Magnetic Nanovectors for the Development of DNA Blood-Stage Malaria Vaccines
Abstract
:1. Introduction
2. Results
2.1. The SPION-Based DNA Vaccine Formulations: Stability and Uptake by Immune Cells
2.2. Antibody Responses Induced by SPIONs/PEI/DNA + HA Complexes
2.3. Antibody Isotypes Induced by the SPIONs/PEI/DNA + HA Complexes
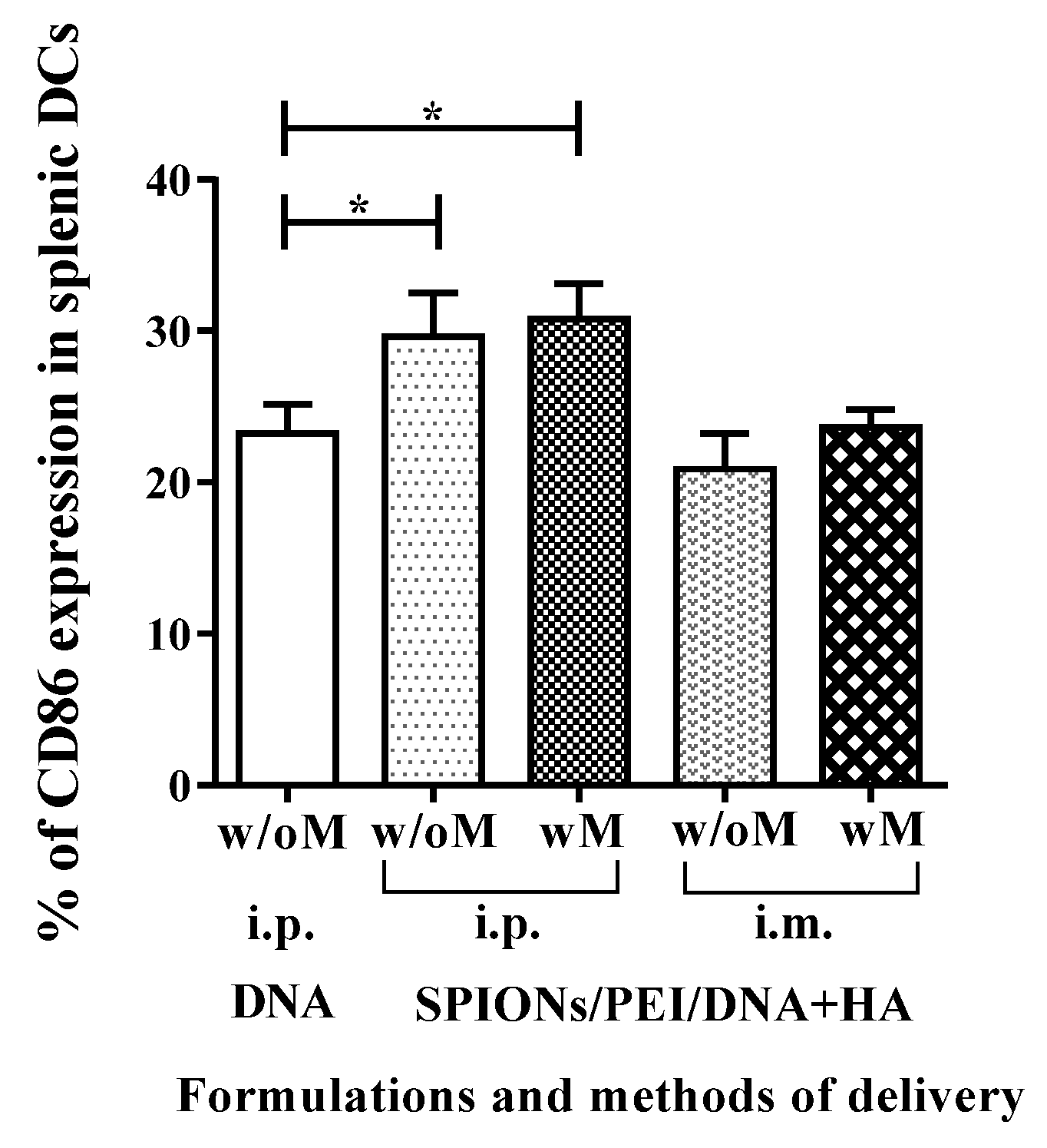
2.4. In Vivo Maturation of Splenic Dendritic Cells Induced by SPIONs/PEI/DNA + HA Complexes Injection
2.5. Cytokine Production Associated with the Cellular-Mediated Immune Responses Induced by the SPIONs/PEI/DNA + HA Complexes
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents
4.2. Preparation of Plasmids DNA
4.3. Preparation of Magnetic Gene Vectors
4.4. Immunisation of Mice
4.5. Antibody Determination by Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Flow Cytometry
4.7. ELISpot Assay
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APC | antigen presenting cells |
| DC | dendritic cells |
| ELISA | enzyme-linked immunosorbent assay |
| ELISpot | enzyme-linked immunospot assay |
| HA | hyaluronic acid |
| i.p. | intraperitoneal |
| i.m. | intramuscular |
| MSP | merozoite surface protein |
| PEI | polyethylenimine |
| SPIONs | superparamagnetic iron oxide nanoparticles |
References
- Delany, I.; Rappuoli, R.; Seib, K.L. Vaccines, reverse vaccinology, and bacterial pathogenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a012476. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Klinman, D.M.; Seder, R.A. DNA vaccines: Immunology, application, and optimization. Annu. Rev. Immunol. 2000, 18, 927–974. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.B.; Zurita-Turk, M.; Saraiva, T.D.L.; De Castro, C.P.; Souza, B.M.; Agresti, P.M.; Lima, F.A.; Pfeiffer, V.N.; Azevedo, M.S.P.; Rocha, C.S. DNA vaccines approach: From concepts to applications. World J. Vaccines 2014, 2014, 44968. [Google Scholar] [CrossRef]
