Evaluating Adverse Effects of Inhaled Nanoparticles by Realistic In Vitro Technology
Abstract
:1. Introduction
2. Airborne Nanoparticles of Concern
3. In Vitro Test System for Inhalation Toxicology
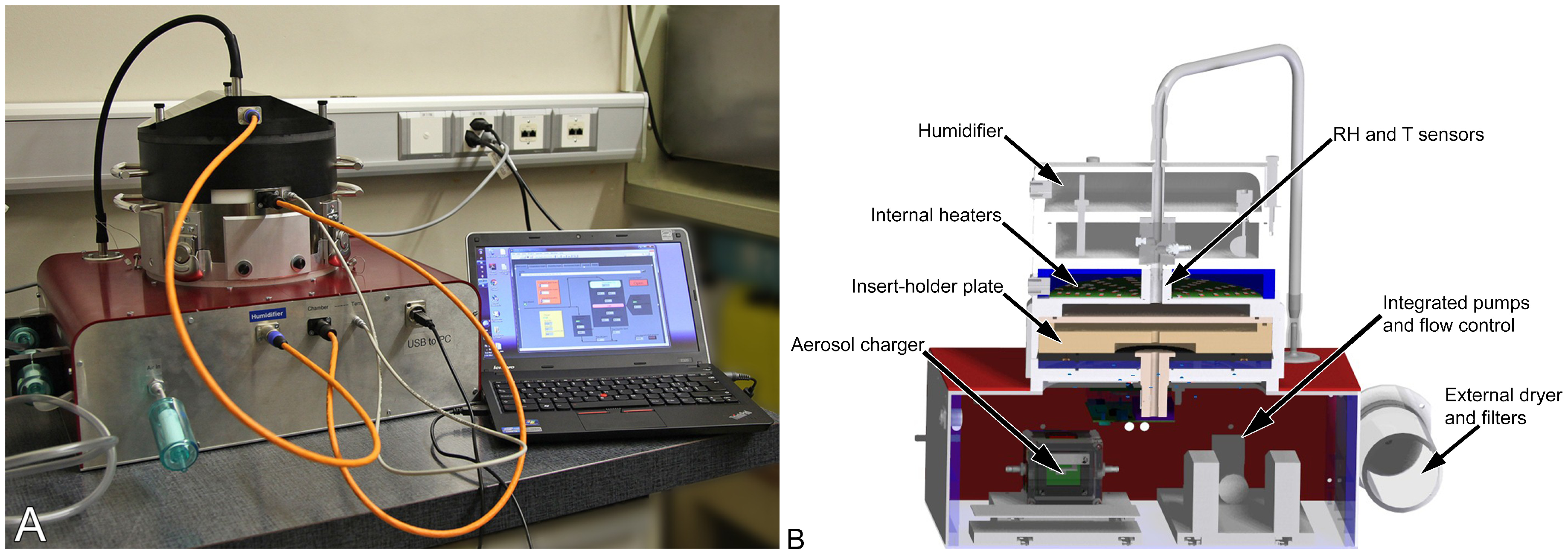
3.1. Aerosol Generation
3.2. Aerosol Deposition Chamber
3.3. The Inner Lung Surface
3.4. Airway Epithelia
3.5. Biological Endpoints
3.6. The Complete In Vitro System and Its Implementation
4. Effects of Selected, Commercial ENP on Normal and Diseased Airway Epithelia
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| Ag | silver |
| ALI | air-liquid interface |
| BAL | bronchoalveolar lavage |
| BEAS-2B | human bronchial epithelial cell line |
| C | carbon |
| COPD | chronic obstructive pulmonary disease |
| CF | cystic fibrosis |
| ENP | engineered nanoparticles |
| HBE | human bronchial epithelia |
| ICP-MS | inductively coupled plasma mass spectrometry |
| IL | interleukin |
| LAORA | Life Alliance Organ Recovery Agency |
| LDH | lactate dehydrogenase |
| MCP | monocyte chemotactic protein |
| NACIVT | Nano Aerosol Chamber for In Vitro Toxicity |
| NP | nanoparticles |
| p-free | particle-free |
| PM | particulate matter |
| PSL | polystyrene latex particles |
| RH | relative humidity |
| SMPS | scanning mobility particle sizer |
| TEM | transmission electron microscope/microscopy |
| TNF | tumor necrosis factor |
References
- Pope, C.A., III. Epidemiology of fine particulate air pollution and human health: Biologic mechanisms and who’s at risk? Environ. Health Perspect. 2000, 108, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.J.; Box, M.; Kalman, D.; Kaufman, J.; Koenig, J.; Larson, T.; Lumley, T.; Sheppard, L.; Wallace, L. Exposure assessment of particulate matter for susceptible populations in Seattle. Environ. Health Perspect. 2003, 111, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Goss, C.H.; Newsom, S.A.; Schildcrout, J.S.; Sheppard, L.; Kaufman, J.D. Effect of ambient air pollution on pulmonary exacerbations and lung function in cystic fibrosis. Am. J. Respir. Crit. Care Med. 2004, 169, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Thurston, G.D. The role of air pollution in asthma and other pediatric morbidities. J. Allergy Clin. Immunol. 2005, 115, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Kreyling, W.G.; Semmler-Behnke, M.; Moller, W. Ultrafine particle-lung interactions: Does size matter? J. Aerosol Med. 2006, 19, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Paur, H.R.; Cassee, F.R.; Teeguarden, J.; Fissan, H.; Diabate, S.; Aufderheide, M.; Kreyling, W.G.; Hänninen, O.; Kasper, G.; Riediker, M.; et al. In Vitro cell exposure studies for the assessment of nanoparticle toxicity in the lung—A dialog between aerosol science and biology. J. Aerosol Sci. 2011, 42, 668–692. [Google Scholar] [CrossRef]
- Aufderheide, M.; Mohr, U. CULTEX a new system and technique for the cultivation and exposure of cells at the air/liquid interface. Exp. Toxicol. Pathol. 1999, 51, 489–490. [Google Scholar] [CrossRef]
