Assembled Carbon Nanostructure Prepared by Spray Freeze Drying for Si-Based Anodes
Abstract
1. Introduction
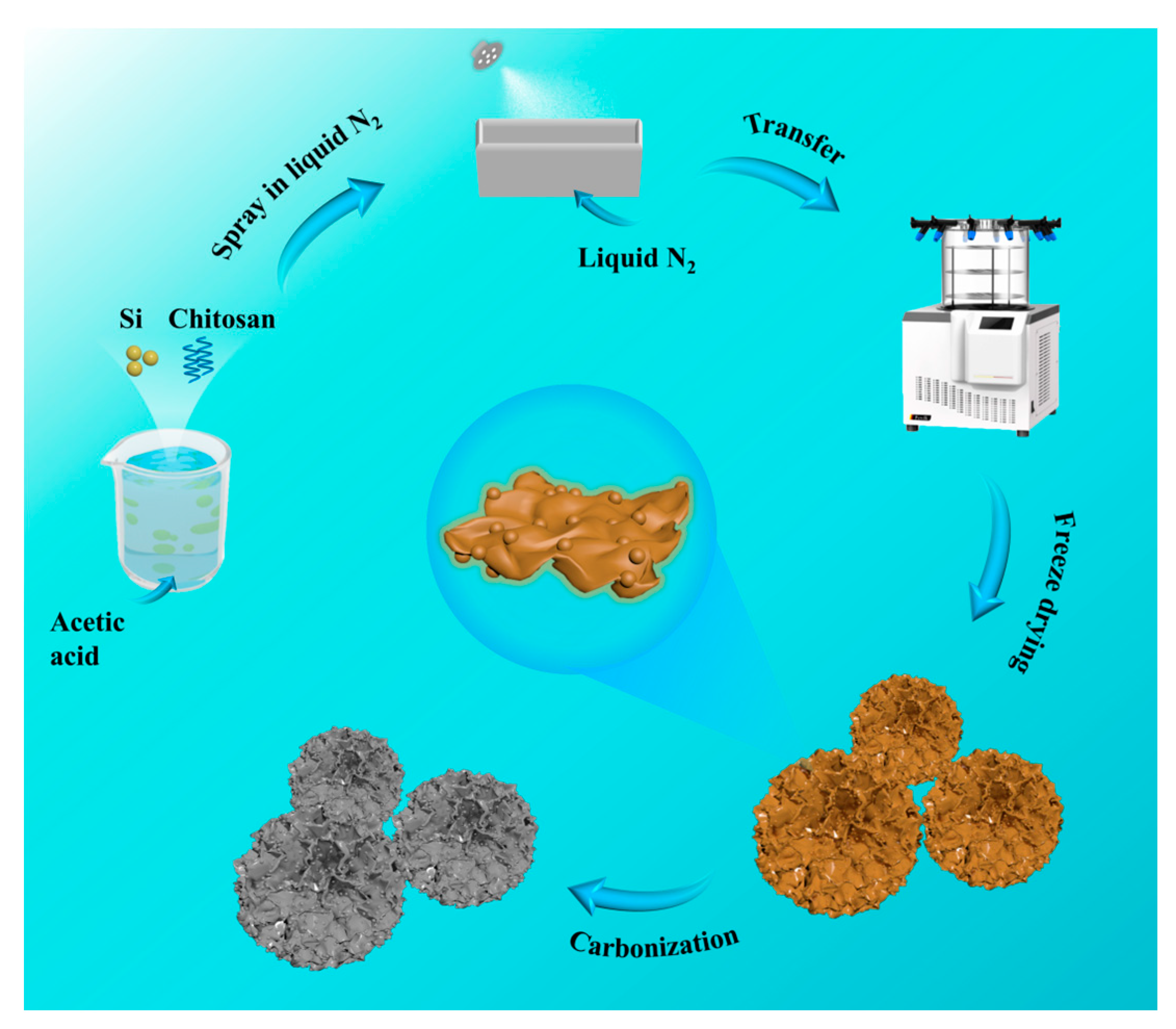
2. Materials and Methods
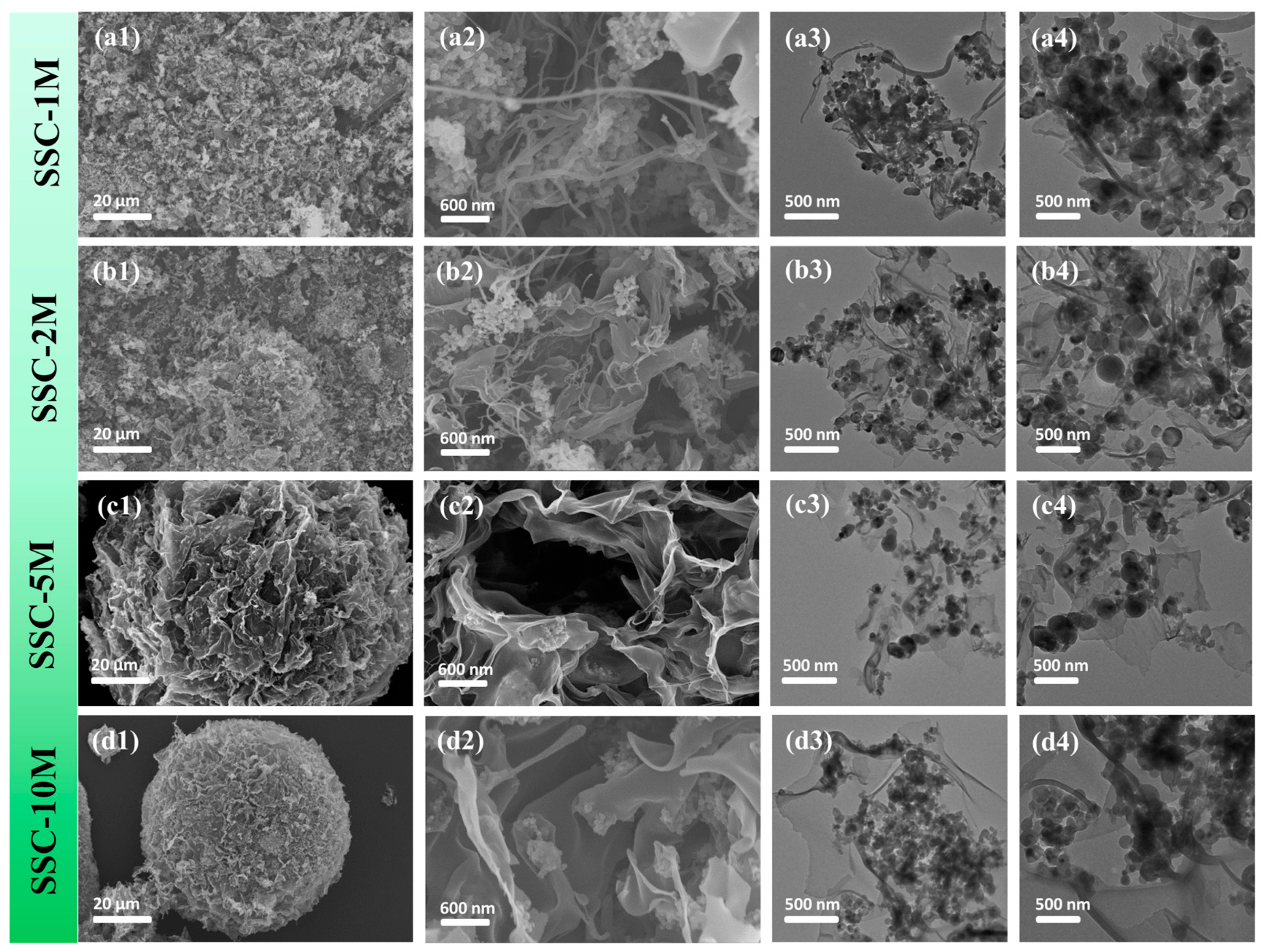
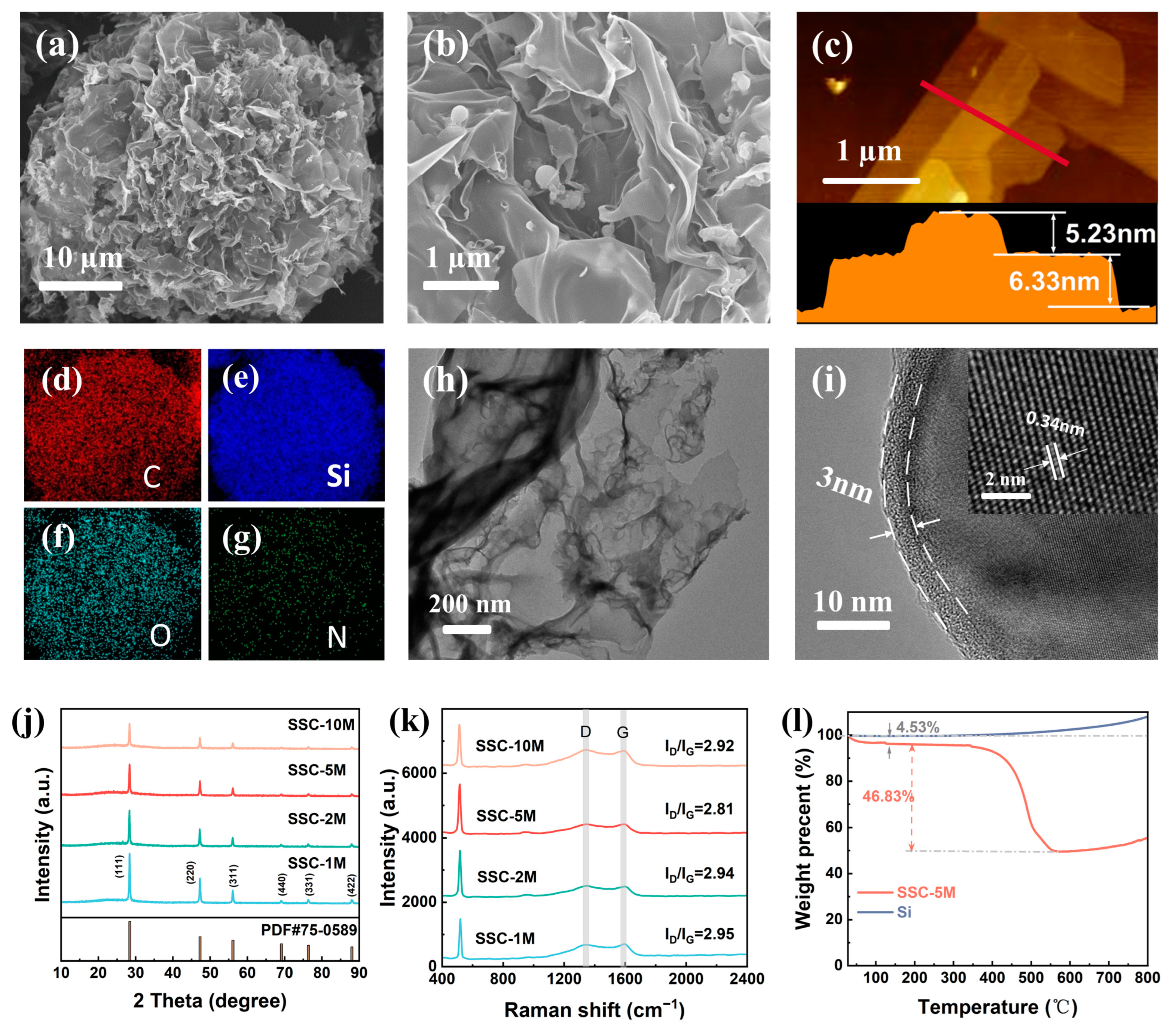
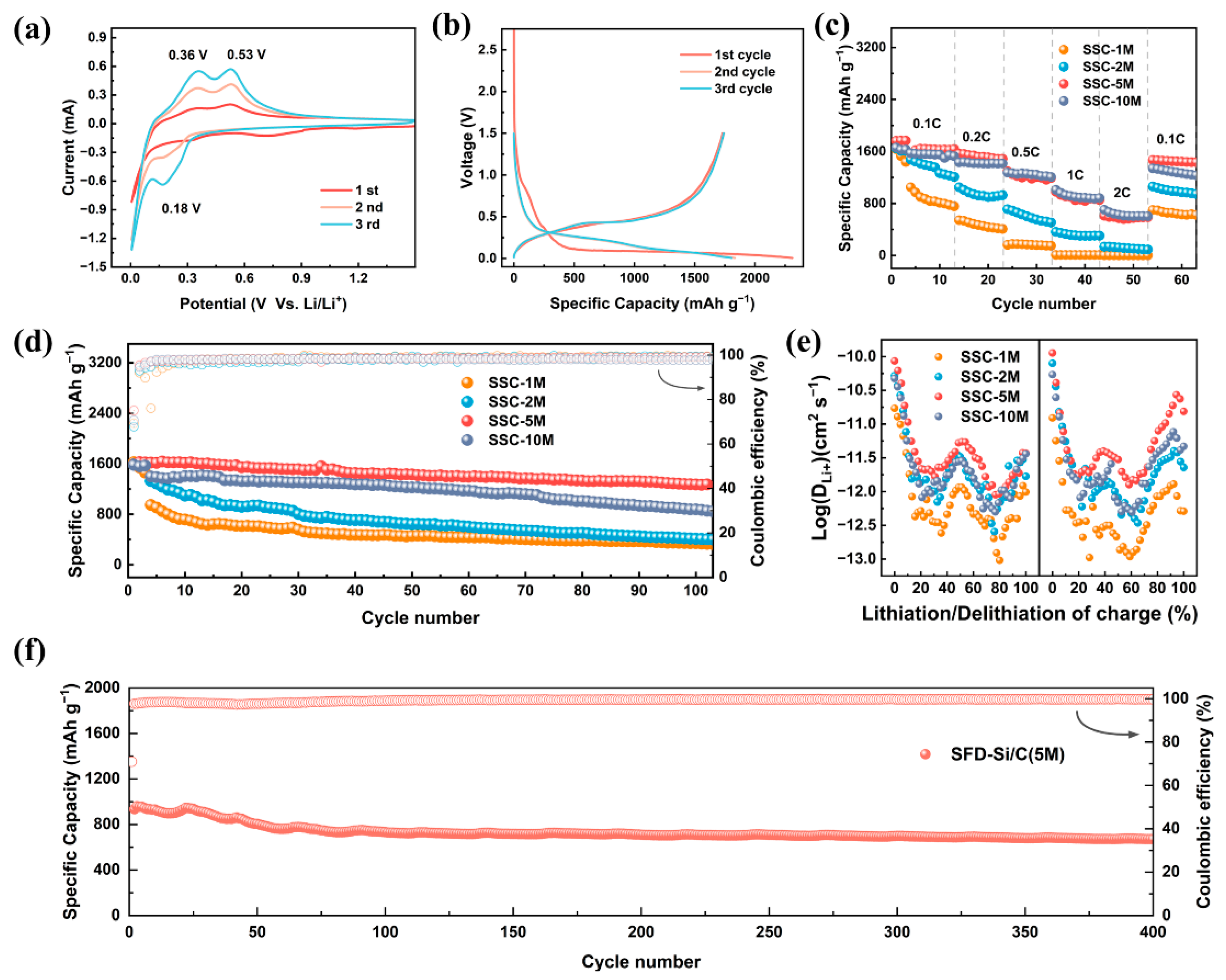
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Luo, W.; Chen, X.; Xia, Y.; Chen, M.; Wang, L.; Wang, Q.; Li, W.; Yang, J. Surface and Interface Engineering of Silicon-Based Anode Materials for Lithium-Ion Batteries. Adv. Energy Mater. 2017, 7, 1701083. [Google Scholar] [CrossRef]
- Wang, D.; Zhou, C.; Cao, B.; Xu, Y.; Zhang, D.; Li, A.; Zhou, J.; Ma, Z.; Chen, X.; Song, H. One-step synthesis of spherical Si/C composites with onion-like buffer structure as high-performance anodes for lithium-ion batteries. Energy Storage Mater. 2020, 24, 312–318. [Google Scholar] [CrossRef]
- Sung, J.; Kim, N.; Ma, J.; Lee, J.H.; Joo, S.H.; Lee, T.; Chae, S.; Yoon, M.; Lee, Y.; Hwang, J.; et al. Subnano-sized silicon anode via crystal growth inhibition mechanism and its application in a prototype battery pack. Nat. Energy 2021, 6, 1164–1175. [Google Scholar] [CrossRef]
- Li, Z.; Han, M.; Yu, P.; Lin, J.; Yu, J. Macroporous Directed and Interconnected Carbon Architectures Endow Amorphous Silicon Nanodots as Low-Strain and Fast-Charging Anode for Lithium-Ion Batteries. Nano-Micro Lett. 2024, 16, 98. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Yuan, R.; Li, X.; Li, J.; Jiang, Z.; Li, A.; Chen, X.; Song, H. Simple Construction of Multistage Stable Silicon–Graphite Hybrid Granules for Lithium-Ion Batteries. Small 2023, 19, e2207167. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Fu, J.; Wang, C.; Wang, J.; Yang, A.; Li, C.; Sun, Q.; Cui, Y.; Li, H. A binder-free high silicon content flexible anode for Li-ion batteries. Energy Environ. Sci. 2020, 13, 848–858. