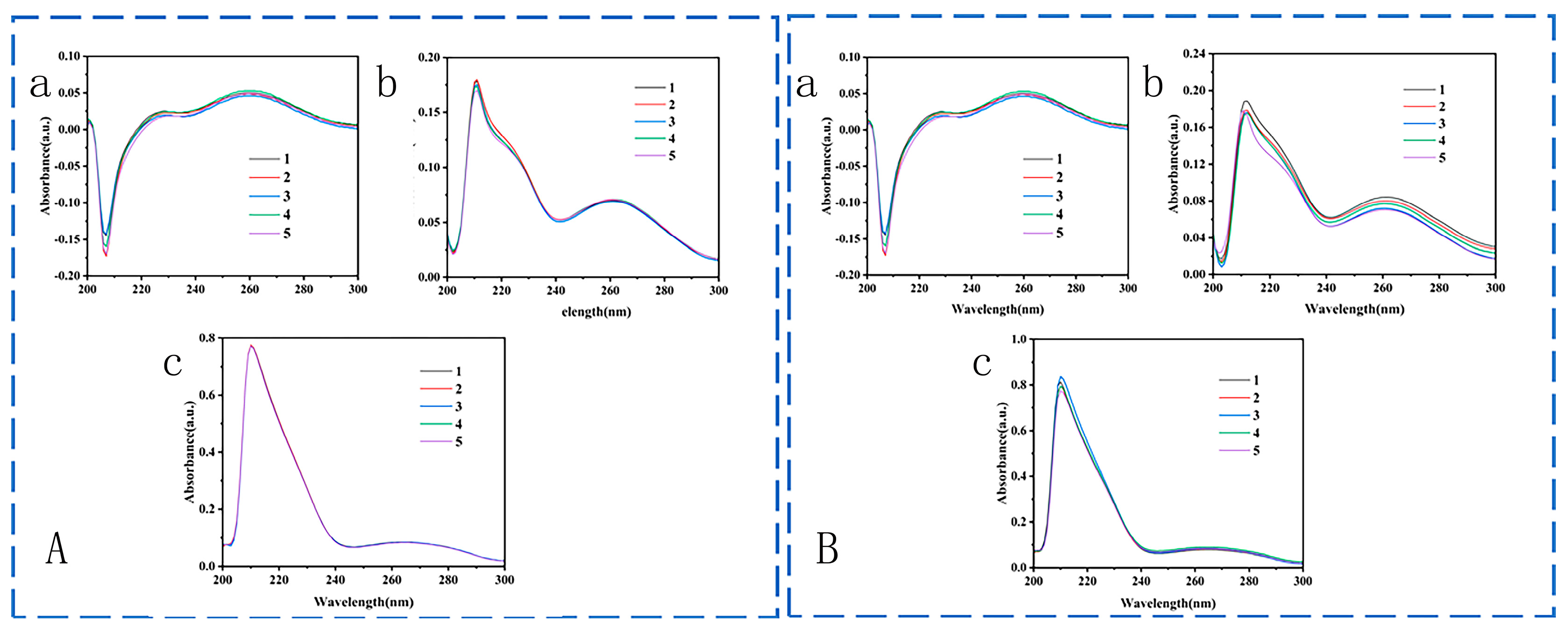
3.2. Preparation of Capture Antibody and Enhance Antibody
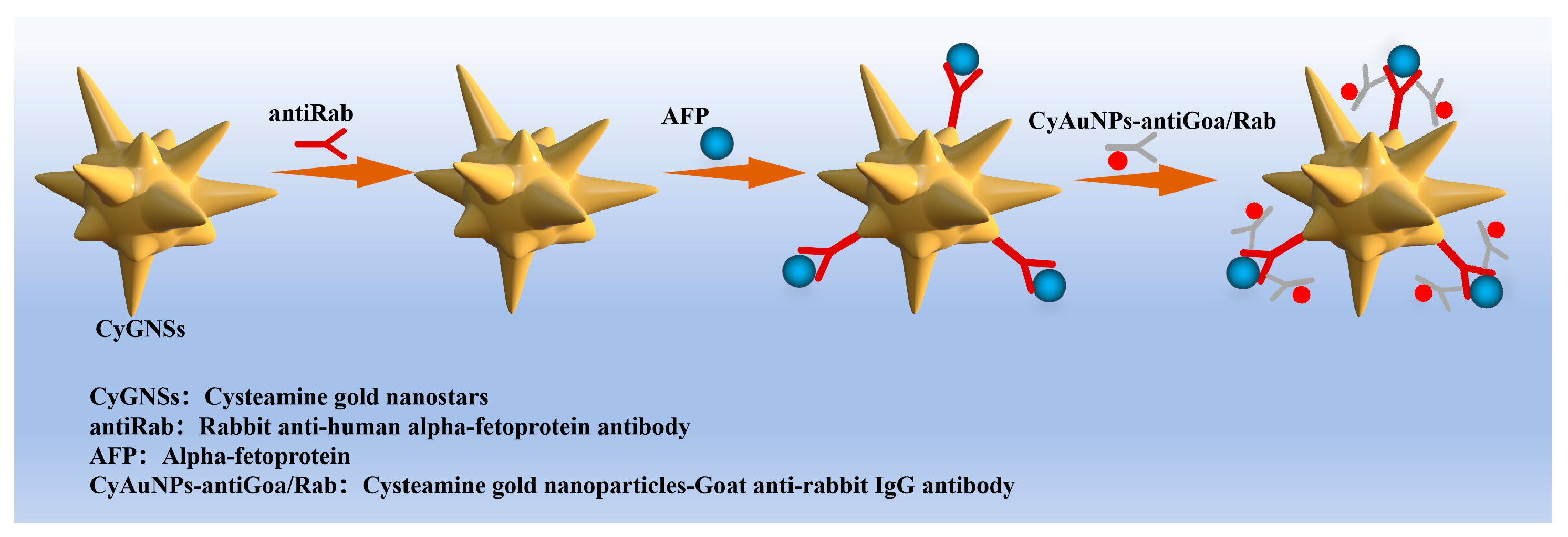
3.3. Establishment of Immunoassay
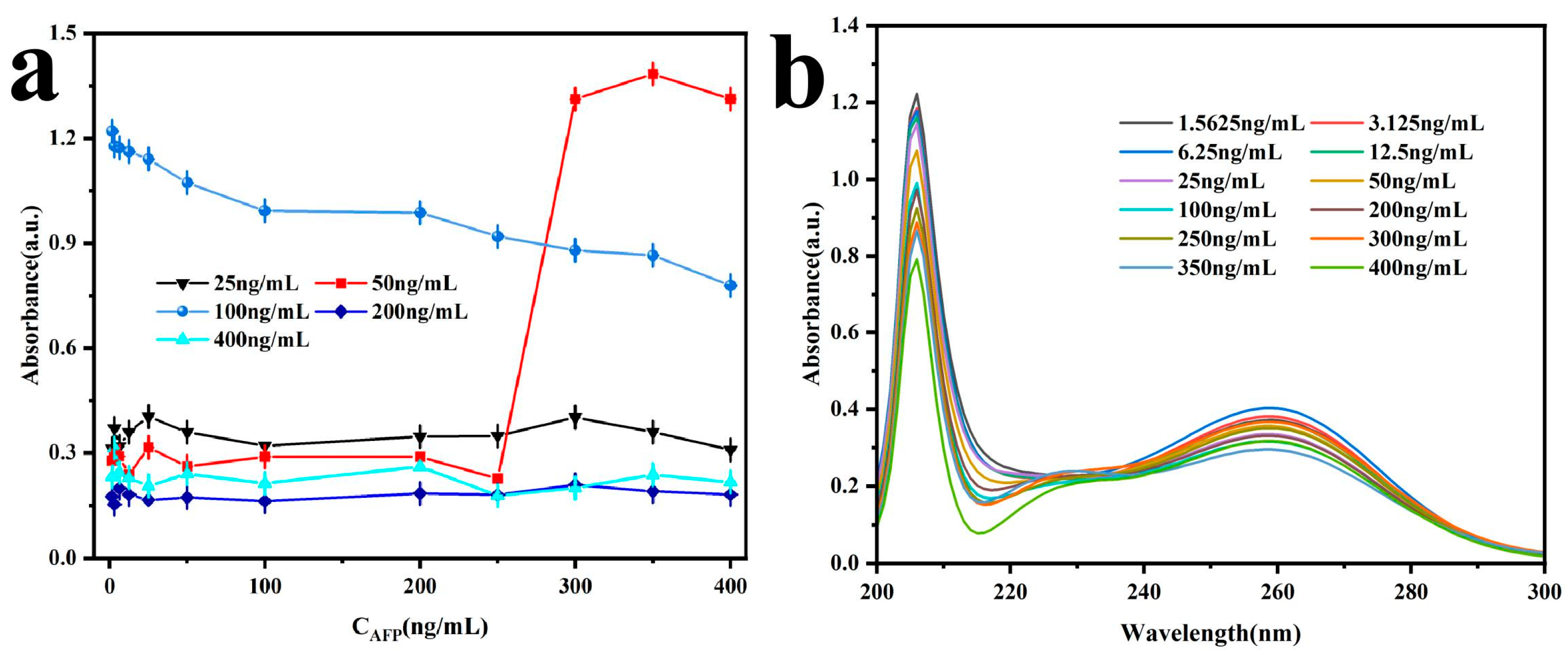
3.3.1. AFP Direct Detection
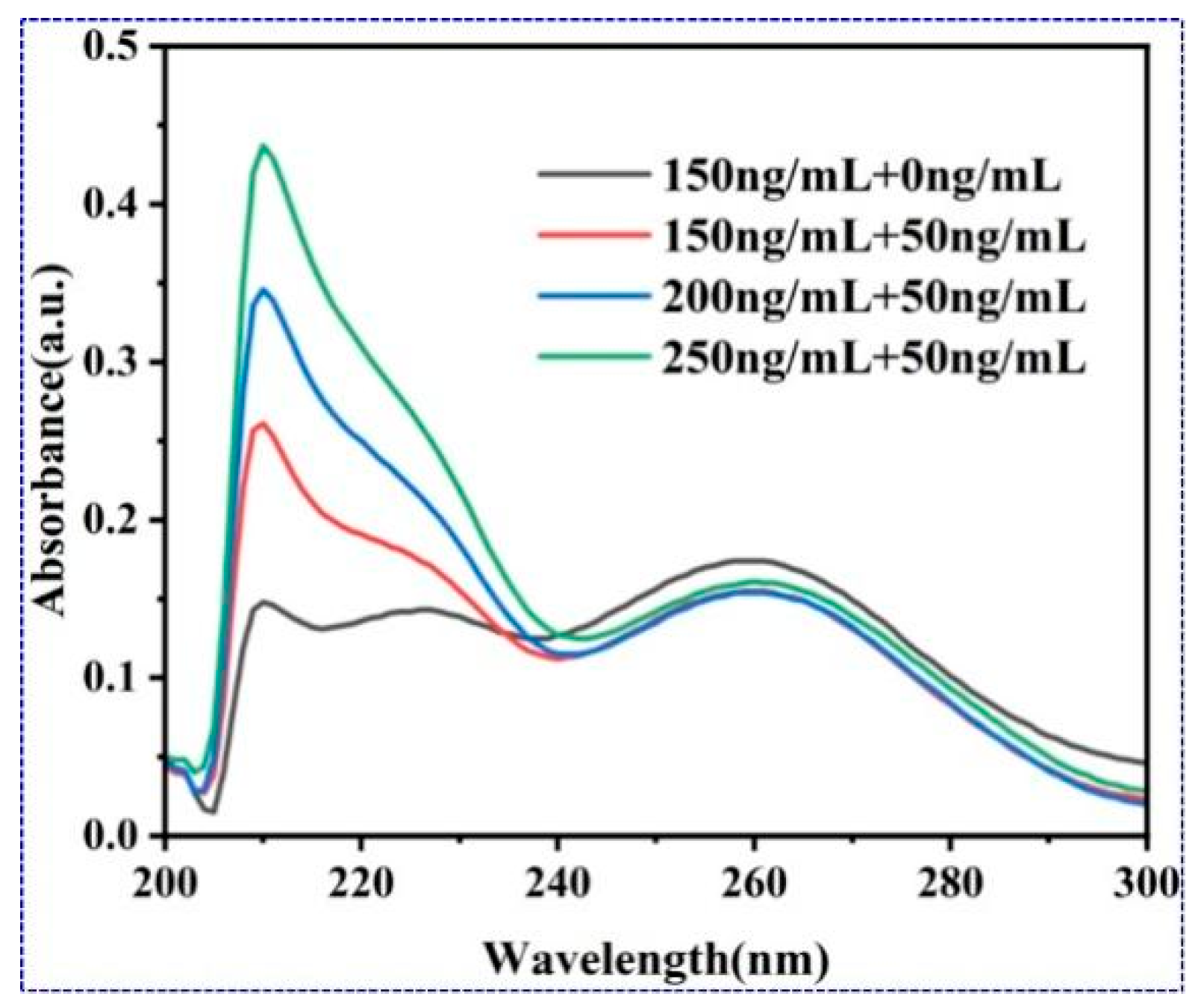
3.3.2. AFP Detection Through Signal Amplification by Enhance Antibody
3.4. Characterization of Analyze Performance
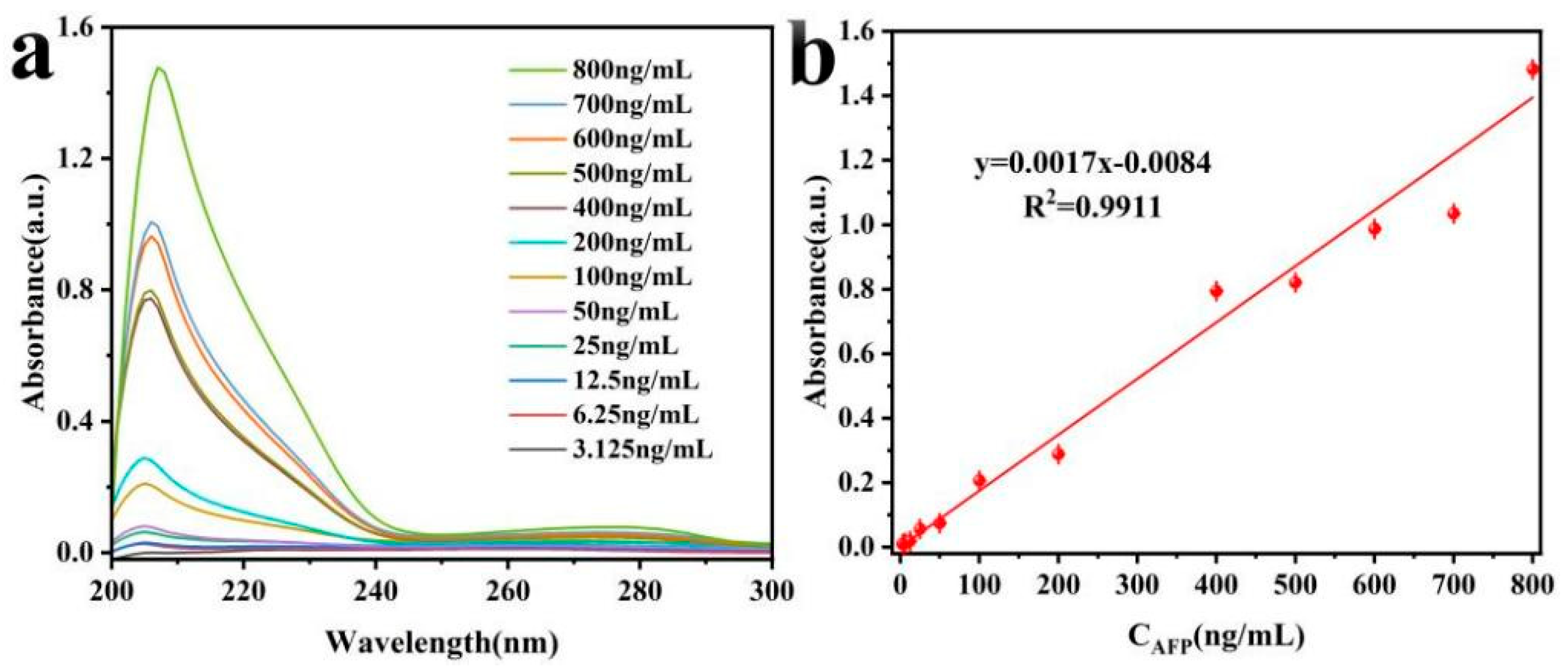
3.4.1. Sensitivity and Detection Range
3.4.2. Precision and Accuracy
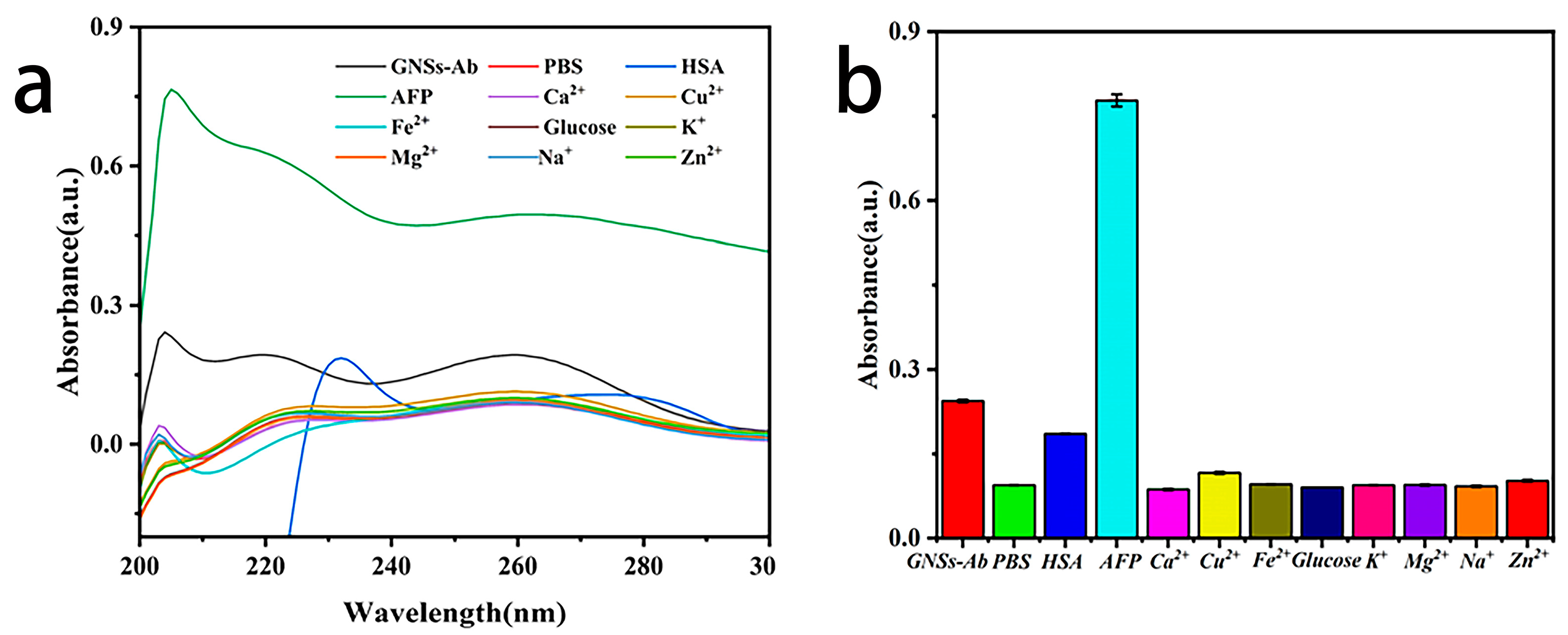
3.4.3. Specificity
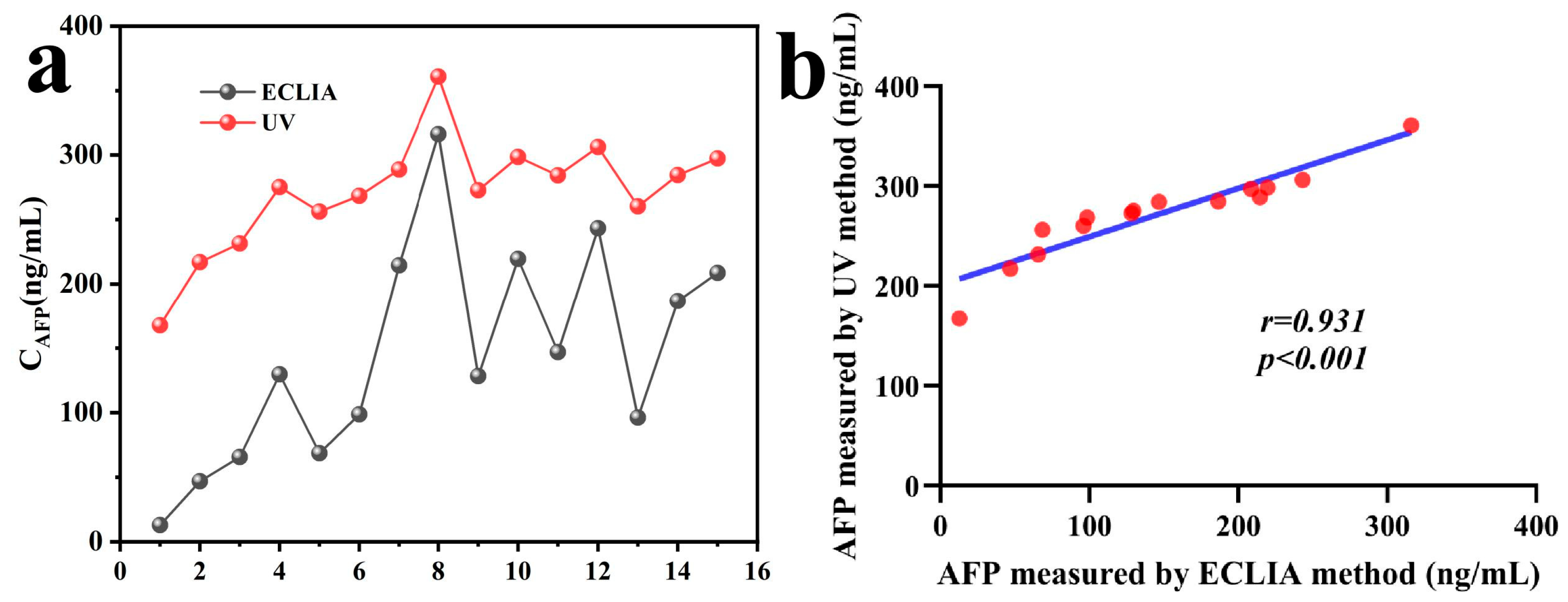
3.5. Methodological Comparison
3.6. Future Work
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bisen, P.S. Cancer Therapy: An Overview. J. Cancer Sci. Ther. 2013, 6, 130. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.-R.; Chen, H.-S.; Mou, D.-C.; Cao, J.; Cong, X.; Qin, L.-L.; Wei, L.; Leng, X.-S.; Wang, Y.; Chen, W.-F. Expression of cancer/testis (CT) antigens in Chinese hepatocellular carcinoma and its correlation with clinical parameters. Cancer Lett. 2005, 219, 223–232. [Google Scholar] [CrossRef]
