Facile Synthesis of Polypyrrole/MnO2/Carbon Cloth Composites for Supercapacitor Electrodes
Abstract
1. Introduction
2. Experiment
2.1. Pretreatment of Carbon Cloth
2.2. Synthesis of MnO2/Polypyrrole on Carbon Cloth
2.2.1. Hydrothermal Growth of MnO2 Nanorods
2.2.2. Vapor-Phase Polymerization (VPP) of Polypyrrole
3. Characterization
4. Results and Discussion
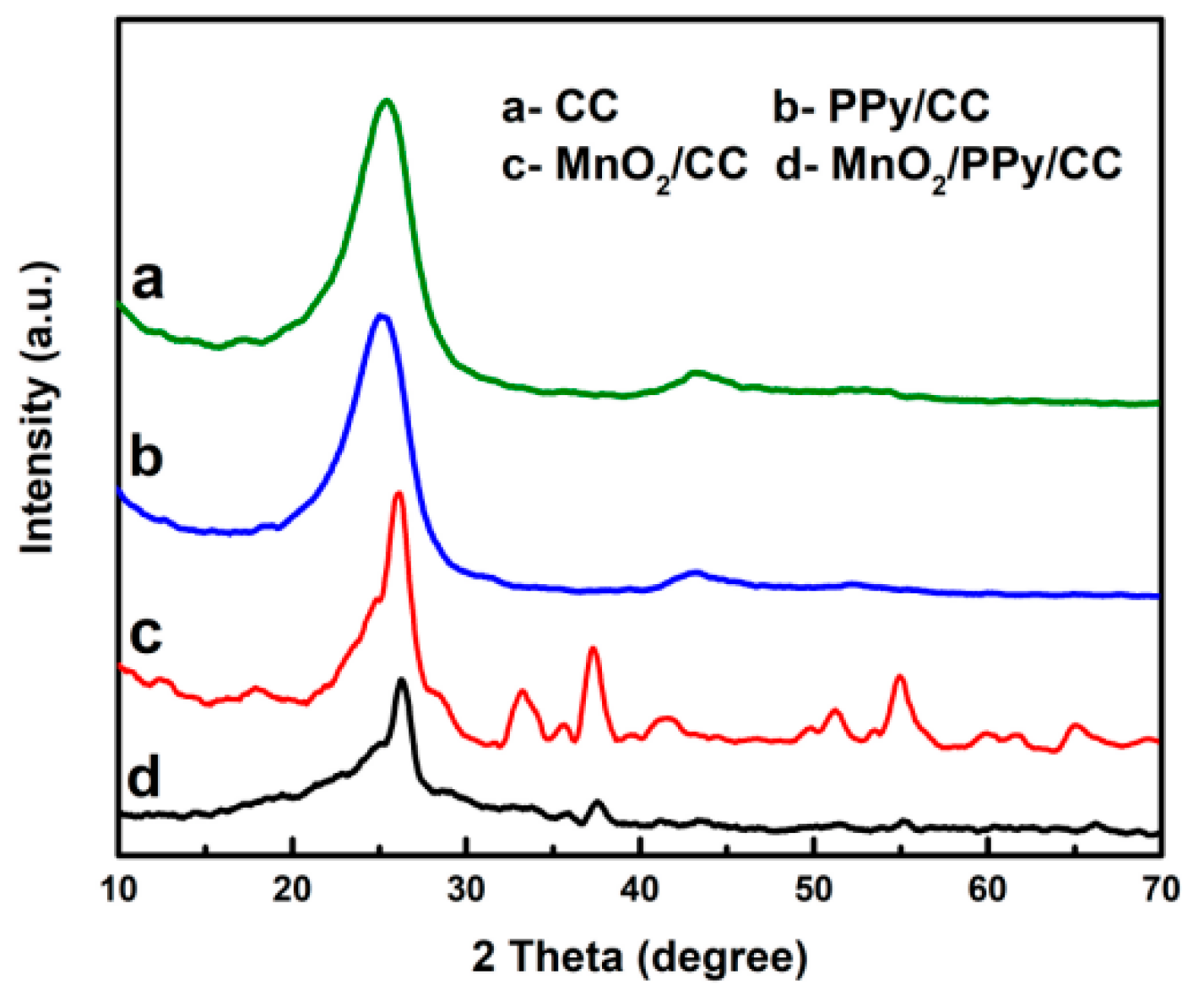
4.1. X-ray Diffraction Analysis
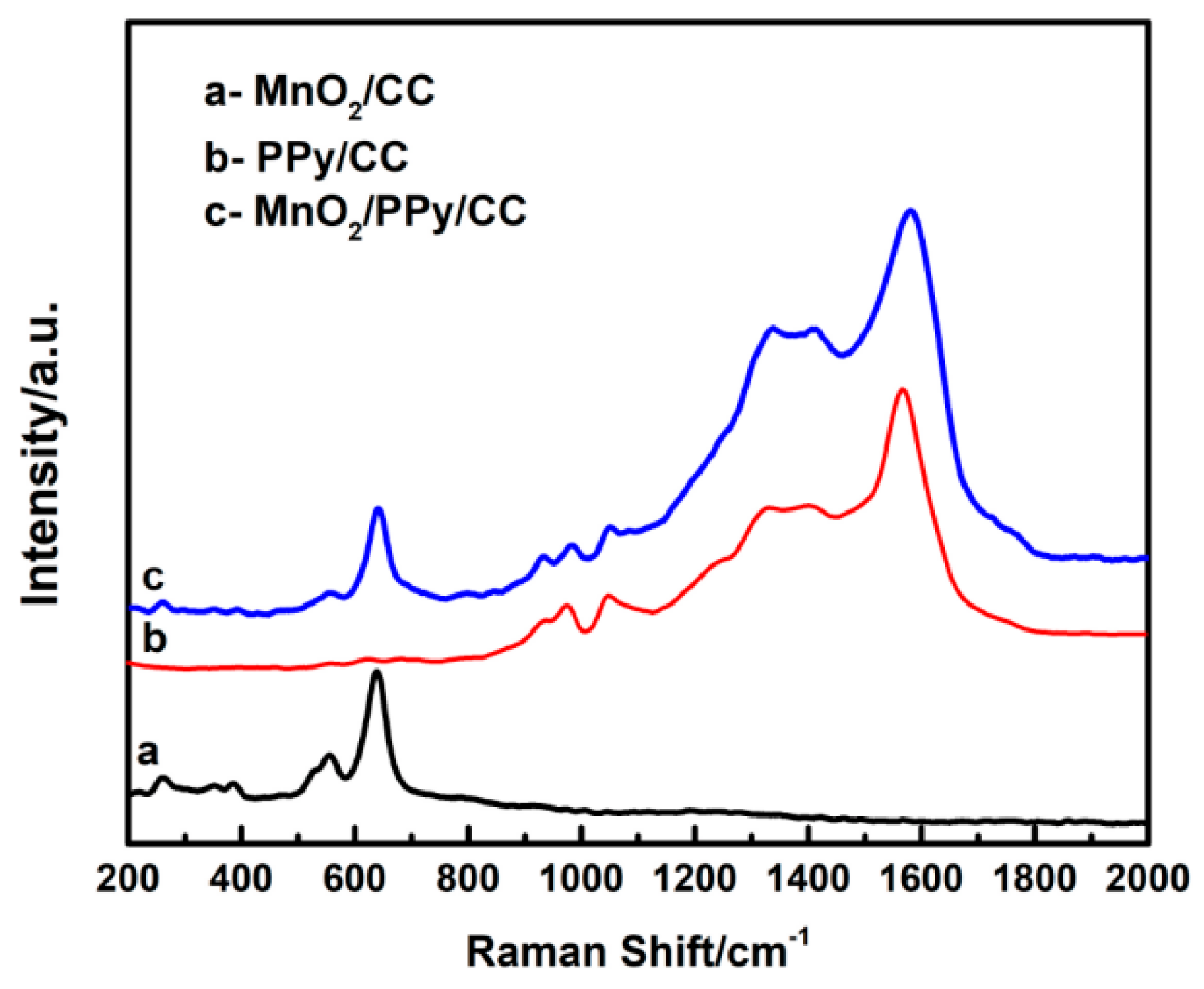
4.2. Raman Spectroscopy Analysis
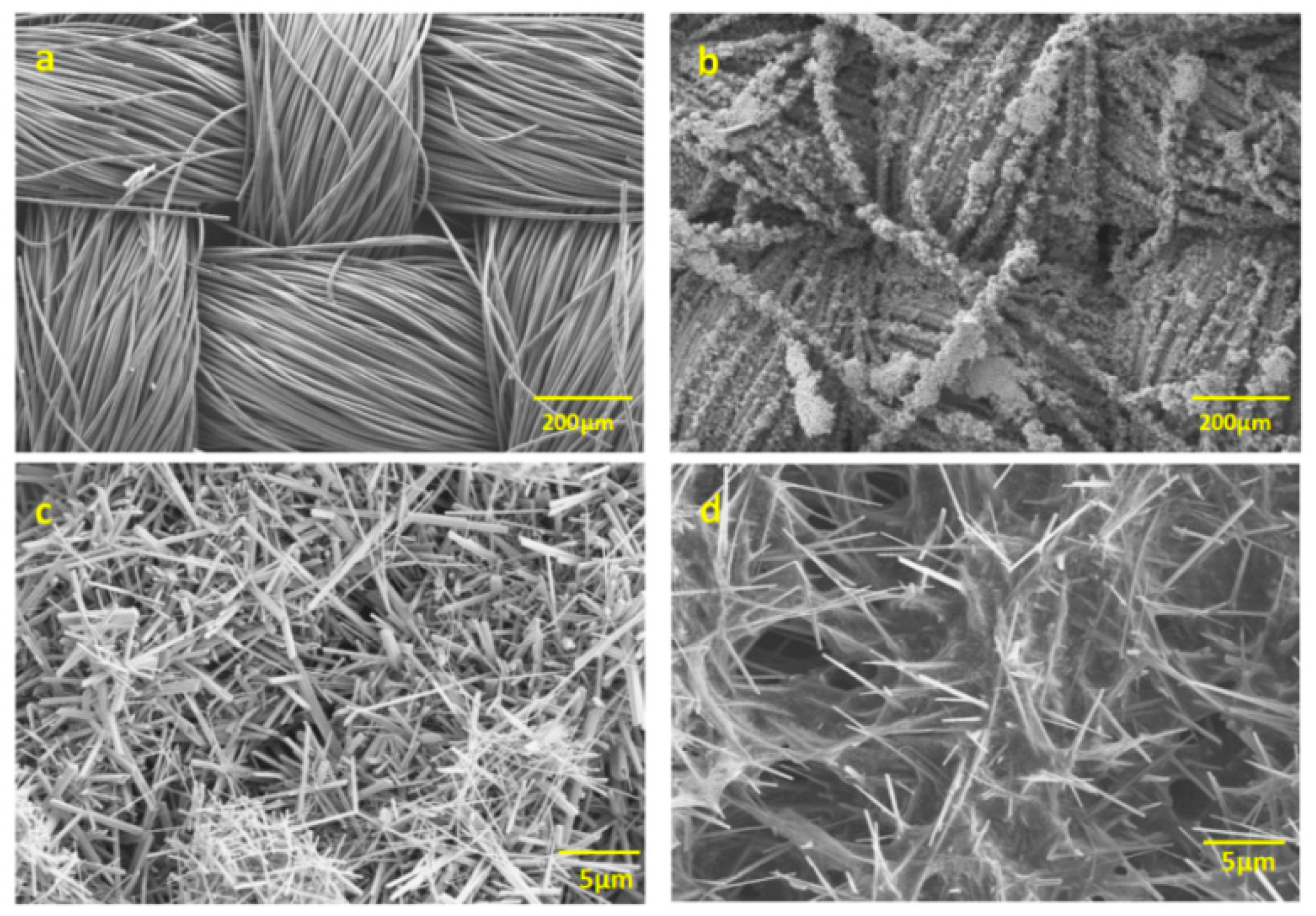
4.3. Morphological Characterization via Scanning Electron Microscopy
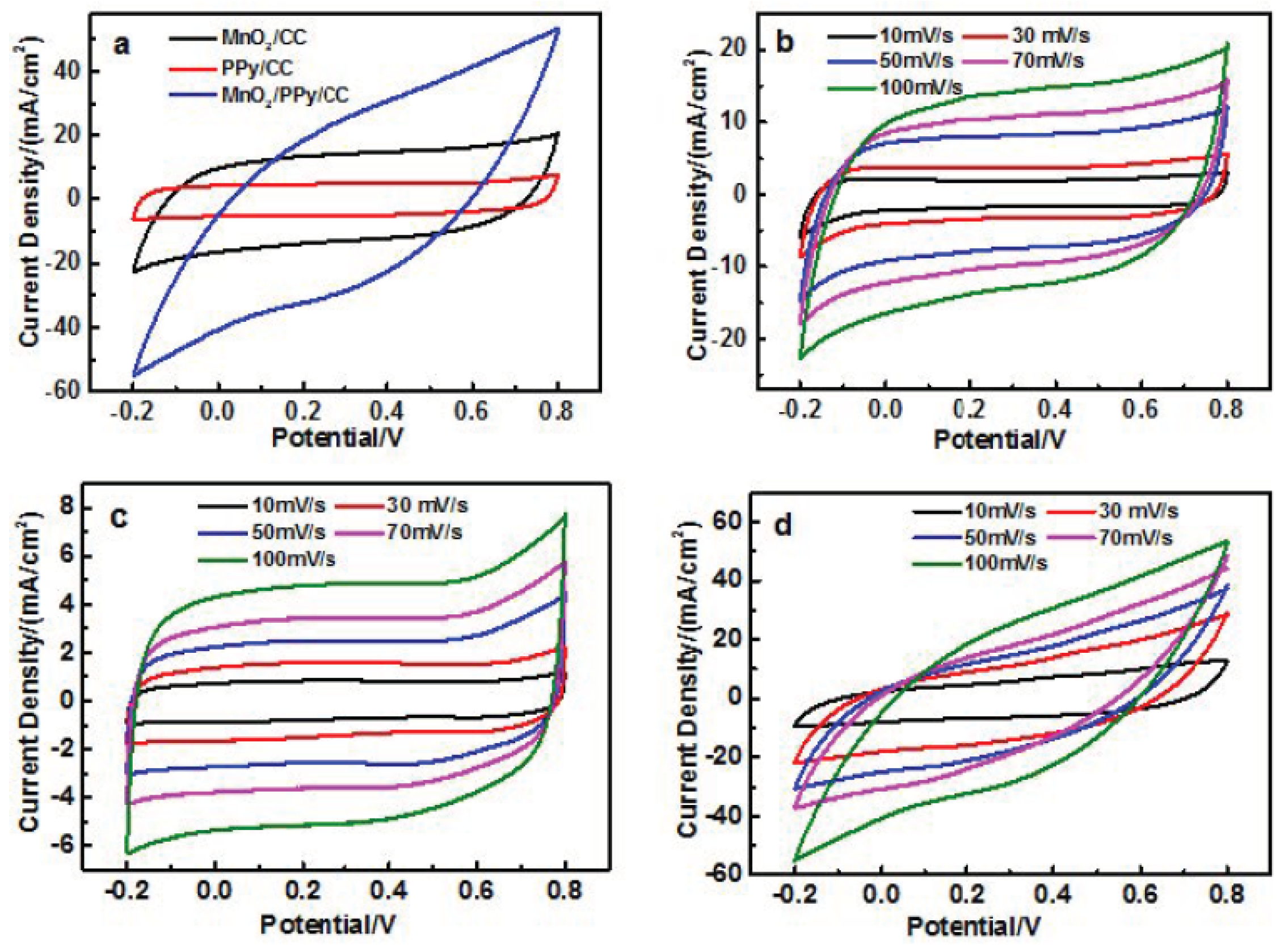
4.4. Electrochemical Performance
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lei, S.; Liu, Y.; Fei, L.; Song, R.; Lu, W.; Shu, L.; Mak, C.L.; Wang, Y.; Huang, H. Commercial Dacron cloth supported Cu(OH)2 nanobelt arrays for wearable supercapacitors. J. Mater. Chem. A 2016, 4, 14781–14788. [Google Scholar] [CrossRef]
- Hu, L.B.; Pasta, M.; Mantia, F.L.; Cui, L.F.; Jeong, S.; Deshazer, H.D.; Choi, J.W.; Han, S.M.; Cui, Y. Stretchable, Porous, and Conductive Energy Textiles. Nano Lett. 2010, 10, 708–714. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Wang, G.; Tong, Y.; Li, Y. Flexible solid-state supercapacitors: Design, fabrication and applications. Energy Environ. Sci. 2014, 7, 2160–2181. [Google Scholar] [CrossRef]
- Zhao, P.; Yao, M.; Ren, H.; Wang, N.; Komarneni, S. Nanocomposites of hierarchical ultrathin MnO2 nanosheets/hollow carbon nanofibers for high-performance asymmetric supercapacitors. Appl. Surf. Sci. 2018, 463, 931–938. [Google Scholar] [CrossRef]
- Yu, N.; Yin, H.; Zhang, W.; Liu, Y.; Tang, Z.; Zhu, M.Q. High-performance fiber-shaped all-solid-state asymmetric supercapacitors based on ultrathin MnO2 nanosheet/carbon fiber cathodes for wearable electronics. Adv. Energy Mater. 2016, 6, 1501458. [Google Scholar] [CrossRef]