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhong, L.; Huang, S.; Mao, S.X.; Zhu, T.; Huang, J.Y. Size-Dependent Fracture of Silicon Nanoparticles During Lithiation. ACS Nano 2012, 6, 1522–1531. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Yan, Z.; Li, X.; Wang, Z.; Ning, P.; Zhang, J.; Huang, J.; Yang, D.; Du, N. Design of phosphorus-doped porous hard carbon/Si anode with enhanced Li-ion kinetics for high-energy and high-power Li-ion batteries. Chem. Eng. J. 2023, 473, 145161. [Google Scholar] [CrossRef]
- Chae, S.; Xu, Y.; Yi, R.; Lim, H.-S.; Velickovic, D.; Li, X.; Li, Q.; Wang, C.; Zhang, J.-G. A Micrometer-Sized Silicon/Carbon Composite Anode Synthesized by Impregnation of Petroleum Pitch in Nanoporous Silicon. Adv. Mater. 2021, 33, 2103095. [Google Scholar] [CrossRef]
- Wang, D.; Ma, Y.; Xu, W.; Zhang, S.; Wang, B.; Zhi, L.; Li, X. Controlled Isotropic Canalization of Microsized Silicon Enabling Stable High-Rate and High-Loading Lithium Storage. Adv. Mater. 2023, 35, 2212157. [Google Scholar] [CrossRef]
- Su, P.; Zhang, Z.; Luo, L.; Zhang, Z.; Lan, C.; Li, Y.; Xu, S.; Han, X.; Lin, G.; Li, C.; et al. Silicon Nanowire Array Weaved by Carbon Chains for Stretchable Lithium-Ion Battery Anode. Small 2024, 20, 2307716. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, Z.; Li, H.; Cheng, L.; Wu, Y.; Liu, X.; Meng, L.; Zhang, Y.; Jiang, S. Recent status, key strategies, and challenging prospects for fast charging silicon-based anodes for lithium-ion batteries. Carbon 2024, 230, 119615. [Google Scholar]
- Xue, X.; Liu, X.; Lou, B.; Yang, Y.; Shi, N.; Wen, F.; Yang, X.; Liu, D. The mitigation of pitch-derived carbon with different structures on the volume expansion of silicon in Si/C composite anode. J. Energy Chem. 2023, 84, 292–302. [Google Scholar] [CrossRef]
