Flexible Solar Interfacial Evaporators with Photocatalytic Function for Purification of High-Salinity Organic Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of g-C3N4
2.3. Fabrication of PCG Evaporators
2.4. Interfacial Solar Evaporation Performance Tests
2.5. MB Removal Experiment Tests
3. Results and Discussion
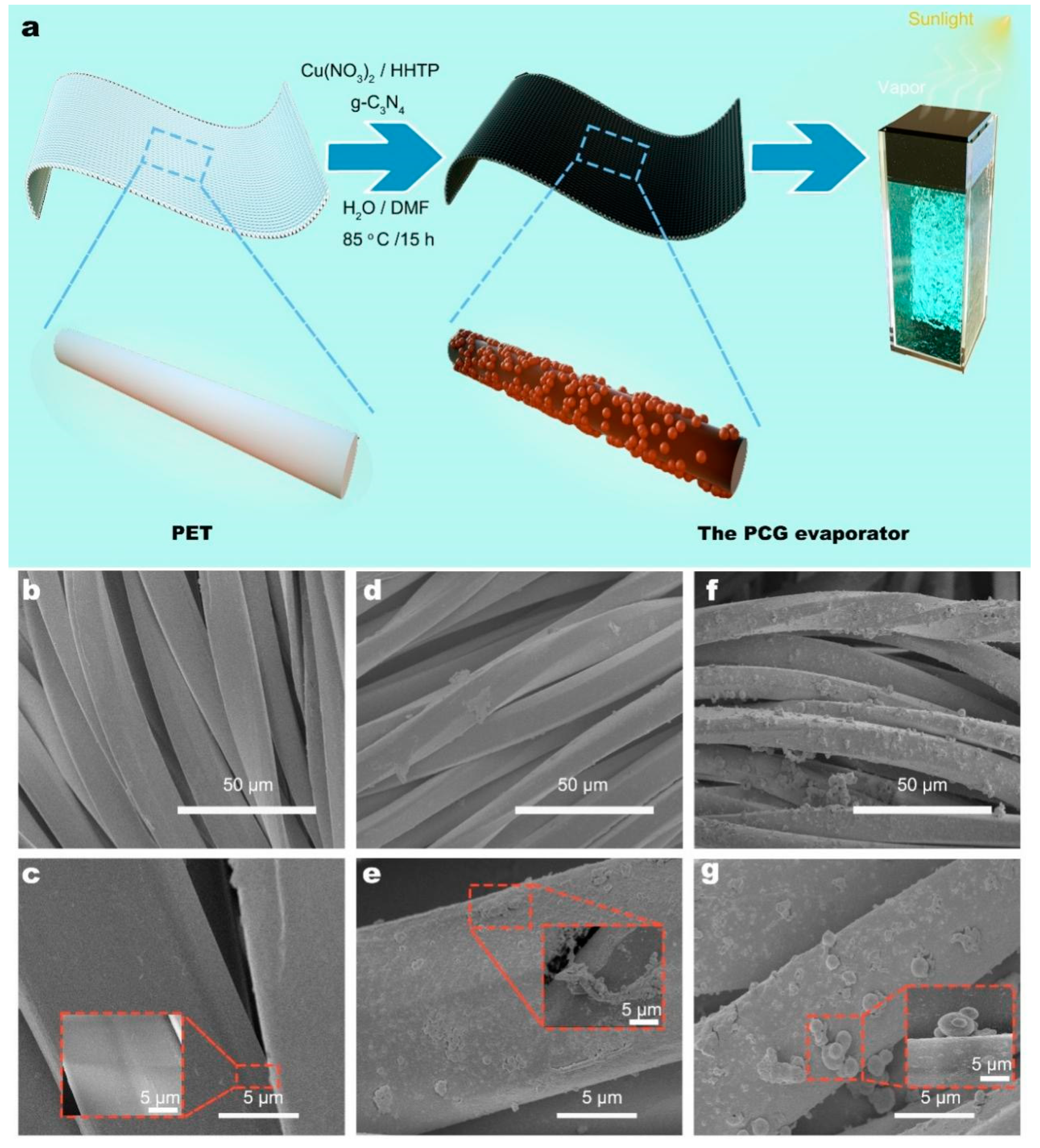
3.1. Fabrication of PCG Evaporators
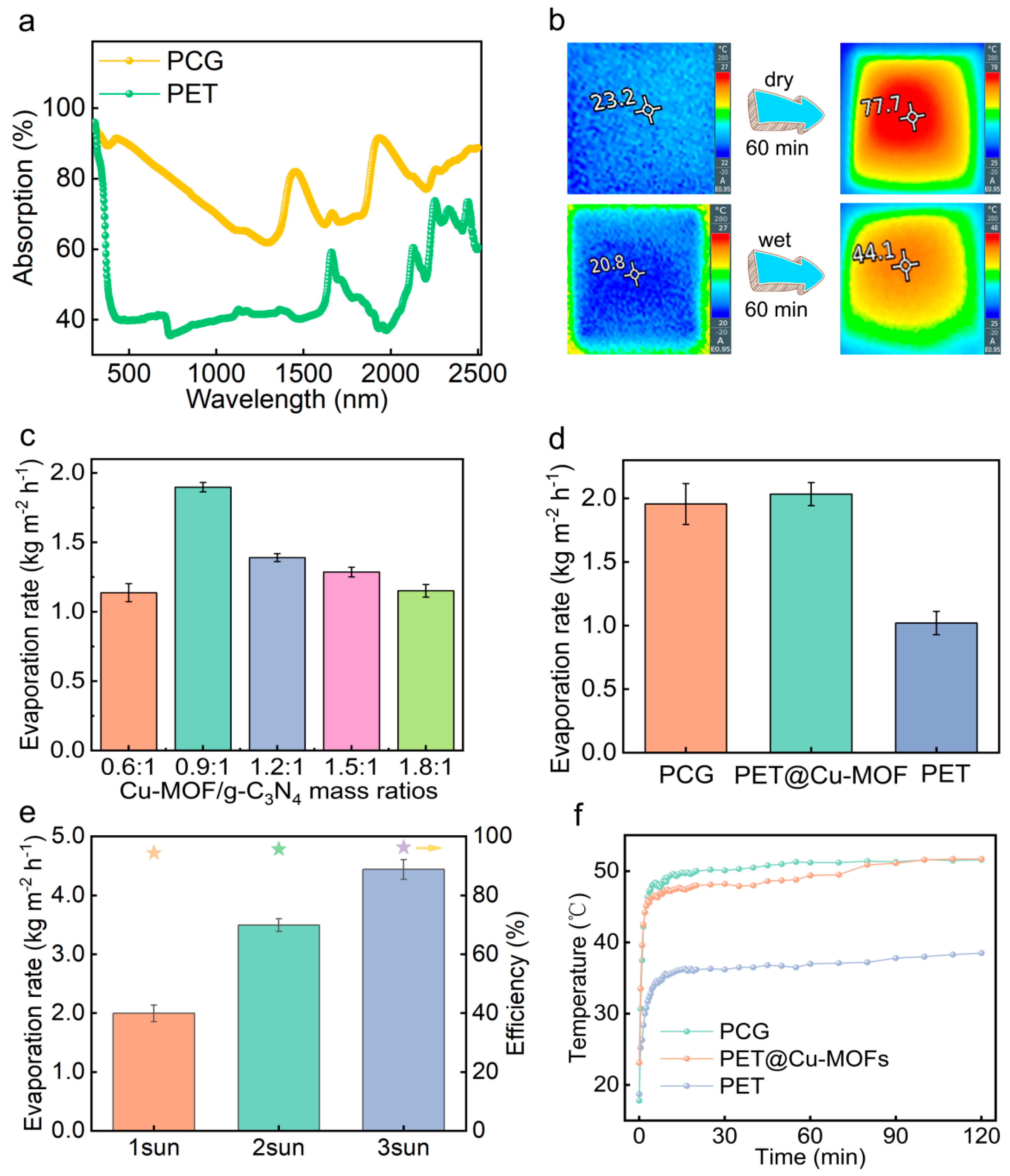
3.2. Solar Absorption and Photothermal Effect of the PCG Evaporators
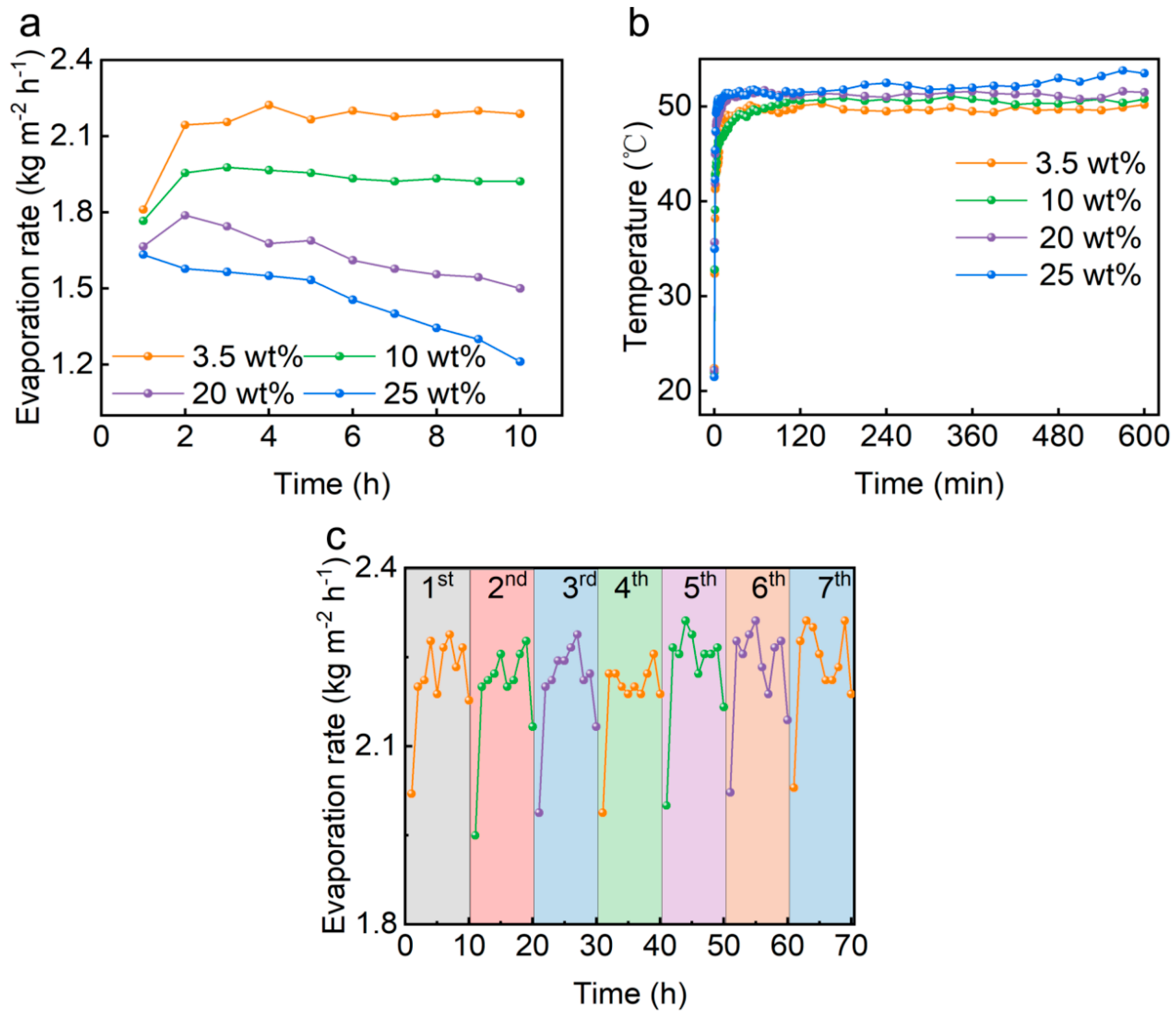
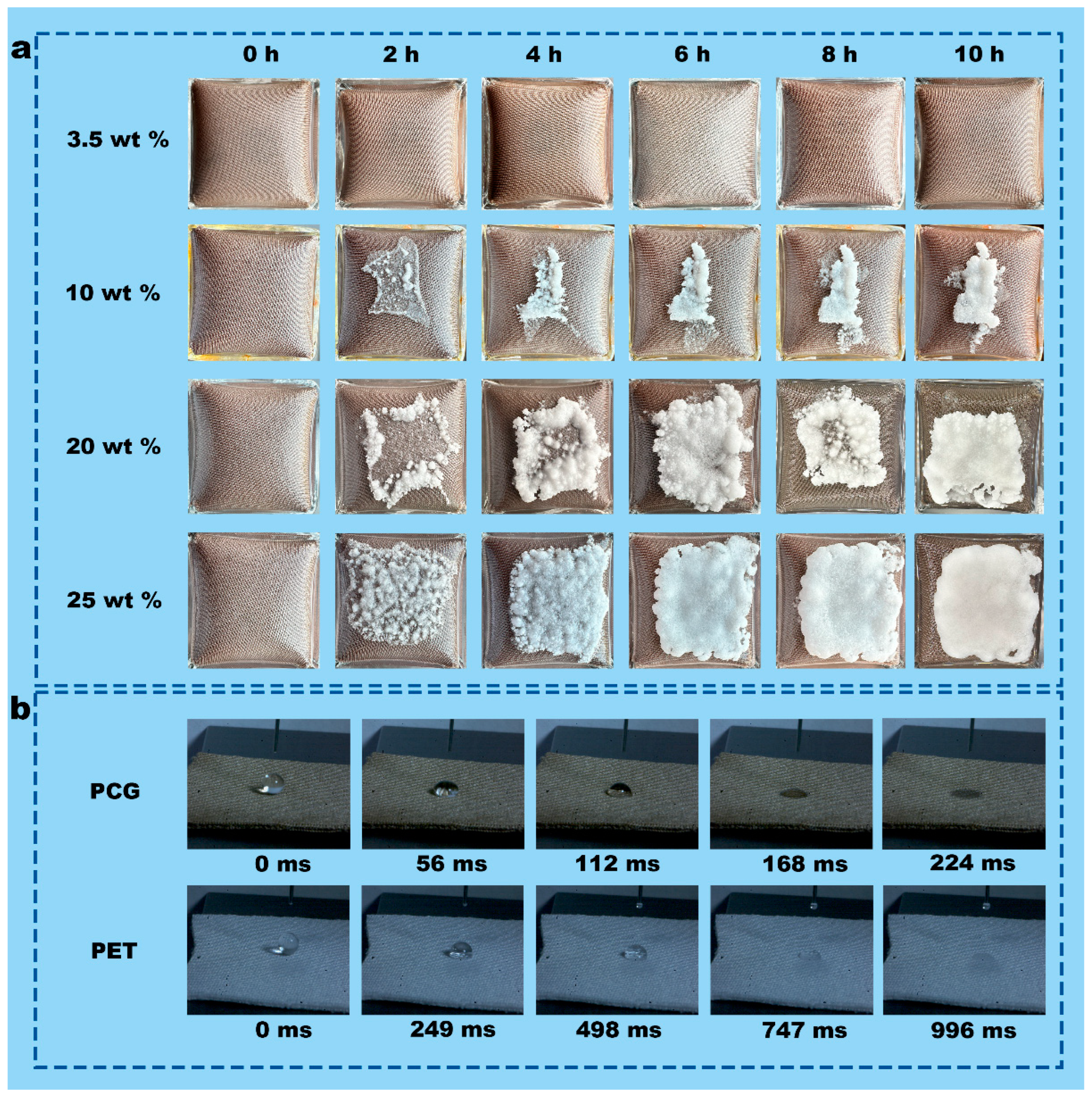
3.3. Salt Resistance of PCG Evaporators
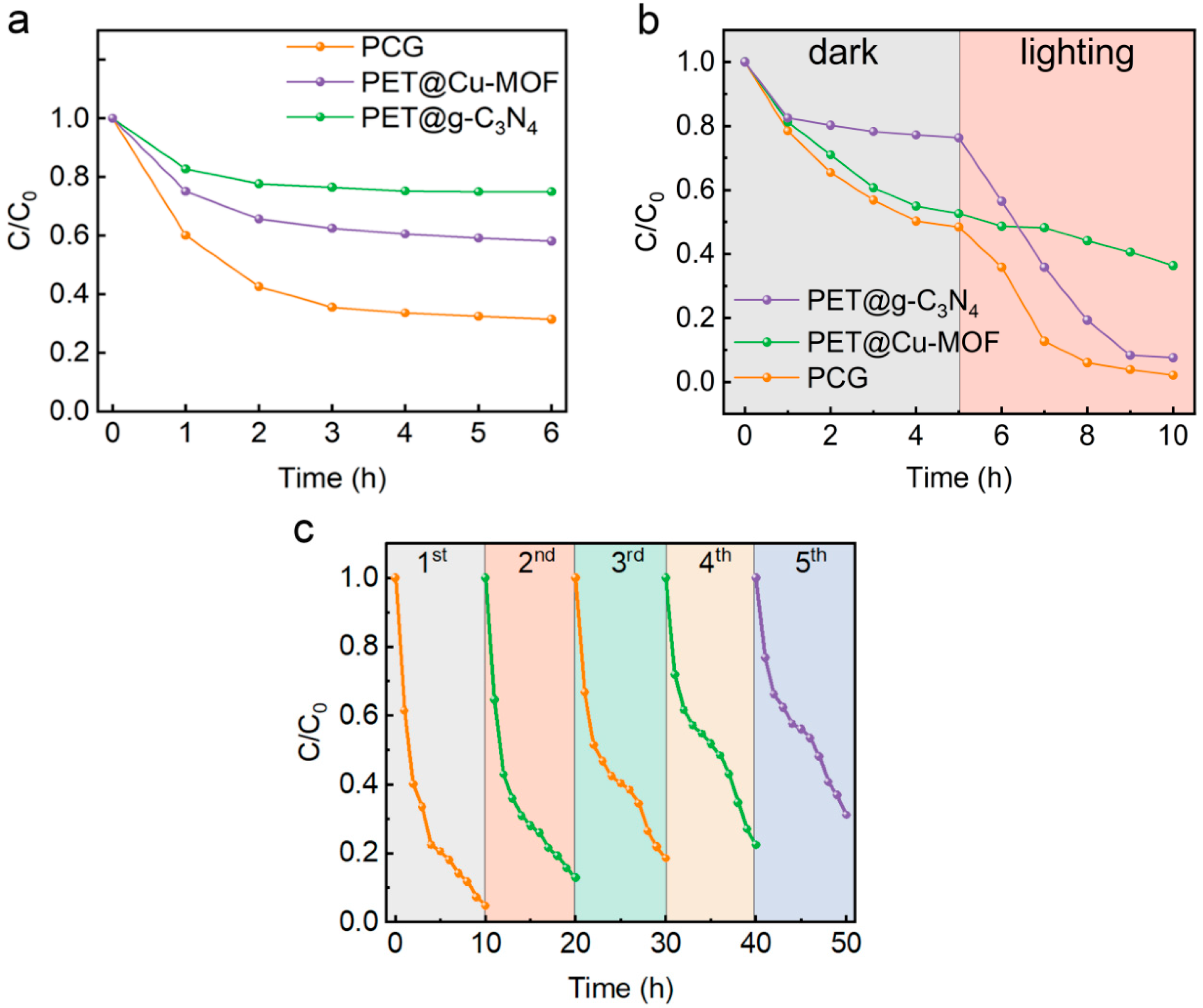
3.4. Photocatalytic Degradation of MB with Simulated Sunlight
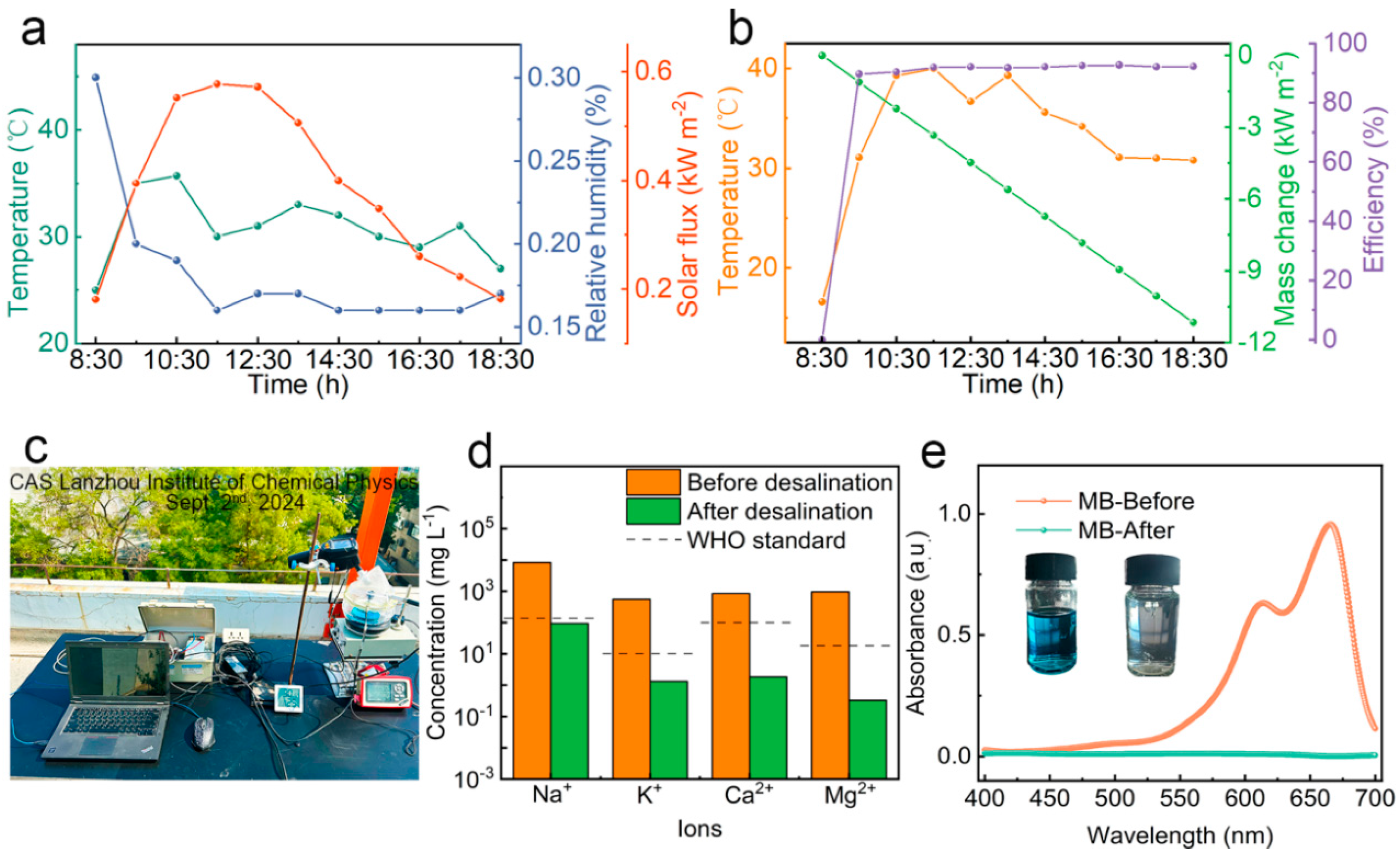
3.5. Freshwater Collection by Simultaneous Solar Evaporation and Photocatalytic Degradation in Outdoor Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.; Naila, A.; Chen, H.; Muhammad, S.I.; Naveed, M.; Shahid, H.; Matiullah, S.; Syed, Z.H.N.; Muhammad, R.; Naeem, S.; et al. Interfacial Photothermal Heat Accumulation for Simultaneous Salt Rejection and Freshwater Generation; an Efficient Solar Energy Harvester. Nanomaterials 2022, 12, 3206. [Google Scholar] [CrossRef] [PubMed]