- Long, X.; Zeng, Z.; Guo, E.; Shi, X.; Zhou, H.; Wang, X. Facile fabrication of all-solid-state flexible interdigitated MnO2 supercapacitor via in-situ catalytic solution route. J. Power Sources 2016, 325, 264–272. [Google Scholar] [CrossRef]
- Wang, Y.M.; Chen, J.C.; Cao, J.Y.; Liu, Y.; Zhou, Y.; Ouyang, J.H.; Jia, D.C. Graphene/carbon black hybrid film for flexible and high rate performance supercapacitor. J. Power Sources 2014, 271, 269–277. [Google Scholar] [CrossRef]
- Abdelkader, A.M.; Karim, N.; Vallés, C.; Afroj, S.; Novoselov, K.S.; Yeates, S.G. Ultraflexible and robust graphene supercapacitors printed on textiles for wearable electronics applications. 2D Mater. 2017, 4, 035016. [Google Scholar] [CrossRef]
- Xiong, S.; Zhang, X.; Chu, J.; Wang, X.; Zhang, R.; Gong, M.; Wu, B. Hydrothermal Synthesis of Porous Sugarcane Bagasse Carbon/MnO2 Nanocomposite for Supercapacitor Application. J. Electron. Mater. 2018, 47, 6575–6582. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, J.; Liu, J.; Wu, J.; Chen, H.; Bi, H. Design and preparation of a ternary composite of graphene oxide/carbon dots/polypyrrole for supercapacitor application: Importance and unique role of carbon dots. Carbon 2017, 115, 134–146. [Google Scholar] [CrossRef]
- Lee, S.; Nam, G.; Sun, J.; Lee, J.S.; Lee, H.W.; Chen, W.; Cho, J.; Cui, Y. Enhanced Intrinsic Catalytic Activity of λ-MnO2 by Electrochemical Tuning and Oxygen Vacancy Generation. Angew. Chem. Int. Ed. 2017, 55, 8599–8604. [Google Scholar] [CrossRef] [PubMed]
