Abstract
Nanotubes (NTs) are nanosized tube-like structured materials made from various substances such as carbon, boron, or silicon. Carbon nanomaterials (CNMs), including carbon nanotubes (CNTs), graphene/graphene oxide (G/GO), and fullerenes, have good interatomic interactions and possess special characteristics, exploitable in several applications because of the presence of sp2 and sp3 bonds. Among NTs, CNTs are the most studied compounds due to their nonpareil electrical, mechanical, optical, and biomedical properties. Moreover, single-walled carbon nanotubes (SWNTs) have, in particular, demonstrated high ability as drug delivery systems and in transporting a wide range of chemicals across membranes and into living cells. Therefore, SWNTs, more than other NT structures, have generated interest in medicinal applications, such as target delivery, improved imaging, tissue regeneration, medication, and gene delivery, which provide nanosized devices with higher efficacy and fewer side effects. SWNTs and multi-walled CNTs (MWCNTs) have recently gained a great deal of attention for their antibacterial effects. Unfortunately, numerous recent studies have revealed unanticipated toxicities caused by CNTs. However, contradictory opinions exist regarding these findings. Moreover, the problem of controlling CNT-based products has become particularly evident, especially in relation to their large-scale production and the nanosized forms of the carbon that constitute them. Important directive rules have been approved over the years, but further research and regulatory measures should be introduced for a safer production and utilization of CNTs. Against this background, and after an overview of CNMs and CNTs, the antimicrobial properties of pristine and modified SWNTs and MWCNTs as well as the most relevant in vitro and in vivo studies on their possible toxicity, have been reported. Strategies and preventive behaviour to limit CNT risks have been provided. Finally, a debate on regulatory issues has also been included.
1. Introduction
Nanotechnology is one of the most exciting 21st century technologies [1,2], with the capability to observe, measure, control, assemble, and manufacture materials at the nanoscale, and it translates the theory of nanoscience into practical applications [3,4,5]. Nanotechnology is a discipline of global interest, which is constantly growing [6,7,8]. It deals with a variety of materials produced at <100 nm through different chemical and physical methods [9]. It is conducted at the nanoscale (1–100 nm) and has unique phenomena that enable novel applications in a wide range of fields, including chemistry, physics, biology, medicine, engineering, and electronics [10,11]. To keep up with the rate of advancement in science and technology, new types of nanomaterials with unconventional edge and innovative properties are incessantly required [12]. Nanotubes (NTs) belong to a promising group of nanomaterials that allow us to approach several new electronic, magnetic, optical, and mechanical properties [13].
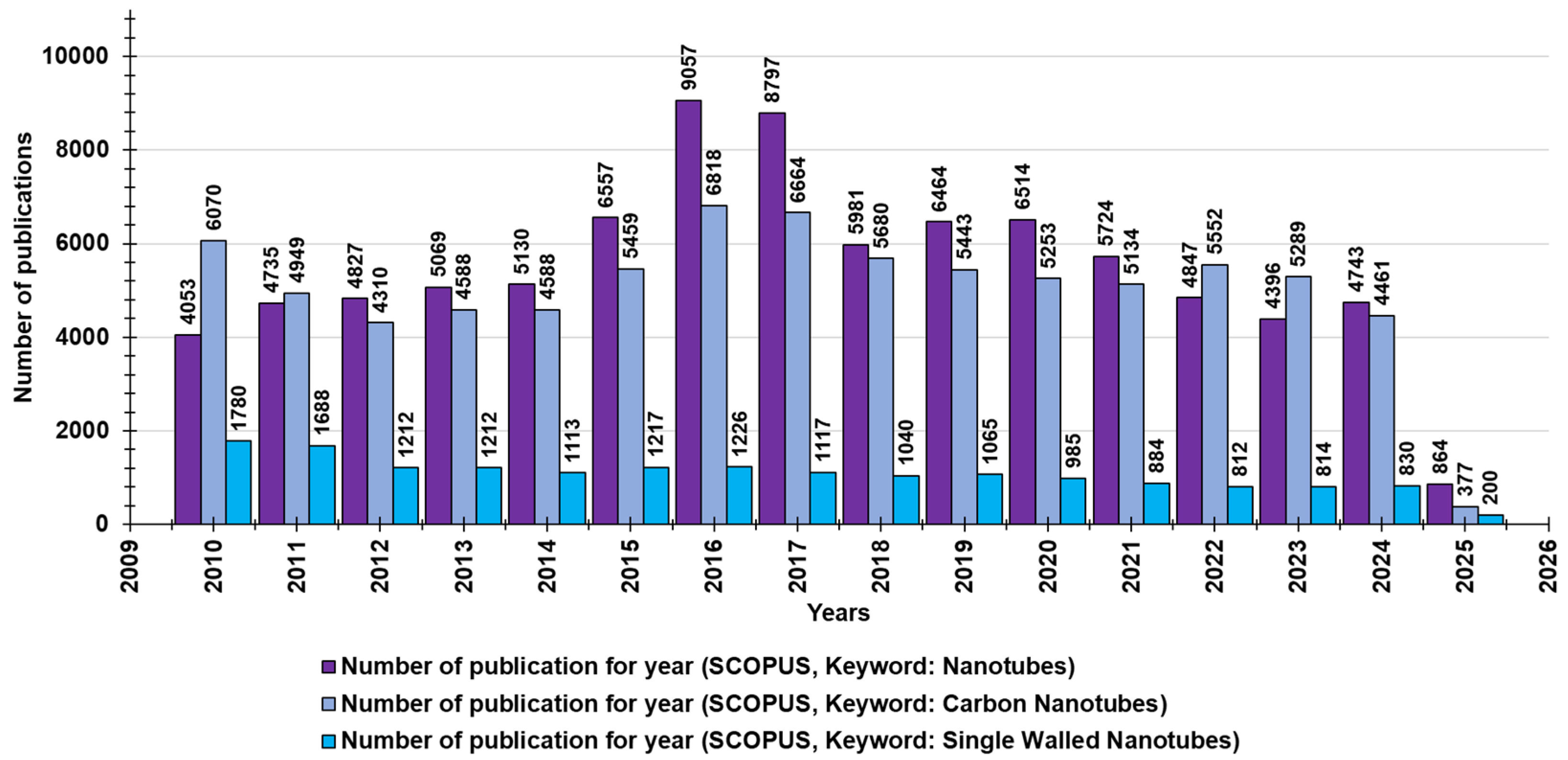
Many of the nanotube structures that have been extensively studied contain boron, silicon, and molybdenum atoms; carbon nanotubes (CNTs) are the most researched group among existing NT structures [14]. In fact, carbon nanomaterials (CNMs), including CNTs, graphene/graphene oxide (G/GO), and fullerenes, are allotropes of sp2 and sp3 hybridized carbon atoms and their sp2 and sp3 bonds and are thought to be responsible for their unique characteristics [12]. They have good interatomic interactions [14] and are very interesting nanostructures in terms of their special properties and possible applications [15]. CNTs are composed of graphite sheets rolled up in an unbreakable and non-stop hexagonal-like lattice structure, in which carbon atoms appear at the tops of the hexagon-type forms. Based on the number of carbon sheets, CNTs are categorized as single-wall carbon nanotubes (SWCNTs), double-wall carbon nanotubes (DWCNTs), and multi-wall carbon nanotubes (MWCNTs) [13]. Although MWCNTs have also been extensively studied for their demonstrated antimicrobial potential, SWCNTs have displayed excellent antibacterial action, according to some research, due to the smaller size of these materials, which play a significant part in the inhibition of microbes [12]. Through a search made on Scopus on 1 March 2025, using “nanotubes” as a keyword, for the last fifty years (2010–2025), we found 87,798 documents concerning “nanotubes” and 80,535 documents concerning “carbon nanotubes” (Figure 1). Through an additional survey and using the keywords “single-walled nanotubes”, we found 17,225 publications (Figure 1).
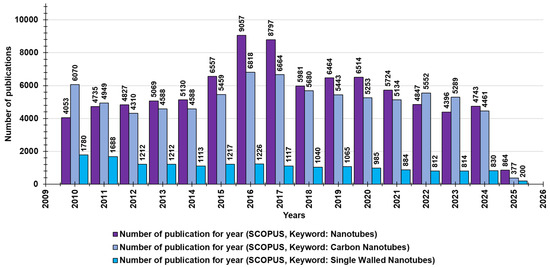
Figure 1.
The number of publications per year during the last 15 years, according to Scopus, concerning nanotubes (purple bars), carbon nanotubes (light purple bars), and SWNTs (blue bars).
Generally, CNMs offer great surface area, mechanical resistance, thermal conductivity, photoluminescence, transparency, and constructional durability, in addition to antibacterial activities against pathogens and remarkable electrical conductivity [16]. These properties increasingly encourage the application of CNMs in several nanocomposites, such as in thin-film transistors, transparent conducting electrodes, photovoltaics, supercapacitors, biosensors, drug delivery systems, tissue engineering, photothermal therapy, and antimicrobial food packaging. As a part of the larger family of CNMs, CNTs are also materials with extraordinary properties that are useful across a variety of new, state-of-the-art applications in sensors, printed electronics, e-readers, flexible displays, energy storage medical treatments, and more. Specifically, since their discovery in 1991 by Ijima [17], SWNTs have influenced a significant amount of activity in both research and industry across the world. Furthermore, SWNTs have stimulated considerable investment in manufacturing methods, characterization techniques, and application development. However, the main drawback of CNTs is immediately recognized; it consists of their scarce solubility in most solvents, which limits their application [16]. To address this issue and amplify CNT use, researchers have focused on surface modifications [14,15,16]. Surface modifications, together with several other factors, including their chemical composition, target bacteria, and reaction environment, can also affect the CNMs’ antimicrobial activity [18]. The antimicrobial effects of CNMs are derived mainly from their capacity to physically isolate pathogen cells from their supportive environment. Anyway, their capacity to penetrate microbial cell walls/membranes, thus causing irreversible structural damage, supports their antimicrobial activity to a large extent [12]. Additionally, the interaction of CNMs with bacterial cells stimulates the generation of noxious substances, including reactive oxygen species (ROS), triggering oxidative stress (OS) in cells and thus promoting their death. Additionally, the interactions between CNMs and microorganisms result in an electron transfer, which induces ROS-independent OS and causes the biological death of pathogens [19]. To confirm that the OS plays an additional role in CNT and SWCNT antimicrobial mechanisms [20], Haung et al. investigated the mechanical effects that influenced the antimicrobial properties of CNTs, including low wear rates, low friction coefficients, favourable tribological characteristics, and high corrosion resistance [21]. Among different types of CNMs and CNTs, the antimicrobial activity of SWCNTs is found to be higher due to their advantageous physicochemical properties [22,23,24,25]. In this regard, Kang and co-workers, in their first report on the antimicrobial activity of purified SWCNTs and MWCNTs, demonstrated that both materials showed significant antimicrobial effects. Moreover, those of SWCNTs seemed to be stronger than those of MWCNTs [26]. They found that CNTs’ antibacterial effect mainly depended on their ruinous impact on the integrity of bacterial membranes upon direct contact; the higher activity of SWCNTs could be due to their small size, which provides a larger surface area to facilitate the membrane perturbation [26,27]. The morphology and metabolic activities of pathogens were also compromised [27]. Also, based on studies conducted by Chen et al., the SWCNTs played a significant role as “nano-darts”, which penetrated bacterial cell walls, reduced membrane potential, released intracellular constituents (DNA and RNA), and ultimately disrupted bacterial membranes [28]. Unfortunately, despite their possible toxicity being initially disregarded due to their harmless carbon-based composition, early and recent investigations have unexpectedly unveiled that CNTs could be toxic to humans, animals, and the environment, depending on their concentrations and exposure times [29]. In fact, even if CNTs are made of normally innocuous and intrinsically safe carbon and do not have nano-dimensioned length, they should be considered as nanomaterials, which, thanks to their nanosized diameter, can penetrate various cells, including those in bacteria, yeast, and mammals [30]. It has been found that, mainly due to their poor biodegradability, accumulation, and persistence in the body, surface structure, and residual impurities, CNTs can be remarkably toxic, genotoxic, and cancerogenic to several cells, organs, and animal models. In this regard, amyloid deposits have been found in the brain, liver, lungs, and kidneys of exposed animals [29,31]. Additionally, investigations prompted by the similarity of the needle-like CNT fibres with those of asbestos evidenced that they can be responsible for pulmonary toxicity, mainly in CNT manufacturers, which results from contact with and/or inhalation of CNT fibres [32,33]. Also, toxicity to the cardiovascular system, immune and reproductive systems, embryos, and neurotoxicity have been reported [33]. Based on these findings, several strategies using advanced synthetic methods, highly efficient purification procedures, post-synthesis chemical modifications, as well as preventive behavioural conducts have already been developed to limit CNTs’ toxic effects [34]. Anyway, more and incessant studies should be implemented to make their production and employment safer. On the other hand, despite significant advancements in terms of regulations concerning CNT production and possible applications for health and safety at the production sites being implemented, several unsolved regulatory issues survive, which need urgent solutions [34]. The increasing production and commercialization of differently structured CNTs and related nanocomposites and their daily application have stimulated the development of more rigid regulatory standards concerning their manufacturing and utilization [34]. Anyway, substantial concern regarding toxicity and environmental safety persists, mainly due to contradictory outcomes of toxicity investigations [35]. However, it must be recognized that CNTs, despite their still not fully clear risk to living organisms and the environment and limited regulation, are paradoxically ubiquitously exploitable for improving the quality of their life. In this regard, with this review, we aimed at stimulating further research on these nonpareil materials to better understand their potential as antimicrobial agents and the mechanisms supporting their cytotoxic effects on pathogens, which could also help to clarify the basis of their toxicity to humans. Such improved knowledge could allow their safer, large-scale production and utilization to enhance environment equilibrium and our quality of life. Against this background, and after an overview of CNMs and CNTs, the antimicrobial properties of pristine and modified SWCNTs and MWCNTs, as well as the most relevant in vitro and in vivo studies on their possible toxicity, have been discussed. The strategies already developed and the suggested preventive behaviour to limit CNT toxicity have also been provided and discussed. Finally, an extensive debate on the regulatory issues and the currently available standard guidelines by relevant International Organizations to produce, characterize, and manage CNTs and related nanocomposites have also been included.
This work is a thorough and contemporary review, which thoroughly explores and discusses different aspects concerning CNTs in a single document, including the synthesis, structure, applications, antimicrobial properties, functionalization strategies, toxicological concerns, and regulatory issues. This review affords systematic and well-organized information on CNTs and their potential applications. This study is supported by a substantial body of literature research and several case-specific tables, which enhance its readability and clarity, showing the double-sided impact that CNT use could have on our lives and providing future perspectives for the most challenging aspects of CNTs.
2. Approaching Carbon Nanotubes
Carbon is a chemical element that has atomic number 6. It is one of the most abundant elements in the Universe by mass, capable of providing approximately 10 million different pure organic compounds. Such compounds possess the uncommon ability to form polymers at earth temperatures, thus being the chemical basis of all known life [35].
CNTs are thin and long cylinders made of carbon, which were found for the first time in 1991 by Sumio Iijima [17]. Many physical properties of CNTs are still obscure and need to be disputed, as well as technical and not technical hurdles, which still limit the CNTs’ success and their extensive application. Anyway, it is generally recognized that CNTs possess a plethora of thermal, electronic, mechanical, and structural properties that can vary based on their different existing forms [35]. In this regard, CNTs differ mainly in their diameter, length, chirality, or twist. Table A1, in Appendix A and reproduced from our previous work [35], collects the key properties and potential uses of CNTs, as well as the current remaining technical and non-technical hurdles. References in Table A1 do not belong to this work but are those contained in Alfei and Schito, 2022 [35]. We have decided on this solution so as not to overly burden the already significantly long list of references in this work. In addition to those reported in Table A1, a plethora of other possible applications for CNTs exist, including solar collectors, conductive and/or waterproof paper, catalyst supports, nano-porous filters, and coatings of all types. Since CNTs possess the capability to absorb infrared light, they may be applied to the I/R Optics Industry. We are confident that many unexpected applications for these nonpareil materials will be found in the next years, which will reinforce the belief that CNTs could be the most important and valuable nanomaterial ever used until now.
2.1. Synthesis of CNTs
Table A2 in Appendix A, reproduced by our previous work [35] collects the descriptions of the three most used methods to synthetize CNTs. Briefly, they include arc discharge (AD) method [36,37], finalized to the production of fullerenes, before 1991 [17], laser ablation (LA) [38,39], developed by Dr. Richard Smalley and co-workers at Rice University, and chemical vapor deposition (CVD) [40,41,42,43,44,45,46], which is the most widely used method to produce CNTs [35]. An advanced CVD technique is named plasma-enhanced chemical vapor deposition (PECVD) and consists of generating plasma by the application of a strong electric field during CNTs growth [47]. By adapting the reactor’s geometry, it is possible to synthesize vertically aligned CNTs (VACNTs) [35], whose morphology is of interest to researchers attracted by electron emission from CNTs. Other methods have been described in detail in our previous work [35]. They include high-pressure carbon monoxide (HiPco) process, optimized at Rice University [35], super-growth CVD (SGCVD) [48,49], introduced by Kenji Hata, Sumio Iijima and col-leagues at AIST (Japan), plasma torch (PT) [50,51], an invention by Olivier Smiljanic in the year 2000, working at Institut National de la Recherche Scientifique (INRS) (Varennes, Canada), subsequently modified in IPT procedure by researchers from Sherbrooke University and the National Research Council of Canada and liquid electrolysis method (LEM) [52,53], which allowed to obtain MWCNTs by electrolysis of molten carbonates [35]. In addition to all these artificial inventions by human re-searcher, CNTs can form naturally in commonly originated flames emitted by burning methane [54], ethylene [55], and benzene [56]. Highly irregular in dimensions and low-quality CNT-based structures can form in smoke from both indoor and outdoor air [57]. Anyway, lacking the high degree of uniformity, due to uncontrolled conditions, necessary to satisfy the many needs of both research and industry, their practical ap-plication is hampered. Despite this, efforts focus on the strategies to control environ-mental flames by theoretical models to produce less irregular CNTs [58,59,60,61,62], thus going towards large-scale, low-cost CNTs synthesis competitive with large scale CVD pro-duction.
2.2. Architecture and Other Features of CNTs
CNTs deserved the prefix “nano” due to their very small size of 1/50,000th of the diameter of a human hair.
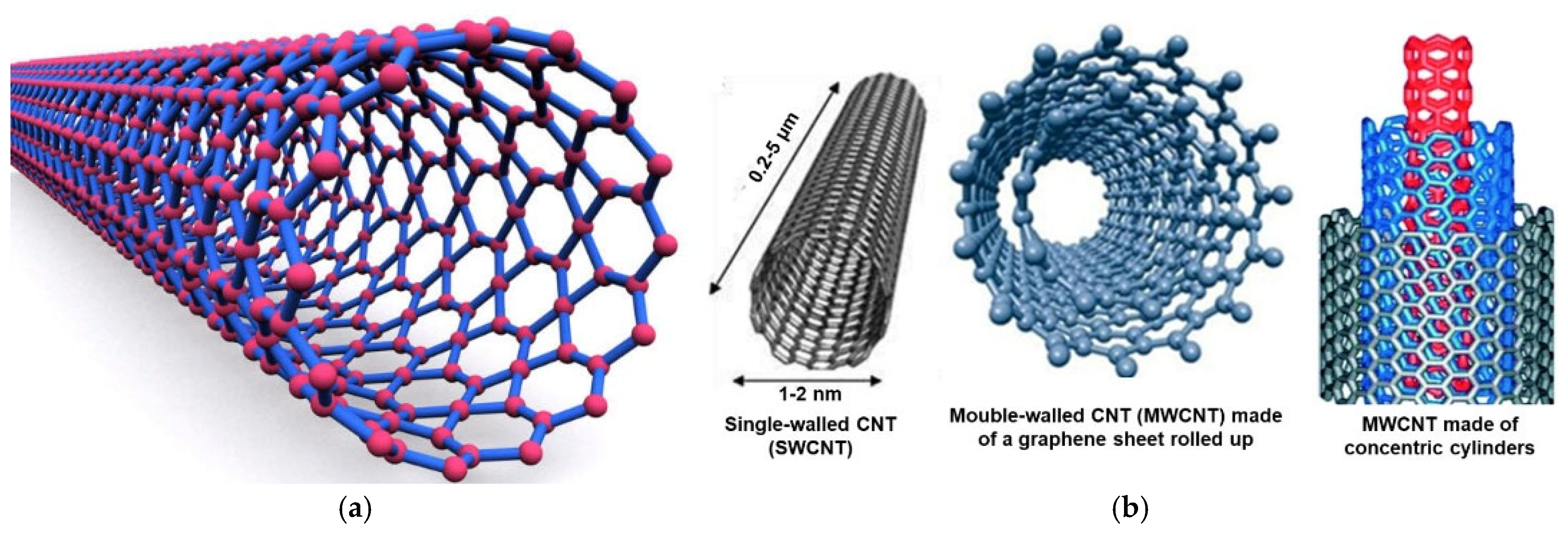
Pure carbon can develop in several carbon-based architectures, and CNTs are one of them. For example, diamond is the tetrahedron crystalline structure of carbon, while graphite or graphene owns a planar structure, with linked carbon atoms forming hexagons [35]. Buckyballs were named after F. Buckminister Fuller, who first designed geodesic spheres; they are also called fullerenes [63]. They are made of hexagons of pure carbon atoms linked to form a sphere. The typical structure of CNTs instead derives from the rolling up of a sheet of carbon atoms associated to form hexagons (graphene), thus forming cylinders (Figure 2a).
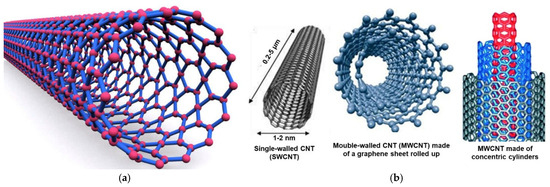
Figure 2.
Structure of CNTs (a); structures of a SWCNT (left), of two different shapes of a MWCNT (center and right) (b). Specifically, an MWCNT made of a single graphene sheet rolled up (center) and an MWCNT made of more than two graphene cylinders with one inside the other (right). These images, by an unknown author, are licensed under CC BY and have been reproduced from our previous work [35].
Different structures that CNTs can assume are represented in Figure 2b. While SWCNTs (left side) are not naturally formed CNTs, MWCNTs are more complex CNTs formed naturally [63].
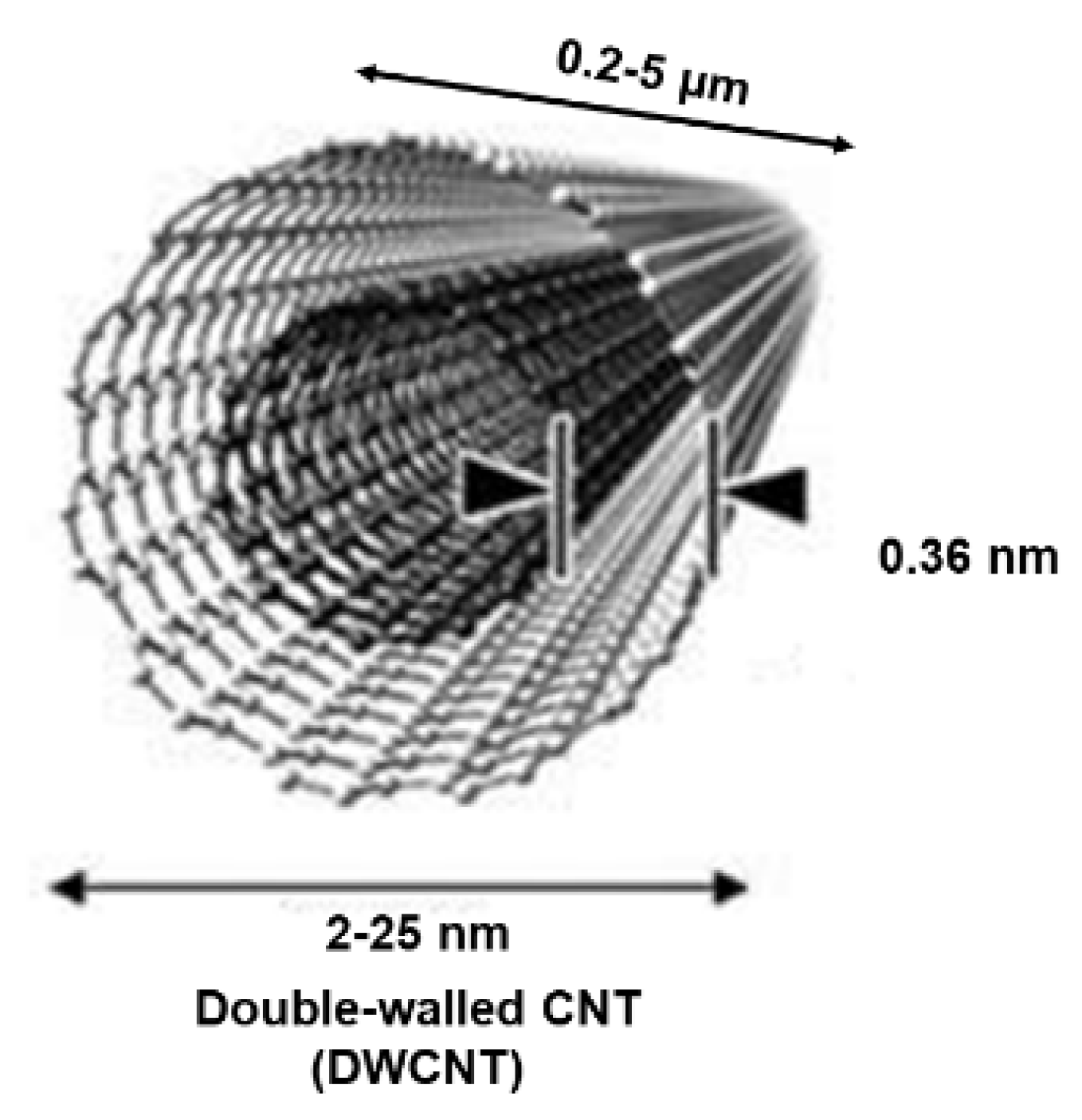
Additionally, when CNTs consist of two or three graphene complete cylinders with one inside the other, they are termed double- and triple-walled carbon nanotubes (DWCNTs and TWCNTs). Figure 3 shows the representative structure of a DWCNT.