- Xu, X.; Ying, Y.; Li, Y. Gold Nanorods Based LSPR Biosensor for Label-Free Detection of Alpha-Fetoprotein. Procedia Eng. 2011, 25, 67–70. [Google Scholar] [CrossRef]
- Huang, X.; El-Sayed, I.H.; Qian, W.; El-Sayed, M.A. Cancer Cells Assemble and Align Gold Nanorods Conjugated to Antibodies to Produce Highly Enhanced, Sharp, and Polarized Surface Raman Spectra: A Potential Cancer Diagnostic Marker. Nano Lett. 2007, 7, 1591–1597. [Google Scholar] [CrossRef]
- Wang, Y.; Zeng, S.; Crunteanu, A.; Xie, Z.; Humbert, G.; Ma, L.; Wei, Y.; Brunel, A.; Bessette, B.; Orlianges, J.-C.; et al. Targeted Sub-Attomole Cancer Biomarker Detection Based on Phase Singularity 2D Nanomaterial-Enhanced Plasmonic Biosensor. Nano-Micro Lett. 2021, 13, 96. [Google Scholar] [CrossRef] [PubMed]
- Cancelliere, R.; Paialunga, E.; Grattagliano, A.; Micheli, L. Label-free electrochemical immunosensors: A practical guide. Trends Anal. Chem. 2024, 180, 117949. [Google Scholar] [CrossRef]
- Shen, Q.; Ding, J.; Guo, Z.; Wang, C.; Zhang, Y.; Lin, C.; Sun, Y.; Hang, L. Sandwich-type electrochemical immunosensor based on Au NPs/3D hierarchical porous carbon network and Au NPs/Cu9S8 nanocages for the detection of alpha-fetoprotein. Colloids Surf. B Biointerfaces 2025, 248, 114471. [Google Scholar] [CrossRef]
