Synthesis of Copper Nanowires Using Monoethanolamine and the Application in Transparent Conductive Films
Abstract
:1. Introduction
2. Experimental Methods
2.1. Materials
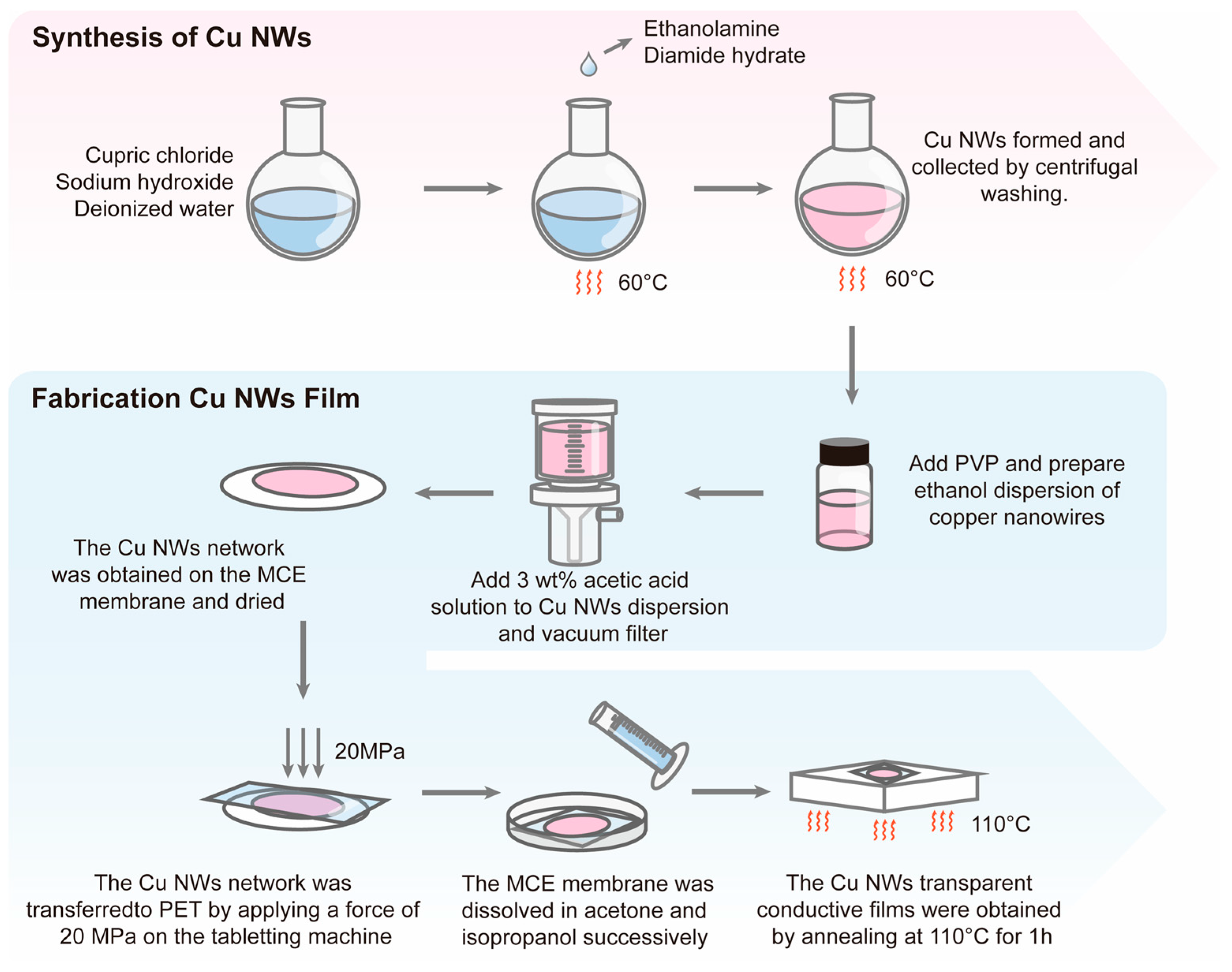
2.2. Preparation of Copper Nanowires
2.3. Preparation of Transparent Conductive Films
2.4. Material Characterization
3. Results and Discussion
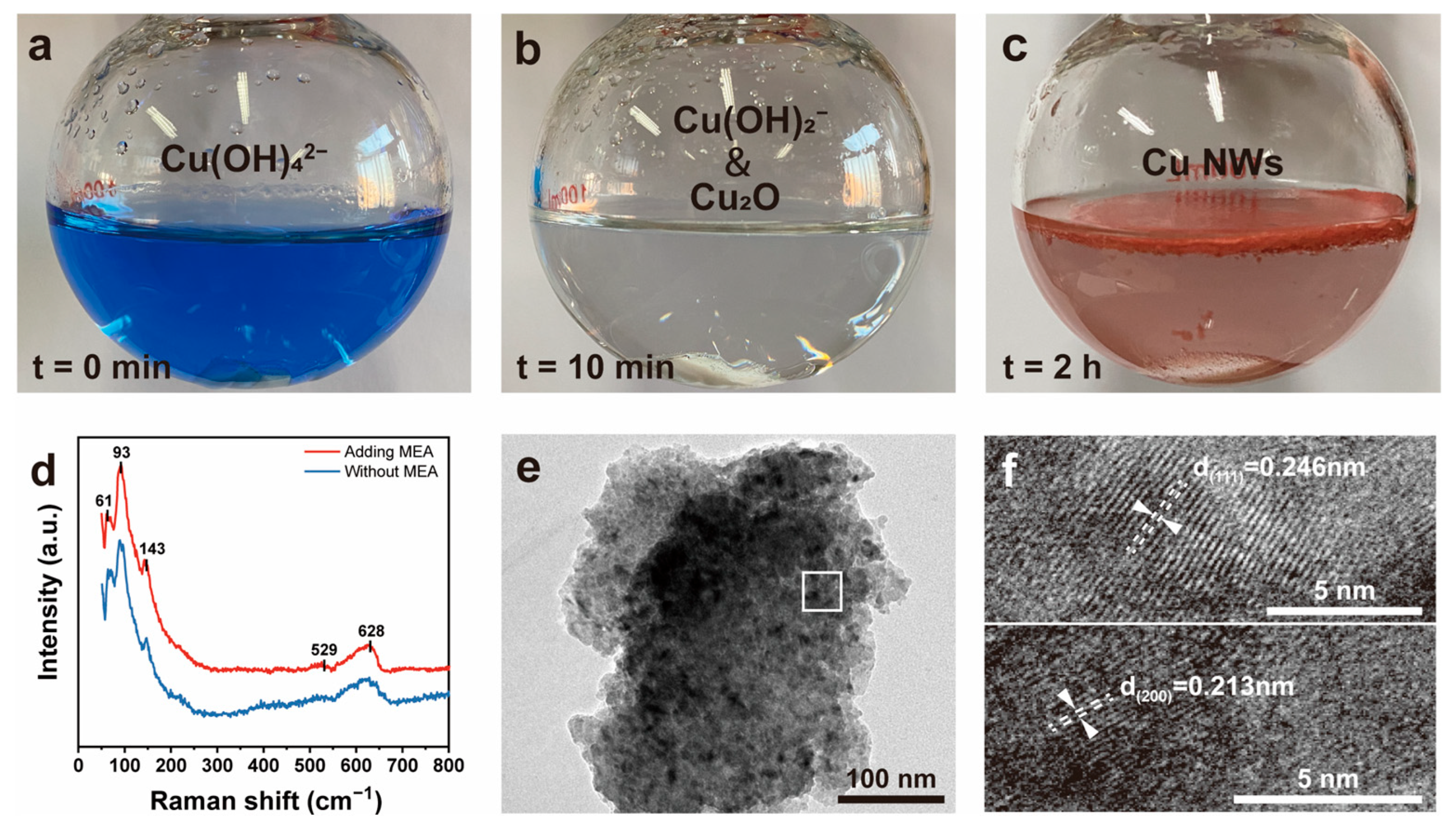
3.1. Synthesis of Cu NWs and Their Morphology and Structure
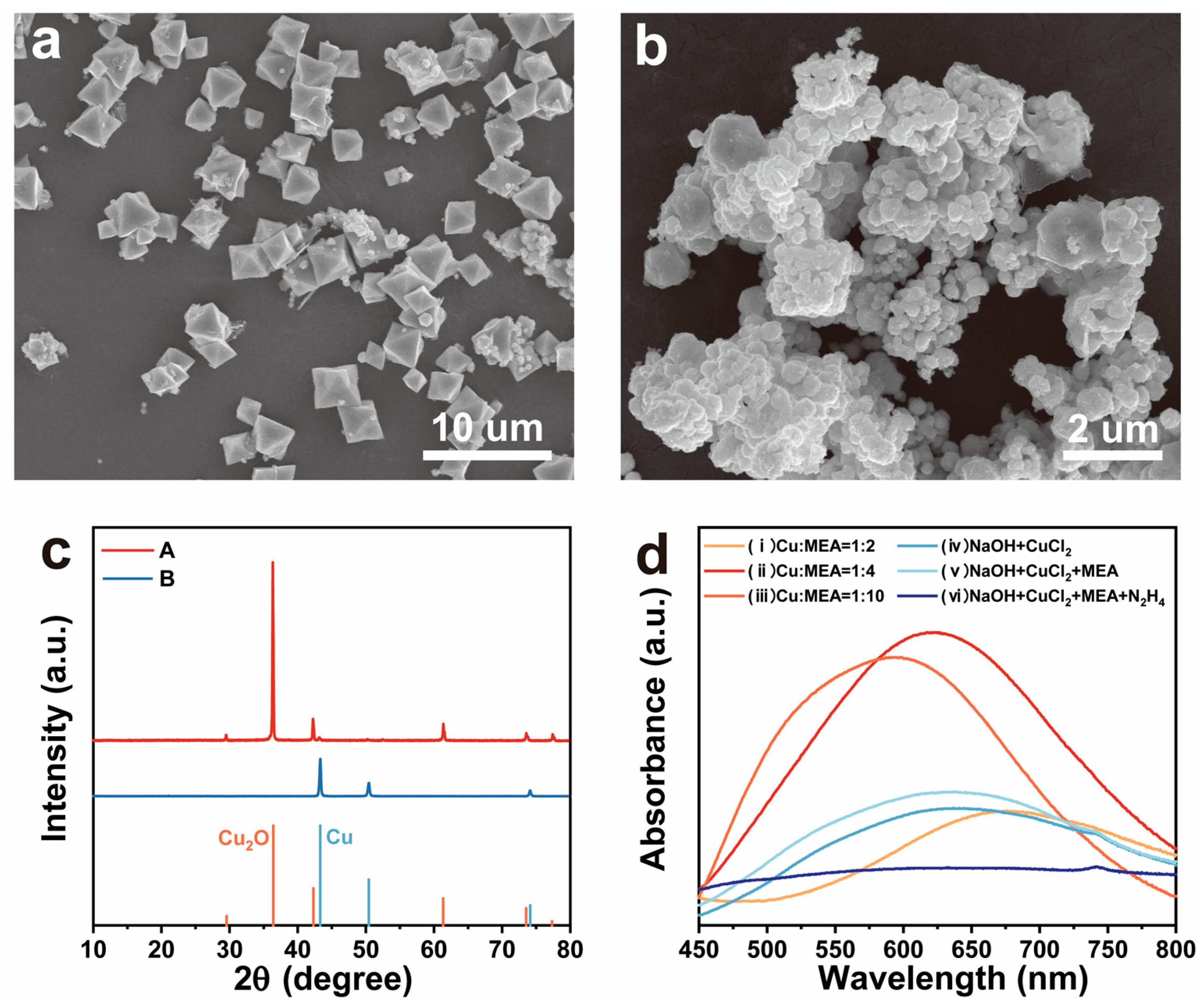
3.2. Study on the Synthesis Process of Cu NWs
3.3. Investigation of the Mechanism of MEA as a Complexing Agent
3.4. Cu NWs Transparent Conductive Film
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kang, S.; Cho, S.; Shanker, R.; Lee, H.; Park, J.; Um, D.-S.; Lee, Y.; Ko, H. Transparent and Conductive Nanomembranes with Orthogonal Silver Nanowire Arrays for Skin-Attachable Loudspeakers and Microphones. Sci. Adv. 2018, 4, eaas8772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ng, S.-W.; Lu, X.; Zheng, Z. Solution-Processed Transparent Electrodes for Emerging Thin-Film Solar Cells. Chem. Rev. 2020, 120, 2049–2122. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Peng, Z.; Li, M.; Wang, Y. Stretchable Transparent Conductive Films from Long Carbon Nanotube Metals. Small 2018, 14, 1802625. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wu, Y.; Li, Y.; Shi, Y.; Pan, Q.; Yang, D.; Wang, T. Novel Facile Synthesis and Room-Temperature Sintering of Copper Nanowires for Solution-Processed Transparent Conductive Films. Chem. Eng. J. 2023, 472, 145030. [Google Scholar] [CrossRef]
- Rosental, T.; Ottomaniello, A.; Mizrahi, S.; Menda, N.; Moshkovitz Douvdevany, M.Y.; Larush, L.; Savraeva, D.; Jarach, N.; Mattoli, V.; Magdassi, S. Additive Manufacturing of Transparent Conductive Indium Tin Oxide by Sol-Gel and Multiphoton Absorption Polymerization. Addit. Manuf. 2024, 92, 104388. [Google Scholar] [CrossRef]
- Liu, S.; Xu, X.; Jiang, J. Flexible Transparent ITO Thin Film with High Conductivity and High-Temperature Resistance. Ceram. Int. 2024, 50, 47649–47654. [Google Scholar] [CrossRef]
- Reynaud, O.; Nasibulin, A.G.; Anisimov, A.S.; Anoshkin, I.V.; Jiang, H.; Kauppinen, E.I. Aerosol Feeding of Catalyst Precursor for CNT Synthesis and Highly Conductive and Transparent Film Fabrication. Chem. Eng. J. 2014, 255, 134–140. [Google Scholar] [CrossRef]