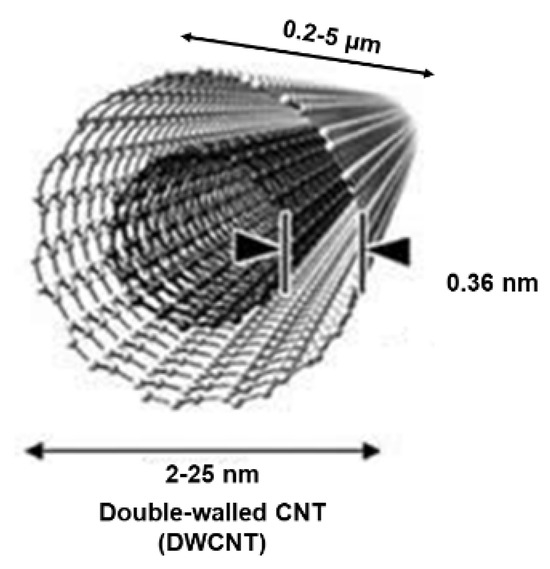
Figure 3.
Structure of a DWCNT. This image by an unknown author is licensed under CC BY and was reproduced from our previous work [35].
CNTs are, atomically speaking, very stable, while SWCNTs are more stable than MWCNTs. Researchers in the field consider tubes with diameters less than 100 nm as CNTs [64]. However, no intrinsic limit exists on how long CNTs can develop [35]. However, their length is usually much larger than their diameter [35]. Table A3 in Appendix A summarizes the physical limits of CNTs. The references in the table do not belong to this work but are those contained in Alfei and Schito, 2022 [35]. We have decided on this solution for the reasons previously described. The production of SWCNTs is more difficult than that of MWCNTs, which can exhibit behaviours different from SWCNTs. In this regard, despite the dispersion of SWCNTs to obtain nanocomposites being more difficult than that of MWCNTs, generally, SWCNTs demonstrated better properties than MWCNTs. For this reason, scientists are focused mainly in finding more practical ways to mass-produce SWCNTs. Concerning the production of CNT-based nanocomposites, the possible junctions between two or more CNTs have been widely discussed theoretically [65,66], while those between CNTs and graphene have been considered both theoretically [67] and experimentally [68] (Table A4, Appendix A, references in the Table do not belong to this work but are those contained in Alfei and Schito, 2022 [35], according to reasons previously described). Junctions between CNTs were observed in CNTs prepared by AD or CVD methods. Lambin et al. were the first experts who studied theoretically the electronic properties of such junctions, starting from the assumption that a connection between a metallic tube and a semiconducting one could form a component of a CNT-based electronic circuit [69]. CNTs-graphene junctions are instead the basis of pillared graphene, characterized by three-dimensional (3D)-carbon nanotube (3D-CNTs) architectures, studied as building blocks to fabricate three-dimensional macroscopic structures [70]. Using these materials, it is possible to obtain free-standing, porous scaffolds made only of carbon, possessing macro-, micro-, and nano-structured pores and tailorable porosity with several applications. They could be used for the fabrication of the next-generation energy storages, supercapacitors, field emission transistors, high-performance catalysts, photovoltaics, and biomedical devices, such as implants and biosensors [71,72,73].
Relationships Structure/Properties
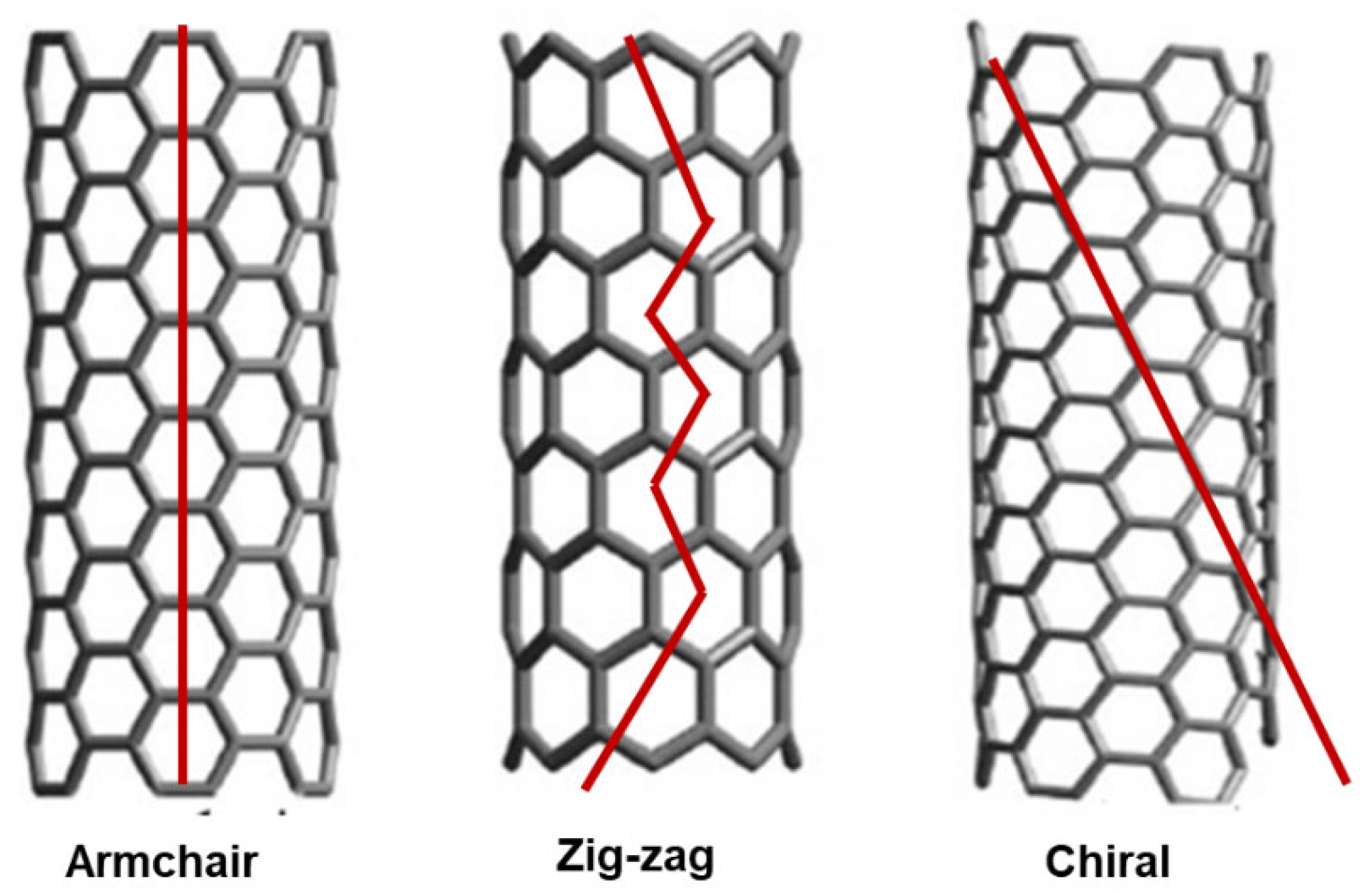
The study of CNT formations remains a cutting-edge field where new discoveries are being made regularly. Among the possible intimate structures that a SWCNT can assume, the armchair, zigzag, and chiral configurations are extensively recognized, based on the graphene mode to package into the CNTs cylinder (Figure 4).
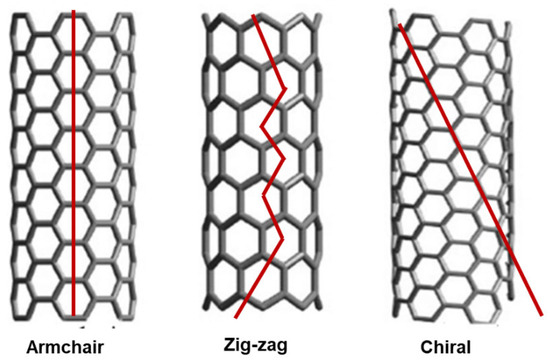
Figure 4.
Armchair, zigzag, and chiral structures of SWCNTs. These images by unknown author are licensed under CC BY and are reproduced by our previous work [35].
The structural conformation of CNTs has a direct effect on their properties, mainly on the mechanical and electrical ones. For example, in MWCNTs, the outer walls of MWCNTs can protect the inner carbon tubes from chemical interactions with outside materials. Over the years, possible structure-related mechanical properties and structure-related electrical and thermal properties of CNTs have been reported in several studies. Structure-related mechanical investigations established that CNTs are the greatest nanomaterials discovered so far. Particularly, CNTs were better than other nanomaterials in terms of elastic modulus and tensile strength [35,74,75,76]. Anyway, it has also been reported that defects in the structure of CNTs due to atomic vacancies or rearrangements of the carbon bonds can cause weak points in small segments of the CNT, which reduce their elasticity and weaken their tensile strength [35]. On the other hand, structure-related electrical and thermal investigations showed that the structure of CNTs affect their conductive potency, both in terms of electrical and thermal conductivity [35,77,78,79,80,81,82].
3. Biomedical Applications of CNTs
CNTs belonging to all categories, including SWCNTs, DWCNTs, and MWCNTs, can differ in purity, length, and functionality. Anyway, all own a plethora of properties, such as high electrical conductivity, high tensile strength, light weight [83,84,85], and high biocompatibility [86]. All possess the capability to load molecules for their transport and delivery, large surface area, and chemical inertness. All can be enriched with functional groups and demonstrated good elasticity, thermal conductivity, capability to expand, electron emission capacity, and high aspect ratio [83,84,85].
These properties, added to peculiar composition and geometry, make CNTs suitable for numerous potential applications, including energy storage, biomedical uses, and air and water filtration. Also, CNTs could become molecular electronics, thermal materials, structural materials, electrical conductors, fabrics and fibres, catalyst supports, conductive plastics, conductive adhesives, as well as ceramics [83].
For this reason, scientists working in this sector are involved in efforts aimed at increasingly lowering the costs of CNT production to commercially viable levels by scaling up their synthesis.
Interestingly, CNTs have great potential to be applied in nanomedicine for disease diagnosis and drug targeting, as well as to transport various biomolecules such as proteins, DNA, RNA, immune-active compounds, and lectins [87,88,89].
CNTs are also extensively applied to develop electrochemical sensors, DNA-based sensors, as well as piezoelectric and gas sensors [83]. Additionally, opportunely structured CNTs, such as cationic CNTs, have been revealed to possess interesting antibacterial and antifungal activity [83]. Table A5 in Appendix A, reproduced from our previous article [35] summarizes the several applications that CNTs could have in the biomedical area. References in the Table do not belong to this work but are those contained in Alfei and Schito, 2022 [35]. We have decided on this solution for the reasons previously described.
3.1. Antimicrobial Properties of Carbon Nanotubes
3.1.1. Mechanisms of Action and Influencing Factors
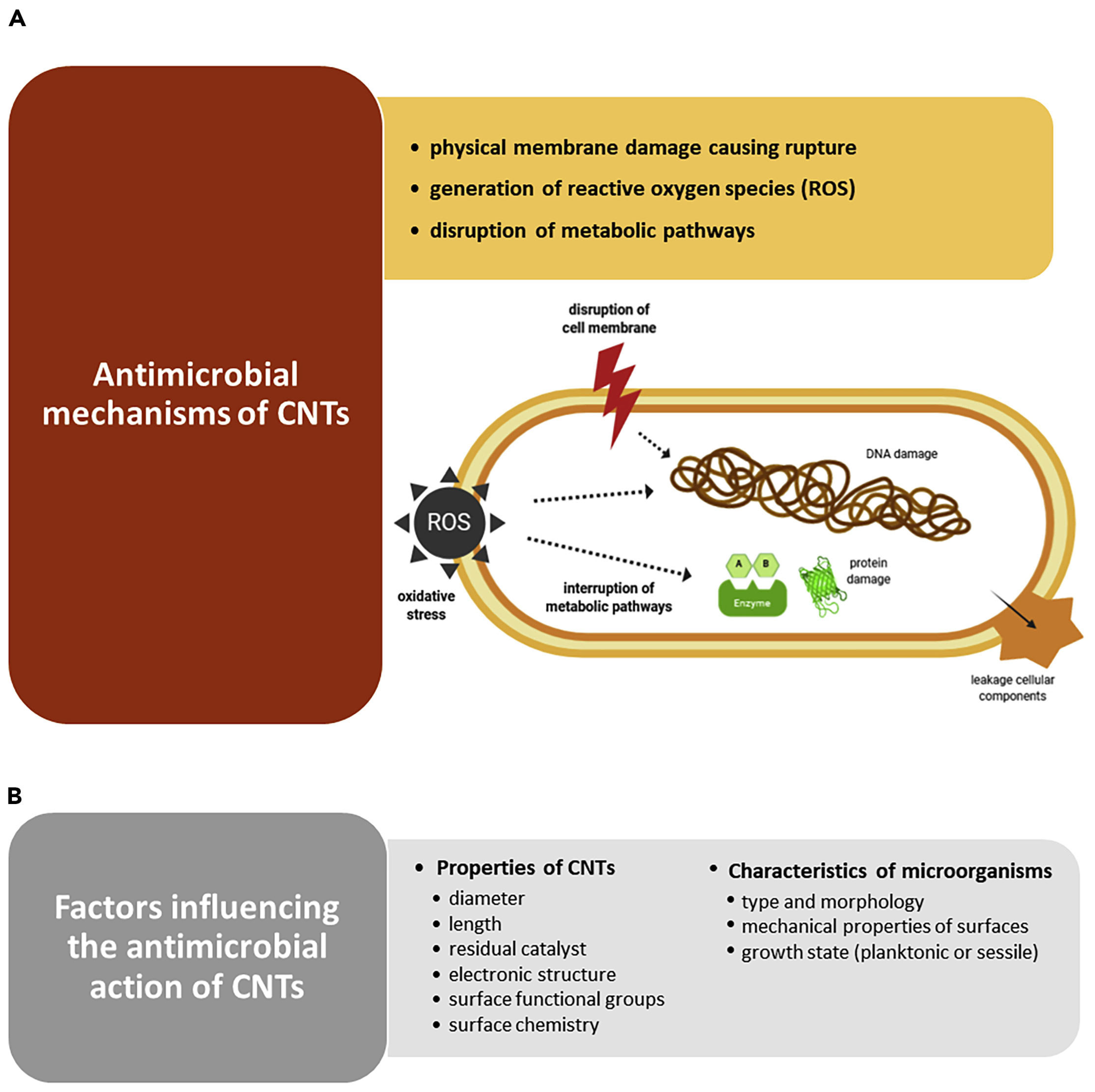
It has been reported that CNTs, especially SWCNTs and MWCNTs, possess excellent antibacterial and antifungal activity. Several mechanisms have been suggested to justify the CNTs’ toxicity against bacteria. Figure 5A shows the most recognized antimicrobial mechanisms [90].
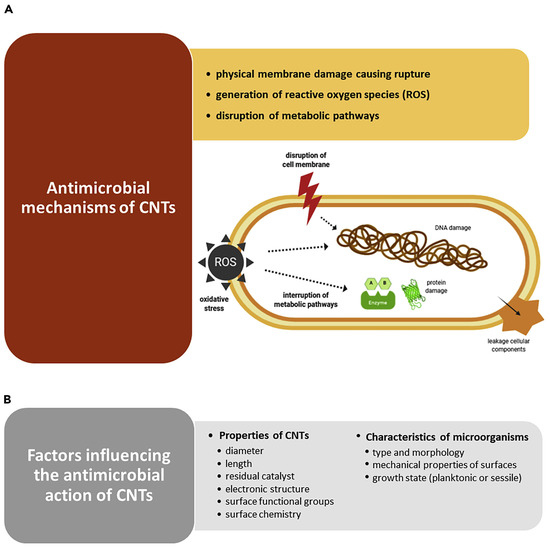
Figure 5.
(A) Possible mechanisms by which CNTs exert antimicrobial effects: physically piercing of outer membrane, with irreversible membrane damage, cell lysis and release of cytoplasmic content; reactive oxygen species (ROS) overproduction causing oxidative stress (OS), which leads to DNA damage, protein and lipid peroxidation, and impairment of metabolic pathways, which results in cell death. (B) Factors influencing the antimicrobial performance of CNTs. This image has been reproduced by an article published in open access [90] and available under the Creative Commons CC-BY-NC-ND (CC BY-NC-ND 4.0, https://creativecommons.org/licenses/by-nc-nd/4.0/, accessed on 18 March 2025) license and permits non-commercial use of the work as published, without adaptation or alteration provided the work is fully attributed.
Kang et al. reported, for the first time in 2007, that SWCNTs demonstrated strong antibacterial activity against Escherichia coli, by cell membrane impairment via direct contact, thus inhibiting 80% of bacterial cells [26]. Another study by the same authors, using E. coli, on the gene expression analysis, disclosed that the potency of antibacterial activity of CNTs mainly depended on their size and that the a-specific disruption of the bacterial membrane was the main mechanism of their antibacterial effects [27]. In the same year, the same authors demonstrated that when exposed to MWCNTs, E. coli revealed significant oxidative stress (OS), complemented by cell membrane disruption, cell lysis, and release of intracellular contents [20]. Nagai and Toyokuni, who studied the differences and similarities between carbon nanotubes and asbestos fibres’ effects on cells during mesothelial carcinogenesis, also reported that the mechanism by which CNTs enter non-phagocytic cells was mainly based on the impairment of cell membrane by direct pore formation on their surface [91]. On the other hand, Kang et al. reported that the length of CNTs is pivotal for their interactions with bacterial membranes, where shorter tubes showed higher toxicity against bacteria [27]. Additionally, Aslan et al. remarked that shorter SWCNTs were more toxic to target cells due to the higher density of open tube ends [92]. A similar observation was also reported by Johnson, showing that nanotubes with smaller diameters manage to impair membranes of target cells via easier interactions with their surface. More specifically, concerning the inhibition of some bacteria and fungi, including E. coli, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans caused by exposure to SWNT, DWNT, and MWNT, it was demonstrated that their death was induced by the accumulation of CNTs following their entrapment onto the microbial cell surface [93,94]. Differently, Arias et al. observed that SWCNTs functionalized with -OH and -COOH groups used in buffered suspension caused Salmonella cell aggregation and the formation of SWCNTs-pathogens aggregates with an increasing size depending on SWCNTs concentrations [95]. Although the authors admitted that additional mechanisms for the toxicity of SWCNTs to bacterial cells could exist, this was assumed to be the main one. The toxicity of SWCNTs to bacterial cells was also dependent on a buffer of suspensions [95]. Many studies have confirmed that SWCNTs are more toxic to different pathogens than MWCNTs and cause their membrane disruption [20,28]. Kang et al. found that while SWCNTs kill most E. coli bacterial cells after one hour of treatment, incubation with MWCNTs leaves bacteria alive [20,27]. The authors also evidenced that E. coli exposed to SWCNTs displayed higher levels of stress-related genes compared to MWCNT treatments. On the contrary, Young et al. demonstrated that the MWCNTs are more toxic than SWCNTs to E. coli [96]. Also, Saleemi et al., who have assessed the antimicrobial effects of DWCNTs and MWCNTs against S. aureus, P. aeruginosa, Klebsiella pneumoniae, and C. albicans, demonstrated that non-covalently dispersed MWCNTs exhibited higher antimicrobial activity than DWCNTs [97]. Despite the contradictory reports, which need further investigations, CNTs are very competitive compared to other nanomaterials in inhibiting a broad range of microorganisms, including both Gram-positive and Gram-negative species and fungi, such as S. aureus, E. coli, Enterococcus faecalis, Lactobacillus acidophilus, and Bifidobacterium adolescentis [20,26,28]. As reported in Figure 5B, several key factors can affect the antimicrobial efficacy of CNTs, including their composition, geometry, surface modification, intrinsic properties, and electronic structure [90]. In this regard, metallic SWCNTs possess higher antimicrobial effects than semiconducting CNTs due to their capability to cause oxidation of pathogens intracellular components [98]. Anyway, the antibacterial activity of CNTs may also depend on the species and morphology of microorganisms [28,99], the mechanical properties of cell surfaces [99], and their growth state (planktonic or sessile) (Figure 5B) [100]. Gram-positive bacteria, such as B. subtilis and S. aureus, are more susceptible to CNTs piercing due to their softer surface, thus leading to higher bacteria death rates [28,99]. Chen et al. demonstrated that CNTs work better against spherical-shaped pathogens than rod-shaped ones [28]. Moreover, as observed for traditional or innovative antibiotics, when microorganisms protect themselves within the structure of biofilm, they are difficult to reach, thus becoming less susceptible also to the effects of CNTs [100]. Anyway, it has been reported that longer immobilized CNTs prevent bacterial settlement and biofilm growth [101], while vertically aligned arrays of carbon nanotubes (VACNTs), consisting of tubes much smaller than the usual size of a bacterial cell, can reduce biofilm formation [102]. However, since the overall mechanisms by which CNTs prevent biofilm establishment and maturation have not yet been fully clarified, more extensive research is required to design robust and effective CNT-based coating materials to impede biofilm formation [90]
3.1.2. Most Relevant Case Studies on the Antimicrobial Effects of Non-Modified CNTs
Table 1 below collects some relevant case studies reported over the years about the antimicrobial properties of non-modified CNTs.

Table 1.
The antimicrobial performance of pristine carbon nanotubes in different studies.
Collectively, the results from studies reported in Table 1 established that both pristine SWCNTs and MWCNTs prepared by different methods possess moderate to remarkable antimicrobial and microbicide effects (1.5–250 µg/mL) against several species of Gram-positive and Gram-negative bacteria and C. albicans. As shown above in Figure 5A, most studies have confirmed that CNTs inhibit pathogens mainly by causing irreversible damage to their outer membrane. Upon aggregation on their surface via electrostatic interactions, severe impairments of microbial cell integrity occur by length-dependent wrapping and diameter-dependent piercing, determining cell lysis and lethal release of intracellular contents. Additionally, CNTs have shown the capability to impede the adhesion of bacteria on surfaces, thus paving the way for their promising application to prevent biofilm formation. Generally, the antimicrobial activity of MWCNTs was higher than that of DWCNTs but lower than that of SWCNTs.
3.1.3. Most Relevant Case Studies on the Antimicrobial Effects of Modified CNTs
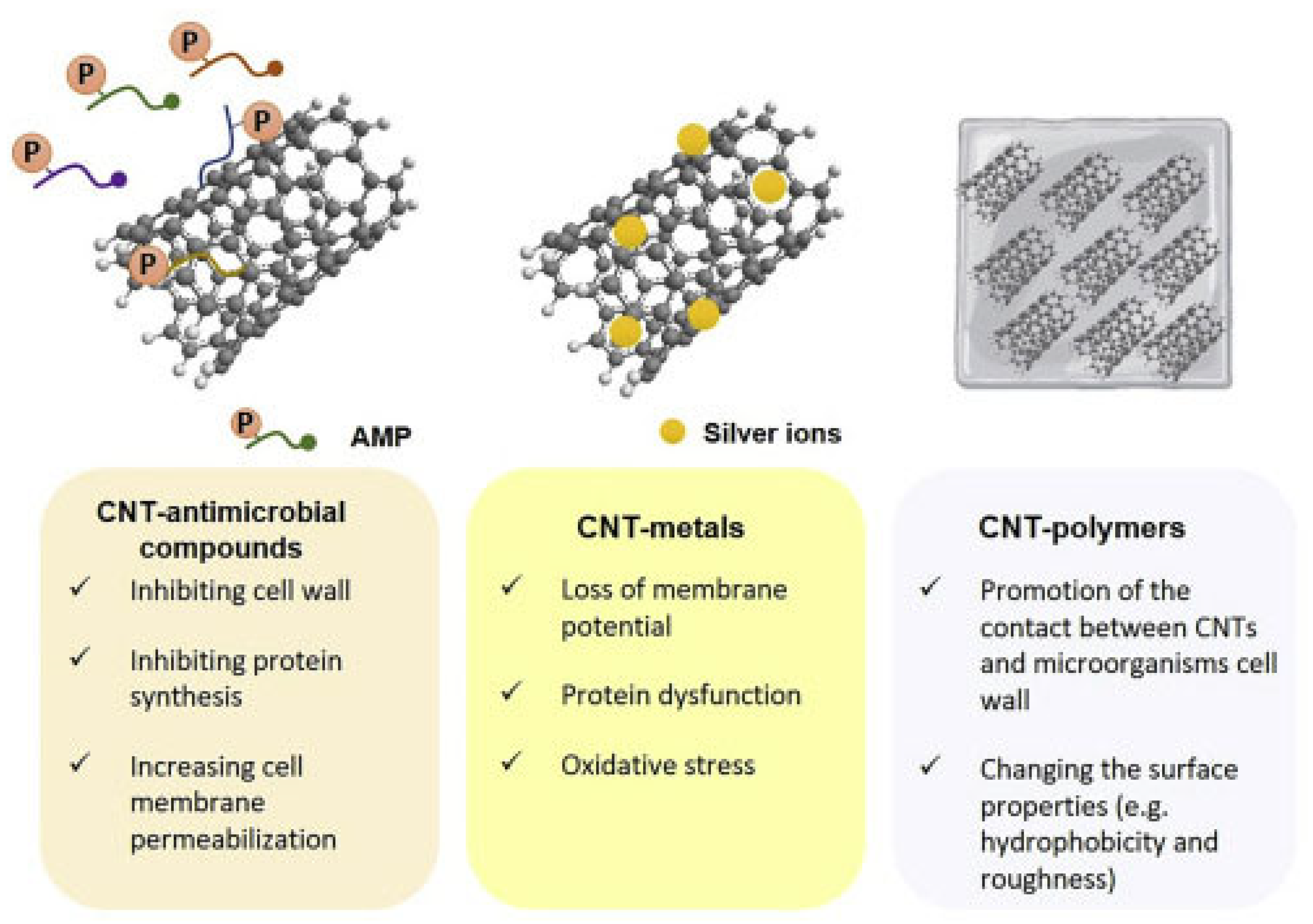
Several other studies investigating the antimicrobial properties of SWCNTs and MWCNTs, have been focused specifically on assessing the effect of their functionalization with different chemical groups and/or their modification by combination with metal nanoparticles (MNPs), polymers, antimicrobial peptides (AMPs), dendrimers, antibodies, etc., on their antimicrobial ability toward various bacterial and fungi strains. In this regard, Figure 6 shows the main synergistic effects observed by associating AMPs, MNPs, and polymers with CNTs [90].