- Miao, H.; Muneerah, A.; Areej, S.A.; Naila, A.; Muhammad, A.; Muhammad, Y.; Muhammad, S.I.Y.-Z.L.; Qiang, L. Strontium-Cobaltite-Based Perovskite (SrCoO3) for Solar-Driven Interfacial Evaporation Systems for Clean Water Generation. Nanomaterials 2023, 13, 1420. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhao, Q.; Liu, Q.; Qiu, J.; Yuan, S.; Wu, Z.; Yang, R.; Cao, J.; Wang, L.; Xu, J.; et al. A Bilayered Wood-Poly(3,4-ethylenedioxythiophene):Polystyrene Sulfonate Hydrogel Interfacial Evaporator for Sustainable Solar-Driven Sewage Purification and Desalination. Nanomaterials 2023, 13, 2321. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, N.; Syakir Ishak, M.I.; Bhawani, S.A.; Umar, K. Various Natural and Anthropogenic Factors Responsible for Water Quality Degradation: A Review. Water 2021, 13, 2660. [Google Scholar] [CrossRef]
- Dong, X.; Gao, S.; Li, S.; Zhu, T.; Huang, J.; Chen, Z.; Lai, Y. Bioinspired structural and functional designs towards interfacial solar steam generation for clean water production. Mater. Chem. Front. 2021, 5, 1510–1524. [Google Scholar] [CrossRef]