- Gorkina, A.L.; Tsapenko, A.P.; Gilshteyn, E.P.; Koltsova, T.S.; Larionova, T.V.; Talyzin, A.; Anisimov, A.S.; Anoshkin, I.V.; Kauppinen, E.I.; Tolochko, O.V.; et al. Transparent and Conductive Hybrid Graphene/Carbon Nanotube Films. Carbon 2016, 100, 501–507. [Google Scholar] [CrossRef]
- Ye, S.; Rathmell, A.R.; Chen, Z.; Stewart, I.E.; Wiley, B.J. Metal Nanowire Networks: The Next Generation of Transparent Conductors. Adv. Mater. 2014, 26, 6670–6687. [Google Scholar] [CrossRef]
- Li, Y.; Ai, Q.; Mao, L.; Guo, J.; Gong, T.; Lin, Y.; Wu, G.; Huang, W.; Zhang, X. Hybrid Strategy of Graphene/Carbon Nanotube Hierarchical Networks for Highly Sensitive, Flexible Wearable Strain Sensors. Sci. Rep. 2021, 11, 21006. [Google Scholar] [CrossRef]
- Suriani, A.B.; Muqoyyanah; Mohamed, A.; Alfarisa, S.; Mamat, M.H.; Ahmad, M.K.; Birowosuto, M.D.; Soga, T. Synthesis, Transfer and Application of Graphene as a Transparent Conductive Film: A Review. Bull. Mater. Sci. 2020, 43, 310. [Google Scholar] [CrossRef]
- Ai, P.; Mai, X.; Xue, B.; Xie, L. Core-Shell Nickel@copper Nanowires Associated with Multilayered Gradient Architecture Design towards Excellent Absorption-Dominant Electromagnetic Interference Shielding. J. Mater. Sci. Technol. 2025, 230, 21–31. [Google Scholar] [CrossRef]
- Zhang, D.; Wang, R.; Wen, M.; Weng, D.; Cui, X.; Sun, J.; Li, H.; Lu, Y. Synthesis of Ultralong Copper Nanowires for High-Performance Transparent Electrodes. J. Am. Chem. Soc. 2012, 134, 14283–14286. [Google Scholar] [CrossRef]
- Qin, S.; Zhao, X.; Lin, J.; Liu, H.; Zhao, L.; Yu, S. Self-Assembled SnO2 Nanoshells Grown on Copper Nanowires after Electroplating Soldering for Stable and Transparent Conductors. Appl. Surf. Sci. 2025, 688, 162348. [Google Scholar] [CrossRef]
- Kwon, D.-K.; Lee, S.J.; Myoung, J.-M. High-Performance Flexible ZnO Nanorod UV Photodetectors with a Network-Structured Cu Nanowire Electrode. Nanoscale 2016, 8, 16677–16683. [Google Scholar] [CrossRef]
- Sun, Y.; Mayers, B.; Herricks, T.; Xia, Y. Polyol Synthesis of Uniform Silver Nanowires: A Plausible Growth Mechanism and the Supporting Evidence. Nano Lett. 2003, 3, 955–960. [Google Scholar] [CrossRef]
- Kumar, D.V.R.; Woo, K.; Moon, J. Promising Wet Chemical Strategies to Synthesize Cu Nanowires for Emerging Electronic Applications. Nanoscale 2015, 7, 17195–17210. [Google Scholar] [CrossRef]