- Maddipatla, R.; Loka, C.; Lee, K.-S. Exploring the Potential of Carbonized Nano-Si within G@C@Si Anodes for Lithium-Ion Rechargeable Batteries. ACS Appl. Mater. Interfaces 2023, 15, 58437–58450. [Google Scholar] [CrossRef]
- Muruganantham, R.; Yang, C.-W.; Wang, H.-J.; Huang, C.-H.; Liu, W.-R. Industrial Silicon-Wafer-Wastage-Derived Carbon-Enfolded Si/Si-C/C Nanocomposite Anode Material through Plasma-Assisted Discharge Process for Rechargeable Li-Ion Storage. Nanomaterials 2022, 12, 659. [Google Scholar] [CrossRef]
- Xu, Q.; Li, J.-Y.; Sun, J.-K.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Watermelon-Inspired Si/C Microspheres with Hierarchical Buffer Structures for Densely Compacted Lithium-Ion Battery Anodes. Adv. Energy Mater. 2017, 7, 1601481. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, C.; Dou, Y.; Cheng, N.; Cui, D.; Du, Y.; Liu, P.; Al-Mamun, M.; Zhang, S.; Zhao, H. A Yolk–Shell Structured Silicon Anode with Superior Conductivity and High Tap Density for Full Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 8824–8828. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, R.; Wang, D.; Li, J.; Yao, X.; Chen, L.; Li, X.; Jiang, Z.; Liu, H.; Hou, Y.; et al. Orchestrating a Controllable Engineering of Dual-Model Carbon Structure in Si/C Anodes. Adv. Funct. Mater. 2025, 2423700. [Google Scholar] [CrossRef]
- Ou, S.; Liu, C.; Yang, R.; Fan, W.; Xie, Z.; Li, B.; Meng, T.; Zou, C.; Shu, D.; Tong, Y. Supramolecular-driven construction of multilayered structure by modified hummers method for robust silicon anode. Energy Storage Mater. 2024, 73, 103814. [Google Scholar] [CrossRef]
- Hou, Y.; Sheng, Z.; Zhang, M.; Lin, K.; Kong, J.; Zhang, X. Hierarchically Porous Carbon Colloidal Aerogels for Highly Efficient Flow Cells. Adv. Funct. Mater. 2024, 2418721. [Google Scholar] [CrossRef]