- Cao, S.; Jiang, Q.; Wu, X.; Ghim, D.; Gholami, D.H.; Chou, P.-I.; Jun, Y.-S.; Singamaneni, S. Advances in solar evaporator materials for freshwater generation. J. Mater. Chem. A 2019, 7, 24092–24123. [Google Scholar] [CrossRef]
- Pistocchi, A.; Bleninger, T.; Breyer, C.; Caldera, U.; Dorati, C.; Ganora, D.; Millan, M.M.; Paton, C.; Poullis, D.; Herrero, F.S.; et al. Can seawater desalination be a win-win fix to our water cycle? Water Res. 2020, 182, 115906. [Google Scholar] [CrossRef]
- Hu, R.; Zhang, J.; Kuang, Y.; Wang, K.; Cai, X.; Fang, Z.; Huang, W.H.; Chen, G.; Wang, Z. A Janus evaporator with low tortuosity for long-term solar desalination. J. Mater. Chem. A 2019, 7, 15333–15340. [Google Scholar] [CrossRef]
- Ni, G.; Zandavi, S.H.; Javid, S.M.; Boriskina, S.V.; Cooper, T.A.; Chen, G. A salt-rejecting floating solar still for low-cost desalination. Energy Environ. Sci. 2018, 11, 1510–1519. [Google Scholar] [CrossRef]
- Zhao, L.; Yang, Q.; Guo, W.; Liu, H.; Ma, T.; Qu, F. Co2.67S4-Based Photothermal Membrane with High Mechanical Properties for Efficient Solar Water Evaporation and Photothermal Antibacterial Applications. ACS Appl. Interfaces 2019, 11, 20820–20827. [Google Scholar] [CrossRef]