- El-Deab, M.S.; Ohsaka, T. Manganese Oxide Nanoparticles Electrodeposited on Platinum Are Superior to Platinum for Oxygen Reduction. Angew. Chem. Int. Ed. 2006, 45, 5963–5966. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Lu, G.; Wu, F.; Xu, C.; Kang, X.; Cheng, Z. Facile synthesis of a nitrogen-doped graphene flower-like MnO2 nanocomposite and its application in supercapacitors. Appl. Surf. Sci. 2018, 427, 986–993. [Google Scholar] [CrossRef]
- Yu, Z.N.; Duong, B.; Abbitt, D.; Thomas, J. Highly ordered MnO2 nanopillars for enhanced supercapacitor performance. Adv. Mater. 2013, 25, 3302–3306. [Google Scholar] [CrossRef] [PubMed]
- Han, X.L.; Zhang, J.; Wang, Z.S.; Younus, H.A.; Wang, D.W. Engineering the Microstructures of Manganese Dioxide Coupled with Oxygen Vacancies for Boosting Aqueous Ammonium-ion Storage in Hybrid Capacitors. Rare Met. 2024, 43, 5734–5746. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, S.; Cui, W.; Lv, J.; Geng, Y.; Li, H.; Deng, J. Interconnected network of ultrafine MnO2 nanowires on carbon cloth with weed-like morphology for high-performance supercapacitor electrodes. Electrochim. Acta 2018, 268, 340–346. [Google Scholar] [CrossRef]
- Makgopa, K.; Ejikeme, P.M.; Jafta, C.J.; Raju, K.; Zeiger, M.; Presser, V.; Ozoemena, K.I. A high-rate aqueous symmetric pseudocapacitor based on highly graphitized onion-like carbon/birnessite-type manganese oxide nanohybrids. J. Mater. Chem. A 2015, 3, 3480–3490. [Google Scholar] [CrossRef]
- Yun, T.G.; Hwang, B.I.; Kim, D.; Hyun, S.; Han, S.M. Polypyrrole–MnO2-Coated Textile-Based Flexible-Stretchable Supercapacitor with High Electrochemical and Mechanical Reliability. ACS Appl. Mater. Interfaces 2015, 7, 9228–9234. [Google Scholar] [CrossRef]
- An, J.; Liu, J.; Ma, Y.; Li, R.; Li, M.; Yu, M.; Li, S. Fabrication of graphene/polypyrrole nanotube/MnO2 nanotube composite and its supercapacitor application. Eur. Phys. J. Appl. Phys. 2012, 58, 30403. [Google Scholar] [CrossRef]