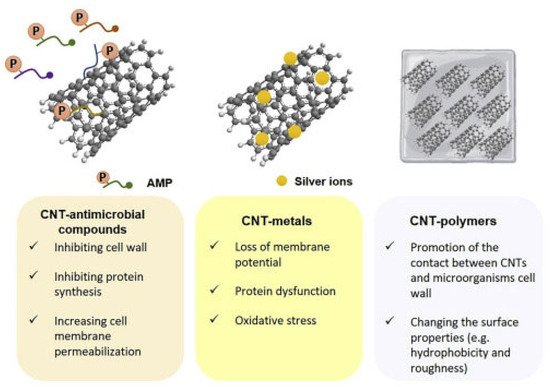
Figure 6.
Synergistic effects observed by associating CNTs to AMPs, MNPs, and polymers. This image has been reproduced by an article published in open access [90] and available under the Creative Commons CC-BY-NC-ND (CC BY-NC-ND 4.0, https://creativecommons.org/licenses/by-nc-nd/4.0/, accessed on 18 March 2025) license and permits non-commercial use of the work as published, without adaptation or alteration provided the work is fully attributed.
In the following, a brief description of the most relevant results observed using modified CNTs is reported.
Modified SWCNTs
CNTs can be functionalized by both covalent and non-covalent methods. The functionalization of the surface of CNTs could have different goals, including the avoidance of desorption processes and of undesired absorption of molecules from the biological media, as well as enhancing the effectiveness of their antimicrobial activity [107,108,109,110,111]. Correlations between the toxicity to bacteria and the physicochemical properties or the agglomeration status of functionalized SWCNTs (f-SWCNTs) were studied by Pasquini et al., discovering that no direct correlation was identified between the bacterial cytotoxicity and thermal, physicochemical, and structural properties of f-SWCNTs [112]. On the contrary, they found that the aggregation of nanoparticles had more incidence than the single chemical and physical properties of functional groups on the f-SWCNTs cytotoxicity [112]. Arias et al. functionalized SWCNTs with different groups and tested them in suspension to evaluate the possible increase in their interaction with pathogens and the effects on their antimicrobial activity [95]. Specifically, the authors studied the effects of various surface functional groups, including -NH2, -COOH, and -OH ones, on their antimicrobial effects against S. aureus, B. subtilis, and Salmonella typhimurium. SWCNTs bearing the cationic -NH2 group inhibited bacterial growth only at high concentrations. On the contrary, SWCNTs bearing anionic -COOH and neutral -OH groups reduced the viability of pathogens by 7 log CFU/g. To explain this fact, the authors assumed that the long carbon chain used to attach the NH2 groups to SWCNTs surface impedes the cylindrical shape of SWCNTs from being in close direct contact with microbial cell walls, thus probably reducing their inhibitory effects. On the contrary, -COOH and -OH groups were derived directly from the surface of SWCNTs, thus allowing the direct contact of bacteria with SWCNTs surface, thus sustaining and enhancing their antimicrobial potency. Several authors have reported on the antimicrobial activity of silver nanoparticles (AgNPs) and other metal oxides together with their inhibitory effects on the infections [113]. Chaudhari et al. observed that the antimicrobial properties of silver-coated SWCNTs on S. aureus, when applied in a skin model, can be modified with antimicrobial peptides (AMPs) [114]. In this regard, they observed that the proliferation of bacteria was reduced by 105 CFU/g by silver-coated functionalized CNT after skin treatment [114]. Generally, AgNPs bind and penetrate the bacterial cell membrane, causing cell death by altering the permeability of the membrane and increasing ROS induction [115], while AMPs have shown antimicrobial effects on various fungi, bacteria, and viruses by the same mechanisms [116]. Therefore, the synergistic effects of AgNPs with AMPs increase the toxic effects of SWCNTs, thus allowing for the possible development of novel antimicrobial therapies [114]. The same authors tested their functionalized SWCNTs against S. aureus, Streptococcus pyogenes, Salmonella enterica serovar Typhimurium, and E. coli [117], finding that the conjugation led to a strong synergistic antibacterial effect of TP359 with SWCNTs-adsorbed AgNPs. Kumar et al. reported the excellent antibacterial potency of SWCNTs enriched with AgNPs inserted in cotton fabrics against S. aureus and E. coli [118]. Moreover, AgNPs embedded in the silica-coated SWCNT substrate inactivated E. coli growth better, with respect to AgNPs plasma-treated SWCNT substrates [119]. Eco-designed biohybrids based on liposomes containing cholesterol, mint–nano-silver, and carbon nanotubes demonstrated antioxidant and antimicrobial properties against S. aureus, E. coli, and E. faecalis [120]. Chang et al. used a simple one-step procedure to synthesize nanocomposites encompassing CNTs, graphene oxide, and AgNPs effective against E. coli and S. aureus, exhibiting high disinfection properties [121]. Further investigation proved that the nanocomposite of Chang and colleagues was able to induce O2-based OS on bacteria, which damaged cell membrane integrity, thus triggering cell death. SWCNTs coated with Ag-doped TiO2 nanoparticles demonstrated strong antibacterial activity against both E. coli and S. aureus, although S. aureus was more tolerant than E. coli under illumination by UV light [107]. Park et al. manufactured pegylated SWCNTs (pSWCNTs) covered AgNPs, and their antibacterial effects were investigated on foodborne pathogenic bacteria [122]. A significant reduction in proteins associated with bacterial biofilm formation and quorum sensing, as well as of proteins necessary for the conservation of cellular structure, were observed. This was associated with a decrease in cell motility in the foodborne pathogens that remained alive. Also, by a simple and low-cost one-pot synthetic procedure, Singh et al. produced a SWCNTs/Ag/PPy-based nanocomposite which succeed in fully inhibiting the growth of S. aureus, P. aeruginosa, E. coli, and B. cereus completely within 24 h treatment [123]. A new antimicrobial nano system made of mesoporous silica and AgNP-coated SWCNTs (SWCNTs@mSiO2-TSD@Ag), which demonstrated strong antimicrobial activity against multidrug-resistant (MDR) bacterial strains by impairing cell membrane and releasing of Ag ions, was projected by Zhu et al. [124]. Yun et al. manufactured CNTs-Ag and GO-Ag, which demonstrated antibacterial effects against both Gram-positive and Gram-negative species, even if the effects of CNTs-Ag were higher than those of GO-Ag nanocomposites [125]. Additionally, a carbon-Ag nanosized complex inhibited the microbial growth of methicillin-resistant S. aureus, K. pneumoniae, Acinetobacter baumannii, Yersinia pestis, and Burkholderia cepacian [126]. SWCNTs were also modified with natural enzymes, such as lysozyme (LSZ) succeeding in enhancing their antibacterial impact against different bacterial species, including S. aureus and Micrococcus lysodeikticus, by causing cell wall lysis via hydrolytic breck of the β-1, 4 bonds in peptidoglycan [127,128,129]. Chemical modifications of SWCNTs were mainly finalized to enhance their dissolution properties and chemical compatibility. On the contrary, the functionalization of SWCNTs with polymers, achieving deposited aggregates and membrane coatings, improved their dispersibility and solubility and increased the interfacial interaction to polymeric matrices in their composites, thus demonstrating high chemical stability and high toxicity toward the microbes. In contrast, pristine SWCNTs, polymer-modified SWCNTs are less expensive and provide an enlarged range of mechanical, degradation, and structural properties, thus being suitable for developing ideal antimicrobial nanomaterials. Several case studies have been available in the literature reporting on the antibacterial activity of these carbon-based nanocomposites. Aslan et al. ideated SWCNTs with poly-(lactic-co-glycolic acid) (PLGA) and explored their antibacterial effects against S. epidermidis and E. coli, observing a 98% viability reduction and a significant decrease in the metabolic activity of the bacteria [92]. On the other hand, the SWCNTs modified with polyvinyl-N-carbazole demonstrated a 90 and 94% inhibition of planktonic cells for B. subtilis and E. coli, respectively, while reducing their biofilm formation [130]. Nanocomposite deriving by refashioning SWCNTs with poly-(L-glutamic acid) and poly-(L-lysine) resulted in a 90% inhibition rate of E. coli and S. epidermidis [131]. Goodwin et al. synthesized a SWCNTs-poly-(vinyl alcohol) nanosized composite, which gradually inactivated P. aeruginosa cells depending on increasing concentrations of SWCNTs [132]. SWCNTs reformulated with porphyrin, which possessed appreciable antibacterial effects against S. aureus in the presence of visible light and used a tungsten-halogen lamp, were reported by Sah et al. [133]. The activity of these materials was based on a photochemical reaction, which ultimately transferred an electron to the atmospheric molecular oxygen to form ROS, which destructed the bacterial cell wall, thus driving bacterial death [133]. Also, SWCNTs covalently bound with polyamide membranes showed a 66% inactivation of bacteria, translating into a delay in membrane biofouling [134]. The well-known poly-(ethylene glycol) (PEG) coating agents, recognized for their high hydrophilicity and biocompatibility, were attached in their linear and branched form by Cajero-Zul et al. to the surface of CNTs, achieving a nanoplatform suitable to manufacture medical devices [135]. Even if SWCNTs-copolymer of star-shaped PEG and poly-(ε caprolactone) (PCL) did not possess antimicrobial activity, they demonstrated thermal and mechanical characteristics superior to those of polymeric matrix. On the contrary, the star-shaped PCL-PEG copolymer structure prevented bacterial growth [135].
Modified MWCNTs
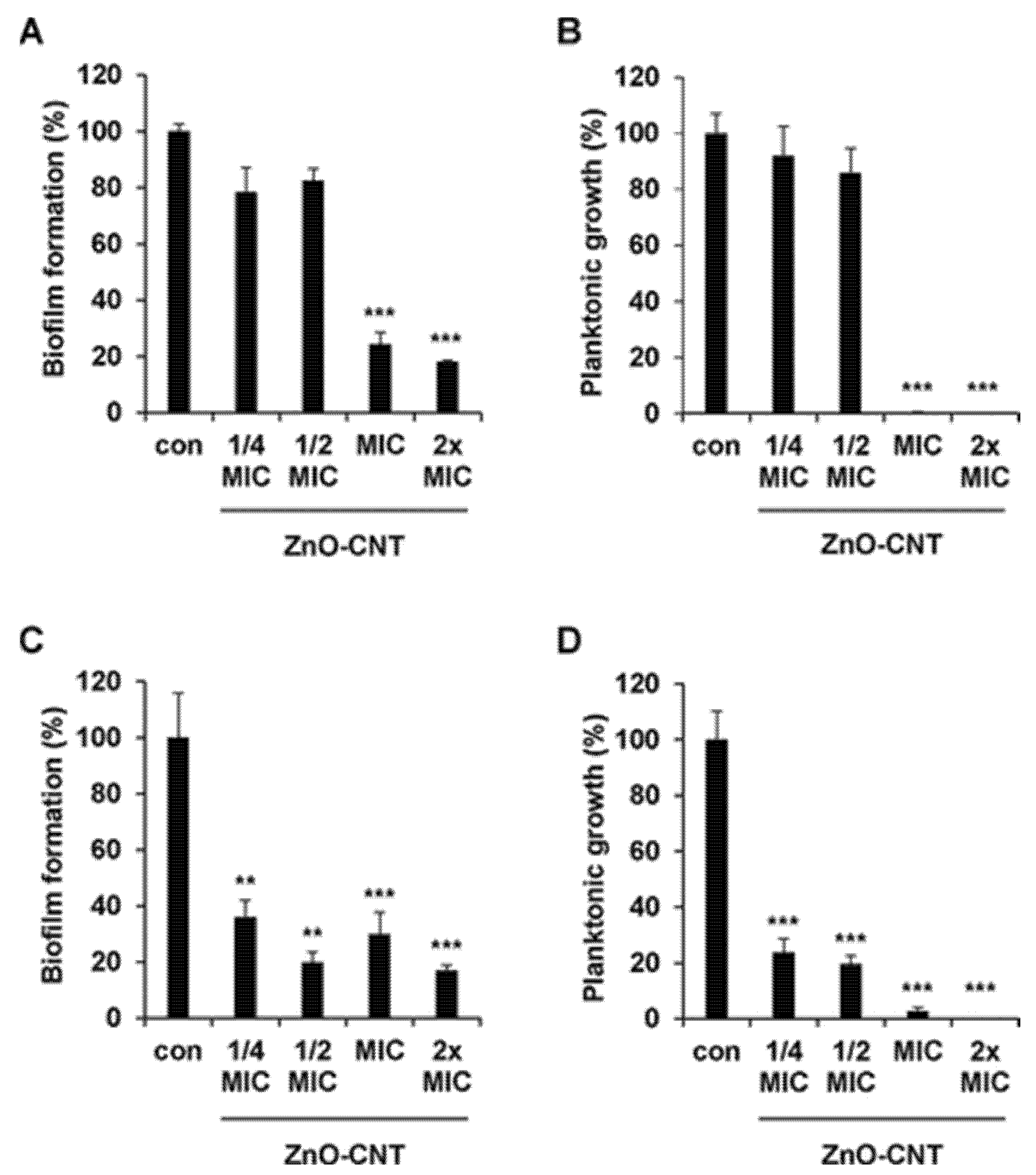
MWCNTs with surface enriched with -COOH group caused a 30, 27, 20–40, and 15~50% reduction in the viability of bacteria, including B. subtilis, P. aeruginosa, E. coli, and S. aureus [136,137,138]. Chen et al. showed that MWCNTs possessing -OH, or -COOH functional groups showed a significant dose-dependent antimicrobial effect on microbes such as E. coli, S. aureus, Enterococcus faecalis, Lactobacillus acidophilus, and B. adolescentis [28]. Ding et al. observed similar properties against Vibrio parahaemolyticus [139], while Arias et al. discovered that MWCNTs functionalized with -COOH, -NH2, and -OH did not exert appreciable antimicrobial effects with respect to analogous SWCNTs [95]. Various studies evaluated the antimicrobial action of non-covalently dispersed DWCNTs and MWCNTs against S. aureus, K. pneumoniae, P. aeruginosa, and C. albicans, finding time- and concentration-dependent mechanisms [97]. MWCNTs decorated with ethanolamine suppressed the microorganism’s growth to a large extent, compared with non-modified MWCNTs [140]. Another study showed that MWCNTs modified with oxygen groups could increase their antimicrobial properties [141]. MWCNTs added with Ag NPs revealed significant antimicrobial performance, which is like the SWCMT counterpart. Specifically, silver/MWCNTs complex caused 94–99, 57, 100 and 70% inactivation of S. epidermidis and E. coli, S. aureus, Sphingomonas spp., and Methylobacterium spp., as well as P. aeruginosa, respectively [138,142,143]. Silver-coated-MWCNTs complexed with amphiphilic poly-(propylene imine) (PPIs) dendrimers inactivated >90% of S. aureus, B. subtilis, and E. coli strains [136]. When the silver/MWCNTs complex was functionalized with polymer colloids, it displayed strong antimicrobial effect against S. aureus and E. coli [144]. Also, silver sulphide (Ag2S) quantum dots associated to poly-(amidoamine) (PAMAMs)-grafted MWCNTs showed better antimicrobial activity than cadmium sulphide quantum dot-coated-MWCNTs resulting in a microbial growth inhibition by 56, 98 and 79% when S. aureus, E. coli, and P. aeruginosa were exposed [138]. Aiming at more favourable results, researchers also amalgamated MWCNTs with copper nanoparticles, thus decreasing the bacteria viability by 75% [145]. Zinc oxide evidenced strong antimicrobial activity against E. coli [146], titanium, and gold is observed to have remarkable microbial growth inhibition against B. subtilis, K. pneumoniae, S. aureus, C. albicans, Streptococcus pneumoniae, Proteus vulgaris, and Shigella dysenteriae [147]. Very recently, Kim et al. modified MWCNTs with ZnO NPs, obtaining an antibacterial nanocomposite that demonstrated remarkably antibacterial effects against E. coli, P. aeruginosa, E. faecalis, S. aureus (Figure 7B,D). When incorporated in a urinary catheter, it inhibited E. coli and P. aeruginosa biofilm formation by 53.42 and 56.44% after 120 h of incubation, respectively (Figure 7A,C) [148].
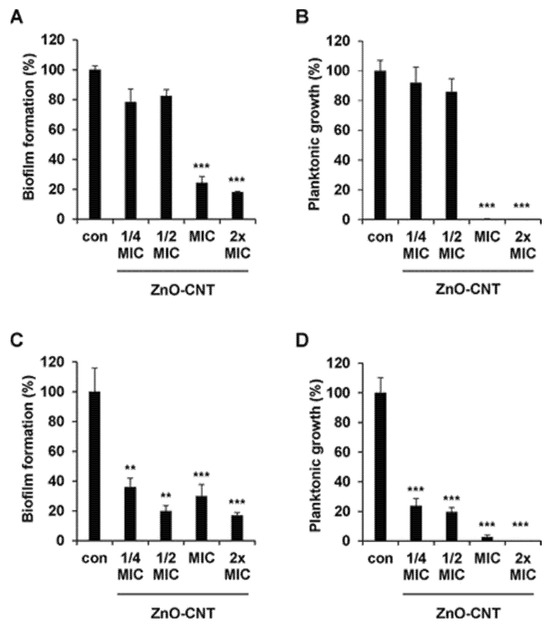
Figure 7.
ZnO-CNT nanocomposite with different concentrations, 1/4, 1/2, 1, and 2× MIC, showed biofilm inhibition against (A) E. coli and (C) P. aeruginosa. Planktonic growth inhibitions against (B) E. coli and (D) P. aeruginosa were determined. Error bars represent the mean ± SD from the average of triplicate experiments. *** p < 0.001, ** p < 0.01. Reprinted (adapted) with permission from “Copyright 2025 American Chemical Society” [148].
Moreover, titanium alloy-coated-MWCNTs combined with rifampicin inhibited the formation of biofilm for up to 5 days [149]. As in the case of SWCNTs, enzymes like chloroperoxidase (CPO) and laccase were impaled on the MWCNTs’ surface, resulting in nanocomplexes capable of reducing the viability of S. aureus and E. coli by 99%. It was observed that the laccase-MWCNTs were even capable of reducing the microbial growth and spore formation of B. cereus and B. anthracis by >99% [150]. Additionally, it was demonstrated that CPO in CPO-MWCNTs oxidated chloride into HOCl by H2O2 in acidic conditions, driving the formation of singlet oxygen. It and HOCl functioned as strong oxidants capable of inhibiting, controlling, or reducing microbial growth of E. coli and S. aureus [150]. On the contrary, it was understood that the main antimicrobial agent in the laccase-methyl syringate (MS) system was the hydroxyl radical produced mainly by the Haber–Weiss reaction starting from H2O2 and superoxide radical, which instigated detrimental and irreversible impairments in the bacterial cells leading to their destruction [150]. When MWCNTs merged with polymers, the antimicrobial activity was maintained. The bacterial growth of E. coli, B. subtilis, and S. aureus was inhibited by 87% and 97% using MWCNTs modified with amphiphilic PPIs dendrimer synthesized by Murugan et al. [136]. Similarly, Neelgund et al. formulated MWCNTs with an aromatic polyamide dendrimer, which exerted good antimicrobial effects on P. aeruginosa (65.2%) and E. coli by inhibiting their growth by 65 and 73%, respectively [137]. Also, MWCNTs functionalized with PAMAMs inhibited the growth of several selected bacteria in an NT concentration-dependent way [138], as also reported by Goodwin et al. for MWCNT-poly (vinyl alcohol) nanocomposites [132]. A current trend is increasingly driving the interest of researchers toward the evaluation of the antimicrobial activity of MWCNT-based chitosan hydrogels due to the biocompatibility of the hydrogel-based materials. In this regard, a robust antimicrobial activity of MWCNT-based chitosan hydrogels was confirmed against S. aureus, E. coli, and C. tropicalis [151], while Mohamed et al. informed that MWCNT-based chitosan possessed a broad-spectrum antimicrobial activity [152]. The following tables, Table 2 and Table 3, schematically collect the most relevant case studies concerning the antimicrobial effects of modified SWCNTs and MWCNTs, respectively.

Table 2.
Overview of the antimicrobial activity of functionalized SWCNT-based nanocomposites in different studies.

Table 3.
Overview of the antimicrobial activity of functionalized MWCNT-based nanocomposites in different studies.
From data reported in Table 2, we noted that the functionalization of SWCNTs with oxygenated groups (-OH, -COOH) translated to more enhanced bactericidal activity, while the improvement derived by amine group insertion was inferior. Combination of SWCNTs in nanocomposites led to remarkable antibacterial effects (>90% bacteria inactivation) when PLGA, PVK, and PLG/PLL polymers were used, while the antibacterial activity was moderate when PEG/poly-ε-caprolactone or polyamide polymers were utilized. Interestingly, SWCNTs-PVK induced 90% and 94% of B. subtilis and E. coli inhibition in the planktonic cells and a significant reduction of biofilm formation, thus being promising to counteract this difficult-to-treat form of pathogens resistance, causing orthopaedic implants-associated severe infections. Concerning modified MWCNTs, they generally demonstrated a time- and concentration-dependent antimicrobial activity toward both bacteria and fungi. In some cases, modified MWCNTs did not pierce the pathogen’s membrane or the non-penetrated cells, but they wrapped around their surface, causing severe injury without lysis. Anyway, in most cases, including MWCNTs modified with gold (Au) NPs, upon contact via electrostatic interactions, MWCNTs inhibited microbes by damaging their external membrane up to its disruption and cell penetration. Functionalization to provide MWCNTs-OH and -COOH materials did not significantly induce antimicrobial activity or caused a moderate to scarce inactivation of 15–50% for B. subtilis, E. coli, P. aeruginosa, and S. aureus. Better antibacterial activity was observed against S. aureus, B. subtilis, and E. coli when MWCNTs were merged with PPI (87–97% inactivation). Differently, limited antibacterial effects were displayed by MWCNTs against E. coli, P. aeruginosa, and S. aureus when modified with an aromatic polyamide dendrimer (36–73% bacteria inactivation) or a PAMAM one (23–60% bacteria inactivation). Generally, modified CNTs exhibited higher antimicrobial activity against Gram-positive bacteria due to their softer surface and lower resistance to piercing and damage.
4. Impediments to the Extensive Application of CNTs: Toxicity Issues
Since the invention of CNTs in 1991 [17], researchers have not thought about the possible negative effects of their use and contact with humans and animals or about their environmental impact, and so they were not investigated [159]. This attitude was mostly due to the limited production processes available at that time, which forced researchers to generate CNTs only on a laboratory level and at a high cost [160]. Only when novel methods, such as CVD, made their production possible on a massive scale since 2000 did the impact of CNTs on health become progressively studied through toxicological studies [161]. It was mainly their shape, like that of asbestos fibres, that prompted the very first health warnings. In fact, asbestos fibres were already identified as material causing inflammation and leading to lung cancer, which starts when macrophage cells try to absorb the needle-like asbestos fibres but fail because they are too long, thus causing the activation of the so-called giant cells. The constant activation of these cells can drive to the establishment of a nodular tissue (granuloma), which may translate into mesothelioma over a long latency period of thirty to forty years. On the other hand, since a huge range of CNTs exists, their possible negative biological effects depend mainly on their shape. To check this assumption, most studies included and include experiments on cells and animal models using rats or mice. Animals were exposed to CNTs and inhaled them through the nose or were subjected to fibres application directly to their lungs or thoraxes. The surface characteristics of CNTs also affect the possible biological outcomes deriving from cells and animals’ exposure to these materials. An injection of 400 μg/mouse of water-soluble SWCNTs has been shown to be quickly expelled through the kidneys with a half-life of 3 h. Similarly, other researchers reported that hydroxylated SWCNTs, regardless of the form of administration, were quickly removed from the body through the urine [162], thus establishing that the contact of the body with these non-bio persistent CNTs could be very short and not worrying [159].
4.1. Introduction to Environmental and Human Safety
Generally, the results from some reported case studies concerning the impact of exposure of humans and the environment to CNTS are contradictory. Indeed, fruit fly larvae nourished with a CNTs-rich diet developed normally [35], while, despite the fish foetus appearing normal, CNTs delayed embryo development in zebrafish [163]. Inflammation of the lung like that caused by asbestos fibres was perceived for a few months in mice exposed to CNTs [164]. Certain human tumour cells proliferated more rapidly when exposed to CNTs [165]. CNT-based solar cells need a cadmium-telluride mixture coating, which is extremely toxic, thus impeding the widespread use of such solar devices [165]. This establishes that frequently, coatings applied to the CNTs, rather than CNTs themselves, are environmentally dangerous. A highly worrying concern about the massive use of CNTs regards their slow biodegradation, which leads to the release of tubes in the environment, their passage into our food supply, and from food into our bodies, with consequences not fully clear until now. It must, however, be considered that CNT applications in electronics are unlikely to be very risky because of the small volumes involved. Collectively, more inspections and regulation measures are necessary in this field, and it would be suggestable to treat CNTs as a new chemical material rather than as an allotrope of inert carbon [165].
Other Hurdles Are Close to Solution
Although the potential of CNTs is huge, obstacles to their production and application on a large scale endure. First, CNT-based devices were manufactured at prohibitive costs for typical consumers [83]. For years, both researchers in industry and academic laboratories have been making efforts to develop automated systems for growing CNTs endowed with uniform and predictable properties, thus making it possible to reduce their unclear toxicity. Indeed, until they realize a method of ideally structurally perfect CNT production on a huge scale, most of their possible applications will remain in the research laboratory, and silicon will manage most technologies, including the computing one [83]. Fortunately, nowadays, this era is closer than we might think. Very recently, a carbon copilot (CARCO), an artificial intelligence (AI)-driven platform that integrates transformer-based language models, robotic chemical vapor deposition (CVD), and data-driven machine learning models, has been developed by Li et al. [166]. Employing CARCO, the authors found a new titanium-platinum bimetallic catalyst for high-density horizontally aligned carbon nanotube (HACNT) array synthesis. This catalyst outperformed traditional ones and was treasured in millions of virtual experiments; an unprecedented 56% precision in synthesizing predetermined densities of HACNT arrays was achieved [166].