- Lin, W.; Ghulam Nabi, A.; Palma, M.; Di Tommaso, D. Copper Nanowires for Electrochemical CO2 Reduction Reaction. ACS Appl. Nano Mater. 2024, 7, 27883–27898. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Huh, Y.-D. Synthesis of Ultralong Copper Nanowires by Reduction of Copper-Amine Complexes. Mater. Lett. 2009, 63, 227–229. [Google Scholar] [CrossRef]
- Chen, Z.-X.; Song, Y.-J.; Li, R.-Z.; Guo, S.-J.; Shi, L.; Yang, Z.-R.; Xue, X.-M.; Zhang, T. Advances in Copper Nanocrystals: Synthesis, Anti-Oxidation Strategies, and Multiple Applications. Coord. Chem. Rev. 2025, 529, 216455. [Google Scholar] [CrossRef]
- Cui, F.; Yu, Y.; Dou, L.; Sun, J.; Yang, Q.; Schildknecht, C.; Schierle-Arndt, K.; Yang, P. Synthesis of Ultrathin Copper Nanowires Using Tris(Trimethylsilyl)Silane for High-Performance and Low-Haze Transparent Conductors. Nano Lett. 2015, 15, 7610–7615. [Google Scholar] [CrossRef] [PubMed]
- Amorim, E.P.M.; da Silva, E.Z. Helical [110] Gold Nanowires Make Longer Linear Atomic Chains. Phys. Rev. Lett. 2008, 101, 125502. [Google Scholar] [CrossRef] [PubMed]
- Osako, T.; Ueno, Y.; Tachi, Y.; Itoh, S. Structures and Redox Reactivities of Copper Complexes of (2-Pyridyl)Alkylamine Ligands. Effects of the Alkyl Linker Chain Length. Inorg. Chem. 2003, 42, 8087–8097. [Google Scholar] [CrossRef]
- Amorim, E.P.M.; da Silva, A.J.R.; Fazzio, A.; da Silva, E.Z. Short Linear Atomic Chains in Copper Nanowires. Nanotechnology 2007, 18, 145701. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Liang, J.; Hu, Z.; Li, S.; Peng, S.; Qian, Y. Synthesis of Copper Nanowires via a Complex-Surfactant-Assisted Hydrothermal Reduction Process. J. Phys. Chem. B. Condens. Matter Mater. Surf. Interfaces Biophys. 2003, 107, 12658–12661. [Google Scholar] [CrossRef]
- Xu, S.; Sun, X.; Ye, H.; You, T.; Song, X.; Sun, S. Selective Synthesis of Copper Nanoplates and Nanowires via a Surfactant-Assisted Hydrothermal Process. Mater. Chem. Phys. 2009, 120, 1–5. [Google Scholar] [CrossRef]
- Jin, M.; He, G.; Zhang, H.; Zeng, J.; Xie, Z.; Xia, Y. Shape-Controlled Synthesis of Copper Nanocrystals in an Aqueous Solution with Glucose as a Reducing Agent and Hexadecylamine as a Capping Agent. Angew. Chem. Int. Ed. 2011, 50, 10560–10564. [Google Scholar] [CrossRef]