- Qu, B.; Li, P.; Bai, L.; Qu, Y.; Li, Z.; Zhang, Z.; Zheng, B.; Sun, J.; Jing, L. Atomically Dispersed Zn-N5 Sites Immobilized on g-C3 N4 Nanosheets for Ultrasensitive Selective Detection of Phenanthrene by Dual Ratiometric Fluorescence. Adv. Mater. 2023, 35, 2211575. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xu, X.; Sheng, X.; Lin, P.; Tang, J.; Pan, L.; Kaneti, Y.V.; Yang, T.; Yamauchi, Y. Solar-Powered Sustainable Water Production: State-of-the-Art Technologies for Sunlight-Energy-Water Nexus. ACS Nano 2021, 15, 12535–12566. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ding, Z.; Fu, X.; Wang, X. Construction of conjugated carbon nitride nanoarchitectures in solution at low temperatures for photoredox catalysis. Angew. Chem. Int. Ed. Engl. 2021, 51, 11814–11818. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Chen, L.; Qu, N.; Yu, J.; Jiang, X.; Xiao, C.; Luo, X.; Hasi, Q. MXene/polypyrrole coated melamine-foam for efficient interfacial evaporation and photodegradation. J. Colloid Interface Sci. 2023, 636, 291–304. [Google Scholar] [CrossRef]
- Rtimi, S.; Pulgarin, C.; Kiwi, J. Recent Developments in Accelerated Antibacterial Inactivation on 2D Cu-Titania Surfaces under Indoor Visible Light. Coatings 2017, 7, 20. [Google Scholar] [CrossRef]