4.2. In Vitro and In Vivo Studies
4.2.1. In Vitro Studies
Over the years, increasing in vitro investigations about the cytotoxicity and genotoxicity of CNTs have been reported. Following is a collection of early studies developed in the years 2003–2011. The cytotoxic effect of titanium oxide (TiO2) nanoparticles (NPs) and MWCNTs was studied in A549 human pneumocytes by Simon-Deckers et al., who evaluated cell viability and intracellular accumulation of both nanomaterials and compared results [167]. Both CNTs and TiO2 NPs were capable of entering cells and passing into the cytoplasm. Anyway, TiO2 NPs showed lower toxicity. Moreover, it was found that neither the presence of metallic impurities nor the length of CNTs influenced their cytotoxicity [167]. The capability of CNTs to penetrate the cellular membrane of both NR8383 rat macrophages and human pneumocytes A549, thus causing alteration of physiology and cellular functions, was reported by Pulskamp et al. [168]. Disfunctions are derived from a dose-dependent increase in intracellular reactive oxygen species (ROS) and from a decrease in the potential of the mitochondrial membrane in both rat NR8383 cells and human A549 lung ones. Interestingly, CNTs with deprived or with reduced metal content due to purification work-up demonstrated few or none of these undesired effects [168]. Collectively, several researchers proposed that the main response to the negative biological effects of commercial CNTs could be metal impurities. Moreover, it was reported that the cytotoxic effects of CNTs could heavily depend on their different degrees of agglomeration. Studies on human MSTO-211H cells revealed that the dispersion of CNTs in surfactant was less toxic compared to agglomerated CNTs, especially when CNTs agglomerated in string forms, which are more voluminous, more rigid, and more solid [169]. In this regard, Montero et al. studied the possible per se toxicity of different surfactants, finding that Pluronic F127 was less toxic [170]. MWCNTs, as well as MWCNTs coated with Pluronic F127, were tested on human keratinocytes, evidencing that the uncoated MWCNTs were found to be more cytotoxic [170]. Based on results from several studies, which confirmed the capability of CNTs to cross cell membranes [167,171], the intracellular distribution of modified SWCNTs was analysed in human fibroblasts 3T6 and murine 3T3 cells by Pantarotto et al. [171]. The results of this study demonstrated that functionalized CNTs could cross the cytoplasmic membrane and accumulate in cytosol or reach the nucleus without toxicity for the cells up to 10 µM concentrations [171]. Pantarotto et al. and Manna et al. investigated the toxicity of SWCNTs in HaCaT, HeLa, H1299, and A549 cells at different concentrations and exposure timings, finding a major loss of cell viability in keratinocytes (HaCaT), probably due to the activation of keratinocyte-specific transcription factor NF-κB by increased oxidative stress (OS) [171,172]. Anyway, Shvedova et al. have already explained that the unpleasant consequences demonstrated by SWCNTs in HaCaT and bronchial epithelial (BEAS-2B) cells, including alterations in cell ultrastructure and morphology, loss of cellular integrity and cellular apoptosis, OS, and depletion of antioxidants in the cells is specifically based on the alteration of certain genes following exposure to SWCNTs. Additionally, the authors declared that exposure to SWCNTs can trigger cutaneous and pulmonary toxicity [173]. Later, the same authors administered mouse macrophages (RAW 264.7) with different concentrations of SWCNTs, finding that even well-dispersed SWCNTs penetrated through the alveolar epithelium, caused interstitial fibrosis and alveolar wall thickening [174]. The production of TGF-β1 was triggered similarly to the effect of zymosan. Anyway, neither oxidative response nor nitric oxide production or cellular apoptosis was induced by CNTs [174]. The uptake of SWCNTs enriched with fluorescein-isothiocyanate by lymphocytes and macrophages reported by Dumortier et al. [175], upon in vitro experiments, did not lead to any changes in cell viability. These findings confirmed previous reports on in vitro cytotoxicity studies using human dermal fibroblasts exposed to modified SWCNTs, which demonstrated toxic effects lower than those observed using not modified SWCNTs [176]. Unexpectedly, while streptavidin-conjugated SWCNTs exhibited low toxicity in HL60 cells, the SWCNT–biotin–streptavidin complex caused cell death [177]. However, despite the functionalization of SWCNTs generally enhancing their potential by reducing their toxic effects, a general increase in toxicity was observed for the MWCNTs bearing carbonyl (C=O), carboxyl (COOH), and/or hydroxyl (OH) groups [178]. Moreover, indirect cytotoxicity of CNTs was reported deriving from their capability to stimulate immune-mediated cytotoxicity in various human cells, even at low concentrations (0.001–0.1 mg/mL). In this regard, it was reported that CNTs at lower concentrations may stimulate the secretion of cytokines with consequent activation of lymphocytes and upregulation of the NF-κB expression in immune cells [179]. The effect of SWCNTs on human epidermal keratinocytes (HEK293T) was investigated by Cui et al. using several techniques [180]. A dose- and time-dependent decrease in cell proliferation and adhesive ability was observed, with the formation of nodular structures, induction of G1 arrest, and apoptosis. SWCNTs (10–20 nm in diameter) showed higher toxicity than MWCNTs of the same dimensions, while fullerenes did not show any cytotoxicity when they were administered to alveolar macrophages in guinea pigs. These results suggested that the geometric structures of carbon nanomaterials are discriminant for cytotoxicity [181]. Pacurari et al. [182] demonstrated that SWCNTs induced ROS generation, increased cell death, and enhanced DNA damage and H2AX phosphorylation due to their fibrous characteristics involved in ROS generation. The DNA damage occurring upon exposure of lung V79 fibroblasts to acid-purified SWCNTs was confirmed by Kisin et al. [183]. According to Francis et al., Patapovich found that, while iron-rich SWCNTs activated the macrophages and catalysed the transformation of extracellular O2– into detrimental hydroxyl radicals, thus encouraging IL-6 production, and causing OS and inflammation, the iron-deprived SWCNTs stimulated TGF-β production, thus promoting apoptosis [184]. MWCNTs were found capable of causing the release of proinflammatory cytokines (TNF-α) in rat peritoneal macrophages [185]. The cytotoxic effects of MWCNTs in rat and mouse peritoneal macrophages (J774.1) were assessed by Muller et al. and Hirano et al. They found higher cytotoxicity of CNTs with respect to that of crocidolite in mouse macrophages due to the action of MWCNTs with MARCO (macrophage receptor with collagenous structure), resulting in the disruption of the plasma membrane [185,186]. Human embryonic kidney 293 cells (HEK-293) exposed for prolonged times to high concentrations of MWCNTs showed reduced viability, a significant increase in IL-8, and altered expression of several proteins, supported by an increasing penetration of MWCNTs via cell membrane over time [187,188]. Also, MWCNTs induced cytotoxicity through GSH depletion, ROS generation, OS, cell inflammation, membrane leakage, lipid peroxidation, and protein release [189]. When human aortic endothelial cells were treated with CNTs, an increase in the messenger RNA of MCP-1, VCAM-1, and IL-8 was observed [184], while when human skin fibroblasts (HSF42) were exposed to MWCNTs and multi-walled carbon nano-onions (MWCNO), inhibition of the cellular cycle and an increase in apoptosis and necrosis, were detected [190]. Several intracellular signalling pathways were altered after exposure to CNTs, depending on the material tested and the dose, establishing that MWCNTs are more toxic than MWCNO by an interferon and p38/ERK-MAPK mediated toxicity. Soto et al., using chrysotile as a positive control, explained that the cellular response to MWCNTs aggregates was like that observed for the chrysotile-induced response [191]. Similarly, Murr et al. showed that SWCNTs aggregates, two types of MWCNTs aggregates, and the chrysotile aggregate provoked cell death starting at 2.5 µg/mL with similar cytotoxic response in mouse alveolar macrophages [192]. Also, Bottini et al. compared the time- and dose-dependent toxicity to T-lymphocytes caused by not modified MWCNTs- and oxidized MWCNTs finding that the latter induced significant apoptosis, being more toxic than hydrophobic pristine MWCNTs, which can result toxic only at high concentrations [193]. The studies reported on the cytotoxicity of CNTs are summarized in Table 4.

Table 4.
In vitro cytotoxicity of CNTs for early experiment of years 2003–2011.
4.2.2. In Vivo Studies: Pulmonary Toxicity
Over the years, experts in the field have developed increasing specialization in devising synthetic and characterization methods for obtaining improved CNTs in large scale. At the same time, the application possibilities of CNTs have grown exponentially to the point of making more studies on their possible cytotoxicity and genotoxicity imperative, also in vivo. The almost daily use of CNTs in novel nanotube-based products greatly increased the possibility of contact with humans, animals, and the environment. CNTs can enter the body through various routes of exposure, including derma, mouth, and nose. Among the possible contact opportunities, CNTs can enter the respiratory airways of the workers and accumulate in the lungs first during manufacture. Also, since CNTs are used as fillers in food packaging products, they can reach the gastrointestinal tract (GIT) of the consumers. Several reports from in vivo studies on rodents have demonstrated that the ingestion of SWCNTs and MWCNTs is toxic. Based on these results, the possibility that CNTs could also be risky to humans has become an increasingly worrying reality, and confirming or refuting it is a necessity. Pulmonary toxicity of raw CNTs, containing iron impurities, acid-purified CNTs, and CarboLex CNTs-rich in nickel and yttrium impurities, was investigated by Lam et al. They dispersed all samples in the serum and in carbon black, or quartz particles were used as negative and positive controls [194]. The samples were administered intratracheally to mice at different concentrations. After 7 days, in the mice group treated with CNTs, epithelioid granulomas and interstitial inflammation were observed in a dose-dependent way, which augmented after 90 days. Moreover, peri-bronchial inflammation and necrosis were also discovered in the lungs of animals that were treated with CNTs via alveolar septa, thus establishing that CNTs are more toxic than carbon black and quartz, which led to negative health effects only in chronic inhalation exposures [194]. Pulmonary toxicity of pristine SWCNTs was assessed by Warheit et al., which used quartz particles as positive control and carbonyl iron particles as negative control, using rodents as animal models [195]. Rats were administered intratracheally with SWCNTs at 1 and 5 mg/kg. Upon administration of the latter dose, 15% of rats died within 24 h, probably due to the mechanical obstruction of the airways by the SWCNTs. Anyway, transient inflammation and cell damage were found in surviving animals, while multifocal granulomas, attributable to tissue reactions against the foreign body, were observed. These early findings confirmed the cytotoxic power of SWCNTs while demonstrating the huge necessity for more chronic study. Shvedova et al. showed that pharyngeal aspiration of SWCNTs induced anomalous pulmonary effects in C57BL/6 mice, which brought acute inflammation, rapid progressive fibrosis, granuloma, and alveolar wall thickening in the lungs [174]. These pathological lesions were associated with functional respiratory deficiencies and decreased bacterial clearance. To complete this worrying scenario, increased clinical marker values were detected in bronchoalveolar lavage (BAL) fluid, which endorsed that the SWCNTs were more toxic than crystalline silica [174]. Even higher inflammatory response, OS, collagen deposition, and fibrosis were observed in C57BL/6 mice when SWCNTs were inhaled rather than pharyngeal aspirated. Anyway, pharyngeal aspiration of SWCNTs provoked faster atherosclerotic plaque formation in ApoE mice [196]. Mutlu et al. administered intratracheally raw aggregated and highly dispersed SWCNTs in 1% Pluronic F 108NF to mice at a 40-µg dose, which was higher than the dose used by Shvedova et al. [174] to induce pulmonary fibrosis in mice [197]. Lung inflammation was induced by aggregated SWCNTs in PBS, while highly dispersed SWCNTs do not cause any adverse phenomena. Muller et al. reported the respiratory toxicity of both MWCNTs and ground MWCNTs suspended in sterile saline (0.9% NaCl), which was observed after 2 months of exposure in the form of pulmonary lesions characterized by collagen-rich granulomas, dose-related pulmonary fibrosis, and increased production of TNF-α [185]. The dangerousness of the inflammatory effect produced by MWCNTs was anyway halved with respect to asbestos and carbon black [185]. Mitchel et al. reported that the exposure of mice to MWCNTs at high doses caused immunosuppression after 14 days of whole-body inhalation. On the contrary, inflammation and granuloma formation were not observed, which was different from what was previously reported [198]. Later, Muller et al. administered intratracheally to rats (2 mg/rat) MWCNTs in the forms of both tubes heated to 600 °C and 2400 °C and ground MWCNTs heated at 2400 °C [199]. Observations revealed that the pulmonary toxicity of MWCNTs was less compared to those of ground MWCNTs, thus indicating that the toxicity of the tubes mostly depends on the imperfect sites in their carbon architecture. However, the group of Donaldson showed that the toxicity of ground MWCNTs may depend either on their larger dispersion in the lungs or on the effects of the released metals upon grinding [200]. Poland and his group reported that mice showed an asbestos-like pathogenic behaviour after exposition to long MWCNTs, which translated into inflammation and granuloma formation. Such a pathogenic scenario was also observed in the mice administered directly into the abdominal cavity with CNTs [32]. The additional exposure of lungs, with allergen-based inflammation, to SWCNTs caused increased pulmonary toxicity characterized by increased lung protein levels of T helper cytokines and chemokines, augmented the level of OS-related biomarkers and accessory allergen-specific IgG1 and IgE activity [201]. By light microscopic examination technique, Kobayashi et al. showed that MWCNT aggregates, accumulated in the lungs, were phagocytized by alveolar macrophages and remained up to 6 months post-exposure [202]. Granulomatous lesions or collagen accumulations were observed only in animals exposed to highly dispersed MWCNTs through intratracheal administration but not in those administered with MWCNTs by inhalation, thus demonstrating that MWCNTs produce pulmonary lesions, based on the route of administration and dose [203]. Also, Grubek-Jaworska et al. showed that four different commercial MWCNTs and SWCNTs, characterized by very low content of iron intratracheally administered, caused pneumonia with an interstitial non-specific focal reaction in guinea pigs, treated for 3 months [204]. Significantly increased concentrations of IL-8 in the bronchoalveolar lavage (BAL) fluids and augmented macrophages and eosinophils were instead caused by other types of CNTs. Additionally, carbon sediments were found mainly in the bronchioles, while the alveolar ducts and the alveoli were free. No granulomas were detected in lungs, while CNT aggregates and mechanical obstructions were found in certain airways of some animals. More recently, Francis et al. showed that the single exposure of rats to MWCNTs per se induced inflammation, epithelial cell membrane damage, and cell lysis, confirmed by augmented inflammation markers concentrations, including TNF-α, IL-4, LDH, WBC, and increased ALP activity [205]. Histopathological experiments revealed that dispersion of MWCNT in the lungs caused fibrosis and granuloma. Since occupational and intentional exposure to MWCNTs has exponentially increased over the years with large-scale production of CNTs, it is mandatory to investigate their toxicity upon prolonged exposure timings. For an easier understanding by the readers of the scenario of experiments carried out to accredit the possible pulmonary toxicity of CTNs, the early pulmonary toxicity studies discussed in this section on CNTs have been summarized in Table 5.

Table 5.
In vivo case studies on pulmonary toxicity of CNTs.
CNTs Cytotoxic Effects to Other Tissues
Initial animal studies reported previously in this section evidenced that exposure of rodents to different forms of CNTs can cause acute and chronic pulmonary inflammation, based on increasing OS and on the assumption of inflammatory effects of atherosclerosis and air pollution [174,185,194,195]. Based on these results, it was presumed that the toxic effects of CNTs could also affect other tissues. In this regard, some authors, such as Simenova and Erdely [206], as well as Li et al. [196], investigated the cardiovascular toxicity of CNTs in rodents by different ways of administration. The studies revealed that CNTs are capable of activating the blood cells via inflammatory markers release, which leads to adverse cardiovascular effects. Damage to the mitochondrial DNA of the aorta was observed in a stress-dependent and dose-dependent mode. The effects caused by CNTs may predispose to atherogenesis. An activated oxidative marker was found in the aorta and cardiac tissues of mice with high blood cholesterol levels exposed to SWCNTs, which also developed aortic DNA damage. Atherosclerotic plaques on the surface of the aorta and increased atherosclerotic lesions in the brachiocephalic arteries were observed in mice that had been exposed to SWCNTs [184,196,206]. Pietroiusti et al. [207], Bai et al. [208] and Philbrook et al. [209] studied the toxic effects of CNTs on the reproductive and developmental system. It was reported that functionalized CNTs administered at low doses were capable of triggering high resorption processes, progressive malformations in the survived infant rodents, and induced ROS generation in the placentas of exposed animals. A higher percentage of resorptions, as well as huge morphological defects and skeletal abnormalities in foetuses, were discovered in Drosophila melanogaster and CD-1 mice exposed to functionalized CNTs. CNTs accumulated in the testicles, causing OS, tissue damage, and alterations, which raised concerns about possible adverse effects on male fertility [207,208,209]. A collection of more recent case studies on in vitro and in vivo CNT toxicity has been reported in Table 6, while Table 7 collects case studies on CMT toxicity in various organs. Table 6 and Table 7 already contain detailed explanations of observed outcomes and do not need additional discussion.

Table 6.
In vitro and in vivo studies for assessing the possible cytotoxicity and genotoxicity by exposure to CNTs.

Table 7.
A quick overview of CNTs’ toxicity on various organs by in vivo and in vitro studies.
5. Possible Strategies to Moderate CNTs’ Toxic Effects: Future Perspective and Preventive Actions
CNTs cause a cascade of events that are noxious to human and animal body tissues. In this regard, Lettiero et al. and Moghimi et al. have reported that inhibitors of such events, achievable by CNTs surface modifications and functionalization, could prevent the activation of the toxic system in the body tissues [232,233]. Generally, PEG-modified SWCNTs were not toxic and biocompatible to mice, achieving the longest blood circulation in 1 day and being fully eliminated from the vital tissues of mice in about 2 months [234]. Longer circulation time, minor uptake in the reticule-endothelial system, and reduced accumulation in the spleen and liver were observed for PEG-CNTs by Yang et al. [235]. Coating CNTs with C1q recombinant globular proteins and adding functional groups of different types to their side walls are other methods to minimize CNT toxicity [176,236]. Silva et al. reported that the onset of CNT toxicity is strictly correlated with the route of administration [237]. CNTs administered by inhalation provided no sign of inflammation after 1 day, but inflammation appeared after 21 days. On the contrary, instilled CNTs caused inflammation after 1 day of exposure, which disappeared after 21 days [238].
Adding catalytic horseradish peroxidase that promoted -COOH CNTs degradation in an acidic medium led to no toxic or inflammatory effects on the lungs of mice used for toxicity experiments.
Collectively, limiting the toxicity of CNTs remains a serious aspect of their safer use in various applications. Table 8 collects some relevant strategies proposed over the years up until now to mitigate potential harmful effects, which could arise from an extensive exposure to CNTs.

Table 8.
Strategies to reduce CNT toxicity.
Proper modification of the surface of CNTs with biocompatible materials or selected molecules can enhance their dispersion in biological fluids and reduce toxicity. Functionalization can also influence cellular uptake and interactions [252,253]. One of the main causes of the hazardous effects of CNTs on the human and animal body is their poor water solubility. This is a problem that may be solved by CNT surface modification [254,255]. A current trend followed by scientists in the sector consists of searching for biocompatible substances that may be smeared with CNTs to improve their characteristics [256]. Much research has been done on the detrimental effects of CNTs on pulmonary systems, with confirming results on A549 cells [257,258]. By covering MWCNTs with curcumin, their propensity to induce OS, inflammation, and cell death was significantly reduced [243]. Wu et al. enriched MWCNTs-COOH and pegylated MWCNTs with oxaliplatin, a cisplatin-derived chemotherapeutic, by both their surface functionalization with the drug and its encapsulation in CNTs cavities [244]. The authors demonstrated that, in an aqueous environment, the entrapped oxaliplatin could easily exit MWCNT-COOH and MWCNT-polyethylene glycol (PEG) and that Pegylated MWCNTs were capable of an oxaliplatin-controlled release, thus augmenting its therapeutic effects.
Shorter and well-dispersed CNTs with a high specific surface area and robust transmembrane mobility showed lower toxicity compared to longer aggregates [245]. These substances can harm proteins and cellular components, leading to macrophage malfunction or even death [222]. Sohaebuddin et al. demonstrated that MWCNTs with large diameters provided minimal harm to lysosomes, while those with tiny diameters might create membrane instability [246]. Highly pure CNTs that do not contain residual metals or catalysts induce lower harmful effects [250]. Considerable progress has been achieved in the purification of CNTs [259]. Also, CNTs with structures that favour biodegradation over time could reduce their persistence in circulation, thus reducing tissue toxicity [260]. Concerning CNT-based delivery systems, the choice of alternative administration routes, including topical or transdermal ones, can minimize the direct exposure of sensitive organs or tissues to CNTs, thus reducing their systemic harmful impact.
Quercetin has good bioavailability and possesses formidable analgesic, antioxidant, anti-inflammatory, and anti-atherosclerotic properties [261]. Additionally, quercetin reduces reactive oxygen species (ROS) generation and increases the antioxidant enzyme activity [262]. It could be exploited as an adjuvant to prevent oxidative damage, inflammation, and immune-toxic effects of MWCNTs [251].
Despite the several strategies developed so far to limit the toxic outcomes of extensive use of CNTs to humans and the environment, it is mandatory to conduct preventive behaviour to limit the large diffusion of their possible cytotoxic impacts. Biocompatibility assays using appropriate in vitro and in vivo models are always necessary before a CNT’s widespread use. Knowing the individual biological responses to different types of CNTs is critical to assessing their specific risk [263]. Although a more specific regulation is urgently needed for these nonpareil nanomaterials, adhering to existing regulations and guidelines on nanomaterial safety is obligatory. The responsible development and use of CNTs could be assured only by ensuring compliance with regulatory standards [264]. Education training for workers who produce, handle, and apply CNTs, thus making them aware of potential risks which could derived by CNTs identified until now and implementing proper safety protocols can help to minimize harmless exposure. Concerning the environment, monitoring programs to evaluate the dispersion and potential impact of CNTs in workplaces and surrounding environments can help update risk management strategies. The invention and development of environmentally friendly or “green” synthesis procedures for creating CNTs as alternative options to the complicated machineries in use could reduce the usage of dangerous chemicals and toxic precursors and can lead to CNTs with fewer impurities and lower toxicity. Duraia et al. recently proposed a straightforward, simple method to generate CNTs on graphitic layers using corn seeds [265]. Alternative nanomaterials to CNTs with similar functionalities but lower toxicity risks should be considered. It is the case of graphene, which could be less hazardous than CNTs [266]. Indeed, as graphene is more robust than CNTs to immune cell destruction, thus limiting the possible release of hazardous compounds deriving from such degradation, it could be a promising option for CNTs. In particular, graphene oxide (GO) was less vulnerable to macrophage destruction than SWCNTs. Unfortunately, efficient standardized techniques to assess the toxicity of these nanomaterials are still challenged by difficulties and restrictions [267]. Permanent research and collaboration between scientists, engineers, and regulators are pivotal to developing safe routines for CNT production and use in various industries.
6. Regulatory Considerations
The National Institute for Occupational Safety and Health (NIOSH) is the most important federal agency in the United States, which conducts research and offers supervision for occupational safety and the consequences on human health from the extensive applications of and exposure to nanomaterials. Studies have revealed that nanoparticles (NPs) may pose a greater health risk than bulk materials due to their greater surface area/unit mass ratio [35]. In 2013, NIOSH published a Current Intelligence Bulletin detailing the potential hazards of CNTs and recommended the exposure limit for CNTs and nanosized fibres [268]. Subsequently, in October 2016, SWCNTs were registered within the European Union’s Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH) regulations as compounds to be studied concerning their possible dangerous effects. As a direct consequence, the commercialization of SWCNT in UE was permitted up to 10 metric tons. REACH has a centralized database that records all chemicals produced or imported into the EU in quantities of one ton per year or more per supplier or importer. A chemical safety report must be submitted to REACH when the quantity of its production is ten tonnes or more. There have been frequent complaints about REACH not having taken into account the nano-specific characteristics of materials, but only the amount of their production. Fundamentally, substances of a specific interest may be subjected to REACH restrictions. If such restraints can be fulfilled by the European Chemicals Agency (ECHA), a thorough comment and consultation procedure must be carried out, and the approval of the Member States’ committee must be pursued for that substance. A Candidate List of Very High Concern Substances (SVHC) containing almost 40 substances was compiled in 2010 by ECHA, but no compound was a nanomaterial. This was mainly due to the high thresholds of production quantity for nanomaterials—1 ton per year or more to impose their registration and 10 tonnes per year or more to make it necessary to submit a chemical safety report. Chemical Regulation in the EU and the US (TSCA; Toxic Substances Control Act) used the chemical composition of a material to define regulation measures without considering particle size or nano-specific features. The problem of controlling carbon-based products has become particularly apparent, especially with the advent of their large-scale production. In REACH, carbon was initially considered a non-issue (“minimum risk owing to its intrinsic features”). This status of immunity was eliminated at the end of the year 2008 when a clear differentiation was made between carbon and carbon in its nano-scale forms. In this regard, C60-fullerene received a distinct CAS (Chemical Abstracts Service) registration number according to the worldwide norms of the chemical denomination in relation to carbon, carbon black, and graphite since these materials, despite their same molecular character, necessitate to be dissociated from each other. (Nano)tubes that would have a diameter smaller than 1 nm and a length exceeding 100 nm were not considered as nanomaterials until 2011 in EU. To address this potential omission, Recommendation 2011/696/EU included in the definition both fullerenes, graphene flakes, and single-wall carbon nanotubes, with one or more external dimensions below 1 nm, as nanomaterials [269]. Unlike the EU, the US introduced CNTs under the Toxic Substances Control Act to strict notification requirements in October 2010. Their production, import, or usage (also as a continuation of ongoing activity) shall be notified to the authority, which will decide whether to import or process the substance within 90 days. The only exclusion was agreed to CNTs stalwartly integrated into a matrix. Concerning health and safety on the job, in 2011, the European Agency of Safety at Work (EU-OSHA) compiled a literature review in the field of CNTs, dealing with occupational exposure, toxicology, and protective measures in the work environment [270]. It was also a compilation of advice and guidelines for technical occupational safety and personal protective equipment. The toxicological data available for SWCNTs and MWCNTs have also been summarized [270]. The purpose of the overview was to provide the Swedish Work Environment Authority with information and a basis for various types of measures [270]. In the United Kingdom—the Health and Safety Executive (HSE), referred to the “10 Recommendations” to be considered for health and safety purposes at the job, published by the Royal Society and the Royal Academy of Engineering, 25 years earlier on the application of nanotechnologies. Based on these rules, the HSE advised on precautionary measures and risk avoidance practices. In 2009, HSE considered CNTs as “extremely concerned substances”. The British Standards Institution (BSI) suggested an exposure limit of 0.01 fibres/mL, even if investigations to monitor this limit test were time-consuming [159]. In the US, the National Institute for Occupational Safety and Health (NIOSH) recommended an occupational exposure limit of less than 7 μg CNTs per m3 of air [159]. This exposure limit is the smallest concentration that can be correctly measured [271]. On the other hand, the exposure limit for 5 mg/m3 of airborne graphite dust set by the EU Occupational Safety and Health Administration (EU-OSHA) is about 1000 times higher [159]. It should also be stressed that dust particles are bigger and, therefore, heavier. Anyway, despite only a few types of CNTs being utilized, the precautionary principles should provide indications for health, safety, and risk limitations to be applied to all types of CNT-based existing products. Indeed, since CNT handling cannot be fully prevented, it should aim at a high standard of safety and control as well [272]. Although the determination of a correct occupational limit for CNT exposure is mainly contingent on exposure data and measurement technology, the US limits concentrations dictated for CNTs are considered by NIOSH high-risk to develop adverse effects on respiratory health. In this context, the promotion of research in this area is essential and mandatory. Also, measures to limit hazards by CNTs need improvement, and measures to maintain airborne CNTs to a minimum level and below the limits of exposure should be developed. Studies have demonstrated that high concentrations of needle-shaped, long, thin, and bio-persistent CNTs have a serious adverse effect on the respiratory tract. In this context, the mechanisms of action and the dose–effect relationship for as high a number of CNT types as possible need to be clarified to be capable of compiling a dangerous chart. Also, the life cycle of CNTs in the environment still has not been thoroughly assessed, and argumentative information on CNT ecotoxicity and credible exposure data are limited and need improvement to better assess their environmental risk. Although legislation on hazardous chemicals and regulations on occupational health and safety exists, it still does not offer specific requirements for handling CNTs. Even though specifications of REACH on the volume thresholds for registration (1 JT) and for the obligatory chemical safety report (10 JT) exist, they have been set at levels that do not include all CNT manufacturers and do not consider CNT-specific features. Although efforts are proceeding at international levels in occupational health and safety to set limits for occupational airborne exposure to CNTs, specific regulations are still not adopted. Analytical and detection methods need improvements through further research and development. Lawmakers, regulators, and environmental activists’ interest in nanomaterials has incessantly increased over the years, and the interrogation of the correct nanotechnology regulations is a daily challenge. The incessant development of nanomaterials and the daily application of nanotechnology have led to rigid regulatory standards in both animal and human manufacturing and utilization. The US Environmental Protection Agency (EPA) reported that CNTs encompass substantial regulatory concerns regarding toxicity and environmental safety [159]. The US National Environmental Policy Act (NEPA) has published a series of rules regarding the main factors for controlling nanomaterials, including quality control and manufacturing and product safety evaluation, like toxicity, clearance, biodistribution, and metabolism. In the following sections, we have briefly introduced the most relevant international organizations that work to develop and publish technical standards, which cover procedures for testing, classification, and characterization of several sorts of materials. Then, we have focused on the main standards concerning nanomaterials and CNTs published by the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM) International Organization.