- Donnelly, J.; Berry, K.; Ulmer, J.B. Technical and regulatory hurdles for DNA vaccines. Int. J. Parasitol. 2003, 33, 457–467. [Google Scholar] [CrossRef]
- Donnelly, J. DNA vaccines. In New Bacterial Vaccines; Ellis, R.W., Brodeur, B.R., Eds.; Springer: New York, NY, USA, 2012; pp. 30–41. [Google Scholar]
- Hasson, S.S.; Al-Busaidi, J.K.; Sallam, T.A. The past, current and future trends in DNA vaccine immunisations. Asian Pac. J. Trop. Biomed. 2015, 5, 344–353. [Google Scholar] [CrossRef]
- Plotkin, S.A. Vaccines: Past, present and future. Nat. Med. 2005, 11, S5–S11. [Google Scholar] [CrossRef] [PubMed]
- Williams, J. Vector design for improved DNA vaccine efficacy, safety and production. Vaccines 2013, 1, 225–249. [Google Scholar] [CrossRef] [PubMed]
- Ingolotti, M.; Kawalekar, O.; Shedlock, D.J.; Muthumani, K.; Weiner, D.B. DNA vaccines for targeting bacterial infections. Expert Rev. Vaccines 2010, 9, 747–763. [Google Scholar] [CrossRef] [PubMed]
- Redding, L.; Werner, D.B. DNA vaccines in veterinary use. Expert Rev.Vaccines 2009, 8, 1251–1276. [Google Scholar] [CrossRef] [PubMed]
- Wahren, B.; Liu, M.A. DNA vaccines: Recent developments and the future. Vaccines 2014, 2, 785–796. [Google Scholar] [CrossRef]
- Vijayanathan, V.; Thomas, T.; Thomas, T.J. DNA nanoparticles and development of DNA delivery vehicles for gene therapy. Biochemistry 2002, 41, 14085–14094. [Google Scholar] [CrossRef] [PubMed]
- Krotz, F.; Sohn, H.Y.; Gloe, T.; Plank, C.; Pohl, U. Magnetofection potentiates gene delivery to cultured endothelial cells. J. Vasc. Res. 2003, 40, 425–434. [Google Scholar] [CrossRef]
- Al-Deen, F.N.; Ho, J.; Selomulya, C.; Ma, C.; Coppel, R. Superparamagnetic nanoparticles for effective delivery of malaria DNA vaccine. Langmuir 2011, 27, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- World Malaria Report 2015—Summary; World Health Organisation: Geneva, Switzerland, 2015; p. 5.