- Liu, H.; Li, X.; Ma, Z.; Sun, M.; Li, M.; Zhang, Z.; Zhang, L.; Tang, Z.; Yao, Y.; Huang, B.; et al. Atomically Dispersed Cu Catalyst for Efficient Chemoselective Hydrogenation Reaction. Nano Lett. 2021, 21, 10284–10291. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofiltration Membrane for Water Purification. Adv. Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Liu, F.; Fan, M.; Liu, X.; Chen, J. One-Pot Synthesis of Cellulose-Based Carbon Aerogel Loaded with TiO2 and g-C3N4 and Its Photocatalytic Degradation of Rhodamine B. Nanomaterials 2024, 14, 1141. [Google Scholar] [CrossRef]
- Li, J.; Yu, X.; Zhu, Y.; Fu, X.; Zhang, Y. 3D-2D-3D BiOI/porous g-C3N4/graphene hydrogel composite photocatalyst with synergy of adsorption-photocatalysis in static and flow systems. J. Alloys Compd. 2021, 850, 156778. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, C.; Zheng, L.; Chen, F.; Qian, J.; Meng, X.; Chen, Z.; Zhong, S.; He, B. N3C-Defect-Tuned g-C3N4 Photocatalysts: Structural Optimization and Enhanced Tetracycline Degradation Performance. Nanomaterials 2025, 15, 466. [Google Scholar] [CrossRef]