6.1. International Standards Concerning Nanomaterials and CNTs
Different international organizations, including ISO (1946), ASTM, BSI (1901), IEC (1906), DIN (1917), ANSI (1918), and AFNOR (1926), were created to develop and publish key standards to chemically and physically characterize materials of every sort. In the following, we consider ISO and ASTM.
6.1.1. ISO Standards
The International Organization for Standardization (ISO) brings global experts together to agree on the best way of operating in several sectors, including the preparation methods to achieve a product, its characterization, and how to manage a process. As one of the oldest non-governmental international organizations, ISO has enabled trade and cooperation between people and companies all over the world since 1946. The International Standards published by ISO must be approved by all ISO members and serve to make life easier, safer, and better (available online at https://www.iso.org/about, accessed 15 April 2025). ISO has provided several standards, guidelines, methods, and requirements concerning differently structured nanomaterials, CNTs, and CNT-containing nanocomposites. The most recent ones concerning CNTs and CNT-containing nanocomposites are available online at https://www.iso.org/search.html?PROD_isoorg_en%5Bquery%5D=carbon%20nanotubes (accessed on 15 April 2025) and have been included in the following Table 9.

Table 9.
Published and under development * ISO standards concerning CNTs and CNT-containing nanocomposites.
6.1.2. ASTM Standards
American Society for Testing and Materials (ASTM) international organization is a nonprofit organization that develops and publishes approximately 12,000 technical standards covering the procedures for testing and classification of materials of every sort. It encompasses 30,000 members from 135 countries. ASTM also serves as the administrator for the US TAGs (United States Technical Advisory Group) to an enormous amount of ISO/TCs (International Organization for Standardization/Technical Committee) and to their subcommittees. Specifically, ASTM Committee E56 on Nanotechnology was formed in 2005. Nowadays, it comprises one hundred and eighty members from twenty-two countries and is formed by nine sub-committees with twenty-eight standards developed, which are available in Volume 14.02 of the Annual Book of ASTM standards. This Committee addresses issues related to standards and guidance materials for nanotechnology and nanomaterials, as well as the coordination of existing ASTM standardization related to nanotechnology needs. These international standards address topics including nano-enabled consumer products, physical and chemical characterization, and intellectual property issues (available online at https://www.astm.org/membership-participation/technical-committees/committee-e56, accessed on 15 April 2025). As an example, some key standards comprise the following:
- E2490 Standard Guide for Measurement of Particle Size Distribution of Nanomaterials in Suspension by Photon Correlation Spectroscopy (PCS);
- E2865 Standard Guide for Measurement of Electrophoretic Mobility and Zeta Potential of Nanosized Biological Materials;
- E2864 Standard Test Method for Measurement of Airborne Metal and Metal Oxide Nanoparticle Surface Area Concentration in Inhalation Exposure Chambers;
- E2524 Standard Test Method for Analysis of Haemolytic Properties of Nanoparticles;
- E2526 Standard Test Method for Evaluation of Cytotoxicity of Nanoparticulate Materials in Porcine Kidney Cells and Human Hepatocarcinoma Cells;
- E2535 Standard Guide for Handling Unbound Engineered Nanoscale Particles in Occupational Settings;
- E3025 Standard Guide for Tiered Approach to Detection and Characterization of Silver Nanomaterials in Textiles;
- E3275-21 Standard Guide for Visualization and Identification of Nanomaterials in Biological and Nonbiological Matrices Using Darkfield Microscopy/Hyperspectral Imaging (DFM/HSI) Analysis;
- E3172-18 Standard Guide for Reporting Production Information and Data for Nano-Objects;
- D8208-19 Standard Practice for Collection of Non-Fibrous Nanoparticles Using a Nanoparticle Respiratory Deposition (NRD) Sampler.
Anyway, we have only found a recent document specifically concerning CNTs: ASTM-D8526. This is the Standard Test Method for Analytical Procedure Using Transmission Electron Microscopy for the Determination of the Concentration of Carbon Nanotubes and Carbon Nanotube-containing Particles in Ambient Atmosphere (available online at https://www.document-center.com/standards/show/ASTM-D8526, accessed on 15 April 2025).
Despite ASTM also serving as the administrator to a huge amount of ISO/TCs and to their sub-committees, and its involvement in the development of standards about nanomaterials, our findings have evidenced that its specific interest in CNTs is extremely limited compared to that of ISO, thus confirming the great gap between the work and opinion of the different organizations worldwide about CNTs, which limit their regulation and hamper their safer production and utilization.
7. Conclusions and Perspectives
In this article, the main knowledge and findings gathered so far about CNTs have been reviewed, and we find that these tube-like nanomaterials, which are made only of carbon atoms or functionalized carbonaceous tubes, possess nonpareil properties, which enable them to be applied in several sectors with excellent results and fantastic improvements. Particular attention has been paid to their antimicrobial effects and toxic aspects on humans, animals, and the environment. These characteristics were confirmed by several case studies reporting in vitro and in vivo experiments developed over the years, which were inserted in reader-friendly tables. First, by a survey on the Scopus database, we have shown that research interest for CNTs in the years 2010–2025 has remained constant, with peaks of publications in 2016 and 2017. This is a feature probably due to the passage of CNTs’ larger-scale production and the development of more efficient production procedures. Our specific research about their biomedical applications has revealed that both variants of CNTs, referred to as SWCNTs and MWCNTs, find application in numerous areas of nanomedicine. Also, concerning the more restricted field of their applicability as antimicrobial agents, our study has highlighted that these carbon-based nanomaterials (CNMs) are very promising as novel antimicrobial agents due to their extensively reported detrimental effects against both Gram-positive and Gram-negative bacteria, fungi, and biofilm. Due to their inhibiting effects on biofilm and excellent mechanical properties, CNTs could represent new optional ingredients for orthopaedical implant construction. Unfortunately, although the results from toxicological tests are often contrasting, several early and recent investigations have unveiled that CNTs could be toxic to humans, animals, and the environment. CNT toxicity is mainly due to their surface construct, nanosized diameters, needle-like structures, and residual metal impurities, and it depends on concentration and exposure times. Initially, the CNTs’ possible toxicity was disregarded because they were made mainly of normally harmless carbon. Anyway, investigations prompted by the similarity of the needle-like CNT fibres with those of asbestos evidenced that they can be remarkably toxic and genotoxic to several cells, organs, and animal models. As with asbestos, the higher toxicity has resulted in pulmonary injuries, which is particularly worrying for CNT manufacturers who have long-time exposure to them and are at risk of inhaling their fibres. Toxicity to the cardiovascular system, as well as immune and reproductive systems, embryos, and neurotoxicity, have also been reported. Fortunately, several strategies, including preventive behaviours, which have been discussed here, have already been developed to limit the CNTs’ toxic effects, but more and incessant studies should be implemented to make their production and employment safer. This study has also shown that despite the significant advancements in terms of regulations concerning CNT production and possible applications, as well as about health and safety at the production sites, several unsolved regulatory issues remain, which need urgent solutions. The incessant development of nanomaterials and CNTs and their daily application has led to rigid regulatory standards developed by different international organizations in both animal and human manufacturing and utilization, but standards and guidelines specific to CNTs are still very limited. Substantial regulatory concerns regarding CNT toxicity and environmental safety remain. Future perspectives should consider improved research finalized to the development of standardized protocols for toxicity assessment, which would allow for more dependable and comparable outcomes. More sophisticated in vitro and in vivo models that closely mimic human biological systems are needed. These will enable more precise forecasts of the behaviour and toxicity of CNTs in real-world situations, as well as the predictions of the long-term health effects resulting from exposure to CNTs. Surely, incessant CNT safety evaluations, including toxicity, clearance, biodistribution, and metabolism, can help in CNT inspection and in reducing conflicting results, thus allowing for a more reliable toxicological assessment. Additionally, the practical challenges of scaling up CNT-based technologies still remain unsolved. Low-cost, eco-friendly, and sustainable synthetic methods for preparing CNTs with precise and pure architecture on a macroscopic scale are necessary. Currently, the fabrication of CNTs requires expensive and energy-intensive professional methods like CVD or AD, generates a combination of different kinds of CNTs, and necessitates long secondary purifying procedures. High-purity, homogenous CNTs can be challenging to produce, thus resulting in harmful materials to human health. Despite these drawbacks, current research also uses artificial intelligence (AI) and strives to resolve these problems and realize the full potential of CNTs in various applications.
In fact, it must be recognized that CNTs, despite their still unclear toxicity and insufficient regulation, are ubiquitously exploitable for improving the quality of human life. SWCNT catalyst support can enhance the selectivity of copper (Cu)-based electrocatalysts in the CO2 conversion into valuable chemicals, which is a key strategy for achieving net-zero carbon emissions. Moreover, CNTs can be used as co-catalysts, as well as catalyst supports, for enhanced fuel cell applications and water splitting, which are excellent techniques for alternative clean energy systems due to zero carbon dioxide release, recyclability, and high gravimetric energy densities. Additionally, CNT-based single-atom catalysts (SACs) for electrochemical nitrogen reduction reactions (NRR) have received increasing attention due to their sustainable, efficient, and green advantages. In this regard, we hope that this review could stimulate further research on these materials, which are aimed at allowing their safer large-scale production and utilization.
Author Contributions
Conceptualization, investigation, writing—original draft preparation, supervision, project administration, S.A. Writing—review and editing, S.A. and G.C.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A

Table A1.
Key properties and potential uses of CNTs, as well as the residual current hurdles that limit CNTs’ extensive applications.
Table A1.
Key properties and potential uses of CNTs, as well as the residual current hurdles that limit CNTs’ extensive applications.
| Key Properties | Potential Uses | Current Hurdles |
|---|---|---|
| Size [61] | Nano-electronics [64] | Electronic heterogeneity [76] |
| Crystallographic defects | ||
| Stone–Wales defects | ||
| Ø < 100 nm Thickness = 1–2 nm Ideally infinite length | Conductors (SWCNTs) Superconductors Semi-conductors 1 Supercapacitors | Not tunable conductivity |
| Electrical and electrochemical properties | Interconnects | ⇩ Controllable orientation |
| ⇧ Electrical conductivity Constant resistively [63] Electrons emission capacity | Chips manufacture [65] Conductivity-enhancing components | ⇩ Organized configuration |
| Thermal Properties | ⇧ Variation in size and density [76] | |
| ⇧ Heat conductivity Expansion | Optics and photonics | |
| Mechanical properties | Light-emitting diodes (LEDs) [66,67] 2 | Export restrictions |
| ⇧ Tensile strength ⇧ Elastic modulus | Photodetectors [68] 2 | |
| Optical Properties | Bolometers [69] 2 | |
| Absorption properties | Optoelectronic memory devices [70] 2 | |
| Photoluminescence (fluorescence) | ||
| Raman spectroscopy properties | ||
| Others | Batteries [71] | Environmental concerns [76] |
| Easily modifiable structure | ||
| Presence of functional groups | Supercapacitors production Ultra-thin flexible batteries Implantable medical devices | |
| Chemical inertness | ||
| Easily optimizable solubility and dispersion | Cleaning up polluted environments | |
| Water filtration Air filters, such as smokestacks [72] | ||
| Others | ||
| Transistors production [73,74] | ||
| Energy production | ||
| Solar cells [75] | ||
| Light weight | Energy Storage | |
| ⇧ Biocompatibility | CNT based fibers and fabrics | |
| Capability of molecules immobilization | CNT based ceramics | |
| Transport of protein, DNA, RNA | ||
| Large surface area | Sources of light | |
| High capability of absorbing chemicals from their surroundings | Entrenched dominance of other material |
1 Conductive capacity of CNTs depends on the chirality degree, i.e., the twist degree and dimension of the diameter of the actual nanotube; 2 SWCNTs; ⇧ = high, wide; ⇩ = scarcely.

Table A2.
The three most used methods to synthetize CNTs.
Table A2.
The three most used methods to synthetize CNTs.
| Method | Process Type | Products Purity | Conditions Yields (%) | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Arc discharge (AC) | The carbon in the negative electrode sublimates due to the high discharge temperatures (T) | SWCNTs short tubes Ø = 0.6–1.4 nm MWCNTs short tubes Inner Ø = 1–3 nm Outer Ø = 10 nm Medium purity | Argon/N2 500 torr T ≤ 4000 °C 20–100% | Few structural defects | Length ≤ 50 µm |
| Laser ablation (LA) | Graphite samples are vaporized in a reactor at ⇧ T by a pulsed laser in presence of an inert gas CNTs grow up on the cooler reactor’s surfaces as the vaporized carbon condenses | SWCNTs 5–20 µm Ø = 1–2 nm MWCNTs Low purity | 500–1000 °C at ⇧ energy laser beam 25–1000 °C ≤70% | Controllable Ø by the reaction temperature | ⇧ Expensive than AC, CVD |
| Chemical Vapor Deposition (CVD) | Layered metal catalyst particles are heated in a reactor where a process gas and a carbon-containing gas are bled into | SWCNTs long tubes Ø = 0.6–4 nm MWCNTs long tubes Ø = 10–240 nm Medium-High purity | Low pressure inner gas (argon) 500–1200 °C 60–90% | ⇧ Economic and simple than AD and LA Synthesis at ⇩ temperature and AP ⇧ Yield and purity than AD and LA Versatile in the control of CNTs structure/architecture Suitable for scale up | ⇩ Crystallinity than that by AD and LA Removal of the catalyst support |
⇧ Denotes high, higher, increase, major; ⇩ denotes low, lower, decrease, minor; AP = ambient pressure.

Table A3.
Physical limits of CNTs produced so far.
Table A3.
Physical limits of CNTs produced so far.
| Physical Property | Largest | Smallest | Refs. |
|---|---|---|---|
| Diameter | N.R. | 0.30 nm (MWCNT) 1 0.43 nm (SWCNT) | [148] |
| Length | 0.5 m 2 | Cycloparaphenylenes (2.49 Å) 3 | [149,150] |
| Density | 1.6 g/cm * | N.R. | [151,152] |
1 Armchair structure; 2 [149]; 3 [150]; * ohmic conductivity, lowest resistance of 22 kΏ.

Table A4.
Possible associations between the different known allotropes of carbon and particular morphologies of CNTs.
Table A4.
Possible associations between the different known allotropes of carbon and particular morphologies of CNTs.
| Associations Morphologies | Type of Interaction Arrangement | Possible Products | Applications/Properties | Refs. |
|---|---|---|---|---|
| CNTs/CNTs * | Junctions | Metallic CNTs/semiconducting CNTs | Component of NT-based electronic circuits | [153,154,157] |
| CNTs/GF * | Junctions | Pillared GF (3D-CNTs) | Energy storage Supercapacitors Field emission Transistors High-performance catalysis Photovoltaics Biomedical devices Implants Sensors | [158,159,160,161] |
| 2 CNTs/fullerenes * | Covalent Bond | Fullerene-like nanobuds | Field emitters | [162] |
| CNT/fullerenes * | Entrapment | Carbon peapods (CPPs) | Heating devices Irradiating devices Oscillators | [163,164,165,166] |
| Doughnut shape ** | CNTs twisted into a toroid (Annulus shape) | Nano tori (NTRs) | ⇧ magnetic moment ⇧ stability | [167,168] |
| GF/MWCNTs * | GFs integrated MWCNTs | GFCNTs | ⇧ Surface area 3D-framework ⇧ Total charge capacity per unit of nominal area | [169,170] |
| 1D carbon structures ** | Stacking microstructure of GF layers | Cup-stacked CNTs (CSCNTs) | Semiconductors | [171] |
GF = graphene; * Associations; ** particular morphologies; ⇧ denotes high, higher, increase, major.

Table A5.
Main biomedical application of CNTs.
Table A5.
Main biomedical application of CNTs.
| Applications | Principle | Detection Target | Year Refs. | |
|---|---|---|---|---|
| Sensors | Electrochemical sensors | Made of AuNPs + MWCNTs Mannan-Os Adducts | Dopamine | 2015 [9] |
| Made of glassy carbon electrodes modified with MWCNTs and CuMPs dispersed in PEIs | Amino acids and glucose | 2016 [10] | ||
| Based on MWCNTs | Clostridium tetani | 2016 [11] | ||
| Based on AgNPs/Bi NPs/MWCNTs nafion modified | Lead and Cadmium | 2020 [12] | ||
| Based on carbon sensor fabricated with coalesced Ru–TiO2 NPs and MWCNTs | Cetrizine | 2019 [13] | ||
| Based on glassy carbon sensor modified with MWCNT in pH 9.0 PBS | Methdilazine | 2019 [14] | ||
| Based on (Ru–TiO2) NPs and MWCNTs | Flufenamic acid (FFA) Mefenamic acid (MFA) | 2019 [15] | ||
| Based on Bi-MWCNT/MCPE at physiological pH | Gallic Acid (GA) | 2020 [16] | ||
| Function | ||||
| Piezoelectric sensors | Based on MWCNTs on PDMS as substrate | N.R. | 2017 [17] | |
| Based on graphene, CNTs and graphene-CNTs composite | N.R. | 2018 [18] | ||
| Based on MWCNT on PDMS as substrate | For developing robotic hands (rehabilitation) Strain-detecting needle for tissue characterization | 2019 [19] | ||
| Based on MWCNTs on thermoplastic urethane as substrate | Sensors integrated into gloves and bandages for assessing specific human functions | 2019 [20] | ||
| Based on MWCNTs on PDMS substrate | To measure pressure directly without the use of deformation materials. | 2019 [21] | ||
| Detection Target | ||||
| Gas sensors | Based on a resonant electromagnetic transducer in microstrip technology | Volatile Organic Compounds (VOCs) | 2019 [22] | |
| Based on dye functionalized matrix anchored onto MWCNTs ammonia | Sulfur dioxide and chlorine | 2018 [23] | ||
| Based on WxOy nano-bricks and MWCNTs | Ammonia gas | 2019 [24] | ||
| Transported Drugs | ||||
| Drug Targeting | Based on MWCNT and pH-responsive gel of chitosan-coated magnetic nanocomposites | Doxorubicin (DOX) to U-87 Glioblastoma cells | 2019 [25] | |
| Based on stimuli-responsive CNTs using Ag nanowires to stimulate the drug release from the core of NTs | Cisplatin | 2017 [26] | ||
| Based on electro-responsive polymer-MWCNT hybrid hydrogel | Sucrose | 2013 [27] | ||
| Based on MWCNTs biodegradable, biocompatible nanocomposite hydrogel | Diclofenac sodium | 2016 [28] | ||
| Cancer diagnosis and treatment | Based on MTX-loaded MWCNTs releasing drugs by enzymatic cleavage | Methotrexate to in vitro breast cells | 2010 [29] | |
| Based on DOX loaded dendrimer modified MWCNTs releasing drugs at low pH | DOX | 2013 [30] | ||
| Based on cationic MWCNTs-NH3þ for direct intertumoral injections | Apoptotic siRNA against polo-like kinase (siPLK1) in calu6 tumor xenografts | 2015 [31] | ||
| Target bacteria/Applications | ||||
| Antibacterial agents | Based on chitosan/MWCNTs nanocomposites | Enterococcus faecalis Staphylococcus epidermidis Escherichia coli | [32,33,34,35,36,37,38] | |
| Based on Fe3O4/SWCNTs | E. coli | 2018 [39] | ||
| Based on Ag–Fe3O4/SWCNTs | N.R. | 2018 [40] | ||
| Based on CDX/Ag/MWCNTs | N.R. | 2014 [41] | ||
| Based on MWCNTs containing carboxylic functions | N.R. | 2015 [42] | ||
| Based on PA/graphene/CNTs | Staphylococcus aureus, E. coli | 2018, 2012 [43,44] | ||
| Based on MWCNTs | Treatment of drinking water through removal and inactivation of viruses and bacteria | 2011 [45] | ||
| Based on MWCNTs functionalized with mono-, di-, and tri-ethanolamine | E. coli, Klebsiella pneumonia Pseudomonas aeruginosa Salmonella typhimurium, Bacillus subtilis S. aureus, Bacillus cereus Streptococcus pneumonia | 2014 [46] | ||
| Based on dispersion SWCNTs/TABM derivative with carboxy groups | E. coli, S. aureus | 2019 [47] | ||
| Target fungi | ||||
| Antifungal agents | Based on chitosan/MWCNTs | Aspergillus niger, Candida trobicalis C. neoformans | 2000 [48] | |
| Based on functionalized CNTs | A. niger, C. albicans, A. fumigatus, Penicillium chrysogenum Saccharomyces cerevisiae, Fusarium culmorum Microsporum canis, Trichophyton mentagrophytes T. rubrum, P. lilacinum | 2013 [49] | ||
| Based on dispersion SWCNTs/TABM derivative with carboxy groups | C. albicans | 2019 [47] | ||
AuNPs = silver nanoparticles (AuNPs); CuMPs = copper microparticles; PEIs = polyethylenimine; Bi = bismuth; Ru–TiO2 = ruthenium-doped titanium dioxide; PBS = phosphate buffer solution; Bi-MWCNT/MCPE = BiNPs decorated MWCNTs modified carbon paste electrode; PDMS = polydimethylsiloxane; WxOy = tungsten oxide; MTX = methotrexate; DOX = doxorubicin; Fe3O4 = iron oxide; CDX = cyclodextrin; PA = polyaniline; TABM = tetra-arylbimesityl.