- Mohl, M.; Pusztai, P.; Kukovecz, A.; Konya, Z.; Kukkola, J.; Kordas, K.; Vajtai, R.; Ajayan, P.M. Low-Temperature Large-Scale Synthesis and Electrical Testing of Ultralong Copper Nanowires. Langmuir 2010, 26, 16496–16502. [Google Scholar] [CrossRef]
- Chiu, J.-M.; Wahdini, I.; Shen, Y.-N.; Tseng, C.-Y.; Sharma, J.; Tai, Y. Highly Stable Copper Nanowire-Based Transparent Conducting Electrode Utilizing Polyimide as a Protective Layer. ACS Appl. Energy Mater. 2023, 6, 5058–5066. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Tai, M.-C.; Liao, C.-N. Mechanistic Understanding of Enhanced Thermal Stability of Twinned Copper Nanowires. Mater. Today Phys. 2024, 43, 101407. [Google Scholar] [CrossRef]
- Anand Omar, R.; Ranavare, S.B.; Verma, N. Cu Nanowire—Impregnated Activated Carbon Fiber for Antibacterial Applications. Chem. Eng. Sci. 2024, 299, 120489. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, W.; Cheng, D. Facile Synthesis of Cu/NiCu Electrocatalysts Integrating Alloy, Core–Shell, and One-Dimensional Structures for Efficient Methanol Oxidation Reaction. ACS Appl. Mater. Interfaces 2017, 9, 19843–19851. [Google Scholar] [CrossRef]
- Guo, H.; Jin, J.; Chen, Y.; Liu, X.; Zeng, D.; Wang, L.; Peng, D.-L. Controllable Synthesis of Cu–Ni Core–Shell Nanoparticles and Nanowires with Tunable Magnetic Properties. Chem. Commun. 2016, 52, 6918–6921. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Bergin, S.M.; Hua, Y.-L.; Li, Z.-Y.; Wiley, B.J. The Growth Mechanism of Copper Nanowires and Their Properties in Flexible, Transparent Conducting Films. Adv. Mater. 2010, 22, 3558–3563. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Wiley, B.J. The Synthesis and Coating of Long, Thin Copper Nanowires to Make Flexible, Transparent Conducting Films on Plastic Substrates. Adv. Mater. 2011, 23, 4798–4803. [Google Scholar] [CrossRef]
- Ye, S.; Rathmell, A.R.; Stewart, I.E.; Ha, Y.-C.; Wilson, A.R.; Chen, Z.; Wiley, B.J. A Rapid Synthesis of High Aspect Ratio Copper Nanowires for High-Performance Transparent Conducting Films. Chem. Commun. 2014, 50, 2562–2564. [Google Scholar] [CrossRef]
- Ye, S.; Chen, Z.; Ha, Y.-C.; Wiley, B.J. Real-Time Visualization of Diffusion-Controlled Nanowire Growth in Solution. Nano Lett. 2014, 14, 4671–4676. [Google Scholar] [CrossRef]