- Chen, X.; Dai, Q.; Qiu, X.; Luo, X.; Li, Y. New nanosensor fabricated on single nanopore electrode filled with prussian blue and graphene quantum dots coated by polypyrrole for hydrogen peroxide sensing. Talanta 2024, 274, 126043. [Google Scholar] [CrossRef]
- Shui, Z.; Zhao, J.; Zheng, J.; Luo, H.; Ma, Y.; Hou, C.; Huo, D. Pattern-based colorimetric sensor array chip for discrimination of Baijiu aromas. Food Chem. 2024, 446, 138845. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Xiong, S.; Li, X.; Zhan, S.; Zeng, L.; Xiong, Y. Dual-mode fluorescent and colorimetric immunoassay for the ultrasensitive detection of alpha-fetoprotein in serum samples. Anal. Chim. Acta 2018, 1038, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Lee, S.; Liao, H.; Rostro, B.C.; Fuentes, A.; Scully, T.P.; Nehl, C.L.; Hafner, J.H. A Label-Free Immunoassay Based Upon Localized Surface Plasmon Resonance of Gold Nanorods. ACS Nano 2008, 2, 687–692. [Google Scholar] [CrossRef]
- Huang, H.; Tang, C.; Zeng, Y.; Yu, X.; Liao, B.; Xia, X.; Yi, P.; Paul, K.C. Label-free optical biosensor based on localized surface plasmon resonance of immobilized gold nanorods. Colloids