- Bahloul, A.; Nessark, B.; Briot, E.; Groult, H.; Mauger, A.; Zaghib, K.; Julien, C.M. Polypyrrole-covered MnO2 as electrode material for supercapacitor. J. Power Sources 2013, 240, 267–272. [Google Scholar] [CrossRef]
- Sidhu, N.K.; Rastogi, A.C. Nanoscale Blended MnO2 Nanoparticles in Electro-polymerized Polypyrrole Conducting Polymer for Energy Storage in Supercapacitors. MRS Proc. 2013, 1552, 11–16. [Google Scholar] [CrossRef]
- Shivakumara, S.; Munichandraiah, N. In-situ preparation of nanostructured α-MnO2/polypyrrole hybrid composite electrode materials for high performance supercapacitors. J. Alloys Compd. 2019, 787, 1044–1050. [Google Scholar] [CrossRef]
- Yalovega, G.E.; Brzhezinskaya, M.; Dmitriev, V.O.; Shmatko, V.A.; Ershov, I.V.; Ulyankina, A.A.; Chernysheva, D.V.; Smirnova, N.V. Interfacial Interaction in MeOx/MWNTs (Me-Cu,Ni) Nanostructures as Efficient Electrode Materials for High-Performance Supercapacitors. Nanomaterials 2024, 14, 947. [Google Scholar] [CrossRef]
- Liu, X.; Guan, C.; Hu, Y.; Zhang, L.; Elshahawy, A.M.; Wang, J. 2D Metal-Organic Frameworks Derived Nanocarbon Arrays for Substrate Enhancement in Flexible Supercapacitors. Small 2017, 14, 1702641. [Google Scholar] [CrossRef]
- Dubal, D.P.; Kim, J.G.; Kim, Y.; Holze, R.; Lokhande, C.D.; Kim, W.B. Supercapacitors Based on Flexible Substrates: An Overview. Energy Technol. 2014, 2, 325–341. [Google Scholar] [CrossRef]
- Wang, W.; Liu, W.; Zeng, Y.; Han, Y.; Yu, M.; Lu, X.; Tong, Y. A Novel Exfoliation Strategy to Significantly Boost the Energy Storage Capability of Commercial Carbon Cloth. Adv. Mater. 2015, 27, 3572–3578. [Google Scholar] [CrossRef]
- Zou, N.; Nie, Q.; Zhang, X.; Zhang, G.; Wang, J.; Zhang, P. Electrothermal regeneration by Joule heat effect on carbon cloth based MnO2 catalyst for long-term formaldehyde removal. Chem. Eng. J. 2018, 357, 1–10. [Google Scholar] [CrossRef]