- Kim, M.J.; Flowers, P.F.; Stewart, I.E.; Ye, S.; Baek, S.; Kim, J.J.; Wiley, B.J. Ethylenediamine Promotes Cu Nanowire Growth by Inhibiting Oxidation of Cu(111). J. Am. Chem. Soc. 2017, 139, 277–284. [Google Scholar] [CrossRef]
- Zhang, J.; Li, X.; Liu, D.; Wang, S.; Yan, J.; Lu, M.; Xie, X.; Huang, L.; Huang, W. Stirring Revealed New Functions of Ethylenediamine and Hydrazine in the Morphology Control of Copper Nanowires. Nanoscale 2019, 11, 11902–11909. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chen, H.-Y.T.; Liao, C.-N. Mechanistic Understanding of Self-Limiting Oxidation Behavior of Copper Nanowires with Dense Twin Boundaries. Appl. Surf. Sci. 2025, 692, 162753. [Google Scholar] [CrossRef]
- Yu, H.; Lanot, A.; Mao, N. The Relationship between Molecular Weight of Bacterial Cellulose and the Viscosity of Its Copper (II) Ethylenediamine Solutions. Cellulose 2024, 31, 7973–7992. [Google Scholar] [CrossRef]
- Fisher, J.F.; Hall, J.L. A Polarographic Study of the Copper(II) Complexes of Mono-, Di, and Triethanolamine. Anal. Chem. 1962, 34, 1094–1098. [Google Scholar] [CrossRef]
- Cody, I.A.; Woodburn, S.I.; Blackmore, M.W.; Magee, R.J. Some Copper(II) Ethanolamine Complexes. J. Inorg. Nucl. Chem. 1970, 32, 3263–3269. [Google Scholar] [CrossRef]
- Ellsworth, A.A.; Walker, A.V. Effect of Ethanolamines on the Electroless Deposition of Copper on Functionalized Organic Surfaces. Langmuir 2018, 34, 4142–4149. [Google Scholar] [CrossRef]
- Chang, Y.; Lye, M.L.; Zeng, H.C. Large-Scale Synthesis of High-Quality Ultralong Copper Nanowires. Langmuir 2005, 21, 3746–3748. [Google Scholar] [CrossRef]
- Aherne, D.; Ledwith, D.M.; Gara, M.; Kelly, J.M. Optical Properties and Growth Aspects of Silver Nanoprisms Produced by a Highly Reproducible and Rapid Synthesis at Room Temperature. Advanced Funct. Mater. 2008, 18, 2005–2016. [Google Scholar] [CrossRef]
- Radwan, S.; El-Khabeary, H. Influence of High-Energy Particles on Copper and Stainless Steel in Fusion Reactor Materials. Can. J. Phys. 2021, 99, 954–968. [Google Scholar] [CrossRef]
- Beverskog, B.; Puigdomenech, I. Revised Pourbaix Diagrams for Copper at 25 to 300 °C. J. Electrochem. Soc. 1997, 144, 3476. [Google Scholar] [CrossRef]
- Ye, S.; Rathmell, A.R.; Ha, Y.-C.; Wilson, A.R.; Wiley, B.J. The Role of Cuprous Oxide Seeds in the One-Pot and Seeded Syntheses of Copper Nanowires. Small 2014, 10, 1771–1778. [Google Scholar] [CrossRef]