Surf. B Biointerfaces 2009, 71, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Lu, D.; You, R.; Shen, H.; Zhu, L.; Lin, Q.; Lu, Y. Surface-Enhanced Raman Scattering Biosensor Based on Self Assembled Gold Nanorod Arrays for Rapid and Sensitive Detection of Tyrosinase. J. Phys. Chem. C 2022, 126, 12651–12659. [Google Scholar] [CrossRef]
- Li, N.-S.; Lin, W.-L.; Hsu, Y.-P.; Chen, Y.-T.; Shiue, Y.-L.; Yang, H.-W. Combined Detection of CA19−9 and MUC1 Using a Colorimetric Immunosensor Based on Magnetic Gold Nanorods for Ultrasensitive Risk Assessment of Pancreatic Cancer. ACS Appl. Bio Mater. 2019, 2, 4847–4855. [Google Scholar] [CrossRef]
- Kou, X.; Sun, Z.; Yang, Z.; Chen, H.; Wang, J. Curvature-directed assembly of gold nanocubes, nanobranches, and nanospheres. Langmuir 2009, 25, 1692–1698. [Google Scholar] [CrossRef]
- Hassan, H.; Sharma, P.; Hasan, M.R.; Singh, S.; Thakur, D.; Narang, J. Gold nanomaterials—The golden approach from synthesis to applications. Mater. Sci. Energy Technol. 2022, 5, 375–390. [Google Scholar] [CrossRef]
- Rodríguez-Lorenzo, L.; de la Rica, R.; Álvarez-Puebla, R.A.; Liz-Marzán, L.M.; Stevens, M.M. Plasmonic nanosensors with inverse sensitivity by means of enzyme-guided crystal growth. Nat. Mater. 2012, 11, 604–607. [Google Scholar] [CrossRef]