- Chen, W.-T.; Muruganantham, R.; Liu, W.-R. Construction of 3D porous graphene aerogel wrapped silicon composite as anode materials for high-efficient lithium-ion storage. Surf. Coat. Technol. 2022, 434, 128147. [Google Scholar] [CrossRef]
- Wang, H.-C.; Muruganantham, R.; Hsieh, C.-T.; Liu, W.-R. Electrochemical elucidation of phosphorus-doped and 3D graphene aerogel surface-modified SiOx porous nanocomposite electrode material for high-performance lithium-ion batteries. Electrochim. Acta 2024, 477, 143775. [Google Scholar] [CrossRef]
- Ishwarya, S.P.; Anandharamakrishnan, C.; Stapley, A.G.F. Spray-freeze-drying: A novel process for the drying of foods and bioproducts. Trends Food Sci. Technol. 2015, 41, 161–181. [Google Scholar] [CrossRef]
- Liao, S.; Zhai, T.; Xia, H. Highly adsorptive graphene aerogel microspheres with center-diverging microchannel structures. J. Mater. Chem. A 2016, 4, 1068–1077. [Google Scholar] [CrossRef]
- Casper, J.M. Physical chemistry of surfaces (3rd Ed.), Arthur W. Adamson, Wiley-Interscience, New York, 1976, 698 pp. $24.95. J. Polym. Sci. Polym. Lett. Ed. 1977, 15, 632–633. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, X.; Cheng, Z.; Wen, Z.; Yu, J.; Wang, F.; Ding, Y. Preparation and electrochemical properties of Si@C/CNTs composites derived from crosslinked chitosan. J. Alloys Compd. 2022, 912, 165212. [Google Scholar] [CrossRef]
- Li, L.; Fang, C.; Wei, W.; Zhang, L.; Ye, Z.; He, G.; Huang, Y. Nano-ordered structure regulation in delithiated Si anode triggered by homogeneous and stable Li-ion diffusion at the interface. Nano Energy 2020, 72, 104651. [Google Scholar] [CrossRef]