- He, X.; Zhao, Y.; Chen, R.; Zhang, H.; Liu, J.; Liu, Q.; Song, D.; Li, R.; Wang, J. Hierarchical FeCo2O4@polypyrrole core/shell nanowires on carbon cloth for high-performance flexible all-solid-state asymmetric supercapacitors. ACS Sustain. Chem. Eng. 2018, 6, 14945–14954. [Google Scholar] [CrossRef]
- He, S.; Chen, W. Application of biomass-derived flexible carbon cloth coated with MnO2 nanosheets in supercapacitors. J. Power Sources 2015, 294, 150–158. [Google Scholar] [CrossRef]
- Guo, X.; Bai, N.; Tian, Y.; Gai, L. Free-standing reduced graphene oxide/polypyrrole films with enhanced electrochemical performance for flexible supercapacitors. J. Power Sources 2018, 408, 51–57. [Google Scholar] [CrossRef]
- Gupta, S. Hydrogen bubble-assisted syntheses of polypyrrole micro/nanostructures using electrochemistry: Structural and physical property characterization. J. Raman Spectrosc. 2008, 39, 1343–1355. [Google Scholar] [CrossRef]
- Sun, Y.; Jia, D.; Zhang, A.; Tian, J.; Zheng, Y.; Zhao, W.; Cui, L.; Liu, J. Synthesis of polypyrrole coated melamine foam by in-situ interfacial polymerization method for highly compressible and flexible supercapacitor. J. Colloid Interface Sci. 2019, 557, 617–627. [Google Scholar] [CrossRef]
- Rabchinskii, M.K.; Sysoev, V.; Ryzhkov, S.A.; Eliseyev, I.; Stolyarova, D.Y.; Antonov, G.A.; Struchkov, N.S.; Brzhezinskaya, M.; Kirilenko, D.A.; Pavlov, S.I.; et al. A Blueprint for the Synthesis and Characterization of Thiolated Graphene. Nanomaterials 2022, 12, 45. [Google Scholar] [CrossRef]
- Fan, X.; Wang, X.; Li, G.; Yu, A.; Chen, Z. High-performance flexible electrode based on electrodeposition of polypyrrole/MnO2 on carbon cloth for supercapacitors. J. Power Sources 2016, 326, 357–364. [Google Scholar] [CrossRef]