- Wilson, D.W.; Fowkes, F.J.; Gilson, P.R.; Elliott, S.R.; Tavul, L.; Michon, P.; Dabod, E.; Siba, P.M.; Mueller, I.; Crabb, B.S.; et al. Quantifying the importance of msp1-19 as a target of growth-inhibitory and protective antibodies against plasmodium falciparum in humans. PLoS ONE 2011, 6, e27705. [Google Scholar] [CrossRef] [PubMed]
- Hodder, A.N.; Crewther, P.E.; Matthew, M.L.; Reid, G.E.; Moritz, R.L.; Simpson, R.J.; Anders, R.F. The disulfide bond structure of plasmodium apical membrane antigen-1. J. Biol. Chem. 1996, 271, 29446–29452. [Google Scholar] [PubMed]
- Daly, T.M.; Long, C.A. Humoral response to a carboxyl-terminal region of the merozoite surface protein-1 plays a predominant role in controlling blood-stage infection in rodent malaria. J. Immunol. 1995, 155, 236–243. [Google Scholar] [PubMed]
- Kumar, S.; Yadava, A.; Keister, D.B.; Tian, J.H.; Ohl, M.; Perdue-Greenfield, K.A.; Miller, L.H.; Kaslow, D.C. Immunogenicity and in vivo efficacy of recombinant plasmodium falciparum merozoite surface protein-1 in aotus monkeys. Mol. Med. 1995, 1, 325–332. [Google Scholar] [PubMed]
- O’Donnell, R.A.; de Koning-Ward, T.F.; Burt, R.A.; Bockarie, M.; Reeder, J.C.; Cowman, A.F.; Crabb, B.S. Antibodies against merozoite surface protein (msp)-1(19) are a major component of the invasion-inhibitory response in individuals immune to malaria. J. Exp. Med. 2001, 193, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Nawwab Al-Deen, F.; Ma, C.; Xiang, S.D.; Selomulya, C.; Plebanski, M.; Coppel, R.L. On the efficacy of malaria DNA vaccination with magnetic gene vectors. J. Control. Release 2013, 168, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.A.; Kong, J.H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ponta, H.; Sherman, L.; Herrlich, P.A. Cd44: From adhesion molecules to signalling regulators. Nat. Rev. Mol. Cell Biol. 2003, 4, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.; Hascall, V.C.; Markwald, R.R.; Ghatak, S. Interactions between hyaluronan and its receptors (cd44, rhamm) regulate the activities of inflammation and cancer. Front. Immunol. 2015, 6, 201. [Google Scholar] [CrossRef] [PubMed]
- Al-Deen, F.N.; Selomulya, C.; Williams, T. On designing stable magnetic vectors as carriers for malaria DNA vaccine. Coll. Surf. B Biointerfaces 2013, 102, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Nawwab Al-Deen, F.M.; Selomulya, C.; Kong, Y.Y.; Xiang, S.D.; Ma, C.; Coppel, R.L.; Plebanski, M. Design of magnetic polyplexes taken up efficiently by dendritic cell for enhanced DNA vaccine delivery. Gene Ther. 2014, 21, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.J.; Wagner, B.; Woof, J.M. The different effector function capabilities of the seven equine IgG subclasses have implications for vaccine strategies. Mol. Immunol. 2008, 45, 818–827. [Google Scholar] [CrossRef] [PubMed]
- Cherif, M.S.; Shuaibu, M.N.; Kurosaki, T.; Helegbe, G.K.; Kikuchi, M.; Yanagi, T.; Tsuboi, T.; Sasaki, H.; Hirayama, K. Immunogenicity of novel nanoparticle-coated msp-1 c-terminus malaria DNA vaccine using different routes of administration. Vaccine 2011, 29, 9038–9050. [Google Scholar] [CrossRef] [PubMed]
- Mehrizi, A.A.; Zakeri, S.; Rafati, S.; Salmanian, A.H.; Djadid, N.D. Immune responses elicited by co-immunization of plasmodium vivax and p. falciparum msp-1 using prime-boost immunization strategies. Parasite Immunol. 2011, 33, 594–608. [Google Scholar] [CrossRef] [PubMed]
- Bouharoun-Tayoun, H.; Druilhe, P. Plasmodium falciparum malaria: Evidence for an isotype imbalance which may be responsible for delayed acquisition of protective immunity. Infect. Immun. 1992, 60, 1473–1481. [Google Scholar] [PubMed]