- Li, X.; Chen, Z.; Liu, X.; Guo, L.; Li, A.; Chen, X.; Song, H. Efficient Lithium Transport and Reversible Lithium Plating in Silicon Anodes: Synergistic Design of Porous Structure and LiF-Rich SEI for Fast Charging. Adv. Funct. Mater. 2024, 34, 2401686. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Dang, L.; Yu, K.; Yang, R.; Fu, A.; Liu, X.; Guo, Y.G.; Li, H. Precursor Induced Assembly of Si Nanoparticles Encapsulated in Graphene/Carbon Matrices and the Influence of Al2O3 Coating on their Properties as Anode for Lithium-Ion Batteries. Small 2023, 20, e2307722. [Google Scholar] [CrossRef]
- Yu, P.; Li, Z.; Han, M.; Yu, J. Growth of Vertical Graphene Sheets on Silicon Nanoparticles Well-Dispersed on Graphite Particles for High-Performance Lithium-Ion Battery Anode. Small 2024, 20, 2307494. [Google Scholar] [CrossRef]
- Hong, J.; Zhang, J.; Li, X.; Guo, Y.; Zhou, X.; Liu, Z. Graphene-Wrapped Composites of Si Nanoparticles, Carbon Nanofibers, and Pyrolytic Carbon as Anode Materials for Lithium-Ion Batteries. ACS Appl. Nano Mater. 2023, 6, 10138–10147. [Google Scholar] [CrossRef]
- Zhou, H.P.; Yang, B.; Zhang, Z.D.; Zhang, H.; Zhang, S.; Feng, T.T.; Xu, Z.Q.; Gao, J.; Wu, M.Q. Fastly PECVD-Grown vertical carbon nanosheets for a composite SiOx-C anode material. Appl. Surf. Sci. 2022, 605, 154627. [Google Scholar] [CrossRef]
- Yang, S.H.; Kim, J.K.; Jung, D.-S.; Kang, Y.C. Facile fabrication of Si-embedded amorphous carbon@graphitic carbon composite microspheres via spray drying as high-performance lithium-ion battery anodes. Appl. Surf. Sci. 2022, 606, 154799. [Google Scholar] [CrossRef]
- Ren, J.; Xia, L.; Zhou, Y.; Zheng, Q.; Liao, J.; Lin, D. A reduced graphene oxide/nitrogen, phosphorus doped porous carbon hybrid framework as sulfur host for high performance lithium-sulfur batteries. Carbon 2018, 140, 30–40. [Google Scholar] [CrossRef]