- Nagaraju, G.; Kakarla, R.; Cha, S.M.; Yu, J.S. Highly flexible conductive fabrics with hierarchically nanostructured amorphous nickel tungsten tetraoxide for enhanced electrochemical energy storage. Nano Res. 2015, 8, 3749–3763. [Google Scholar] [CrossRef]
- Wang, J.G.; Yang, Y.; Huang, Z.H.; Kang, F. Rational synthesis of MnO2/conducting polypyrrole@carbon nanofiber triaxial nano-cables for high-performance supercapacitors. J. Mater. Chem. 2012, 22, 16943–16949. [Google Scholar] [CrossRef]
- Lin, Z.; Xiang, X.; Chen, K.; Peng, S.; Jiang, X.; Hou, L. Facile synthesis of MnO2 nanorods grown on porous carbon for supercapacitor with enhanced electrochemical performance. J. Colloid Interface Sci. 2019, 540, 466–475. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, X.; Yang, D.; Wangyang, P.; Zeng, B.; Sun, H. A novel design of poly (3,4-ethylenedioxythiophene):poly (styrenesulfonate)/molybdenum disulfide/poly (3,4-ethylenedioxythiophene) nanocomposites for fabric micro-supercapacitors with favourable performances. Electrochim. Acta 2018, 298, 297–304. [Google Scholar] [CrossRef]
- Wang, Y.; Huo, W.C.; Yuan, X.Y.; Zhang, Y.X. Composite of Manganese Dioxide and Two-dimensional Materials Applied to Supercapacitors. Acta Phys. Chim. Sin. 2020, 36, 1904007. [Google Scholar] [CrossRef]
- Zhou, H.; Yan, Z.; Yang, X.; Lv, J.; Kang, L.; Liu, Z.H. RGO/MnO2/polypyrrole ternary film electrode for supercapacitor. Mater. Chem. Phys. 2016, 177, 40–47. [Google Scholar] [CrossRef]
- Xue, Y.J.; Huo, J.H.; Wang, X.; Zhao, Y.Z. ZnxMnO2/PPy Nanowires Composite as Cathode Material for Aqueous Zinc-Ion Hybrid Supercapacitors. Battery Energy 2024, 3, 20240035. [Google Scholar] [CrossRef]