References
- Ifijen, I.H.; Maliki, M.; Omorogbe, S.O.; Ibrahim, S.D. Incorporation of Metallic Nanoparticles Into Alkyd Resin: A Review of Their Coating Performance. In TMS 2022 151st Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2022; pp. 338–349. [Google Scholar]
- Omorogbe, S.O.; Ikhuoria, E.U.; Ifijen, H.I.; Simo, A.; Aigbodion, A.; Maaza, M. Fabrication of Monodispersed Needle-Sized Hollow Core Polystyrene Microspheres. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2019; pp. 155–164. [Google Scholar]
- Ifijen, H.I.; Ikhuoria, E.U.; Omorogbe, S.O. Correlative Studies on the Fabrication of Poly(Styrene-Methyl-Methacrylate-Acrylic Acid) Colloidal Crystal Films. J. Dispers. Sci. Technol. 2019, 40, 1023–1030. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Ikhuoria, E.U.; Omorogbe, S.O.; Aigbodion, A.I. Ordered Colloidal Crystals Fabrication and Studies on the Properties of Poly (Styrene–Butyl Acrylate–Acrylic Acid) and Polystyrene Latexes. In Nanocomposites VI: Nanoscience and Nanotechnology in Advanced Composites; Springer: Cham, Switzerland, 2019; pp. 125–135. [Google Scholar]
- Ifijen, I.H.; Maliki, M.; Ovonramwen, O.B.; Aigbodion, A.I.; Ikhuoria, E.U. Brilliant Coloured Monochromatic Photonic Crystals Films Generation from Poly (Styrene-Butyl Acrylate-Acrylic Acid) Latex. J. Appl. Sci. Environ. Manag. 2019, 23, 1661. [Google Scholar] [CrossRef]
- Omorogbe, S.O.; Aigbodion, A.I.; Ifijen, H.I.; Simo, A.; Ogbeide-Ihama, N.L.; Ikhuoria, E.U. Low-Temperature Synthesis of Superparamagnetic Fe3O4 Morphologies Tuned Using Oleic Acid as Crystal Growth Modifiers. In TMS 2020 149th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2020; pp. 619–631. [Google Scholar]
- Ifijen, I.H.; Ikhuoria, E.U.; Maliki, M.; Otabor, G.O.; Aigbodion, A.I. Nanostructured Materials: A Review on Its Application in Water Treatment. In TMS 2022 151st Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2022; pp. 1172–1180. [Google Scholar]
- Ifijen, I.H.; Aghedo, O.N.; Odiachi, I.J.; Omorogbe, S.O.; Olu, E.L.; Onuguh, I.C. Nanostructured Graphene Thin Films: A Brief Review of Their Fabrication Techniques and Corrosion Protective Performance. In TMS 2022 151st Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2022; pp. 366–377. [Google Scholar]
- Ciambelli, P.; La Guardia, G.; Vitale, L. Nanotechnology for Green Materials and Processes. In Studies in Surface Science and Catalysis; Elsevier Science: Amsterdam, The Netherlands, 2020; pp. 97–116. [Google Scholar]
- Omorogbe, S.O.; Aigbodion, A.I.; Ikhuoria, E.U.; Ifijen, I.H. Impact of Varying the Concentration of Tetraethyl-OrthoSilicate on the Average Particle Diameter of Monodisperse Colloidal Silica Spheres. Chem. Sci. J. 2018, 9, 1. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Maliki, M.; Odiachi, I.J.; Aghedo, O.N.; Ohiocheoya, E.B. Review on Solvents Based Alkyd Resins and Water Borne Alkyd Resins: Impacts of Modification on Their Coating Properties. Chem. Afr. 2022, 5, 211–225. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Omonmhenle, S.I. Antimicrobial Properties of Carbon Nanotube: A Succinct Assessment. Biomed. Mater. Devices 2024, 2, 113–120. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Su, Q.; Gan, L.; Liu, J.; Yang, X. Carbon Dots Derived from Pea for Specifically Binding with Cryptococcus Neoformans. Anal. Biochem. 2020, 589, 113476. [Google Scholar] [CrossRef]
- Salari, S.; Jafari, S.M. Application of Nanofluids for Thermal Processing of Food Products. Trends Food Sci. Technol. 2020, 97, 100–113. [Google Scholar] [CrossRef]
- Abd-Elsalam, K.A. Carbon Nanomaterials: 30 Years of Research in Agroecosystems. In Carbon Nanomaterials for Agri-Food and Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–18. [Google Scholar]
- Iijima, S. Helical Microtubules of Graphitic Carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Rahman, M.A.; Abul Barkat, H.; Harwansh, R.K.; Deshmukh, R. Carbon-based Nanomaterials: Carbon Nanotubes, Graphene, and Fullerenes for the Control of Burn Infections and Wound Healing. Curr. Pharm. Biotechnol. 2022, 23, 1483–1496. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.; Ge, C.; Fang, G.; Wu, R.; Zhang, H.; Chai, Z.; Chen, C.; Yin, J.-J. Light-Enhanced Antibacterial Activity of Graphene Oxide, Mainly via Accelerated Electron Transfer. Environ. Sci. Technol. 2017, 51, 10154–10161. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Mauter, M.S.; Elimelech, M. Physicochemical Determinants of Multiwalled Carbon Nanotube Bacterial Cytotoxicity. Environ. Sci. Technol. 2008, 42, 7528–7534. [Google Scholar] [CrossRef]
- Haung, C.-F.; Chan, Y.-H.; Chen, L.-K.; Liu, C.-M.; Huang, W.-C.; Ou, S.-F.; Ou, K.-L.; Wang, D.-Y. Preparation, Characterization, and Properties of Anticoagulation and Antibacterial Films of Carbon-Based Nanowires Fabricated on Surfaces of Ti Implants. J. Electrochem. Soc. 2013, 160, H392–H397. [Google Scholar] [CrossRef]
- Lee, E.-S.; Kim, Y.-O.; Ha, Y.-M.; Lim, D.; Hwang, J.Y.; Kim, J.; Park, M.; Cho, J.W.; Jung, Y.C. Antimicrobial Properties of Lignin-Decorated Thin Multi-Walled Carbon Nanotubes in Poly(Vinyl Alcohol) Nanocomposites. Eur. Polym. J. 2018, 105, 79–84. [Google Scholar] [CrossRef]
- Azizi-Lalabadi, M.; Hashemi, H.; Feng, J.; Jafari, S.M. Carbon Nanomaterials against Pathogens; the Antimicrobial Activity of Carbon Nanotubes, Graphene/Graphene Oxide, Fullerenes, and Their Nanocomposites. Adv. Colloid. Interface Sci. 2020, 284, 102250. [Google Scholar] [CrossRef]
- Oyelami, A.O.; Semple, K.T. Impact of Carbon Nanomaterials on Microbial Activity in Soil. Soil. Biol. Biochem. 2015, 86, 172–180. [Google Scholar] [CrossRef]
- Maleki Dizaj, S.; Mennati, A.; Jafari, S.; Khezri, K.; Adibkia, K. Antimicrobial Activity of Carbon-Based Nanoparticles. Adv. Pharm. Bull. 2015, 5, 19–23. [Google Scholar] [CrossRef]
- Kang, S.; Pinault, M.; Pfefferle, L.D.; Elimelech, M. Single-Walled Carbon Nanotubes Exhibit Strong Antimicrobial Activity. Langmuir 2007, 23, 8670–8673. [Google Scholar] [CrossRef]
- Kang, S.; Herzberg, M.; Rodrigues, D.F.; Elimelech, M. Antibacterial Effects of Carbon Nanotubes: Size Does Matter! Langmuir 2008, 24, 6409–6413. [Google Scholar] [CrossRef]
- Chen, H.; Wang, B.; Gao, D.; Guan, M.; Zheng, L.; Ouyang, H.; Chai, Z.; Zhao, Y.; Feng, W. Broad-Spectrum Antibacterial Activity of Carbon Nanotubes to Human Gut Bacteria. Small 2013, 9, 2735–2746. [Google Scholar] [CrossRef]
- Awasthi, S.; Srivastava, A.; Kumar, D.; Pandey, S.K.; Mubarak, N.M.; Dehghani, M.H.; Ansari, K. An Insight into the Toxicological Impacts of Carbon Nanotubes (CNTs) on Human Health: A Review. Environ. Adv. 2024, 18, 100601. [Google Scholar] [CrossRef]
- Kostarelos, K.; Lacerda, L.; Pastorin, G.; Wu, W.; Wieckowski, S.; Luangsivilay, J.; Godefroy, S.; Pantarotto, D.; Briand, J.P.; Muller, S.; et al. Cellular Uptake of Functionalized Carbon Nanotubes Is Independent of Functional Group and Cell Type. Nat. Nanotechnol. 2007, 2, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Noonan, D.M.; Pagani, A.; Pulze, L.; Bruno, A.; Principi, E.; Congiu, T.; Grimaldi, A.; Bassani, B.; De Flora, S.; Gini, E.; et al. Environmental Impact of Multi-Wall Carbon Nanotubes in a Novel Model of Exposure: Systemic Distribution, Macrophage Accumulation, and Amyloid Deposition. Int. J. Nanomed. 2015, 10, 6133–6145. [Google Scholar] [CrossRef] [PubMed]
- Poland, C.A.; Duffin, R.; Kinloch, I.; Maynard, A.; Wallace, W.A.H.; Seaton, A.; Stone, V.; Brown, S.; MacNee, W.; Donaldson, K. Carbon Nanotubes Introduced into the Abdominal Cavity of Mice Show Asbestos-like Pathogenicity in a Pilot Study. Nat. Nanotechnol. 2008, 3, 423–428. [Google Scholar] [CrossRef]
- Ge, C.; Meng, L.; Xu, L.; Bai, R.; Du, J.; Zhang, L.; Li, Y.; Chang, Y.; Zhao, Y.; Chen, C. Acute Pulmonary and Moderate Cardiovascular Responses of Spontaneously Hypertensive Rats after Exposure to Single-Wall Carbon Nanotubes. Nanotoxicology 2012, 6, 526–542. [Google Scholar] [CrossRef]
- Goerzen, D.; Heller, D.A.; Rachel, A. Meidl Balancing Safety and Innovation: Shaping Responsible Carbon Nanotube Policy; Rice Universities’, Baker Institute for Public Policy: Houston, TX, USA, 2024. [Google Scholar]
- Alfei, S.; Schito, G.C. Nanotubes: Carbon-Based Fibers and Bacterial Nano-Conduits Both Arousing a Global Interest and Conflicting Opinions. Fibers 2022, 10, 75. [Google Scholar] [CrossRef]
- Ebbesen, T.W.; Ajayan, P.M. Large-Scale Synthesis of Carbon Nanotubes. Nature 1992, 358, 220–222. [Google Scholar] [CrossRef]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon Nanotubes: Properties, Synthesis, Purification, and Medical Applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Rinzler, A.G.; Tomanek, D.; Colbert, D.T.; Smalley, R.E. Self-Assembly of Tubular Fullerenes. J. Phys. Chem. 1995, 99, 10694–10697. [Google Scholar] [CrossRef]
- Guo, T.; Nikolaev, P.; Thess, A.; Colbert, D.T.; Smalley, R.E. Catalytic Growth of Single-Walled Nanotubes by Laser Vaporization. Chem. Phys. Lett. 1995, 243, 49–54. [Google Scholar] [CrossRef]
- Kumar, M.; Ando, Y. Chemical Vapor Deposition of Carbon Nanotubes: A Review on Growth Mechanism and Mass Production. J. Nanosci. Nanotechnol. 2010, 10, 3739–3758. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Lastres, M.; Chiarella, M.; Li, W.; Su, Q.; Du, G. Synthesis and Field Emission Properties of Vertically Aligned Carbon Nanotube Arrays on Copper. Carbon 2012, 50, 2641–2650. [Google Scholar] [CrossRef]
- Inami, N.; Mohamed, M.A.; Shikoh, E.; Fujiwara, A. Synthesis-Condition Dependence of Carbon Nanotube Growth by Alcohol Catalytic Chemical Vapor Deposition Method. Sci. Technol. Adv. Mater. 2007, 8, 292–295. [Google Scholar] [CrossRef]
- Ishigami, N.; Ago, H.; Imamoto, K.; Tsuji, M.; Iakoubovskii, K.; Minami, N. Crystal Plane Dependent Growth of Aligned Single-Walled Carbon Nanotubes on Sapphire. J. Am. Chem. Soc. 2008, 130, 9918–9924. [Google Scholar] [CrossRef]
- Naha, S.; Puri, I.K. A Model for Catalytic Growth of Carbon Nanotubes. J. Phys. D Appl. Phys. 2008, 41, 065304. [Google Scholar] [CrossRef]
- Banerjee, S.; Naha, S.; Puri, I.K. Molecular Simulation of the Carbon Nanotube Growth Mode during Catalytic Synthesis. Appl. Phys. Lett. 2008, 92, 233121. [Google Scholar] [CrossRef]
- Eftekhari, A.; Jafarkhani, P.; Moztarzadeh, F. High-Yield Synthesis of Carbon Nanotubes Using a Water-Soluble Catalyst Support in Catalytic Chemical Vapor Deposition. Carbon 2006, 44, 1343–1345. [Google Scholar] [CrossRef]
- Ren, Z.F.; Huang, Z.P.; Xu, J.W.; Wang, J.H.; Bush, P.; Siegal, M.P.; Provencio, P.N. Synthesis of Large Arrays of Well-Aligned Carbon Nanotubes on Glass. Science 1998, 282, 1105–1107. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef]
- Futaba, D.N.; Hata, K.; Yamada, T.; Hiraoka, T.; Hayamizu, Y.; Kakudate, Y.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S. Shape-Engineerable and Highly Densely Packed Single-Walled Carbon Nanotubes and Their Application as Super-Capacitor Electrodes. Nat. Mater. 2006, 5, 987–994. [Google Scholar] [CrossRef]
- Smiljanic, O.; Stansfield, B.L.; Dodelet, J.-P.; Serventi, A.; Désilets, S. Gas-Phase Synthesis of SWNT by an Atmospheric Pressure Plasma Jet. Chem. Phys. Lett. 2002, 356, 189–193. [Google Scholar] [CrossRef]
- Kim, K.S.; Cota-Sanchez, G.; Kingston, C.T.; Imris, M.; Simard, B.; Soucy, G. Large-Scale Production of Single-Walled Carbon Nanotubes by Induction Thermal Plasma. J. Phys. D Appl. Phys. 2007, 40, 2375–2387. [Google Scholar] [CrossRef]
- Ren, J.; Li, F.-F.; Lau, J.; González-Urbina, L.; Licht, S. One-Pot Synthesis of Carbon Nanofibers from CO2. Nano Lett. 2015, 15, 6142–6148. [Google Scholar] [CrossRef] [PubMed]
- Service, R.F. Conjuring Chemical Cornucopias out of Thin Air. Science 2015, 349, 1160. [Google Scholar] [CrossRef]
- Yuan, L.; Saito, K.; Pan, C.; Williams, F.A.; Gordon, A.S. Nanotubes from Methane Flames. Chem. Phys. Lett. 2001, 340, 237–241. [Google Scholar] [CrossRef]
- Yuan, L.; Saito, K.; Hu, W.; Chen, Z. Ethylene Flame Synthesis of Well-Aligned Multi-Walled Carbon Nanotubes. Chem. Phys. Lett. 2001, 346, 23–28. [Google Scholar] [CrossRef]
- Duan, H.M.; McKinnon, J.T. Nanoclusters Produced in Flames. J. Phys. Chem. 1994, 98, 12815–12818. [Google Scholar] [CrossRef]
- Murr, L.E.; Bang, J.J.; Esquivel, E.V.; Guerrero, P.A.; Lopez, D.A. Carbon Nanotubes, Nanocrystal Forms, and Complex Nanoparticle Aggregates in Common Fuel-Gas Combustion Sources and the Ambient Air. J. Nanopart. Res. 2004, 6, 241–251. [Google Scholar] [CrossRef]
- Vander Wal, R.L. Fe-Catalyzed Single-Walled Carbon Nanotube Synthesis within a Flame Environment. Combust. Flame 2002, 130, 37–47. [Google Scholar] [CrossRef]
- Saveliev, A.V.; Merchan-Merchan, W.; Kennedy, L.A. Metal Catalyzed Synthesis of Carbon Nanostructures in an Opposed Flow Methane Oxygen Flame. Combust. Flame 2003, 135, 27–33. [Google Scholar] [CrossRef]
- Height, M.J.; Howard, J.B.; Tester, J.W.; Vander Sande, J.B. Flame Synthesis of Single-Walled Carbon Nanotubes. Carbon 2004, 42, 2295–2307. [Google Scholar] [CrossRef]
- Sen, S.; Puri, I.K. Flame Synthesis of Carbon Nanofibres and Nanofibre Composites Containing Encapsulated Metal Particles. Nanotechnology 2004, 15, 264–268. [Google Scholar] [CrossRef]
- Naha, S.; Sen, S.; De, A.K.; Puri, I.K. A Detailed Model for the Flame Synthesis of Carbon Nanotubes and Nanofibers. Proc. Combust. Inst. 2007, 31, 1821–1829. [Google Scholar] [CrossRef]
- Jha, R.; Singh, A.; Sharma, P.K.; Fuloria, N.K. Smart carbon nanotubes for drug delivery system: A comprehensive study. J. Drug Deliv. Sci. Technol. 2020, 58, 101811. [Google Scholar] [CrossRef]
- Monthioux, M.; Kuznetsov, V.L. Who Should Be given the Credit for the Discovery of Carbon Nanotubes? Carbon 2006, 44, 1621–1623. [Google Scholar] [CrossRef]
- Chernozatonskii, L.A. Carbon Nanotube Connectors and Planar Jungle Gyms. Phys. Lett. A 1992, 172, 173–176. [Google Scholar] [CrossRef]
- Menon, M.; Srivastava, D. Carbon Nanotube “T Junctions”: Nanoscale Metal-Semiconductor-Metal Contact Devices. Phys. Rev. Lett. 1997, 79, 4453–4456. [Google Scholar] [CrossRef]
- Ma, K.L.; Yan, X.H.; Guo, Y.D.; Xiao, Y. Electronic Transport Properties of Junctions between Carbon Nanotubes and Graphene Nanoribbons. Eur. Phys. J. B 2011, 83, 487–492. [Google Scholar] [CrossRef]
- Harris, P.J.F.; Suarez-Martinez, I.; Marks, N.A. The Structure of Junctions between Carbon Nanotubes and Graphene Shells. Nanoscale 2016, 8, 18849–18854. [Google Scholar] [CrossRef]
- Lambin, P.; Vigneron, J.P.; Fonseca, A.; Nagy, J.B.; Lucas, A.A. Atomic Structure and Electronic Properties of a Bent Carbon Nanotube. Synth. Met. 1996, 77, 249–252. [Google Scholar] [CrossRef]
- Dimitrakakis, G.K.; Tylianakis, E.; Froudakis, G.E. Pillared Graphene: A New 3-D Network Nanostructure for Enhanced Hydrogen Storage. Nano Lett. 2008, 8, 3166–3170. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Kwaczala, A.T.; Kanakia, S.; Patel, S.C.; Judex, S.; Sitharaman, B. Fabrication and Characterization of Three-Dimensional Macroscopic All-Carbon Scaffolds. Carbon 2013, 53, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, G.; Gopalan, A.; D’Agati, M.; Srinivas Sankaran, J.; Judex, S.; Qin, Y.-X.; Sitharaman, B. Porous Three-Dimensional Carbon Nanotube Scaffolds for Tissue Engineering. J. Biomed. Mater. Res. A 2015, 103, 3212–3225. [Google Scholar] [CrossRef]
- Noyce, S.G.; Vanfleet, R.R.; Craighead, H.G.; Davis, R.C. High Surface-Area Carbon Microcantilevers. Nanoscale Adv. 2019, 1, 1148–1154. [Google Scholar] [CrossRef]
- Yu, M.-F.; Lourie, O.; Dyer, M.J.; Moloni, K.; Kelly, T.F.; Ruoff, R.S. Strength and Breaking Mechanism of Multiwalled Carbon Nanotubes Under Tensile Load. Science 2000, 287, 637–640. [Google Scholar] [CrossRef]
- Peng, B.; Locascio, M.; Zapol, P.; Li, S.; Mielke, S.L.; Schatz, G.C.; Espinosa, H.D. Measurements of Near-Ultimate Strength for Multiwalled Carbon Nanotubes and Irradiation-Induced Crosslinking Improvements. Nat. Nanotechnol. 2008, 3, 626–631. [Google Scholar] [CrossRef]
- Jensen, K.; Mickelson, W.; Kis, A.; Zettl, A. Buckling and Kinking Force Measurements on Individual Multiwalled Carbon Nanotubes. Phys. Rev. B 2007, 76, 195436. [Google Scholar] [CrossRef]
- Mingo, N.; Stewart, D.A.; Broido, D.A.; Srivastava, D. Phonon Transmission through Defects in Carbon Nanotubes from First Principles. Phys. Rev. B 2008, 77, 033418. [Google Scholar] [CrossRef]
- Kumanek, B.; Janas, D. Thermal Conductivity of Carbon Nanotube Networks: A Review. J. Mater. Sci. 2019, 54, 7397–7427. [Google Scholar] [CrossRef]
- Koziol, K.K.; Janas, D.; Brown, E.; Hao, L. Thermal Properties of Continuously Spun Carbon Nanotube Fibres. Phys. E Low. Dimens. Syst. Nanostruct. 2017, 88, 104–108. [Google Scholar] [CrossRef]
- Hong, S.; Myung, S. A Flexible Approach to Mobility. Nat. Nanotechnol. 2007, 2, 207–208. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, Q.; Xie, J.; Jin, Z.; Wang, J.; Li, Y.; Jiang, K.; Fan, S. Fabrication of Ultralong and Electrically Uniform Single-Walled Carbon Nanotubes on Clean Substrates. Nano Lett. 2009, 9, 3137–3141. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, Z. Curved Pi-Conjugation, Aromaticity, and the Related Chemistry of Small Fullerenes (<C60) and Single-Walled Carbon Nanotubes. Chem. Rev. 2005, 105, 3643–3696. [Google Scholar] [CrossRef] [PubMed]
- Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon Nanotube—A Review on Synthesis, Properties and Plethora of Applications in the Field of Biomedical Science. Sens. Int. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Khan, M.U.; Reddy, K.R.; Snguanwongchai, T.; Haque, E.; Gomes, V.G. Polymer Brush Synthesis on Surface Modified Carbon Nanotubes via in Situ Emulsion Polymerization. Colloid. Polym. Sci. 2016, 294, 1599–1610. [Google Scholar] [CrossRef]
- Che, J.; Çagin, T.; Goddard, W.A. Thermal Conductivity of Carbon Nanotubes. Nanotechnology 2000, 11, 65–69. [Google Scholar] [CrossRef]
- Smart, S.K.; Cassady, A.I.; Lu, G.Q.; Martin, D.J. The Biocompatibility of Carbon Nanotubes. Carbon 2006, 44, 1034–1047. [Google Scholar] [CrossRef]
- Negri, V.; Pacheco-Torres, J.; Calle, D.; López-Larrubia, P. Carbon Nanotubes in Biomedicine. Top. Curr. Chem. 2020, 378, 15. [Google Scholar] [CrossRef]
- Lamberti, M.; Pedata, P.; Sannolo, N.; Porto, S.; De Rosa, A.; Caraglia, M. Carbon Nanotubes: Properties, Biomedical Applications, Advantages and Risks in Patients and Occupationally-Exposed Workers. Int. J. Immunopathol. Pharmacol. 2015, 28, 4–13. [Google Scholar] [CrossRef]
- Kumar, S.; Rani, R.; Dilbaghi, N.; Tankeshwar, K.; Kim, K.-H. Carbon Nanotubes: A Novel Material for Multifaceted Applications in Human Healthcare. Chem. Soc. Rev. 2017, 46, 158–196. [Google Scholar] [CrossRef]
