Photocatalytic Response of Flash-Lamp-Annealed Titanium Oxide Films Produced by Oblique-Angle Deposition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Sample Characterization
2.3. Wettability and Photocatalytic Properties
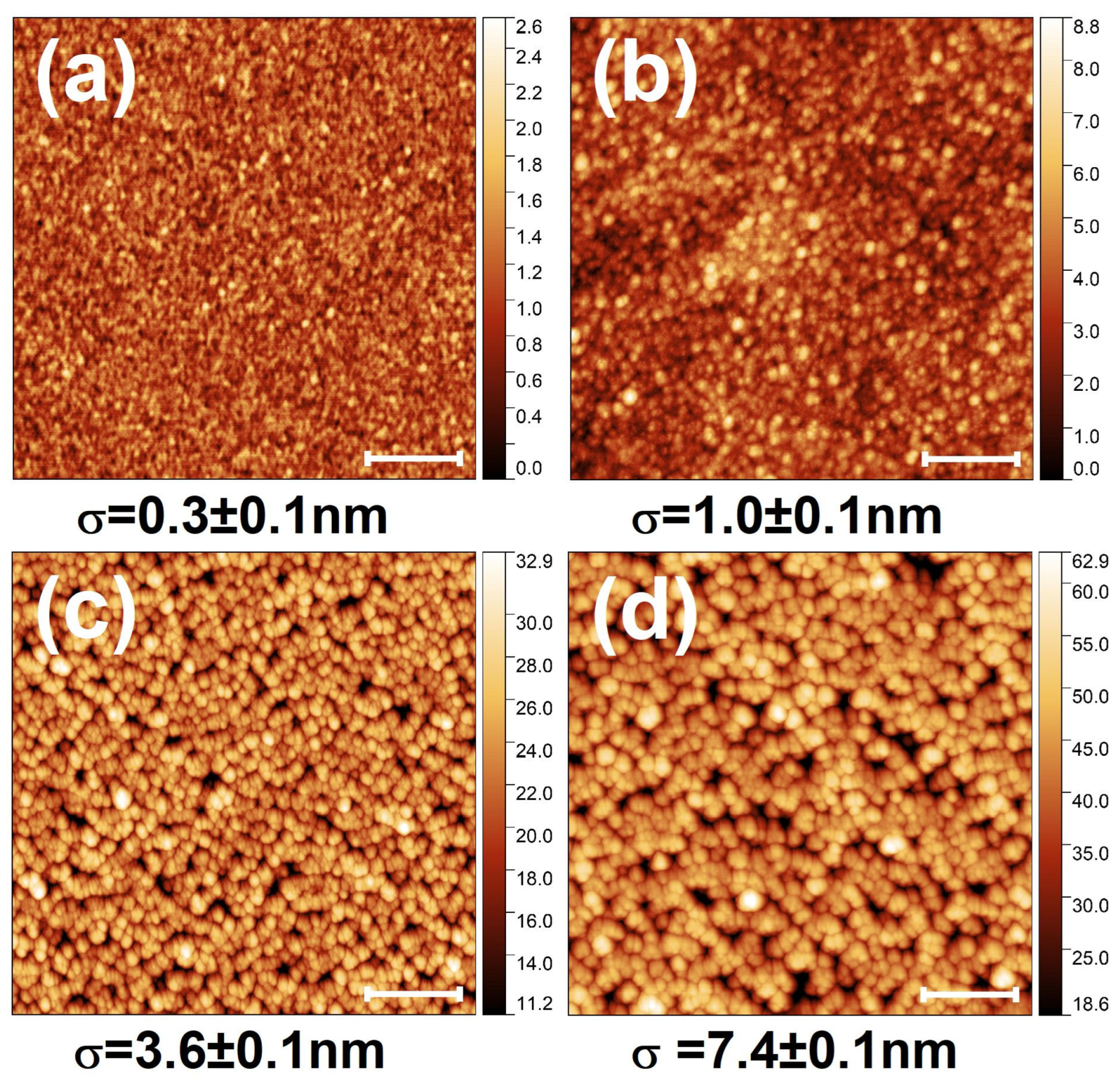
3. Results and Discussion
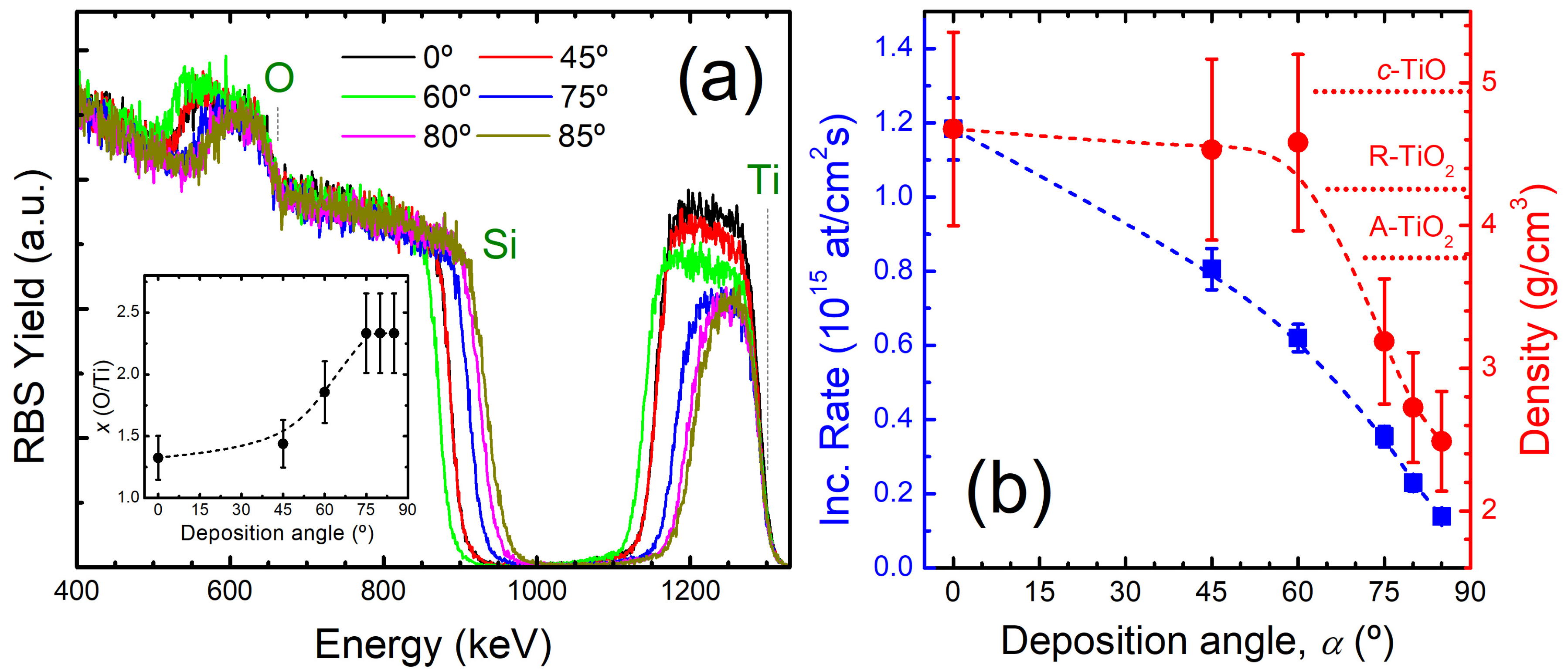
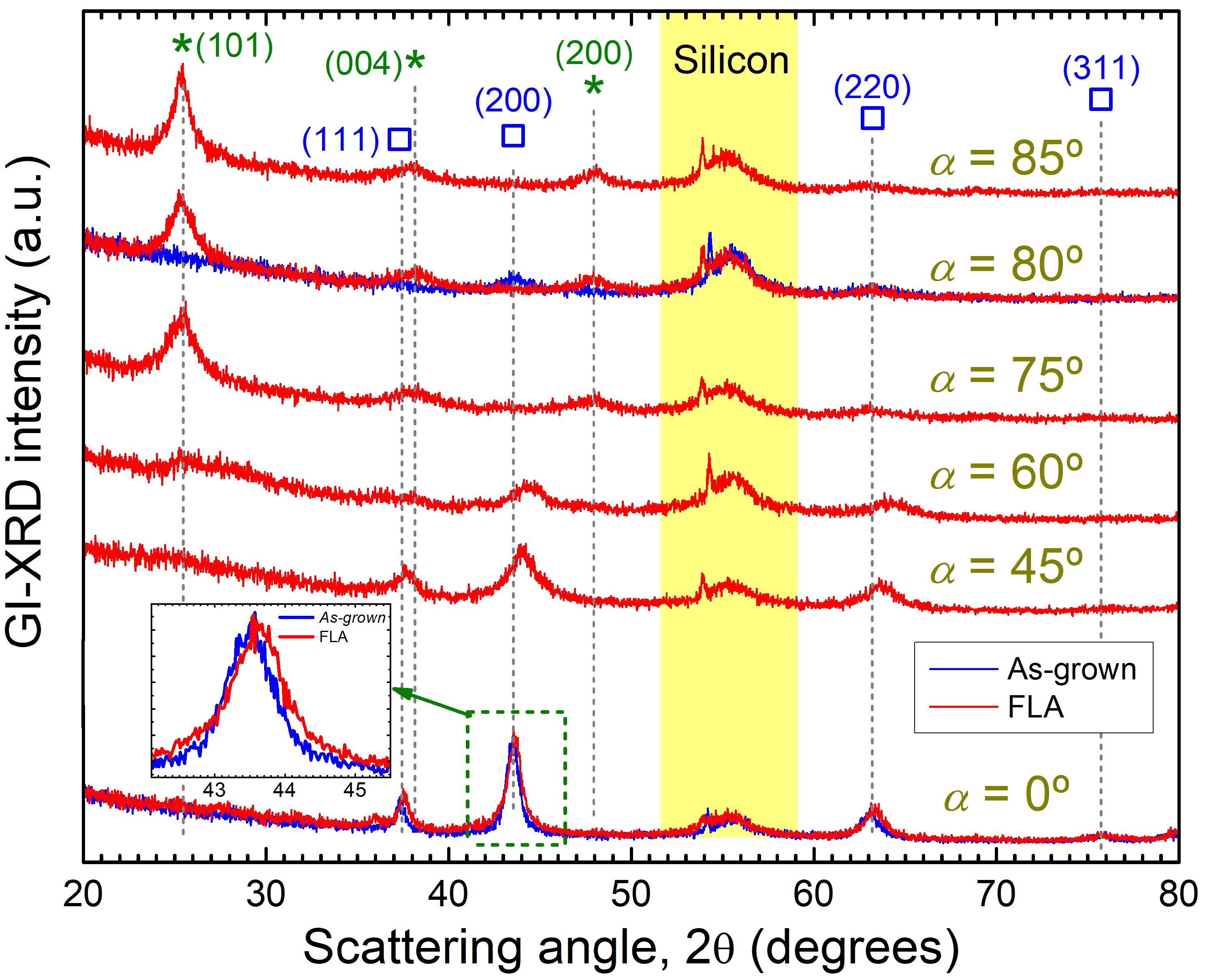
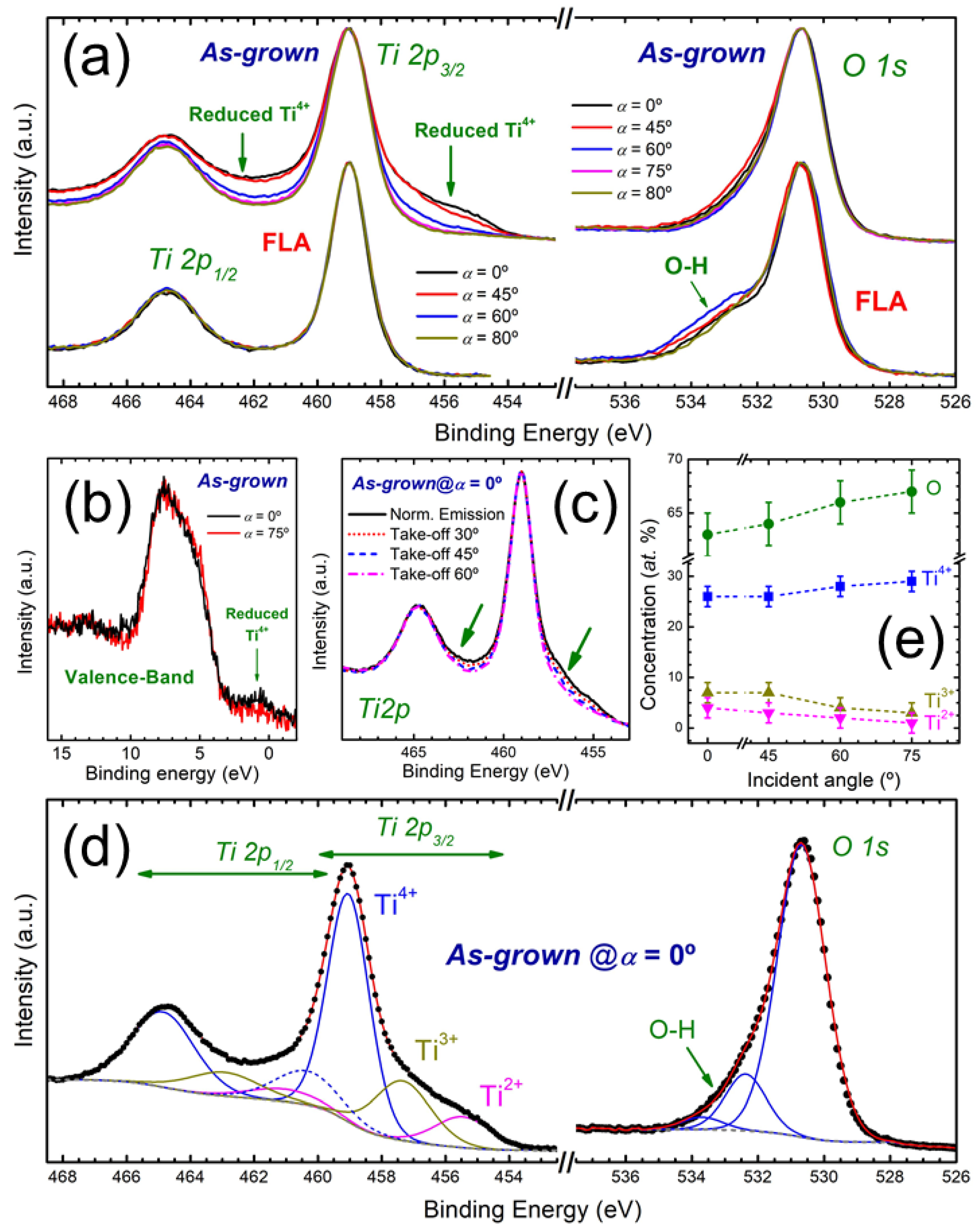
3.1. Microstructure of As-Grown OAD TiOx Films
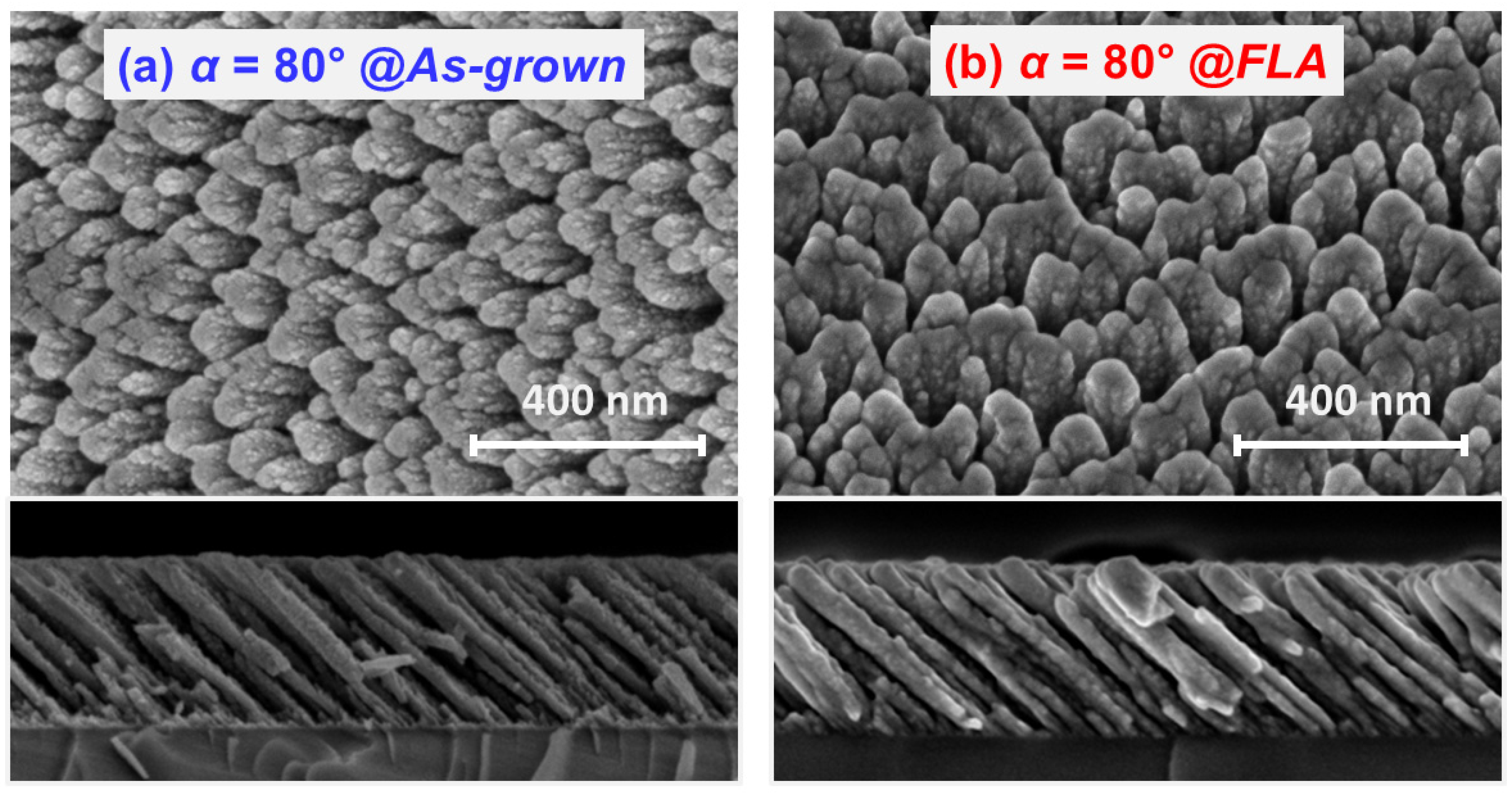
3.2. Effect of FLA in OAD TiOx Films
3.3. Photocatalytic Assesses
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Henderson, M.A. A surface science perspective on TiO2 photocatalysis. Surf. Sci. Rep. 2011, 66, 185–297. [Google Scholar] [CrossRef]
- Linsebigler, A.L.; Lu, G.; Yates, J.T., Jr. Photocatalysis on TiO2 Surfaces: Principles, Mechanisms, and Selected Results. Chem. Rev. 1995, 95, 735–758. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Xiao, W.; Zhou, P.; Jaroniec, M. Enhanced Photocatalytic CO2-Reduction Activity of Anatase TiO2 by Coexposed {001} and {101} Facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Daghrir, R.; Drogui, P.; Robert, D. Modified TiO2 For Environmental Photocatalytic Applications: A Review. Ind. Eng. Chem. Res. 2013, 52, 3581–3599. [Google Scholar] [CrossRef]
- Eidsvåg, H.; Bentouba, S.; Vajeeston, P.; Yohi, S.; Velauthapillai, D. TiO2 as a Photocatalyst for Water Splitting—An Experimental and Theoretical Review. Molecules 2021, 26, 1687. [Google Scholar] [CrossRef]