- Balkanski, M.; Nusimovici, M.A.; Reydellet, J. First Order Raman Spectrum of Cu2O. Solid State Commun. 1969, 7, 815–818. [Google Scholar] [CrossRef]
- Reydellet, J.; Balkanski, M.; Trivich, D. Light Scattering and Infrared Absorption in Cuprous Oxide. Phys. Status Solidi (b) 1972, 52, 175–185. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, D.; Guo, L.; Zhang, R.; Yin, P.; Wang, R. One-Pot Assembly of Cu2O Chain-Like Hollow Structures. J. Nanosci. Nanotechnol. 2008, 8, 6332–6337. [Google Scholar] [CrossRef]
- Min, C.; Li, S.; Shi, Z.; Xie, J.; Ma, R. Effect of pH on the Electrodeposition Nucleation and Growth Mechanism of Cuprous Oxide. J. Solid State Electrochem. 2023, 27, 1085–1093. [Google Scholar] [CrossRef]
- Fertig, D.; Valiskó, M.; Boda, D. Controlling Ionic Current through a Nanopore by Tuning pH: A Local Equilibrium Monte Carlo Study. Mol. Phys. 2019, 117, 2793–2801. [Google Scholar] [CrossRef]
- Ye, S.; Stewart, I.E.; Chen, Z.; Li, B.; Rathmell, A.R.; Wiley, B.J. How Copper Nanowires Grow and How To Control Their Properties. Acc. Chem. Res. 2016, 49, 442–451. [Google Scholar] [CrossRef]
- Li, Z.; Chang, S.; Khuje, S.; Ren, S. Recent Advancement of Emerging Nano Copper-Based Printable Flexible Hybrid Electronics. ACS Nano 2021, 15, 6211–6232. [Google Scholar] [CrossRef]
- Seong, K.; Kim, J.M.; Kang, J.; Hwang, M.; Lee, C.; Piao, Y. An Ultradurable and Uniform Cu Electrode by Blending Carbon Nanotube Fillers in Copper-Based Metal–Organic Decomposition Ink for Flexible Printed Electronics. Adv. Mater. Interfaces 2018, 5, 1800502. [Google Scholar] [CrossRef]
- Feng, Y.; Lv, X.; Ran, X.; Jia, C.; Qin, L.; Chen, M.; Qi, R.; Peng, H.; Lin, H. High-Efficiency Synthesis of Cu Superfine Particles via Reducing Cuprous and Cupric Oxides with Monoethanolamine and Their Antimicrobial Potentials. J. Colloid Interface Sci. 2022, 608, 749–757. [Google Scholar] [CrossRef]
- Wang, Y.; Lü, Y.; Zhan, W.; Xie, Z.; Kuang, Q.; Zheng, L. Synthesis of Porous Cu2O/CuO Cages Using Cu-Based Metal–Organic Frameworks as Templates and Their Gas-Sensing Properties. J. Mater. Chem. A 2015, 3, 12796–12803. [Google Scholar] [CrossRef]
- Koo, J.; Kwon, S.; Kim, N.R.; Shin, K.; Lee, H.M. Ethylenediamine-Enhanced Oxidation Resistivity of a Copper Surface during Water-Based Copper Nanowire Synthesis. J. Phys. Chem. C 2016, 120, 3334–3340. [Google Scholar] [CrossRef]