- Teixeira-Santos, R.; Gomes, M.; Gomes, L.C.; Mergulhão, F.J. Antimicrobial and Anti-Adhesive Properties of Carbon Nanotube-Based Surfaces for Medical Applications: A Systematic Review. iScience 2021, 24, 102001. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Toyokuni, S. Differences and Similarities between Carbon Nanotubes and Asbestos Fibers during Mesothelial Carcinogenesis: Shedding Light on Fiber Entry Mechanism. Cancer Sci. 2012, 103, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Loebick, C.Z.; Kang, S.; Elimelech, M.; Pfefferle, L.D.; Van Tassel, P.R. Antimicrobial Biomaterials Based on Carbon Nanotubes Dispersed in Poly(Lactic-Co-Glycolic Acid). Nanoscale 2010, 2, 1789. [Google Scholar] [CrossRef] [PubMed]
- Olivi, M.; Zanni, E.; De Bellis, G.; Talora, C.; Sarto, M.S.; Palleschi, C.; Flahaut, E.; Monthioux, M.; Rapino, S.; Uccelletti, D.; et al. Inhibition of Microbial Growth by Carbon Nanotube Networks. Nanoscale 2013, 5, 9023. [Google Scholar] [CrossRef]
- Liu, S.; Ng, A.K.; Xu, R.; Wei, J.; Tan, C.M.; Yang, Y.; Chen, Y. Antibacterial Action of Dispersed Single-Walled Carbon Nanotubes on Escherichia Coli and Bacillus Subtilis Investigated by Atomic Force Microscopy. Nanoscale 2010, 2, 2744. [Google Scholar] [CrossRef]
- Arias, L.R.; Yang, L. Inactivation of Bacterial Pathogens by Carbon Nanotubes in Suspensions. Langmuir 2009, 25, 3003–3012. [Google Scholar] [CrossRef]
- Young, Y.-F.; Lee, H.-J.; Shen, Y.-S.; Tseng, S.-H.; Lee, C.-Y.; Tai, N.-H.; Chang, H.-Y. Toxicity Mechanism of Carbon Nanotubes on Escherichia coli. Mater. Chem. Phys. 2012, 134, 279–286. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Fouladi, M.H.; Yong, P.V.C.; Wong, E.H. Elucidation of Antimicrobial Activity of Non-Covalently Dispersed Carbon Nanotubes. Materials 2020, 13, 1676. [Google Scholar] [CrossRef]
- Vecitis, C.D.; Zodrow, K.R.; Kang, S.; Elimelech, M. Electronic-Structure-Dependent Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. ACS Nano 2010, 4, 5471–5479. [Google Scholar] [CrossRef]
- Liu, S.; Wei, L.; Hao, L.; Fang, N.; Chang, M.W.; Xu, R.; Yang, Y.; Chen, Y. Sharper and Faster “Nano Darts” Kill More Bacteria: A Study of Antibacterial Activity of Individually Dispersed Pristine Single-Walled Carbon Nanotube. ACS Nano 2009, 3, 3891–3902. [Google Scholar] [CrossRef]
- Rodrigues, D.F.; Elimelech, M. Toxic Effects of Single-Walled Carbon Nanotubes in the Development of E. Coli Biofilm. Environ. Sci. Technol. 2010, 44, 4583–4589. [Google Scholar] [CrossRef] [PubMed]
- Malek, I.; Schaber, C.F.; Heinlein, T.; Schneider, J.J.; Gorb, S.N.; Schmitz, R.A. Vertically Aligned Multi Walled Carbon Nanotubes Prevent Biofilm Formation of Medically Relevant Bacteria. J. Mater. Chem. B 2016, 4, 5228–5235. [Google Scholar] [CrossRef] [PubMed]
- Yick, S.; Mai-Prochnow, A.; Levchenko, I.; Fang, J.; Bull, M.K.; Bradbury, M.; Murphy, A.B.; Ostrikov, K.K. The Effects of Plasma Treatment on Bacterial Biofilm Formation on Vertically-Aligned Carbon Nanotube Arrays. RSC Adv. 2015, 5, 5142–5148. [Google Scholar] [CrossRef]
- Vassallo, J.; Besinis, A.; Boden, R.; Handy, R.D. The Minimum Inhibitory Concentration (MIC) Assay with Escherichia Coli: An Early Tier in the Environmental Hazard Assessment of Nanomaterials? Ecotoxicol. Environ. Saf. 2018, 162, 633–646. [Google Scholar] [CrossRef]
- Hartono, M.R.; Kushmaro, A.; Chen, X.; Marks, R.S. Probing the Toxicity Mechanism of Multiwalled Carbon Nanotubes on Bacteria. Environ. Sci. Pollut. Res. 2018, 25, 5003–5012. [Google Scholar] [CrossRef]
- Zhang, Q.; Nghiem, J.; Silverberg, G.J.; Vecitis, C.D. Semiquantitative Performance and Mechanism Evaluation of Carbon Nanomaterials as Cathode Coatings for Microbial Fouling Reduction. Appl. Environ. Microbiol. 2015, 81, 4744–4755. [Google Scholar] [CrossRef]
- Kim, J.; Shashkov, E.V.; Galanzha, E.I.; Kotagiri, N.; Zharov, V.P. Photothermal Antimicrobial Nanotherapy and Nanodiagnostics with Self-assembling Carbon Nanotube Clusters. Lasers Surg. Med. 2007, 39, 622–634. [Google Scholar] [CrossRef]
- Mohammad, M.R.; Ahmed, D.S.; Mohammed, M.K.A. Synthesis of Ag-Doped TiO2 Nanoparticles Coated with Carbon Nanotubes by the Sol–Gel Method and Their Antibacterial Activities. J. Sol-Gel Sci. Technol. 2019, 90, 498–509. [Google Scholar] [CrossRef]
- Mocan, T.; Matea, C.T.; Pop, T.; Mosteanu, O.; Buzoianu, A.D.; Suciu, S.; Puia, C.; Zdrehus, C.; Iancu, C.; Mocan, L. Carbon Nanotubes as Anti-Bacterial Agents. Cell. Mol. Life Sci. 2017, 74, 3467–3479. [Google Scholar] [CrossRef]
- Huang, Y.-P.; Lin, I.-J.; Chen, C.-C.; Hsu, Y.-C.; Chang, C.-C.; Lee, M.-J. Delivery of Small Interfering RNAs in Human Cervical Cancer Cells by Polyethylenimine-Functionalized Carbon Nanotubes. Nanoscale Res. Lett. 2013, 8, 267. [Google Scholar] [CrossRef]
- Martincic, M.; Tobias, G. Filled Carbon Nanotubes in Biomedical Imaging and Drug Delivery. Expert. Opin. Drug Deliv. 2015, 12, 563–581. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, H.; Shen, S.; She, X.; Shi, W.; Chen, J.; Zhang, Q.; Hu, Y.; Pang, Z.; Jiang, X. Fibrin-Targeting Peptide CREKA-Conjugated Multi-Walled Carbon Nanotubes for Self-Amplified Photothermal Therapy of Tumor. Biomaterials 2016, 79, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, L.M.; Hashmi, S.M.; Sommer, T.J.; Elimelech, M.; Zimmerman, J.B. Impact of Surface Functionalization on Bacterial Cytotoxicity of Single-Walled Carbon Nanotubes. Environ. Sci. Technol. 2012, 46, 6297–6305. [Google Scholar] [CrossRef] [PubMed]
- Saleemi, M.A.; Kong, Y.L.; Yong, P.V.C.; Wong, E.H. An Overview of Antimicrobial Properties of Carbon Nanotubes-Based Nanocomposites. Adv. Pharm. Bull. 2022, 12, 449–465. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Joshi, S.; Vig, K.; Sahu, R.; Dixit, S.; Baganizi, R.; Dennis, V.A.; Singh, S.R.; Pillai, S. A Three-Dimensional Human Skin Model to Evaluate the Inhibition of Staphylococcus aureus by Antimicrobial Peptide-Functionalized Silver Carbon Nanotubes. J. Biomater. Appl. 2019, 33, 924–934. [Google Scholar] [CrossRef]
- Prabhu, S.; Poulose, E.K. Silver Nanoparticles: Mechanism of Antimicrobial Action, Synthesis, Medical Applications, and Toxicity Effects. Int. Nano Lett. 2012, 2, 32. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, Z.; Li, S.; Yuan, X. Antimicrobial Strategies for Urinary Catheters. J. Biomed. Mater. Res. A 2019, 107, 445–467. [Google Scholar] [CrossRef]
- Chaudhari, A.A.; Ashmore, D.; Nath, S.d.; Kate, K.; Dennis, V.; Singh, S.R.; Owen, D.R.; Palazzo, C.; Arnold, R.D.; Miller, M.E.; et al. A Novel Covalent Approach to Bio-Conjugate Silver Coated Single Walled Carbon Nanotubes with Antimicrobial Peptide. J. Nanobiotechnol. 2016, 14, 58. [Google Scholar] [CrossRef]
- Kumar, A.; Dalal, J.; Dahiya, S.; Punia, R.; Sharma, K.D.; Ohlan, A.; Maan, A.S. In Situ Decoration of Silver Nanoparticles on Single-Walled Carbon Nanotubes by Microwave Irradiation for Enhanced and Durable Anti-Bacterial Finishing on Cotton Fabric. Ceram. Int. 2019, 45, 1011–1019. [Google Scholar] [CrossRef]
- Karumuri, A.K.; Oswal, D.P.; Hostetler, H.A.; Mukhopadhyay, S.M. Silver Nanoparticles Supported on Carbon Nanotube Carpets: Influence of Surface Functionalization. Nanotechnology 2016, 27, 145603. [Google Scholar] [CrossRef]
- Barbinta-Patrascu, M.E.; Ungureanu, C.; Iordache, S.M.; Iordache, A.M.; Bunghez, I.-R.; Ghiurea, M.; Badea, N.; Fierascu, R.-C.; Stamatin, I. Eco-Designed Biohybrids Based on Liposomes, Mint–Nanosilver and Carbon Nanotubes for Antioxidant and Antimicrobial Coating. Mater. Sci. Eng. C 2014, 39, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Gong, J.-L.; Zeng, G.-M.; Ou, X.-M.; Song, B.; Guo, M.; Zhang, J.; Liu, H.-Y. Antimicrobial Behavior Comparison and Antimicrobial Mechanism of Silver Coated Carbon Nanocomposites. Process Saf. Environ. Prot. 2016, 102, 596–605. [Google Scholar] [CrossRef]
- Park, S.B.; Steadman, C.S.; Chaudhari, A.A.; Pillai, S.R.; Singh, S.R.; Ryan, P.L.; Willard, S.T.; Feugang, J.M. Proteomic Analysis of Antimicrobial Effects of Pegylated Silver Coated Carbon Nanotubes in Salmonella enterica serovar Typhimurium. J. Nanobiotechnol. 2018, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Goswami, A.; Nain, S. Enhanced Antibacterial Activity and Photo-Remediation of Toxic Dyes Using Ag/SWCNT/PPy Based Nanocomposite with Core–Shell Structure. Appl. Nanosci. 2020, 10, 2255–2268. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, J.; Wang, Y.; Chen, C.; Gu, H.; Chai, Y.; Wang, Y. Silver Nanoparticles-Decorated and Mesoporous Silica Coated Single-Walled Carbon Nanotubes with an Enhanced Antibacterial Activity for Killing Drug-Resistant Bacteria. Nano Res. 2020, 13, 389–400. [Google Scholar] [CrossRef]
- Yun, H.; Kim, J.D.; Choi, H.C.; Lee, C.W. Antibacterial Activity of CNT-Ag and GO-Ag Nanocomposites Against Gram-Negative and Gram-Positive Bacteria. Bull. Korean Chem. Soc. 2013, 34, 3261–3264. [Google Scholar] [CrossRef]
- Leid, J.G.; Ditto, A.J.; Knapp, A.; Shah, P.N.; Wright, B.D.; Blust, R.; Christensen, L.; Clemons, C.B.; Wilber, J.P.; Young, G.W.; et al. In Vitro Antimicrobial Studies of Silver Carbene Complexes: Activity of Free and Nanoparticle Carbene Formulations against Clinical Isolates of Pathogenic Bacteria. J. Antimicrob. Chemother. 2012, 67, 138–148. [Google Scholar] [CrossRef]
- Nepal, D.; Balasubramanian, S.; Simonian, A.L.; Davis, V.A. Strong Antimicrobial Coatings: Single-Walled Carbon Nanotubes Armored with Biopolymers. Nano Lett. 2008, 8, 1896–1901. [Google Scholar] [CrossRef]
- Upadhyayula, V.K.K.; Gadhamshetty, V. Appreciating the Role of Carbon Nanotube Composites in Preventing Biofouling and Promoting Biofilms on Material Surfaces in Environmental Engineering: A Review. Biotechnol. Adv. 2010, 28, 802–816. [Google Scholar] [CrossRef]
- Levashov, P.A.; Sedov, S.A.; Shipovskov, S.; Belogurova, N.G.; Levashov, A.V. Quantitative Turbidimetric Assay of Enzymatic Gram-Negative Bacteria Lysis. Anal. Chem. 2010, 82, 2161–2163. [Google Scholar] [CrossRef]
- Ahmed, F.; Santos, C.M.; Vergara, R.A.M.V.; Tria, M.C.R.; Advincula, R.; Rodrigues, D.F. Antimicrobial Applications of Electroactive PVK-SWNT Nanocomposites. Environ. Sci. Technol. 2012, 46, 1804–1810. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Deneufchatel, M.; Hashmi, S.; Li, N.; Pfefferle, L.D.; Elimelech, M.; Pauthe, E.; Van Tassel, P.R. Carbon Nanotube-Based Antimicrobial Biomaterials Formed via Layer-by-Layer Assembly with Polypeptides. J. Colloid. Interface Sci. 2012, 388, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, D.G.; Marsh, K.M.; Sosa, I.B.; Payne, J.B.; Gorham, J.M.; Bouwer, E.J.; Fairbrother, D.H. Interactions of Microorganisms with Polymer Nanocomposite Surfaces Containing Oxidized Carbon Nanotubes. Environ. Sci. Technol. 2015, 49, 5484–5492. [Google Scholar] [CrossRef] [PubMed]
- Sah, U.; Sharma, K.; Chaudhri, N.; Sankar, M.; Gopinath, P. Antimicrobial Photodynamic Therapy: Single-Walled Carbon Nanotube (SWCNT)-Porphyrin Conjugate for Visible Light Mediated Inactivation of Staphylococcus aureus. Colloids Surf. B Biointerfaces 2018, 162, 108–117. [Google Scholar] [CrossRef]
- Tiraferri, A.; Vecitis, C.D.; Elimelech, M. Covalent Binding of Single-Walled Carbon Nanotubes to Polyamide Membranes for Antimicrobial Surface Properties. ACS Appl. Mater. Interfaces 2011, 3, 2869–2877. [Google Scholar] [CrossRef]
- Cajero-Zul, L.R.; López-Dellamary, F.A.; Gómez-Salazar, S.; Vázquez-Lepe, M.; Vera-Graziano, R.; Torres-Vitela, M.R.; Olea-Rodríguez, M.A.; Nuño-Donlucas, S.M. Evaluation of the Resistance to Bacterial Growth of Star-Shaped Poly(ε-Caprolactone)-Co-Poly(Ethylene Glycol) Grafted onto Functionalized Carbon Nanotubes Nanocomposites. J. Biomater. Sci. Polym. Ed. 2019, 30, 163–189. [Google Scholar] [CrossRef]
- Murugan, E.; Vimala, G. Effective Functionalization of Multiwalled Carbon Nanotube with Amphiphilic Poly(Propyleneimine) Dendrimer Carrying Silver Nanoparticles for Better Dispersability and Antimicrobial Activity. J. Colloid. Interface Sci. 2011, 357, 354–365. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A. Deposition of Silver Nanoparticles on Dendrimer Functionalized Multiwalled Carbon Nanotubes: Synthesis, Characterization and Antimicrobial Activity. J. Nanosci. Nanotechnol. 2011, 11, 3621–3629. [Google Scholar] [CrossRef]
- Neelgund, G.M.; Oki, A.; Luo, Z. Antimicrobial Activity of CdS and Ag2S Quantum Dots Immobilized on Poly(Amidoamine) Grafted Carbon Nanotubes. Colloids Surf. B Biointerfaces 2012, 100, 215–221. [Google Scholar] [CrossRef]
- Ong, L.C.; Tan, Y.F.; Tan, B.S.; Chung, F.F.; Cheong, S.K.; Leong, C.O. Single-walled carbon nanotubes (SWCNTs) inhibit heat shock protein 90 (HSP90) signaling in human lung fibroblasts and keratinocytes. Toxicol. Appl. Pharmacol. 2017, 15, 329:347–357. [Google Scholar] [CrossRef]
- Zardini, H.Z.; Davarpanah, M.; Shanbedi, M.; Amiri, A.; Maghrebi, M.; Ebrahimi, L. Microbial Toxicity of Ethanolamines—Multiwalled Carbon Nanotubes. J. Biomed. Mater. Res. A 2014, 102, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Gilbertson, L.M.; Goodwin, D.G.; Taylor, A.D.; Pfefferle, L.; Zimmerman, J.B. Toward Tailored Functional Design of Multi-Walled Carbon Nanotubes (MWNTs): Electrochemical and Antimicrobial Activity Enhancement via Oxidation and Selective Reduction. Environ. Sci. Technol. 2014, 48, 5938–5945. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Seo, Y.; Hwang, J.; Kim, J.; Jeong, Y.; Hwang, M. Antibacterial Activity and Cytotoxicity of Multi-Walled Carbon Nanotubes Decorated with Silver Nanoparticles. Int. J. Nanomed. 2014, 9, 4621–4629. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Hwang, G.B.; Lee, J.E.; Bae, G.N. Preparation of Airborne Ag/CNT Hybrid Nanoparticles Using an Aerosol Process and Their Application to Antimicrobial Air Filtration. Langmuir 2011, 27, 10256–10264. [Google Scholar] [CrossRef]
- Rusen, E.; Mocanu, A.; Nistor, L.C.; Dinescu, A.; Călinescu, I.; Mustăţea, G.; Voicu, Ş.I.; Andronescu, C.; Diacon, A. Design of Antimicrobial Membrane Based on Polymer Colloids/Multiwall Carbon Nanotubes Hybrid Material with Silver Nanoparticles. ACS Appl. Mater. Interfaces 2014, 6, 17384–17393. [Google Scholar] [CrossRef]
- Mohan, R.; Shanmugharaj, A.M.; Sung Hun, R. An Efficient Growth of Silver and Copper Nanoparticles on Multiwalled Carbon Nanotube with Enhanced Antimicrobial Activity. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 96B, 119–126. [Google Scholar] [CrossRef]
- Sui, M.; Zhang, L.; Sheng, L.; Huang, S.; She, L. Synthesis of ZnO Coated Multi-Walled Carbon Nanotubes and Their Antibacterial Activities. Sci. Total Environ. 2013, 452–453, 148–154. [Google Scholar] [CrossRef]
- Karthika, V.; Arumugam, A. Synthesis and Characterization of MWCNT/TiO2/Au Nanocomposite for Photocatalytic and Antimicrobial Activity. IET Nanobiotechnol. 2017, 11, 113–118. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, D.Y.; Park, J.S.; Park, M.; Park, M.C. Antimicrobial and Antibiofilm Activities of Urinary Catheter Incorporated with ZnO-Carbon Nanotube. ACS Appl. Bio Mater. 2025, 8, 1397–1405. [Google Scholar] [CrossRef]
- Hirschfeld, J.; Akinoglu, E.M.; Wirtz, D.C.; Hoerauf, A.; Bekeredjian-Ding, I.; Jepsen, S.; Haddouti, E.-M.; Limmer, A.; Giersig, M. Long-Term Release of Antibiotics by Carbon Nanotube-Coated Titanium Alloy Surfaces Diminish Biofilm Formation by Staphylococcus Epidermidis. Nanomedicine 2017, 13, 1587–1593. [Google Scholar] [CrossRef]
- Grover, N.; Borkar, I.V.; Dinu, C.Z.; Kane, R.S.; Dordick, J.S. Laccase- and Chloroperoxidase-Nanotube Paint Composites with Bactericidal and Sporicidal Activity. Enzym. Microb. Technol. 2012, 50, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Jayakumar, R.; Mohandas, A.; Bhatnagar, I.; Kim, S.-K. Antimicrobial Activity of Chitosan-Carbon Nanotube Hydrogels. Materials 2014, 7, 3946–3955. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, N.A.; Al-Harby, N.F.; Almarshed, M.S. Synthesis and Characterization of Novel Trimellitic Anhydride Isothiocyanate-Cross Linked Chitosan Hydrogels Modified with Multi-Walled Carbon Nanotubes for Enhancement of Antimicrobial Activity. Int. J. Biol. Macromol. 2019, 132, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, H.; Liu, D.; Zeng, X.-A.; Mao, Y. Bacteria Capture and Inactivation with Functionalized Multi-Walled Carbon Nanotubes (MWCNTs). J. Nanosci. Nanotechnol. 2020, 20, 2055–2062. [Google Scholar] [CrossRef]
- Levi-Polyachenko, N.; Young, C.; MacNeill, C.; Braden, A.; Argenta, L.; Reid, S. Eradicating Group A Streptococcus Bacteria and Biofilms Using Functionalised Multi-Wall Carbon Nanotubes. Int. J. Hyperth. 2014, 30, 490–501. [Google Scholar] [CrossRef]
- Bai, Y.; Park, I.S.; Lee, S.J.; Bae, T.S.; Watari, F.; Uo, M.; Lee, M.H. Aqueous Dispersion of Surfactant-Modified Multiwalled Carbon Nanotubes and Their Application as an Antibacterial Agent. Carbon 2011, 49, 3663–3671. [Google Scholar] [CrossRef]
- Khazaee, M.; Ye, D.; Majumder, A.; Baraban, L.; Opitz, J.; Cuniberti, G. Non-Covalent Modified Multi-Walled Carbon Nanotubes: Dispersion Capabilities and Interactions with Bacteria. Biomed. Phys. Eng. Express 2016, 2, 055008. [Google Scholar] [CrossRef]
- Akhavan, O.; Azimirad, R.; Safa, S. Functionalized Carbon Nanotubes in ZnO Thin Films for Photoinactivation of Bacteria. Mater. Chem. Phys. 2011, 130, 598–602. [Google Scholar] [CrossRef]
- Amiri, A.; Zardini, H.Z.; Shanbedi, M.; Maghrebi, M.; Baniadam, M.; Tolueinia, B. Efficient Method for Functionalization of Carbon Nanotubes by Lysine and Improved Antimicrobial Activity and Water-Dispersion. Mater. Lett. 2012, 72, 153–156. [Google Scholar] [CrossRef]
- Hasnain, M.S.; Nayak, A.K. Regulatory Considerations of Carbon Nanotubes. In Carbon Nanotubes for Targeted Drug Delivery; Springer: Cham, Switzerland, 2019; pp. 103–106. [Google Scholar]
- Ma-Hock, L.; Treumann, S.; Strauss, V.; Brill, S.; Luizi, F.; Mertler, M.; Wiench, K.; Gamer, A.O.; van Ravenzwaay, B.; Landsiedel, R. Inhalation Toxicity of Multiwall Carbon Nanotubes in Rats Exposed for 3 Months. Toxicol. Sci. 2009, 112, 468–481. [Google Scholar] [CrossRef]
- Wikipedia. Toxicology of Carbon Nanotubes. Available online: https://en.wikipedia.org/wiki/Toxicology_of_carbon_nanomaterials#:~:text=The%20toxicity%20of%20carbon%20nanotubes%20has%20been%20an,in%20evaluating%20the%20toxicity%20of%20this%20heterogeneous%20material (accessed on 7 March 2025).
- Wang, H.; Wang, J.; Deng, X.; Sun, H.; Shi, Z.; Gu, Z.; Liu, Y.; Zhaoc, Y. Biodistribution of Carbon Single-Wall Carbon Nanotubes in Mice. J. Nanosci. Nanotechnol. 2004, 4, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Flahaut, E.; Cheng, S.H. Effect of Carbon Nanotubes on Developing Zebrafish (Danio Rerio) Embryos. Environ. Toxicol. Chem. 2007, 26, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Li, J.G.; Li, Q.N.; Xu, J.Y.; Cai, X.Q.; Liu, R.L.; Li, Y.J.; Ma, J.F.; Li, W.X. The Pulmonary Toxicity of Multi-Wall Carbon Nanotubes in Mice 30 and 60 Days After Inhalation Exposure. J. Nanosci. Nanotechnol. 2009, 9, 1384–1387. [Google Scholar] [CrossRef]
- Scoville, C.; Cole, R.; Hogg, J.; Farooque, O.; Russel, A. Carbon Nanotubes. Available online: https://docslib.org/doc/13279829/carbon-nanotubes-by-chris-scoville-robin-cole-jason-hogg-omar-farooque-and-archie-russell (accessed on 18 March 2025).