- Diebold, U. The Surface Science of Titanium Dioxide. Surf. Sci. Rep. 2003, 48, 53–229. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Žerjav, G.; Žižek, K.; Zavašnik, J.; Pintar, A. Brookite vs. rutile vs. anatase: What`s behind their various photocatalytic activities? J. Environ. Chem. Eng. 2022, 10, 107722. [Google Scholar] [CrossRef]
- Bizarro, M.; Tapia-Rodríguez, M.A.; Ojeda, M.L.; Alonso, J.C.; Ortiz, A. Photocatalytic activity enhancement of TiO2 films by micro and nano-structured surface modification. Appl. Surf. Sci. 2009, 255, 6274–6278. [Google Scholar] [CrossRef]
- Barranco, A.; Borrás, A.; González-Elipe, A.R.; Palmero, A. Perspectives on oblique angle deposition of thin films: From fundamentals to devices. Prog. Mater. Sci. 2016, 76, 59–153. [Google Scholar] [CrossRef]
- Alvarez, R.; García-Martín, J.M.; Garcia-Valenzuela, A.; Macías-Montero, M.; Ferrer, F.J.; Santiso, J.; Rico, V.; Cotrino, J.; Gonzalez-Elipe, A.R.; Palmero, A. Growth dynamics of nanocolumnar thin films deposited by magnetron sputtering at oblique angles. J. Phys. D Appl. Phys. 2016, 49, 045303. [Google Scholar] [CrossRef]
- Li, Z.; Xing, L.; Zhang, Z. Photocatalytic Properties of Columnar Nanostructured TiO2 Films Fabricated by Sputtering Ti and Subsequent Annealing. Adv. Mater. Sci. Eng. 2012, 2012, 413638. [Google Scholar] [CrossRef]