- Coffman, R.L.; Seymour, B.W.; Lebman, D.A.; Hiraki, D.D.; Christiansen, J.A.; Shrader, B.; Cherwinski, H.M.; Savelkoul, H.F.; Finkelman, F.D.; Bond, M.W.; et al. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol. Rev. 1988, 102, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Sin, J.I.; Bagarazzi, M.; Pachuk, C.; Weiner, D.B. DNA priming-protein boosting enhances both antigen-specific antibody and th1-type cellular immune responses in a murine herpes simplex virus-2 gd vaccine model. DNA Cell Biol. 1999, 18, 771–779. [Google Scholar] [CrossRef] [PubMed]
- White, W.I.; Evans, C.B.; Taylor, D.W. Antimalarial antibodies of the immunoglobulin G2a isotype modulate parasitemias in mice infected with plasmodium yoelii. Infect. Immun. 1991, 59, 3547–3554. [Google Scholar] [PubMed]
- Ling, I.T.; Ogun, S.A.; Momin, P.; Richards, R.L.; Garcon, N.; Cohen, J.; Ballou, W.R.; Holder, A.A. Immunization against the murine malaria parasite plasmodium yoelii using a recombinant protein with adjuvants developed for clinical use. Vaccine 1997, 15, 1562–1567. [Google Scholar] [CrossRef]
- Hirunpetcharat, C.; Tian, J.H.; Kaslow, D.C.; van Rooijen, N.; Kumar, S.; Berzofsky, J.A.; Miller, L.H.; Good, M.F. Complete protective immunity induced in mice by immunization with the 19-kilodalton carboxyl-terminal fragment of the merozoite surface protein-1 (msp1[19]) of plasmodium yoelii expressed in saccharomyces cerevisiae: Correlation of protection with antigen-specific antibody titer, but not with effector cd4+ T cells. J. Immunol. 1997, 159, 3400–3411. [Google Scholar] [PubMed]
- Plebanski, M.; Hill, A.V. The immunology of malaria infection. Curr. Opin. Immunol. 2000, 12, 437–441. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Hohlfeld, R.; Engel, A.G. The immunobiology of muscle. Immunol. Today 1994, 15, 269–274. [Google Scholar] [CrossRef]
- Wang, R.; Doolan, D.L.; Charoenvit, Y.; Hedstrom, R.C.; Gardner, M.J.; Hobart, P.; Tine, J.; Sedegah, M.; Fallarme, V.; Sacci, J.B., Jr.; et al. Simultaneous induction of multiple antigen-specific cytotoxic T lymphocytes in nonhuman primates by immunization with a mixture of four plasmodium falciparum DNA plasmids. Infect. Immun. 1998, 66, 4193–4202. [Google Scholar] [PubMed]
- Xiang, S.D.; Selomulya, C.; Ho, J.; Apostolopoulos, V.; Plebanski, M. Delivery of DNA vaccines: An overview on the use of biodegradable polymeric and magnetic nanoparticles. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Scherer, F.; Anton, M.; Schillinger, U.; Henke, J.; Bergemann, C.; Kruger, A.; Gansbacher, B.; Plank, C. Magnetofection: Enhancing and targeting gene delivery by magnetic force in vitro and in vivo. Gene Ther. 2002, 9, 102–109. [Google Scholar] [CrossRef]
- Golos, A.; Lutynska, A. Aluminium-adjuvanted vaccines—A review of the current state of knowledge. Prz. Epidemiol. 2015, 69, 731–734. [Google Scholar]
- Wolff, J.A.; Dowty, M.E.; Jiao, S.; Repetto, G.; Berg, R.K.; Ludtke, J.J.; Williams, P.; Slautterback, D.B. Expression of naked plasmids by cultured myotubes and entry of plasmids into t tubules and caveolae of mammalian skeletal muscle. J. Cell Sci. 1992, 103, 1249–1259. [Google Scholar] [PubMed]
- Zhou, X.F.; Liu, B.; Yu, X.H.; Zha, X.; Zhang, X.Z.; Wang, X.Y.; Jin, Y.H.; Wu, Y.G.; Jiang, C.L.; Chen, Y.; et al. Using magnetic force to enhance immune response to DNA vaccine. Small 2007, 3, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.H.; Good, M.F.; Hirunpetcharat, C.; Kumar, S.; Ling, I.T.; Jackson, D.; Cooper, J.; Lukszo, J.; Coligan, J.; Ahlers, J.; et al. Definition of t cell epitopes within the 19 kda carboxylterminal fragment of plasmodium yoelii merozoite surface protein 1 (msp1(19)) and their role in immunity to malaria. Parasite Immunol. 1998, 20, 263–278. [Google Scholar] [CrossRef] [PubMed]
- Egan, A.; Waterfall, M.; Pinder, M.; Holder, A.; Riley, E. Characterization of human T- and B-cell epitopes in the C terminus of plasmodium falciparum merozoite surface protein 1: Evidence for poor T-cell recognition of polypeptides with numerous disulfide bonds. Infect. Immun. 1997, 65, 3024–3031. [Google Scholar] [PubMed]