- Li, Y.; Wang, S.; Lv, Z.; Wang, Z.; Zhao, Y.; Xie, Y.; Xu, Y.; Qian, L.; Yang, Y.; Zhao, Z.; et al. Transforming the Synthesis of Carbon Nanotubes with Machine Learning Models and Automation. Matter 2025, 8, 101913. [Google Scholar] [CrossRef]
- Simon-Deckers, A.; Gouget, B.; Mayne-L’Hermite, M.; Herlin-Boime, N.; Reynaud, C.; Carrière, M. In Vitro Investigation of Oxide Nanoparticle and Carbon Nanotube Toxicity and Intracellular Accumulation in A549 Human Pneumocytes. Toxicology 2008, 253, 137–146. [Google Scholar] [CrossRef]
- Pulskamp, K.; Diabate, S.; Krug, H. Carbon Nanotubes Show No Sign of Acute Toxicity but Induce Intracellular Reactive Oxygen Species in Dependence on Contaminants. Toxicol. Lett. 2007, 168, 58–74. [Google Scholar] [CrossRef]
- Wick, P.; Manser, P.; Limbach, L.; Dettlaffweglikowska, U.; Krumeich, F.; Roth, S.; Stark, W.; Bruinink, A. The Degree and Kind of Agglomeration Affect Carbon Nanotube Cytotoxicity. Toxicol. Lett. 2007, 168, 121–131. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Inman, A.O.; Wang, Y.Y.; Nemanich, R.J. Surfactant Effects on Carbon Nanotube Interactions with Human Keratinocytes. Nanomedicine 2005, 1, 293–299. [Google Scholar] [CrossRef]
- Pantarotto, D.; Briand, J.-P.; Prato, M.; Bianco, A. Translocation of Bioactive Peptides across Cell Membranes by Carbon Nanotubes. Chem. Commun. 2004, 1, 16–17. [Google Scholar] [CrossRef]
- Manna, S.K.; Sarkar, S.; Barr, J.; Wise, K.; Barrera, E.V.; Jejelowo, O.; Rice-Ficht, A.C.; Ramesh, G.T. Single-Walled Carbon Nanotube Induces Oxidative Stress and Activates Nuclear Transcription Factor-ΚB in Human Keratinocytes. Nano Lett. 2005, 5, 1676–1684. [Google Scholar] [CrossRef]
- Shvedova, A.; Castranova, V.; Kisin, E.; Schwegler-Berry, D.; Murray, A.; Gandelsman, V.; Maynard, A.; Baron, P. Exposure to Carbon Nanotube Material: Assessment of Nanotube Cytotoxicity Using Human Keratinocyte Cells. J. Toxicol. Environ. Health A 2003, 66, 1909–1926. [Google Scholar] [CrossRef] [PubMed]
- Shvedova, A.A.; Kisin, E.R.; Mercer, R.; Murray, A.R.; Johnson, V.J.; Potapovich, A.I.; Tyurina, Y.Y.; Gorelik, O.; Arepalli, S.; Schwegler-Berry, D.; et al. Unusual Inflammatory and Fibrogenic Pulmonary Responses to Single-Walled Carbon Nanotubes in Mice. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2005, 289, L698–L708. [Google Scholar] [CrossRef] [PubMed]
- Dumortier, H.; Lacotte, S.; Pastorin, G.; Marega, R.; Wu, W.; Bonifazi, D.; Briand, J.-P.; Prato, M.; Muller, S.; Bianco, A. Functionalized Carbon Nanotubes Are Non-Cytotoxic and Preserve the Functionality of Primary Immune Cells. Nano Lett. 2006, 6, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Liang, F.; Hudson, J.L.; Mendez, J.; Guo, W.; Beach, J.M.; Moore, V.C.; Doyle, C.D.; West, J.L.; Billups, W.E.; et al. Functionalization Density Dependence of Single-Walled Carbon Nanotubes Cytotoxicity in Vitro. Toxicol. Lett. 2006, 161, 135–142. [Google Scholar] [CrossRef]
- Shi Kam, N.W.; Jessop, T.C.; Wender, P.A.; Dai, H. Nanotube Molecular Transporters: Internalization of Carbon Nanotube−Protein Conjugates into Mammalian Cells. J. Am. Chem. Soc. 2004, 126, 6850–6851. [Google Scholar] [CrossRef]
- Fenoglio, I.; Tomatis, M.; Lison, D.; Muller, J.; Fonseca, A.; Nagy, J.B.; Fubini, B. Reactivity of Carbon Nanotubes: Free Radical Generation or Scavenging Activity? Free Radic. Biol. Med. 2006, 40, 1227–1233. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, Z.; Meng, J.; Meng, J.; Duan, J.; Xie, S.; Lu, X.; Zhu, Z.; Wang, C.; Chen, S.; et al. Carbon Nanotubes Enhance Cytotoxicity Mediated by Human Lymphocytes In Vitro. PLoS ONE 2011, 6, e21073. [Google Scholar] [CrossRef]
- Cui, D.; Tian, F.; Ozkan, C.S.; Wang, M.; Gao, H. Effect of Single Wall Carbon Nanotubes on Human HEK293 Cells. Toxicol. Lett. 2005, 155, 73–85. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of Carbon Nanomaterials: Single-Wall Nanotube, Multi-Wall Nanotube, and Fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef]
- Pacurari, M.; Yin, X.J.; Zhao, J.; Ding, M.; Leonard, S.S.; Schwegler-Berry, D.; Ducatman, B.S.; Sbarra, D.; Hoover, M.D.; Castranova, V.; et al. Raw Single-Wall Carbon Nanotubes Induce Oxidative Stress and Activate MAPKs, AP-1, NF-ΚB, and Akt in Normal and Malignant Human Mesothelial Cells. Environ. Health Perspect. 2008, 116, 1211–1217. [Google Scholar] [CrossRef]
- Kisin, E.R.; Murray, A.R.; Keane, M.J.; Shi, X.-C.; Schwegler-Berry, D.; Gorelik, O.; Arepalli, S.; Castranova, V.; Wallace, W.E.; Kagan, V.E.; et al. Single-Walled Carbon Nanotubes: Geno- and Cytotoxic Effects in Lung Fibroblast V79 Cells. J. Toxicol. Environ. Health A 2007, 70, 2071–2079. [Google Scholar] [CrossRef] [PubMed]
- Francis, A.P.; Devasena, T. Toxicity of Carbon Nanotubes: A Review. Toxicol. Ind. Health 2018, 34, 200–210. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Huaux, F.; Moreau, N.; Misson, P.; Heilier, J.-F.; Delos, M.; Arras, M.; Fonseca, A.; Nagy, J.B.; Lison, D. Respiratory Toxicity of Multi-Wall Carbon Nanotubes. Toxicol. Appl. Pharmacol. 2005, 207, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Kanno, S.; Furuyama, A. Multi-Walled Carbon Nanotubes Injure the Plasma Membrane of Macrophages. Toxicol. Appl. Pharmacol. 2008, 232, 244–251. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Nemanich, R.J.; Inman, A.O.; Wang, Y.Y.; Riviere, J.E. Multi-Walled Carbon Nanotube Interactions with Human Epidermal Keratinocytes. Toxicol. Lett. 2005, 155, 377–384. [Google Scholar] [CrossRef]
- Monteiro-Riviere, N.A.; Inman, A.O. Challenges for Assessing Carbon Nanomaterial Toxicity to the Skin. Carbon 2006, 44, 1070–1078. [Google Scholar] [CrossRef]
- Yang, H.; Liu, C.; Yang, D.; Zhang, H.; Xi, Z. Comparative Study of Cytotoxicity, Oxidative Stress and Genotoxicity Induced by Four Typical Nanomaterials: The Role of Particle Size, Shape and Composition. J. Appl. Toxicol. 2009, 29, 69–78. [Google Scholar] [CrossRef]
- Ding, L.; Stilwell, J.; Zhang, T.; Elboudwarej, O.; Jiang, H.; Selegue, J.P.; Cooke, P.A.; Gray, J.W.; Chen, F.F. Molecular Characterization of the Cytotoxic Mechanism of Multiwall Carbon Nanotubes and Nano-Onions on Human Skin Fibroblast. Nano Lett. 2005, 5, 2448–2464. [Google Scholar] [CrossRef]
- Soto, K.F.; Carrasco, A.; Powell, T.G.; Garza, K.M.; Murr, L.E. Comparative in Vitro Cytotoxicity Assessment of Some Manufacturednanoparticulate Materials Characterized by Transmissionelectron Microscopy. J. Nanopart. Res. 2005, 7, 145–169. [Google Scholar] [CrossRef]
- Murr, L.E.; Garza, K.M.; Soto, K.F.; Carrasco, A.; Powell, T.G.; Ramirez, D.A.; Guerrero, P.A.; Lopez, D.A.; Venzor, J. Cytotoxicity Assessment of Some Carbon Nanotubes and Related Carbon Nanoparticle Aggregates and the Implications for Anthropogenic Carbon Nanotube Aggregates in the Environment. Int. J. Environ. Res. Public. Health 2005, 2, 31–42. [Google Scholar] [CrossRef]
- Bottini, M.; Bruckner, S.; Nika, K.; Bottini, N.; Bellucci, S.; Magrini, A.; Bergamaschi, A.; Mustelin, T. Multi-Walled Carbon Nanotubes Induce T Lymphocyte Apoptosis. Toxicol. Lett. 2006, 160, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.-W. Pulmonary Toxicity of Single-Wall Carbon Nanotubes in Mice 7 and 90 Days After Intratracheal Instillation. Toxicol. Sci. 2003, 77, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B. Comparative Pulmonary Toxicity Assessment of Single-Wall Carbon Nanotubes in Rats. Toxicol. Sci. 2003, 77, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hulderman, T.; Salmen, R.; Chapman, R.; Leonard, S.S.; Young, S.-H.; Shvedova, A.; Luster, M.I.; Simeonova, P.P. Cardiovascular Effects of Pulmonary Exposure to Single-Wall Carbon Nanotubes. Environ. Health Perspect. 2007, 115, 377–382. [Google Scholar] [CrossRef]
- Mutlu, G.M.; Budinger, G.R.S.; Green, A.A.; Urich, D.; Soberanes, S.; Chiarella, S.E.; Alheid, G.F.; McCrimmon, D.R.; Szleifer, I.; Hersam, M.C. Biocompatible Nanoscale Dispersion of Single-Walled Carbon Nanotubes Minimizes in Vivo Pulmonary Toxicity. Nano Lett. 2010, 10, 1664–1670. [Google Scholar] [CrossRef]
- Mitchell, L.A.; Lauer, F.T.; Burchiel, S.W.; McDonald, J.D. Mechanisms for How Inhaled Multiwalled Carbon Nanotubes Suppress Systemic Immune Function in Mice. Nat. Nanotechnol. 2009, 4, 451–456. [Google Scholar] [CrossRef]
- Muller, J.; Huaux, F.; Fonseca, A.; Nagy, J.B.; Moreau, N.; Delos, M.; Raymundo-Piñero, E.; Béguin, F.; Kirsch-Volders, M.; Fenoglio, I.; et al. Structural Defects Play a Major Role in the Acute Lung Toxicity of Multiwall Carbon Nanotubes: Toxicological Aspects. Chem. Res. Toxicol. 2008, 21, 1698–1705. [Google Scholar] [CrossRef]
- Donaldson, K.; Aitken, R.; Tran, L.; Stone, V.; Duffin, R.; Forrest, G.; Alexander, A. Carbon Nanotubes: A Review of Their Properties in Relation to Pulmonary Toxicology and Workplace Safety. Toxicol. Sci. 2006, 92, 5–22. [Google Scholar] [CrossRef]
- Inoue, K.; Yanagisawa, R.; Koike, E.; Nishikawa, M.; Takano, H. Repeated Pulmonary Exposure to Single-Walled Carbon Nanotubes Exacerbates Allergic Inflammation of the Airway: Possible Role of Oxidative Stress. Free Radic. Biol. Med. 2010, 48, 924–934. [Google Scholar] [CrossRef]
- Kobayashi, N.; Naya, M.; Ema, M.; Endoh, S.; Maru, J.; Mizuno, K.; Nakanishi, J. Biological Response and Morphological Assessment of Individually Dispersed Multi-Wall Carbon Nanotubes in the Lung after Intratracheal Instillation in Rats. Toxicology 2010, 276, 143–153. [Google Scholar] [CrossRef]
- Morimoto, Y.; Hirohashi, M.; Ogami, A.; Oyabu, T.; Myojo, T.; Todoroki, M.; Yamamoto, M.; Hashiba, M.; Mizuguchi, Y.; Lee, B.W.; et al. Pulmonary Toxicity of Well-Dispersed Multi-Wall Carbon Nanotubes Following Inhalation and Intratracheal Instillation. Nanotoxicology 2012, 6, 587–599. [Google Scholar] [CrossRef] [PubMed]
- Grubek-Jaworska, H.; Nejman, P.; Czumińska, K.; Przybyłowski, T.; Huczko, A.; Lange, H.; Bystrzejewski, M.; Baranowski, P.; Chazan, R. Preliminary Results on the Pathogenic Effects of Intratracheal Exposure to One-Dimensional Nanocarbons. Carbon 2006, 44, 1057–1063. [Google Scholar] [CrossRef]
- Francis, A.P.; Ganapathy, S.; Palla, V.R.; Murthy, P.B.; Ramaprabhu, S.; Devasena, T. One Time Nose-Only Inhalation of MWCNTs: Exploring the Mechanism of Toxicity by Intermittent Sacrifice in Wistar Rats. Toxicol. Rep. 2015, 2, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Simeonova, P.P.; Erdely, A. Engineered Nanoparticle Respiratory Exposure and Potential Risks for Cardiovascular Toxicity: Predictive Tests and Biomarkers. Inhal. Toxicol. 2009, 21, 68–73. [Google Scholar] [CrossRef]
- Pietroiusti, A.; Massimiani, M.; Fenoglio, I.; Colonna, M.; Valentini, F.; Palleschi, G.; Camaioni, A.; Magrini, A.; Siracusa, G.; Bergamaschi, A.; et al. Low Doses of Pristine and Oxidized Single-Wall Carbon Nanotubes Affect Mammalian Embryonic Development. ACS Nano 2011, 5, 4624–4633. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Y.; Zhang, J.; Mu, Q.; Zhang, W.; Butch, E.R.; Snyder, S.E.; Yan, B. Repeated Administrations of Carbon Nanotubes in Male Mice Cause Reversible Testis Damage without Affecting Fertility. Nat. Nanotechnol. 2010, 5, 683–689. [Google Scholar] [CrossRef]
- Philbrook, N.A.; Walker, V.K.; Afrooz, A.R.M.N.; Saleh, N.B.; Winn, L.M. Investigating the Effects of Functionalized Carbon Nanotubes on Reproduction and Development in Drosophila Melanogaster and CD-1 Mice. Reprod. Toxicol. 2011, 32, 442–448. [Google Scholar] [CrossRef]
- Møller, P.; Wils, R.S.; Di Ianni, E.; Gutierrez, C.A.T.; Roursgaard, M.; Jacobsen, N.R. Genotoxicity of Multi-Walled Carbon Nanotube Reference Materials in Mammalian Cells and Animals. Mutat. Res./Rev. Mutat. Res. 2021, 788, 108393. [Google Scholar] [CrossRef]
- Sargent, L.M.; Hubbs, A.F.; Young, S.-H.; Kashon, M.L.; Dinu, C.Z.; Salisbury, J.L.; Benkovic, S.A.; Lowry, D.T.; Murray, A.R.; Kisin, E.R.; et al. Single-Walled Carbon Nanotube-Induced Mitotic Disruption. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2012, 745, 28–37. [Google Scholar] [CrossRef]
- Lash, L.H. Special Issue: Membrane Transporters in Toxicology. Toxicol. Appl. Pharmacol. 2005, 204, 197. [Google Scholar] [CrossRef]
- Murray, A.R.; Kisin, E.; Leonard, S.S.; Young, S.H.; Kommineni, C.; Kagan, V.E.; Castranova, V.; Shvedova, A.A. Oxidative Stress and Inflammatory Response in Dermal Toxicity of Single-Walled Carbon Nanotubes. Toxicology 2009, 257, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Bedognetti, D.; Sgarrella, F.; Marincola, F.M.; Bianco, A.; Delogu, L.G. Impact of Carbon Nanotubes and Graphene on Immune Cells. J. Transl. Med. 2014, 12, 138. [Google Scholar] [CrossRef]
- Svadlakova, T.; Holmannova, D.; Kolackova, M.; Malkova, A.; Krejsek, J.; Fiala, Z. Immunotoxicity of Carbon-Based Nanomaterials, Starring Phagocytes. Int. J. Mol. Sci. 2022, 23, 8889. [Google Scholar] [CrossRef] [PubMed]
- Kan, H.; Pan, D.; Castranova, V. Engineered Nanoparticle Exposure and Cardiovascular Effects: The Role of a Neuronal-Regulated Pathway. Inhal. Toxicol. 2018, 30, 335–342. [Google Scholar] [CrossRef]
- Alidori, S.; Bowman, R.L.; Yarilin, D.; Romin, Y.; Barlas, A.; Mulvey, J.J.; Fujisawa, S.; Xu, K.; Ruggiero, A.; Riabov, V.; et al. Deconvoluting Hepatic Processing of Carbon Nanotubes. Nat. Commun. 2016, 7, 12343. [Google Scholar] [CrossRef]
- Reddy, A.R.N.; Reddy, Y.N.; Krishna, D.R.; Himabindu, V. Multi Wall Carbon Nanotubes Induce Oxidative Stress and Cytotoxicity in Human Embryonic Kidney (HEK293) Cells. Toxicology 2010, 272, 11–16. [Google Scholar] [CrossRef]
- Facciolà, A.; Visalli, G.; La Maestra, S.; Ceccarelli, M.; D’Aleo, F.; Nunnari, G.; Pellicanò, G.F.; Di Pietro, A. Carbon Nanotubes and Central Nervous System: Environmental Risks, Toxicological Aspects and Future Perspectives. Environ. Toxicol. Pharmacol. 2019, 65, 23–30. [Google Scholar] [CrossRef]
- Zheng, W.; McKinney, W.; Kashon, M.; Salmen, R.; Castranova, V.; Kan, H. The Influence of Inhaled Multi-Walled Carbon Nanotubes on the Autonomic Nervous System. Part. Fibre Toxicol. 2015, 13, 8. [Google Scholar] [CrossRef]
- Aragon, M.J.; Topper, L.; Tyler, C.R.; Sanchez, B.; Zychowski, K.; Young, T.; Herbert, G.; Hall, P.; Erdely, A.; Eye, T.; et al. Serum-Borne Bioactivity Caused by Pulmonary Multiwalled Carbon Nanotubes Induces Neuroinflammation via Blood–Brain Barrier Impairment. Proc. Natl. Acad. Sci. USA 2017, 114, E1968–E1976. [Google Scholar] [CrossRef]
- Gholamine, B.; Karimi, I.; Salimi, A.; Mazdarani, P.; Becker, L.A. Neurobehavioral Toxicity of Carbon Nanotubes in Mice. Toxicol. Ind. Health 2017, 33, 340–350. [Google Scholar] [CrossRef]
- Larner, S.F.; Wang, J.; Goodman, J.; O’Donoghue Altman, M.B.; Xin, M.; Wang, K.K.W. In Vitro Neurotoxicity Resulting from Exposure of Cultured Neural Cells to Several Types of Nanoparticles. J. Cell Death 2017, 10, 117967071769452. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, X.; Nicholas, J.; Humes, S.T.; Loeb, J.C.; Robinson, S.E.; Bisesi, J.H.; Das, D.; Saleh, N.B.; Castleman, W.L.; et al. Single-Walled Carbon Nanotubes Modulate Pulmonary Immune Responses and Increase Pandemic Influenza a Virus Titers in Mice. Virol. J. 2017, 14, 242. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Choi, J.; Kim, J.-H.; Lee, B.-S.; Yoon, C.; Jeong, U.; Kim, Y. Subchronic Immunotoxicity and Screening of Reproductive Toxicity and Developmental Immunotoxicity Following Single Instillation of HIPCO-Single-Walled Carbon Nanotubes: Purity-Based Comparison. Nanotoxicology 2016, 10, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Khang, D.; Lee, S.; Kim, S.-H. High Dispersity of Carbon Nanotubes Diminishes Immunotoxicity in Spleen. Int. J. Nanomed. 2015, 10, 2697–2710. [Google Scholar] [CrossRef]
- Qi, W.; Bi, J.; Zhang, X.; Wang, J.; Wang, J.; Liu, P.; Li, Z.; Wu, W. Damaging Effects of Multi-Walled Carbon Nanotubes on Pregnant Mice with Different Pregnancy Times. Sci. Rep. 2014, 4, 4352. [Google Scholar] [CrossRef]
- Cheng, J.; Cheng, S.H. Influence of Carbon Nanotube Length on Toxicity to Zebrafish Embryos. Int. J. Nanomed. 2012, 7, 3731–3739. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, F.; Sun, X.; Choi, K.-Y.; Niu, G.; Zhang, G.; Guo, J.; Lee, S.; Chen, X. The Genotype-Dependent Influence of Functionalized Multiwalled Carbon Nanotubes on Fetal Development. Biomaterials 2014, 35, 856–865. [Google Scholar] [CrossRef]
- Zhu, B.; Zhu, S.; Li, J.; Hui, X.; Wang, G.-X. The Developmental Toxicity, Bioaccumulation and Distribution of Oxidized Single Walled Carbon Nanotubes in Artemia salina. Toxicol. Res. 2018, 7, 897–906. [Google Scholar] [CrossRef]
- Lindberg, H.K.; Falck, G.C.-M.; Singh, R.; Suhonen, S.; Järventaus, H.; Vanhala, E.; Catalán, J.; Farmer, P.B.; Savolainen, K.M.; Norppa, H. Genotoxicity of Short Single-Wall and Multi-Wall Carbon Nanotubes in Human Bronchial Epithelial and Mesothelial Cells in Vitro. Toxicology 2013, 313, 24–37. [Google Scholar] [CrossRef]
- Lettiero, B.; Andersen, A.J.; Hunter, A.C.; Moghimi, S.M. Complement System and the Brain: Selected Pathologies and Avenues toward Engineering of Neurological Nanomedicines. J. Control. Release 2012, 161, 283–289. [Google Scholar] [CrossRef]
- Moghimi, S.M.; Peer, D.; Langer, R. Reshaping the Future of Nanopharmaceuticals: Ad iudicium. ACS Nano 2011, 5, 8454–8458. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Davis, C.; Cai, W.; He, L.; Chen, X.; Dai, H. Circulation and Long-Term Fate of Functionalized, Biocompatible Single-Walled Carbon Nanotubes in Mice Probed by Raman Spectroscopy. Proc. Natl. Acad. Sci. USA 2008, 105, 1410–1415. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Fernando, K.A.S.; Liu, J.; Wang, J.; Sun, H.; Liu, Y.; Chen, M.; Huang, Y.; Wang, X.; Wang, H.; et al. Covalently PEGylated Carbon Nanotubes with Stealth Character In Vivo. Small 2008, 4, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Pondman, K.M.; Pednekar, L.; Paudyal, B.; Tsolaki, A.G.; Kouser, L.; Khan, H.A.; Shamji, M.H.; ten Haken, B.; Stenbeck, G.; Sim, R.B.; et al. Innate Immune Humoral Factors, C1q and Factor H, with Differential Pattern Recognition Properties, Alter Macrophage Response to Carbon Nanotubes. Nanomedicine 2015, 11, 2109–2118. [Google Scholar] [CrossRef]
- Silva, R.M.; Doudrick, K.; Franzi, L.M.; TeeSy, C.; Anderson, D.S.; Wu, Z.; Mitra, S.; Vu, V.; Dutrow, G.; Evans, J.E.; et al. Instillation versus Inhalation of Multiwalled Carbon Nanotubes: Exposure-Related Health Effects, Clearance, and the Role of Particle Characteristics. ACS Nano 2014, 8, 8911–8931. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Sun, B.; Chen, C. Understanding the Toxicity of Carbon Nanotubes. Acc. Chem. Res. 2013, 46, 702–713. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Li, Q.; Li, Y.; Li, Y.; Zhang, X.; Huang, Q. Effects of Serum Proteins on Intracellular Uptake and Cytotoxicity of Carbon Nanoparticles. Carbon 2009, 47, 1351–1358. [Google Scholar] [CrossRef]
- Dong, X.; Sun, Z.; Wang, X.; Leng, X. An Innovative MWCNTs/DOX/TC Nanosystem for Chemo-Photothermal Combination Therapy of Cancer. Nanomedicine 2017, 13, 2271–2280. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, R.; Hu, Y.; Sun, R.; Song, T.; Shi, X.; Yin, S. Stacking of Doxorubicin on Folic Acid-Targeted Multiwalled Carbon Nanotubes for in Vivo Chemotherapy of Tumors. Drug Deliv. 2018, 25, 1607–1616. [Google Scholar] [CrossRef]
- Awasthi, S.; De, S.; Pandey, S.K. Surface Grafting of Carbon Nanostructures. In Handbook of Functionalized Carbon Nanostructures; Springer International Publishing: Cham, Switzerland, 2024; pp. 1–45. [Google Scholar]
- Rele, S.; Thakur, C.K.; Khan, F.; Baral, B.; Saini, V.; Karthikeyan, C.; Moorthy, N.S.H.N.; Jha, H.C. Curcumin Coating: A Novel Solution to Mitigate Inherent Carbon Nanotube Toxicity. J. Mater. Sci. Mater. Med. 2024, 35, 24. [Google Scholar] [CrossRef]
- Wu, L.; Man, C.; Wang, H.; Lu, X.; Ma, Q.; Cai, Y.; Ma, W. PEGylated Multi-Walled Carbon Nanotubes for Encapsulation and Sustained Release of Oxaliplatin. Pharm. Res. 2013, 30, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.M.; Bourgognon, M.; Wang, J.T.-W.; Al-Jamal, K.T. Functionalised Carbon Nanotubes: From Intracellular Uptake and Cell-Related Toxicity to Systemic Brain Delivery. J. Control. Release 2016, 241, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Sohaebuddin, S.K.; Thevenot, P.T.; Baker, D.; Eaton, J.W.; Tang, L. Nanomaterial Cytotoxicity Is Composition, Size, and Cell Type Dependent. Part. Fibre Toxicol. 2010, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Heras, A.; Colina, A.; López-Palacios, J.; Ayala, P.; Sainio, J.; Ruiz, V.; Kauppinen, E.I. Electrochemical Purification of Carbon Nanotube Electrodes. Electrochem. Commun. 2009, 11, 1535–1538. [Google Scholar] [CrossRef]
- Mercier, G.; Hérold, C.; Marêché, J.-F.; Cahen, S.; Gleize, J.; Ghanbaja, J.; Lamura, G.; Bellouard, C.; Vigolo, B. Selective Removal of Metal Impurities from Single Walled Carbon Nanotube Samples. New J. Chem. 2013, 37, 790. [Google Scholar] [CrossRef]
- Pełech, I.; Narkiewicz, U.; Kaczmarek, A.; Jędrzejewska, A.; Pełech, R. Removal of Metal Particles from Carbon Nanotubes Using Conventional and Microwave Methods. Sep. Purif. Technol. 2014, 136, 105–110. [Google Scholar] [CrossRef]
- Madani, S.Y.; Mandel, A.; Seifalian, A.M. A Concise Review of Carbon Nanotube’s Toxicology. Nano Rev. 2013, 4, 21521. [Google Scholar] [CrossRef]
- Sallam, A.A.; Ahmed, M.M.; El-Magd, M.A.; Magdy, A.; Ghamry, H.I.; Alshahrani, M.Y.; Abou El-Fotoh, M.F. Quercetin-Ameliorated, Multi-Walled Carbon Nanotubes-Induced Immunotoxic, Inflammatory, and Oxidative Effects in Mice. Molecules 2022, 27, 2117. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, X.; Ji, Z.; Shen, X.; Dong, L.; Wu, M.; Gu, T.; Liu, Y. Long-Term Hepatotoxicity of Polyethylene-Glycol Functionalized Multi-Walled Carbon Nanotubes in Mice. Nanotechnology 2010, 21, 175101. [Google Scholar] [CrossRef]
- Pérez-Luna, V.; Moreno-Aguilar, C.; Arauz-Lara, J.L.; Aranda-Espinoza, S.; Quintana, M. Interactions of Functionalized Multi-Wall Carbon Nanotubes with Giant Phospholipid Vesicles as Model Cellular Membrane System. Sci. Rep. 2018, 8, 17998. [Google Scholar] [CrossRef]
- Adenuga, A.A.; Truong, L.; Tanguay, R.L.; Remcho, V.T. Preparation of Water Soluble Carbon Nanotubes and Assessment of Their Biological Activity in Embryonic Zebrafish. Int. J. Biomed. Nanosci. Nanotechnol. 2013, 3, 38. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.L.; Liu, H.Q.; Tan, H.R.; Liu, Y. Delivery of Paclitaxel by Physically Loading onto Poly(Ethylene Glycol) (PEG)-Graftcarbon Nanotubes for Potent Cancer Therapeutics. Nanotechnology 2010, 21, 065101. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, S.T.R.; Rasheed, T.; Hussain, D.; Najam ul Haq, M.; Majeed, S.; Shafi, S.; Ahmed, N.; Nawaz, R. Modification Strategies for Improving the Solubility/Dispersion of Carbon Nanotubes. J. Mol. Liq. 2020, 297, 111919. [Google Scholar] [CrossRef]
- Saleemi, M.A.; Hosseini Fouladi, M.; Yong, P.V.C.; Chinna, K.; Palanisamy, N.K.; Wong, E.H. Toxicity of Carbon Nanotubes: Molecular Mechanisms, Signaling Cascades, and Remedies in Biomedical Applications. Chem. Res. Toxicol. 2021, 34, 24–46. [Google Scholar] [CrossRef]
- Barosova, H.; Karakocak, B.B.; Septiadi, D.; Petri-Fink, A.; Stone, V.; Rothen-Rutishauser, B. An In Vitro Lung System to Assess the Proinflammatory Hazard of Carbon Nanotube Aerosols. Int. J. Mol. Sci. 2020, 21, 5335. [Google Scholar] [CrossRef]
- Hou, P.-X.; Liu, C.; Cheng, H.-M. Purification of Carbon Nanotubes. Carbon 2008, 46, 2003–2025. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, M. Biodegradation of Carbon Nanotubes by Macrophages. Front. Mater. 2019, 6, 225. [Google Scholar] [CrossRef]
- Yang, H.; Yang, T.; Heng, C.; Zhou, Y.; Jiang, Z.; Qian, X.; Du, L.; Mao, S.; Yin, X.; Lu, Q. Quercetin Improves Nonalcoholic Fatty Liver by Ameliorating Inflammation, Oxidative Stress, and Lipid Metabolism in Db/Db Mice. Phytother. Res. 2019, 33, 3140–3152. [Google Scholar] [CrossRef]
- Di Petrillo, A.; Orrù, G.; Fais, A.; Fantini, M.C. Quercetin and Its Derivates as Antiviral Potentials: A Comprehensive Review. Phytother. Res. 2022, 36, 266–278. [Google Scholar] [CrossRef]
- Nakanishi, J.; Morimoto, Y.; Ogura, I.; Kobayashi, N.; Naya, M.; Ema, M.; Endoh, S.; Shimada, M.; Ogami, A.; Myojyo, T.; et al. Risk Assessment of the Carbon Nanotube Group. Risk Anal. 2015, 35, 1940–1956. [Google Scholar] [CrossRef]
- Kumar Babele, P.; Kumar Verma, M.; Kant Bhatia, R. Carbon Nanotubes: A Review on Risks Assessment, Mechanism of Toxicity and Future Directives to Prevent Health Implication. BIOCELL 2021, 45, 267–279. [Google Scholar] [CrossRef]
- Duraia, E.M.; Opoku, M.; Beall, G.W. Efficient Eco-Friendly Synthesis of Carbon Nanotubes over Graphite Nanosheets from Yellow Corn: A One-Step Green Approach. Sci. Rep. 2024, 14, 16405. [Google Scholar] [CrossRef] [PubMed]
- Fisher, C.; Rider, A.E.; Jun Han, Z.; Kumar, S.; Levchenko, I.; Ostrikov, K. Applications and Nanotoxicity of Carbon Nanotubes and Graphene in Biomedicine. J. Nanomater. 2012, 2012, 315185. [Google Scholar] [CrossRef]
- Gendron, D.; Bubak, G. Carbon Nanotubes and Graphene Materials as Xenobiotics in Living Systems: Is There a Consensus on Their Safety? J. Xenobiot. 2023, 13, 740–760. [Google Scholar] [CrossRef]
- Zumwalde, R.D. Current Intelligence Bulletin 65: Occupational Exposure to Carbon Nanotubes and Nanofibers; The National Institute for Occupational Safety and Health (NIOSH): Atlanta, GA, USA, 2013. [Google Scholar]
- European Union Eur-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=OJ:JOC_2022_229_R_0001 (accessed on 13 March 2025).
- EU-OSHA—The Swedish Work Environment Authority. Carbon Nanotubes—Exposure, Toxicology and Protective Measures in the Work Environment; EU-OSHA—The Swedish Work Environment Authority: Solna, Sweden, 2011. [Google Scholar]
- Schulte, P.A.; Murashov, V.; Zumwalde, R.; Kuempel, E.D.; Geraci, C.L. Occupational Exposure Limits for Nanomaterials: State of the Art. J. Nanopart. Res. 2010, 12, 1971–1987. [Google Scholar] [CrossRef]
- Kerr, R.; McHugh, M.; McCrory, M. HSE Management Standards and Stress-Related Work Outcomes. Occup. Med. 2009, 59, 574–579. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).