- Suzuki, M.; Ito, T.; Taga, Y. Photocatalysis of sculptured thin films of TiO2. Appl. Phys. Lett. 2001, 78, 3968–3970. [Google Scholar] [CrossRef]
- Pihosh, Y.; Turkevych, I.; Ye, J.; Goto, M.; Kasahara, A.; Kondo, M.; Tosa, M. Photocatalytic Properties of TiO2 Nanostructures Fabricated by Means of Glancing Angle Deposition and Anodization. J. Electrochem. Soc. 2009, 156, K160–K165. [Google Scholar] [CrossRef]
- Michalcik, Z.; Horakova, M.; Spatenka, P.; Klementova, S.; Zlamal, M.; Martin, N. Photocatalytic Activity of Nanostructured Titanium Dioxide Thin Films. Int. J. Photoenergy 2012, 2012, 689154. [Google Scholar] [CrossRef]
- Riley, M.J.; Williams, B.; Condon, G.Y.; Borja, J.; Lu, T.M.; Gill, W.N.; Plawsky, J.L. Photocatalytic properties of porous titania grown by oblique angle deposition. J. Appl. Phys. 2012, 111, 074904. [Google Scholar] [CrossRef]
- He, Y.P.; Zhang, Z.Y.; Zhao, Y.P. Optical and photocatalytic properties of oblique angle deposited TiO2 nanorod array. J. Vac. Sci. Technol. B 2008, 26, 1350. [Google Scholar] [CrossRef]
- Riley, M.; Wu, V.; Liu, S.; Gill, W.; Lu, T.M.; Plawsky, J. Surface area and porosity in obliquely grown photocatalytic titanium dioxide for air purification. J. Appl. Phys. 2014, 115, 174907. [Google Scholar] [CrossRef]
- Smith, W.; Ingram, W.; Zhao, Y. The scaling of the photocatalytic decay rate with the length of aligned TiO2 nanorod arrays. Chem. Phys. Lett. 2009, 479, 270–273. [Google Scholar] [CrossRef]