- Dhusia, K.; Kesarwani, P.; Yadav, P.K. Epitope prediction for msp119 protein in plasmodium yeolii using computational approaches. Netw. Model. Anal. Health Inf. Bioinf. 2016, 5, 19. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Fuchsberger, M.; Plebanski, M.; Apostolopoulos, V. Alteration of early dendritic cell activation by cancer cell lines predisposes immunosuppression, which cannot be reversed by tlr4 stimulation. Acta Biochim. Biophys. Sin. 2016, 48, 1101–1111. [Google Scholar] [CrossRef] [PubMed]



| Test Conditions | Buffer Conditions | Formulation Configurations | ||
|---|---|---|---|---|
| 1st Configuration (SPIONs/PEI/DNA/HA) | 2nd Configuration (SPIONs/PEI/DNA + HA) | 3rd Configuration (SPIONs/PEI + DNA + HA) | ||
| Size (nm) (with the increase % of charge ratio HA:PEI) | water | 100–300 | 100–300 | 400–200 |
| NaCl | 1000–500 | >1000 | 500–2500 | |
| RPMI | 600–200 | 500–100 | 400–1600 | |
| RPMI + 10% FCS | 150–40 | 150–40 | 180–70 | |
| Charges (with the increase % of charge ratio HA:PEI, reduced the positive charges) | water | ~32 mV to ~−25 mV | ~32 mV to ~−25 mV | ~29 mV |
| NaCl | ~14 mV to ~−10 mV | ~9 mV to ~−10 mV | ~17 mV to ~2 mV | |
| RPMI | ~8 mV to ~−12 mV | ~8 mV to ~−15 mV | ~12 mV to ~−5 mV | |
| RPMI + 10% FCS | increase the positive charge (~−18 mV to ~−10 mV) | increase the positive charge (~−18 mV to ~−10 mV) | increase the positive charge (~−16 mV to ~−8 mV) | |
| DNA retardation (with the increase % of charge ratio HA:PEI) | water | >25% DNA released | partial DNA disassembly, but no free DNA | no DNA release or disassembly |
| NaCl | No release of DNA | No release of DNA | No release of DNA | |
| RPMI | No release of DNA | No release of DNA | No release of DNA | |
| RPMI + 10% FCS | partial DNA disassembly | partial DNA disassembly | no DNA release or disassembly | |
| DNA binding | 5% of charge ratio HA:PEI | 96% ± 3% | 94% ± 1% | 99% ± 0.5% |
| 100% of charge ratio HA:PEI | 95% ± 2% | 93% ± 3% | 99% ± 1% | |
| Stability against extracellular environment | + | +++ | ++ | |
| Stability against nuclease | + | +++ | ++ | |
| HA Molecular Weight (MW) | HA:PEI Ratio | |
|---|---|---|
| Efficiency of particle uptake | increase with higher MW | increase with higher ratio |
| Impact of external magnetic field on uptake efficiency | increase with lower MW formulation | increase with lower ratio formulation |
| CD86 expression | increase with higher MW | increase with higher ratio |
| Major histocompatibility complex I (MHC I) expression | MHC I upregulation only observed in 100% HA under magnetic field | no upregulation at lower ratio either with or without magnetic field |
| Major histocompatibility complex II (MHC II) expression | MHC II upregulation only observed in 100% high MW HA under magnetic field | no upregulation at lower ratio either with or without magnetic field |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Deen, F.M.N.; Xiang, S.D.; Ma, C.; Wilson, K.; Coppel, R.L.; Selomulya, C.; Plebanski, M. Magnetic Nanovectors for the Development of DNA Blood-Stage Malaria Vaccines. Nanomaterials 2017, 7, 30. https://doi.org/10.3390/nano7020030
Al-Deen FMN, Xiang SD, Ma C, Wilson K, Coppel RL, Selomulya C, Plebanski M. Magnetic Nanovectors for the Development of DNA Blood-Stage Malaria Vaccines. Nanomaterials. 2017; 7(2):30. https://doi.org/10.3390/nano7020030
Chicago/Turabian StyleAl-Deen, Fatin M. Nawwab, Sue D. Xiang, Charles Ma, Kirsty Wilson, Ross L. Coppel, Cordelia Selomulya, and Magdalena Plebanski. 2017. "Magnetic Nanovectors for the Development of DNA Blood-Stage Malaria Vaccines" Nanomaterials 7, no. 2: 30. https://doi.org/10.3390/nano7020030
APA StyleAl-Deen, F. M. N., Xiang, S. D., Ma, C., Wilson, K., Coppel, R. L., Selomulya, C., & Plebanski, M. (2017). Magnetic Nanovectors for the Development of DNA Blood-Stage Malaria Vaccines. Nanomaterials, 7(2), 30. https://doi.org/10.3390/nano7020030