- Jin, H.; Zhu, M.; Liu, J.; Gan, L.; Gong, Z.; Long, M. Alkaline chitosan solution as etching phase to design Si@SiO2@N-Carbon anode for Lithium-ion battery. Appl. Surf. Sci. 2021, 541, 148436. [Google Scholar] [CrossRef]
- Liu, H.; Duan, P.; Wu, Z.; Liu, Y.; Yan, Z.; Zhong, Y.; Wang, Y.; Wang, X. Silicon/graphite/amorphous carbon composites as anode materials for lithium-ion battery with enhanced electrochemical performances. Mater. Res. Bull. 2025, 181, 113082. [Google Scholar] [CrossRef]
- Chen, S.; Shen, L.; van Aken, P.A.; Maier, J.; Yu, Y. Dual-Functionalized Double Carbon Shells Coated Silicon Nanoparticles for High Performance Lithium-Ion Batteries. Adv. Mater. 2017, 29, 1605650. [Google Scholar] [CrossRef]
- Wu, H.; Yu, G.; Pan, L.; Liu, N.; McDowell, M.T.; Bao, Z.; Cui, Y. Stable Li-ion battery anodes by in-situ polymerization of conducting hydrogel to conformally coat silicon nanoparticles. Nat. Commun. 2013, 4, 1943. [Google Scholar] [CrossRef]
- Zou, X.; Li, M.; Li, H.; Cao, G.; Jiang, Q.; Duan, R.; Qian, H.; Li, J.; Yang, X.; Cao, Y.; et al. Three-dimensional CNTs boosting the conductive confinement structure of silicon/carbon anodes in lithium-ion batteries. Chem. Eng. J. 2024, 498, 155573. [Google Scholar] [CrossRef]
- Yang, X.; Hou, S.; Xu, D.; Nan, D.; Lv, R.; Shen, W.; Kang, F.; Huang, Z.-H. Nano-silicon embedded in mildly-exfoliated graphite for lithium-ion battery anode materials. Adv. Powder Technol. 2024, 35, 104463. [Google Scholar] [CrossRef]
- Zhao, L.-F.; Hu, Z.; Lai, W.-H.; Tao, Y.; Peng, J.; Miao, Z.-C.; Wang, Y.-X.; Chou, S.-L.; Liu, H.-K.; Dou, S.-X. Hard Carbon Anodes: Fundamental Understanding and Commercial Perspectives for Na-Ion Batteries beyond Li-Ion and K-Ion Counterparts. Adv. Energy Mater. 2021, 11, 2002704. [Google Scholar] [CrossRef]