- Li, Z.; Zhu, Y.; Zhou, Q.; Ni, J.; Zhang, Z. Photocatalytic properties of TiO2 thin films obtained by glancing angle deposition. Appl. Surf. Sci. 2012, 258, 2766–2770. [Google Scholar] [CrossRef]
- Prucnal, S.; Rebohle, L.; Skorupa, W. Doping by flash lamp annealing. Mater. Sci. Semicond. Process. 2017, 62, 115–127. [Google Scholar] [CrossRef]
- Gago, R.; Prucnal, S.; Pérez-Casero, R.; Caretti, I.; Jiménez, I.; Lungwitz, F.; Cornelius, S. Structural impact of chromium incorporation in as-grown and flash-lamp-annealed sputter deposited titanium oxide films. J. Alloys Compd. 2017, 729, 438–445. [Google Scholar] [CrossRef]
- Gago, R.; Prucnal, S.; Azpeitia, J.; Jiménez, I.; Álvarez-Fraga, L. Phase selectivity upon flash-lamp annealing of sputter deposited amorphous titanium oxide films. Ceram. Intern. 2024, 50, 49112–49118. [Google Scholar] [CrossRef]
- Sangwal, K. Phase Transformation and Isothermal Crystallization Kinetics. In Nucleation and Crystal Growth: Metastability of Solutions and Melts; Sangwal, K., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 145–188. [Google Scholar] [CrossRef]
- Prucnal, S.; Gago, R.; Calatayud, D.G.; Rebohle, L.; Liedke, M.O.; Butterling, M.; Wagner, A.; Helm, M.; Zhou, S. TiO2 Phase Engineering by Millisecond Range Annealing for Highly Efficient Photocatalysis. J. Phys. Chem. C 2023, 127, 12686–12694. [Google Scholar] [CrossRef]