- Wu, C.-K.; Yin, M.; O’Brien, S.; Koberstein, J.T. Quantitative Analysis of Copper Oxide Nanoparticle Composition and Structure by X-Ray Photoelectron Spectroscopy. Chem. Mater. 2006, 18, 6054–6058. [Google Scholar] [CrossRef]
- McIntyre, N.S.; Cook, M.G. X-Ray Photoelectron Studies on Some Oxides and Hydroxides of Cobalt, Nickel, and Copper. Anal. Chem. 1975, 47, 2208–2213. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, X.; Xu, J.; Xu, R.; Yang, H.; Kan, C. Comparative Study on Preparation Methods for Transparent Conductive Films Based on Silver Nanowires. Molecules 2022, 27, 8907. [Google Scholar] [CrossRef]
- You, W.; Liao, B.; Wan, S.; Guo, X. Research Progress on the Stability of Transparent Conductive Films for Silver Nanowires. Microelectron. Reliab. 2024, 156, 115394. [Google Scholar] [CrossRef]
- Thomas, N.; Bharathkumar, S.; Koshy, A.M.; Basavaraj, M.G.; Swaminathan, P. Investigating the Optical and Electrical Performance of Rod Coated Silver Nanowire-Based Transparent Conducting Films. Nanotechnology 2024, 35, 465602. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.-Y.; Peumans, P.; Cui, Y. Scalable Coating and Properties of Transparent, Flexible, Silver Nanowire Electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef]
- Zhang, Q.; Nam, J.-S.; Han, J.; Datta, S.; Wei, N.; Ding, E.-X.; Hussain, A.; Ahmad, S.; Skakalova, V.; Khan, A.T.; et al. Large-Diameter Carbon Nanotube Transparent Conductor Overcoming Performance–Yield Tradeoff. Adv. Funct. Mater. 2022, 32, 2103397. [Google Scholar] [CrossRef]
- Da, S.-X.; Wang, J.; Geng, H.-Z.; Jia, S.-L.; Xu, C.-X.; Li, L.-G.; Shi, P.-P.; Li, G. High Adhesion Transparent Conducting Films Using Graphene Oxide Hybrid Carbon Nanotubes. Appl. Surf. Sci. 2017, 392, 1117–1125. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Dong, L.; Huang, M.; Ying, S.; Peng, C. Preparation of Copper Nanowires Conductive Films by Using Cuprous Oxide Nanowire as Template. IOP Conf. Ser. Earth Environ. Sci. 2020, 446, 022027. [Google Scholar] [CrossRef]