- Li, J.; Cai, R.; Kawazoea, N.; Chen, G. Facile preparation of albumin-stabilized gold nanostars for the targeted photothermal ablation of cancer cells. J. Mater. Chem. B 2015, 3, 5806–5814. [Google Scholar] [CrossRef]
- Dondapati, S.K.; Sau, T.K.; Hrelescu, C.; Klar, T.A.; Stefani, F.D.; Feldmann, J. Label-free biosensing based on single gold nanostars as plasmonic transducers. ACS Nano 2010, 4, 6318–6322. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, Z.; Zong, S.; Cui, Y. Highly sensitive SERS-based immunoassay with simultaneous utilization of self-assembled substrates of gold nanostars and aggregates of gold nanostars. J. Mater. Chem. B 2013, 1, 3992–3998. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Shiraki, T.; Niki, M.; Sato, S.; Tanaka, G.; Shimizu, T.; Yamaguchi, T.; Park, K.-H.; Mori, S.; Iso, Y.; et al. Proposal of an integrated staging system using albumin-bilirubin grade and serum alpha-fetoprotein values for predicting postoperative prognosis of recurrent hepatocellular carcinoma. Eur. J. Surg. Oncol. 2024, 50, 108356. [Google Scholar] [CrossRef]
- Yuan, H.; Khoury, C.G.; Hwang, H.; Wilson, C.M.; Grant, G.A.; Vo-Dinh, T. Gold nanostars: Surfactant-free synthesis, 3D modelling, and two-photon photoluminescence imaging. Nanotechnology 2012, 23, 075102. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.; Won, N.; Jin, H.; Chung, H.; Kim, S. pH-Induced Aggregation of Gold Nanoparticles for Photothermal Cancer Therapy. J. Am. Chem. Soc. 2009, 131, 13639–13645. [Google Scholar] [CrossRef]
- Shao, L.; Susha, A.S.; Cheung, L.S.; Sau, T.K.; Rogach, A.L.; Wang, J. Plasmonic Properties of Single Multispiked Gold Nanostars: Correlating Modeling with Experiments. Langmuir 2012, 28, 8979–8984. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, I.G.; Ruenraroengsak, P.; Gonzalez-Carter, D.A.; Jiang, Q.; Yagüe, E.; Aboagye, E.O.; Coombes, R.C.; Porter, A.E.; Ryana, M.P.; Xie, F. Towards multiplexed near-infrared cellular imaging using gold nanostar arrays with tunable fluorescence enhancement. Nanoscale 2019, 11, 2079–2088. [Google Scholar] [CrossRef]
- Jiang, S.; Tang, H.; Fang, Y.; Ma, C.; Chen, H.; Huang, X.; Men, R.; Gao, J.; Huang, C. AuNPs labeled antisera improve the visualization of direct agglutination and sensitivity of agar double immunodiffusion test. Ferroelectrics 2022, 595, 117–125. [Google Scholar] [CrossRef]