- COMSOL Multiphysics® v. 6.1; COMSOL AB: Stockholm, Sweden; Available online: www.comsol.com (accessed on 1 January 2019).
- Skorupa, W.; Gebel, T.; Yankov, R.A.; Paul, S.; Lerch, W.; Downey, D.F.; Arevalo, E.A. Advanced Thermal Processing of Ultrashallow Implanted Junctions Using Flash Lamp Annealing. J. Electrochem. Soc. 2005, 152, G436–G440. [Google Scholar] [CrossRef]
- Mayer, M. SIMNRA User’s Guide 6.05; Max-Planck-Institut für Plasmaphysik: Garching, Germany, 2009; Available online: www.simnra.com (accessed on 1 February 2024).
- González-García, L.; González-Valls, I.; Lira-Cantu, M.; Barranco, A.; González-Elipe, A.R. Aligned TiO2 nanocolumnar layers prepared by PVD-GLAD for transparent dye sensitized solar cells. Energy Environ. Sci. 2011, 4, 3426–3435. [Google Scholar] [CrossRef]
- Álvarez-Fraga, L.; Gago, R.; Araiza, J.d.J.; Azpeitia, J.; Jiménez, I.; Sánchez, O. Impact of Silver on the Structural and Wettability Properties of ZnO Films Grown by Oblique Angle Magnetron Sputtering. Processes 2023, 11, 1428. [Google Scholar] [CrossRef]
- Tait, R.N.; Smy, T.; Brett, M.J. Modelling and characterization of columnar growth in evaporated films. Thin Solid Films 1993, 226, 196–201. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, W.; Larsen, G.K.; Toole, R.; Zhao, Y. Tilting angle of nanocolumnar films fabricated by oblique angle deposition. J. Vac. Sci. Technol. B 2012, 30, 030606. [Google Scholar] [CrossRef]