- Bae, S.; Kim, H.; Lee, Y.; Xu, X.; Park, J.-S.; Zheng, Y.; Balakrishnan, J.; Lei, T.; Ri Kim, H.; Song, Y.I.; et al. Roll-to-Roll Production of 30-Inch Graphene Films for Transparent Electrodes. Nat. Nanotech 2010, 5, 574–578. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, C.; Jia, D.; Li, S.; Ji, S.; Ye, C. Silver Nanowire Transparent Conductive Films with High Uniformity Fabricated via a Dynamic Heating Method. ACS Appl. Mater. Interfaces 2016, 8, 9865–9871. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhou, X.; Huang, W.; Kang, J.; He, G. High-Performance Copper Nanowire Electrode for Efficient Flexible Organic Light-Emitting Diode. Org. Electron. 2023, 113, 106690. [Google Scholar] [CrossRef]
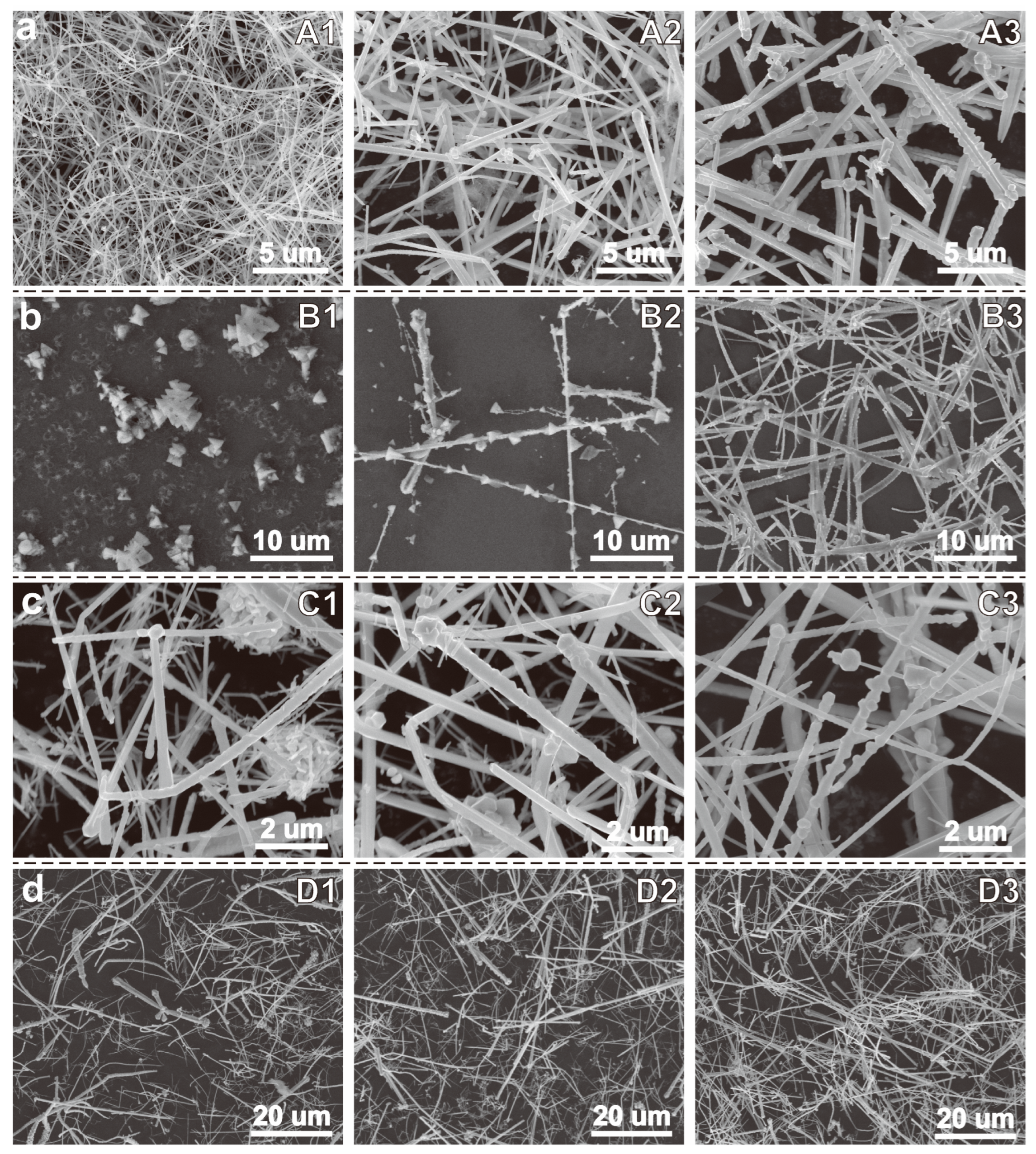

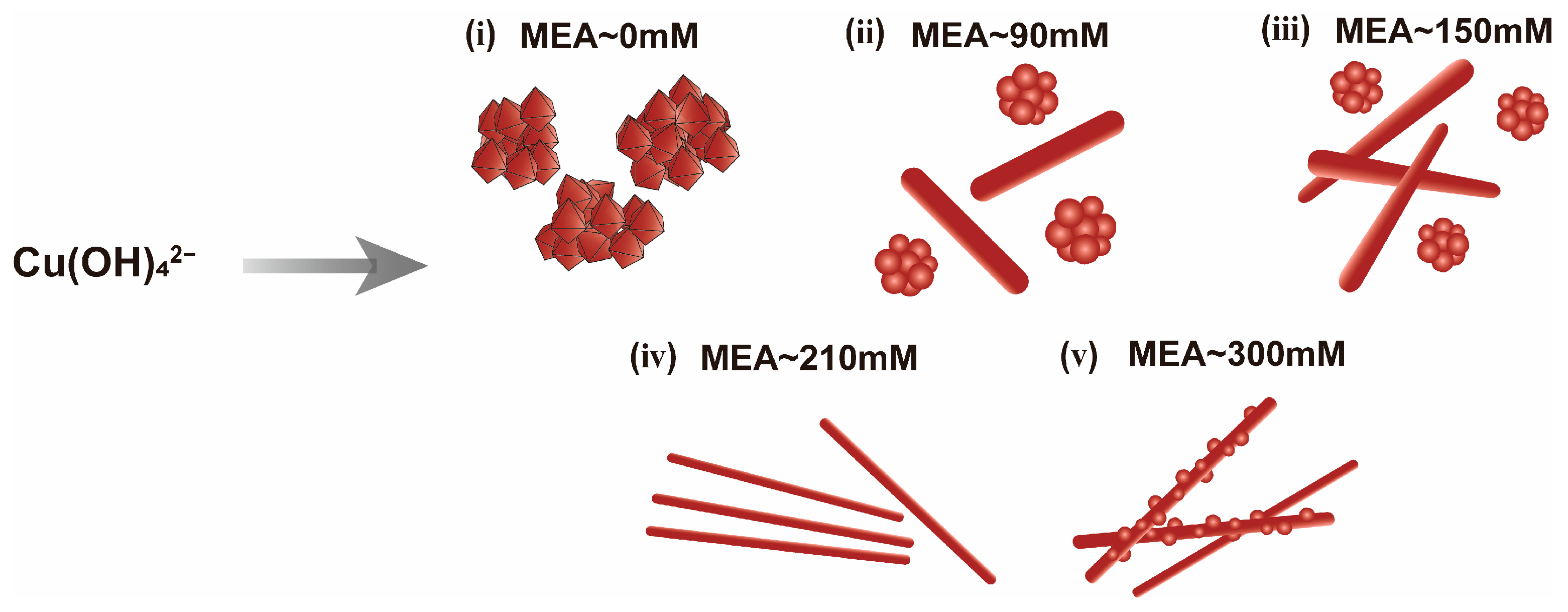

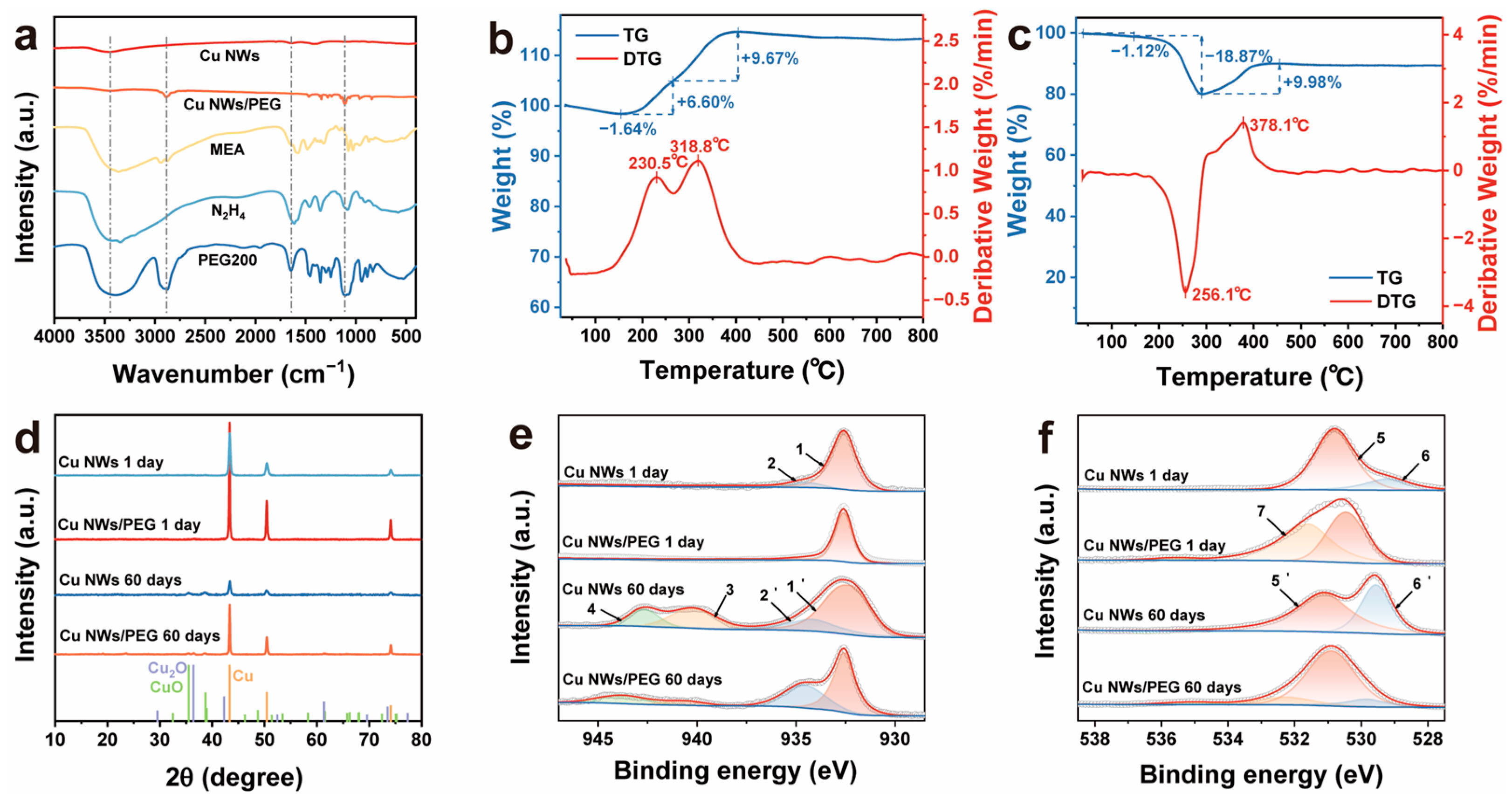
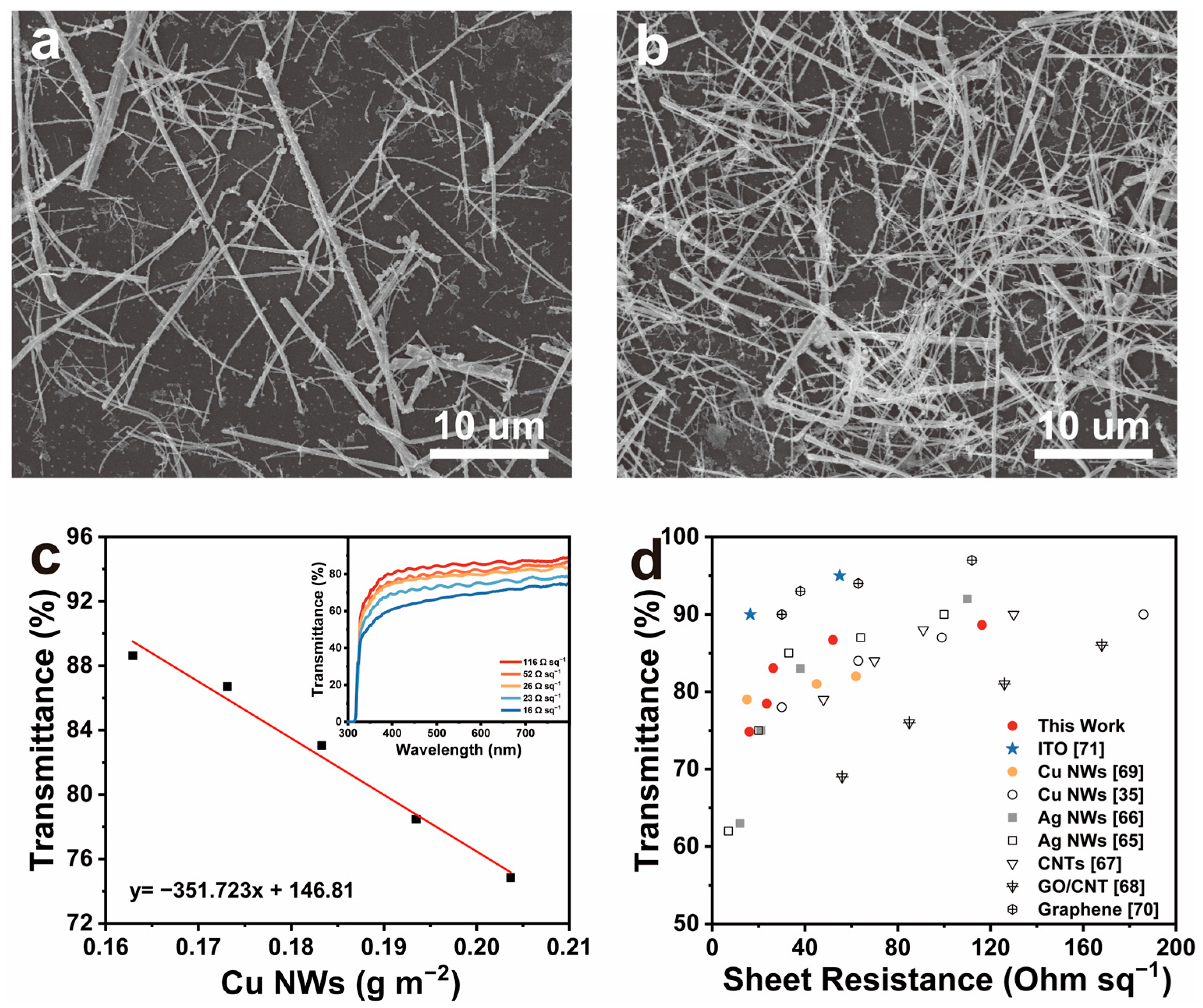
| Sample | Temp./°C | NaOH/M | MEA/mM | N2H4/mM | Product |
|---|---|---|---|---|---|
| A1 | 60 | 15 | 210 | 12.5 | Cu NWs |
| A2 | 70 | 15 | 210 | 12.5 | Mixture of NWs and NRs |
| A3 | 80 | 15 | 210 | 12.5 | Dominated by NRs |
| B1 | 60 | 7.5 | 210 | 12.5 | Cu2O Tetrahedron |
| B2 | 60 | 10 | 210 | 12.5 | Mixture of Cu2O and NWs |
| B3 | 60 | 12.5 | 210 | 12.5 | Dominated by NWs |
| C1 | 60 | 15 | 90 | 12.5 | Mixture of NRs and NPs |
| C2 | 60 | 15 | 150 | 12.5 | Mixture of NRs and NPs |
| C3 | 60 | 15 | 300 | 12.5 | Mixture of NWs and NRs |
| D1 | 60 | 15 | 210 | 7.5 | Dominated by NRs |
| D2 | 60 | 15 | 210 | 10 | Mixture of NWs and NRs |
| D3 | 60 | 15 | 210 | 15 | Mixture of NWs and NRs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zha, X.; Gong, D.; Chen, W.; Wu, L.; Zhang, C. Synthesis of Copper Nanowires Using Monoethanolamine and the Application in Transparent Conductive Films. Nanomaterials 2025, 15, 638. https://doi.org/10.3390/nano15090638
Zha X, Gong D, Chen W, Wu L, Zhang C. Synthesis of Copper Nanowires Using Monoethanolamine and the Application in Transparent Conductive Films. Nanomaterials. 2025; 15(9):638. https://doi.org/10.3390/nano15090638
Chicago/Turabian StyleZha, Xiangyun, Depeng Gong, Wanyu Chen, Lili Wu, and Chaocan Zhang. 2025. "Synthesis of Copper Nanowires Using Monoethanolamine and the Application in Transparent Conductive Films" Nanomaterials 15, no. 9: 638. https://doi.org/10.3390/nano15090638
APA StyleZha, X., Gong, D., Chen, W., Wu, L., & Zhang, C. (2025). Synthesis of Copper Nanowires Using Monoethanolamine and the Application in Transparent Conductive Films. Nanomaterials, 15(9), 638. https://doi.org/10.3390/nano15090638