- Huang, C.; Chen, Y.; Wang, C.; Zhu, W.; Ma, H.; Jin, G. Detection of alpha-fetoprotein through biological signal amplification by biosensor based on imaging ellipsometry. Thin Solid Film. 2011, 519, 2763–2767. [Google Scholar] [CrossRef]
- Cheon, G.; Hwang, D.; Le, T.C.; Lee, Y.; Han, E.; An, S.; Jung, Y.; Chung, H.; Lee, S. Crystal structure of an antibody specifically recognizing 3,4-methyl enedioxy methamphetamine through the epoxide moiety. Biochem. Biophys. Res. Commun. 2024, 733, 150607. [Google Scholar] [CrossRef]
- Luo, L.; Luo, S.-Z.; Jia, B.-Z.; Zhang, W.-F.; Wang, H.; Wei, X.-Q.; Shen, Y.-D.; Lei, H.-T.; Xu, Z.-L.; Yang, J.-Y. A high-resolution colorimetric immunoassay for tyramine detection based on enzyme-enabled growth of gold nanostar coupled with smartphone readout. Food Chem. 2022, 396, 133729. [Google Scholar] [CrossRef]
- Nehl, C.L.; Liao, H.; Hafner, J.H. Optical Properties of Star-Shaped Gold Nanoparticles. Nano Lett. 2006, 6, 683–688. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Yang, F.; Lu, X.; Li, H.; Yang, Z.; Yin, Q.; Zhang, L.; Long, Y.; Shen, C.; Chen, L.; et al. Facile Immunoassay Constructed by Gold Nanostar-Labeled Rabbit-AFP Antibody and Gold Nanoparticle-Conjugated Goat Anti-Rabbit IgG. Nanomaterials 2025, 15, 612. https://doi.org/10.3390/nano15080612
Yang K, Yang F, Lu X, Li H, Yang Z, Yin Q, Zhang L, Long Y, Shen C, Chen L, et al. Facile Immunoassay Constructed by Gold Nanostar-Labeled Rabbit-AFP Antibody and Gold Nanoparticle-Conjugated Goat Anti-Rabbit IgG. Nanomaterials. 2025; 15(8):612. https://doi.org/10.3390/nano15080612
Chicago/Turabian StyleYang, Kang, Fang Yang, Xiaoling Lu, Hao Li, Zeng Yang, Qi Yin, Lin Zhang, You Long, Chao Shen, Liya Chen, and et al. 2025. "Facile Immunoassay Constructed by Gold Nanostar-Labeled Rabbit-AFP Antibody and Gold Nanoparticle-Conjugated Goat Anti-Rabbit IgG" Nanomaterials 15, no. 8: 612. https://doi.org/10.3390/nano15080612
APA StyleYang, K., Yang, F., Lu, X., Li, H., Yang, Z., Yin, Q., Zhang, L., Long, Y., Shen, C., Chen, L., Yao, B., & Huang, C. (2025). Facile Immunoassay Constructed by Gold Nanostar-Labeled Rabbit-AFP Antibody and Gold Nanoparticle-Conjugated Goat Anti-Rabbit IgG. Nanomaterials, 15(8), 612. https://doi.org/10.3390/nano15080612