- Álvarez, R.; González-García, L.; Romero-Gómez, P.; Rico, V.; Cotrino, J.; González-Elipe, A.R.; Palmero, A. Theoretical and experimental characterization of TiO2 thin films deposited at oblique angles. J. Phys. D Appl. Phys. 2011, 44, 385302. [Google Scholar] [CrossRef]
- Gago, R.; Redondo-Cubero, A.; Vinnichenko, M.; Lehmann, J.; Munnik, F.; Palomares, F.J. Spectroscopic evidence of NOx formation and band-gap narrowing in N-doped TiO2 films grown by pulsed magnetron sputtering. Mater. Chem. Phys. 2012, 136, 729–736. [Google Scholar] [CrossRef]
- Gago, R.; Prucnal, S.; Azpeitia, J.; Esteban-Mendoza, D.; Jiménez, I. Soft X-ray Absorption Study of Sputtered Tin Oxide Films. J. Alloys Comp. 2022, 902, 163768. [Google Scholar] [CrossRef]
- Lide, D.R. (Ed.) Physical Constants of Inorganic Compounds, 89th ed.; CRC Handbook of Chemistry and Physics; CRC Press/Taylor and Francis: Boca Raton, FL, USA, 2009; Internet Version. [Google Scholar]
- Grigoriev, F.V.; Sulimov, V.B.; Tikhonravov, A.V. Combined Modeling of the Optical Anisotropy of Porous Thin Films. Coatings 2020, 10, 517. [Google Scholar] [CrossRef]
- Balachandran, U.; Eror, N.G. Raman Spectra of Titanium Dioxide. J. Sol. Stat. Chem. 1982, 42, 276–282. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-Ray Diffraction Procedures for Polycrystalline and Amorphous Materials; Wiley: New York, NY, USA, 1974. [Google Scholar]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Q.; Lv, L.; Wang, Y.; Hu, Y.; Deng, Z.; Lou, Z.; Hou, Y.; Teng, F. Ligand-free