- Lv, B.; Li, S.; Yu, Y.; Liu, Y.; Xu, Y.; Fan, X. A 2.5-Dimensional biomimetic dual-functional bifacial-evaporator for high efficient evaporation and water purification. J. Hazard. Mater. 2024, 476, 134993. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Cen, W.; Zhang, Y.; Dong, F. Bridging the g-C3N4 Interlayers for Enhanced Photocatalysis. ACS Catal. 2016, 6, 2462–2472. [Google Scholar] [CrossRef]
- Zimmerman, J.L.R.W.; Khabashesku, V.N.; Margrave, J.L. Synthesis of Spherical Carbon Nitride Nanostructures. Nano Lett. 2001, 12, 731–734. [Google Scholar] [CrossRef]
- Chen, K.; Li, L.; Li, B.; Yang, Y.; Zhu, K.; Zhang, J. Simultaneous Fresh Water Collection and Li+ Selective Adsorption Enabled by A Salt-Resistant Separated Solar Evaporator. Adv. Func. Mater. 2024, 34, 2402221. [Google Scholar] [CrossRef]
- Gao, Z.; Guan, J.; Wang, M.; Liu, S.; Chen, K.; Liu, Q.; Chen, X. A novel laccase-like Cu-MOF for colorimetric differentiation and detection of phenolic compounds. Talanta 2024, 272, 125840. [Google Scholar] [CrossRef]
- Wang, F.-F.; Sun, W.-Y. Cu-MOF and CuBi Double-Perovskite Composites for Selective CO2 Electroreduction to HCOOH. ACS Sustain. Chem. Eng. 2024, 12, 15651–15658. [Google Scholar] [CrossRef]
- Majdoub, A.; Majdoub, M.; Zaitan, H. g-C3N4/CuO loaded polyester fabric as effective Fenton-like dip-catalyst for the oxidation of dyes. J. Water Process. Eng. 2024, 60, 105167. [Google Scholar] [CrossRef]