- Mohd Abdah, M.A.A.; Mohammed Modawe Aldris Edris, N.; Kulandaivalu, S.; Abdul Rahman, N.; Sulaiman, Y. Supercapacitor with superior electrochemical properties derived from symmetrical manganese oxide-carbon fiber coated with polypyrrole. Int. J. Hydrogen Energy 2018, 43, 17328–17337. [Google Scholar] [CrossRef]
- Ramli, N.I.T.; Abdul Rashid, S.; Sulaiman, Y.; Mamat, M.S.; Mohd Zobir, S.A.; Krishnan, S. Physicochemical and electrochemical properties of carbon nanotube/graphite nanofiber hybrid nanocomposites for supercapacitor. J. Power Sources 2016, 328, 195–202. [Google Scholar] [CrossRef]
- Liang, K.; Gu, T.; Cao, Z.; Tang, X.; Hu, W.; Wei, B. In situ synthesis of SWNTs@MnO2/polypyrrole hybrid film as binder-free supercapacitor electrode. Nano Energy 2014, 9, 245–251. [Google Scholar] [CrossRef]
- Zhang, Z.; Chi, K.; Xiao, F.; Wang, S. Advanced solid-state asymmetric supercapacitors based on 3D graphene/MnO2 and graphene/polypyrrole hybrid architectures. J. Mater. Chem. A 2015, 3, 12828–12835. [Google Scholar] [CrossRef]
- Han, G.; Liu, Y.; Kan, E.; Tang, J.; Zhang, L.; Wang, H.; Tang, W. Sandwich-structured MnO2/polypyrrole/reduced graphene oxide hybrid composites for high-performance supercapacitors. RSC Adv. 2014, 4, 9898–9904. [Google Scholar] [CrossRef]
- Wang, C.; Zhan, Y.; Wu, L.X.; Li, Y.Y.; Liu, J.P. High-voltage and High-rate Symmetric supercapacitor based on MnO2-polypyrrole hybrid nanofilm. Nanotechnology 2014, 25, 305401. [Google Scholar] [CrossRef]
- Nie, G.D.; Zhang, Z.Y.; Liu, Y.Q.; Wang, J.; Fu, C.; Yin, H.Q.; Chen, J.; Zhao, L.; Pan, Z. One-Pot Rational Deposition of Coaxial Double-Layer MnO2/Ni(OH)2 Nanosheets on Carbon Nanofibers for High-Performance Supercapacitors. Adv. Fiber Mater. 2022, 4, 1129–1140. [Google Scholar] [CrossRef]