rutile and anatase TiO2 nanocrystals as electron extraction layers for high performance inverted polymer solar cells. RSC Adv. 2017, 7, 20084–20092. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, X.; Yu, J.; Parkin, I.P.; Fujishima, A.; Nakata, K. Intrinsic intermediate gap states of TiO2 materials and their roles in charge carrier kinetics. J. Photochem. Photobiol. C Photochem. Rev. 2019, 39, 1–57. [Google Scholar] [CrossRef]
- Oku, M.; Matsuta, H.; Wagatsuma, K.; Waseda, Y.; Kohiki, S. Removal of inelastic scattering part from Ti2p XPS spectrum of TiO2 by deconvolution method using O1s as response function. J. Elect. Spectr. Rel. Phen. 1999, 105, 211–218. [Google Scholar] [CrossRef]
- Fernández-Velayos, S.; Recio, F.J.; Palomares, F.J.; Menéndez, N.; Herrasti, P.; Mazarío, E. Highly efficient Cu2O@CuxFeyO4 nanohybrid catalyst for the degradation of emerging pollutants. J. Water Proc. Eng. 2023, 52, 103549. [Google Scholar] [CrossRef]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of photocatalytic degradation of organic compounds: A mini-review and new approach. RSC Adv. 2023, 13, 16915–16925. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.M.; Taschuk, M.T.; Harris, K.D.; Rider, D.A.; Wakefield, N.G.; Sit, J.C.; Buriak, J.M.; Thommes, M.; Brett, M.J. Surface Area Characterization of Obliquely Deposited Metal Oxide Nanostructured Thin Films. Langmuir 2010, 26, 4368–4376. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.J.; Wen, B.; Zhou, C.; Selloni, A.; Liu, L.M. Excess electrons in reduced rutile and anatase TiO2. Surf. Sci. Rep. 2018, 73, 58–82. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gago, R.; Prucnal, S.; Palomares, F.J.; Álvarez-Fraga, L.; Castellanos-Aliaga, A.; Calatayud, D.G. Photocatalytic Response of Flash-Lamp-Annealed Titanium Oxide Films Produced by Oblique-Angle Deposition. Nanomaterials 2025, 15, 662. https://doi.org/10.3390/nano15090662
Gago R, Prucnal S, Palomares FJ, Álvarez-Fraga L, Castellanos-Aliaga A, Calatayud DG. Photocatalytic Response of Flash-Lamp-Annealed Titanium Oxide Films Produced by Oblique-Angle Deposition. Nanomaterials. 2025; 15(9):662. https://doi.org/10.3390/nano15090662
Chicago/Turabian StyleGago, Raúl, Slawomir Prucnal, Francisco Javier Palomares, Leopoldo Álvarez-Fraga, Ana Castellanos-Aliaga, and David G. Calatayud. 2025. "Photocatalytic Response of Flash-Lamp-Annealed Titanium Oxide Films Produced by Oblique-Angle Deposition" Nanomaterials 15, no. 9: 662. https://doi.org/10.3390/nano15090662
APA StyleGago, R., Prucnal, S., Palomares, F. J., Álvarez-Fraga, L., Castellanos-Aliaga, A., & Calatayud, D. G. (2025). Photocatalytic Response of Flash-Lamp-Annealed Titanium Oxide Films Produced by Oblique-Angle Deposition. Nanomaterials, 15(9), 662. https://doi.org/10.3390/nano15090662