- Zeng, L.; Deng, D.; Zhu, L.; Wang, H.; Zhang, Z.; Yao, Y. Biomass photothermal structures with carbonized durian for efficient solar-driven water evaporation. Energy 2023, 273, 127170. [Google Scholar] [CrossRef]
- Zuo, Z.; Zhu, F.; Wang, L.; Wang, Z.; Zhao, J.; Ji, Z.; An, M.; Ye, Y.N.; Yu, W.; Wang, Z.; et al. Trapping waste metal ions in a hydrogel/coal powder composite for boosting sewage purification via solar-driven interfacial water evaporation with long-term durability. Chem. Eng. J. 2024, 481, 148524. [Google Scholar] [CrossRef]
- Huang, X.P.; Li, L.X.; Chen, K.; Zhang, J.P. Scalable Superhydrophilic Solar Evaporators for Long-Term Stable Desalination, Fresh Water Collection and Salt Collection by Vertical Salt Deposition. ChemSusChem 2024, 17, 202400111. [Google Scholar] [CrossRef]
- Huang, X.; Li, L.; Zhao, X.; Zhang, J. Highly Salt-Resistant interfacial solar evaporators based on Melamine@Silicone nanoparticles for stable Long-Term desalination and water harvesting. J. Colloid Interface Sci. 2023, 646, 141–149. [Google Scholar] [CrossRef]