- Ding, N.; Xu, J.; Yao, Y.X.; Wegner, G.; Fang, X.; Chen, C.H.; Lieberwirth, I. Determination of the diffusion coefficient of lithium ions in nano-Si. Solid State Ion. 2009, 180, 222–225. [Google Scholar] [CrossRef]
- Zhou, C.; Gong, X.; Wang, Z.; Liu, J. Ultrathin Carbon Sheet Obtained by Self-Template Method toward Highly Effective Charge Transfer for Si-Based Anodes. ACS Appl. Mater. Interfaces 2024, 16, 4689–4699. [Google Scholar] [CrossRef]
- Zhu, R.; Li, L.; Wang, Z.; Zhang, S.; Dang, J.; Liu, X.; Wang, H. Adjustable Dimensionality of Microaggregates of Silicon in Hollow Carbon Nanospheres: An Efficient Pathway for High-Performance Lithium-Ion Batteries. ACS Nano 2022, 16, 1119–1133. [Google Scholar] [CrossRef]
- Kim, M.J.; Lee, I.; Lee, J.W.; Yoon, D.; Kim, J.H.; Lee, S.; Kim, K.; Kim, P.J.; Choi, J.; Kang, Y.C.; et al. A Novel Structured Si-Based Composite with 2D Structured Graphite for High-Performance Lithium-Ion Batteries. Small 2024, 20, 2405005. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Lou, S.; Sun, B.; Wu, L.; Qian, Z.; Mu, T.; Ma, Y.; Cheng, X.; Gao, Y.; Zuo, P.; et al. Improving electrochemical performance of Nano-Si/N-doped carbon through tunning the microstructure from two dimensions to three dimensions. Electrochim. Acta 2020, 332, 135507. [Google Scholar] [CrossRef]
- Zhang, Q.; Xi, B.; Chen, W.; Feng, J.; Qian, Y.; Xiong, S. Synthesis of carbon nanotubes-supported porous silicon microparticles in low-temperature molten salt for high-performance Li-ion battery anodes. Nano Res. 2022, 15, 6184–6191. [Google Scholar] [CrossRef]
- Lv, D.; Yang, L.; Song, R.; Yuan, H.; Luan, J.; Liu, J.; Hu, W.; Zhong, C. A hierarchical porous hard carbon@Si@soft carbon material for advanced lithium-ion batteries. J. Colloid Interface Sci. 2025, 678, 336–342. [Google Scholar] [CrossRef]