- Zhu, S.J.; Huo, W.C.; Wang, T.; Li, K.L.; Liu, X.Y.; Ji, J.Y.; Yao, H.; Dong, F.; Zhang, Y.; Zhang, L. Compulsive malposition of birnessite slab in 2D-Parallel birnessite on β-MnO2 networks for enhanced pseudocapacitance performances. Nano Mater. Sci. 2021, 3, 404–411. [Google Scholar] [CrossRef]


| Electrodes | Fabrication Method | Capacitance | Current Density/Scan Rate | Ref. |
|---|---|---|---|---|
| MnO2/PPy/CC | Hydrothermal process | 45.6 mF/cm2 | 2.5 mV/s | [47] |
| MnO2/PPy | In situ interfacial redox reaction | 705 F/g | 2 mV/s | [36] |
| MnO2/PPy | Layer-by-layer method | 404 F/g | 1 A/g | [46] |
| SWNTs@MnO2/PPy | Chemical vapor deposition | 351 F/g | 1 mV/s | [44] |
| MnO2/Ni(OH)2 | One-pot method | 1133.3 F/g | 1 A/g | [48] |
| β-MnO2 | Hydrothermal process | 625 F/g | 0.25 A/g | [49] |
| MnO2/PPy/CC | Vapor-phase polymerization | 324.5 mF/cm2 (773 F/g) | 2.5 mA/cm2 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; He, H.; Liu, M.; Xu, H.; Zhang, H.; Zhu, X.; Yang, D. Facile Synthesis of Polypyrrole/MnO2/Carbon Cloth Composites for Supercapacitor Electrodes. Nanomaterials 2025, 15, 641. https://doi.org/10.3390/nano15090641
Chen Y, He H, Liu M, Xu H, Zhang H, Zhu X, Yang D. Facile Synthesis of Polypyrrole/MnO2/Carbon Cloth Composites for Supercapacitor Electrodes. Nanomaterials. 2025; 15(9):641. https://doi.org/10.3390/nano15090641
Chicago/Turabian StyleChen, Yan, Hanyue He, Min Liu, He Xu, Haibo Zhang, Xinghua Zhu, and Dingyu Yang. 2025. "Facile Synthesis of Polypyrrole/MnO2/Carbon Cloth Composites for Supercapacitor Electrodes" Nanomaterials 15, no. 9: 641. https://doi.org/10.3390/nano15090641
APA StyleChen, Y., He, H., Liu, M., Xu, H., Zhang, H., Zhu, X., & Yang, D. (2025). Facile Synthesis of Polypyrrole/MnO2/Carbon Cloth Composites for Supercapacitor Electrodes. Nanomaterials, 15(9), 641. https://doi.org/10.3390/nano15090641