- Wagner, M.; Andrew Lin, K.Y.; Oh, W.D.; Lisak, G. Metal-organic frameworks for pesticidal persistent organic pollutants detection and adsorption—A mini review. J. Hazard. Mater. 2021, 413, 125325. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhao, X.; Hu, T.; Li, L.; Huang, X.; Zhang, J. Flexible Solar Interfacial Evaporators with Photocatalytic Function for Purification of High-Salinity Organic Wastewater. Nanomaterials 2025, 15, 632. https://doi.org/10.3390/nano15080632
Li Y, Zhao X, Hu T, Li L, Huang X, Zhang J. Flexible Solar Interfacial Evaporators with Photocatalytic Function for Purification of High-Salinity Organic Wastewater. Nanomaterials. 2025; 15(8):632. https://doi.org/10.3390/nano15080632
Chicago/Turabian StyleLi, Yucheng, Xia Zhao, Tao Hu, Lingxiao Li, Xiaopeng Huang, and Junping Zhang. 2025. "Flexible Solar Interfacial Evaporators with Photocatalytic Function for Purification of High-Salinity Organic Wastewater" Nanomaterials 15, no. 8: 632. https://doi.org/10.3390/nano15080632
APA StyleLi, Y., Zhao, X., Hu, T., Li, L., Huang, X., & Zhang, J. (2025). Flexible Solar Interfacial Evaporators with Photocatalytic Function for Purification of High-Salinity Organic Wastewater. Nanomaterials, 15(8), 632. https://doi.org/10.3390/nano15080632