- Pei, Y.; Wang, Y.; Chang, A.-Y.; Liao, Y.; Zhang, S.; Wen, X.; Wang, S. Nanofiber-in-microfiber carbon/silicon composite anode with high silicon content for lithium-ion batteries. Carbon 2023, 203, 436–444. [Google Scholar] [CrossRef]
- Wang, D.; Kong, L.; Zhang, F.; Liu, A.; Huang, H.; Liu, Y.; Shi, Z. Porous carbon-coated silicon composites for high performance lithium-ion batterie anode. Appl. Surf. Sci. 2024, 661, 160076. [Google Scholar] [CrossRef]
- Qiao, Y.; Hu, Y.; Qian, Z.; Qu, M.; Liu, Z. An innovative strategy for constructing multicore yolk-shell Si/C anodes for lithium-ion batteries. J. Colloid Interface Sci. 2025, 684, 678–689. [Google Scholar] [CrossRef] [PubMed]
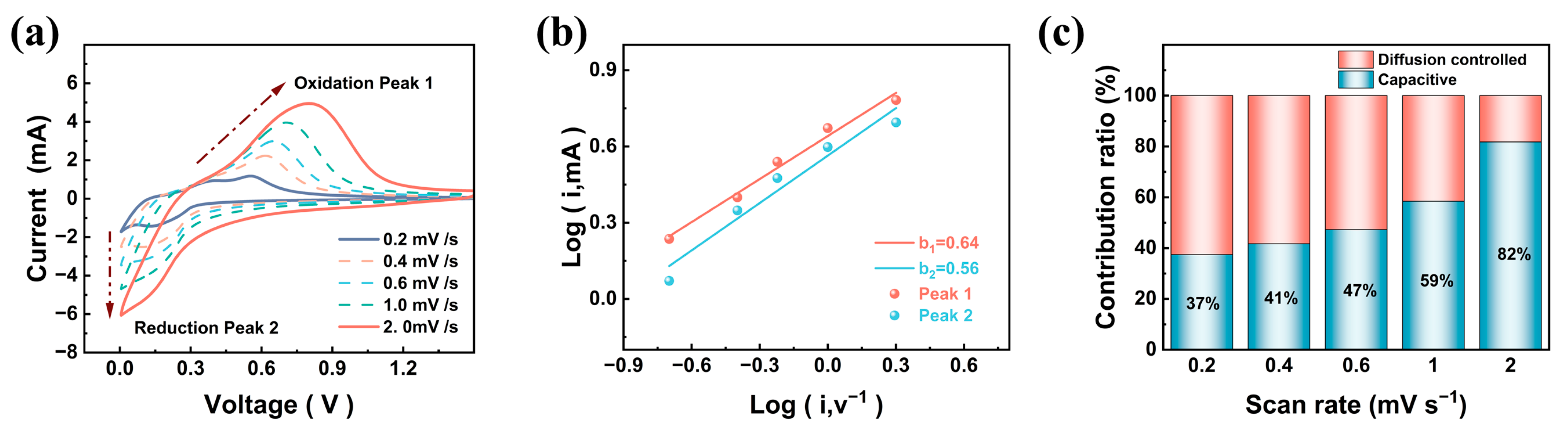
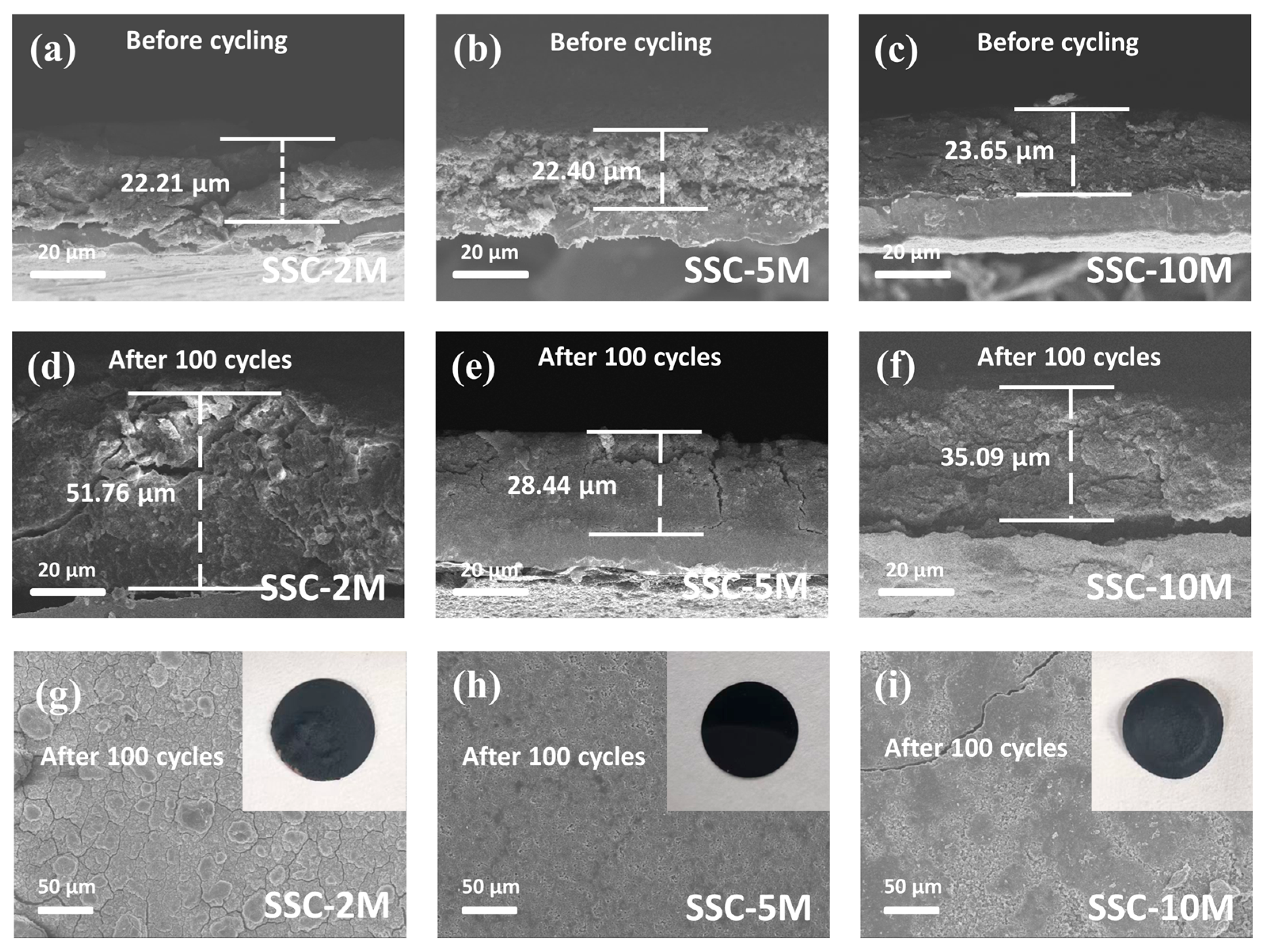
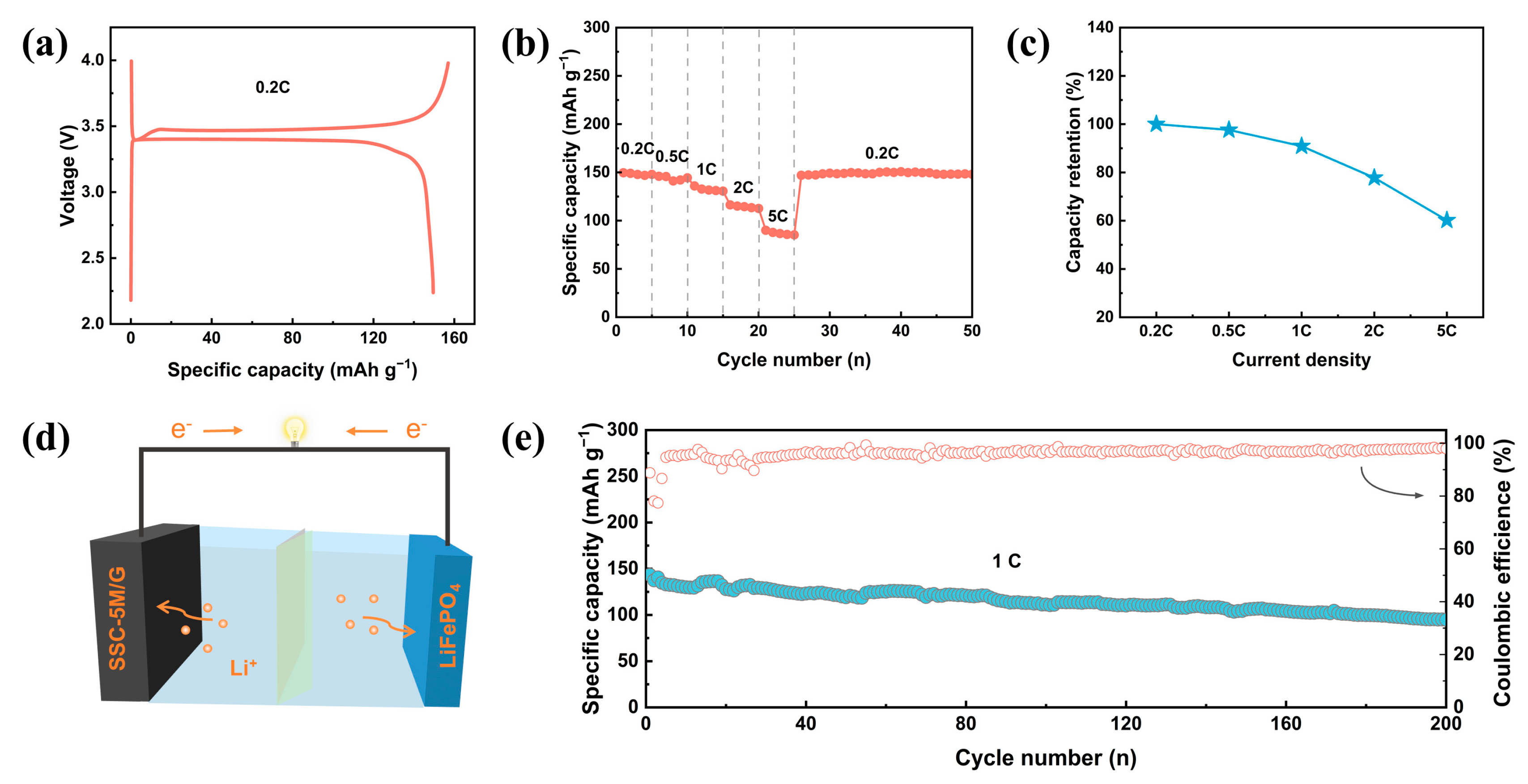
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Guo, L.; Li, K.; Shen, M.; Lu, C.; Jiang, Z.; Song, H.; Li, A. Assembled Carbon Nanostructure Prepared by Spray Freeze Drying for Si-Based Anodes. Nanomaterials 2025, 15, 661. https://doi.org/10.3390/nano15090661
Zhu W, Guo L, Li K, Shen M, Lu C, Jiang Z, Song H, Li A. Assembled Carbon Nanostructure Prepared by Spray Freeze Drying for Si-Based Anodes. Nanomaterials. 2025; 15(9):661. https://doi.org/10.3390/nano15090661
Chicago/Turabian StyleZhu, Wanxiong, Liewen Guo, Kairan Li, Mengxue Shen, Chang Lu, Zipeng Jiang, Huaihe Song, and Ang Li. 2025. "Assembled Carbon Nanostructure Prepared by Spray Freeze Drying for Si-Based Anodes" Nanomaterials 15, no. 9: 661. https://doi.org/10.3390/nano15090661
APA StyleZhu, W., Guo, L., Li, K., Shen, M., Lu, C., Jiang, Z., Song, H., & Li, A. (2025). Assembled Carbon Nanostructure Prepared by Spray Freeze Drying for Si-Based Anodes. Nanomaterials, 15(9), 661. https://doi.org/10.3390/nano15090661