- Phillips, J.; Kluss, B.; Richter, A.; Massey, E. Exposure of bronchial epithelial cells to whole cigarette smoke: Assessment of cellular responses. Altern. Lab. Anim. 2005, 33, 239–248. [Google Scholar] [PubMed]
- Müller, L.; Comte, P.; Czerwinski, J.; Kasper, M.; Mayer, A.C.; Gehr, P.; Burtscher, H.; Morin, J.P.; Konstandopoulos, A.; Rothen-Rutishauser, B. New exposure system to evaluate the toxicity of (scooter) exhaust emissions in lung cells in vitro. Environ. Sci. Technol. 2010, 44, 2632–2638. [Google Scholar]
- Anderson, S.E.; Jackson, L.G.; Franko, J.; Wells, J.R. Evaluation of dicarbonyls generated in a simulated indoor air environment using an in vitro exposure system. Toxicol. Sci. 2010, 115, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Savi, M.; Kalberer, M.; Lang, D.; Ryser, M.; Fierz, M.; Gaschen, A.; Rička, J.; Geiser, M. A novel exposure system for the efficient and controlled deposition of aerosol particles onto cell cultures. Environ. Sci. Technol. 2008, 42, 5667–5674. [Google Scholar] [CrossRef] [PubMed]
- De Bruijne, K.; Ebersviller, S.; Sexton, K.G.; Lake, S.; Leith, D.; Goodman, R.; Jetters, J.; Walters, G.W.; Doyle-Eisele, M.; Woodside, R.; et al. Design and testing of Electrostatic Aerosol in Vitro Exposure System (EAVES): An alternative exposure system for particles. Inhal. Toxicol. 2009, 21, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Aufderheide, M.; Scheffler, S.; Mohle, N.; Halter, B.; Hochrainer, D. Analytical in vitro approach for studying cyto- and genotoxic effects of particulate airborne material. Anal. Bioanal. Chem. 2011, 401, 3213–3220. [Google Scholar] [CrossRef] [PubMed]
- Saffari, H.; Malugin, A.; Ghandehari, H.; Pease, L.F. Electrostatic deposition of nanoparticles into live cell culture using an electrospray differential mobility analyzer (ES-DMA). J. Aerosol Sci. 2012, 48, 56–62. [Google Scholar] [CrossRef]
- Lenz, A.G.; Karg, E.; Lentner, B.; Dittrich, V.; Brandenberger, C.; Rothen-Rutishauser, B.; Schulz, H.; Ferron, G.A.; Schmid, O. A dose-controlled system for air-liquid interface cell exposure and application to zinc oxide nanoparticles. Part. Fibre Toxicol. 2009, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NACIVT, the Nano Aerosol Chamber for In Vitro Toxicology. Available online: http://www.nacivt.ch/ (accessed on 17 February 2017).
- Fulcher, M.L.; Gabriel, S.; Burns, K.A.; Yankaskas, J.R.; Randell, S.H. Well-differentiated human airway epithelial cell cultures. Methods Mol. Med. 2005, 107, 183–206. [Google Scholar] [PubMed]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959. [Google Scholar]
- Mülhopt, S.; Diabaté, S.; Krebs, T.; Weiss, C.; Paur, H.-R. Lung toxicity determination by in vitro exposure at the air-liquid interface with an integrated online dose measurement. J. Phys. Conf. Ser. 2009, 170, 012008. [Google Scholar] [CrossRef]
- Mülhopt, S.; Dilger, M.; Diabaté, S.; Schlager, C.; Krebs, T.; Zimmermann, R.; Buters, J.; Oeder, S.; Wäscher, T.; Weiss, C.; et al. Toxicity testing of combustion aerosols at the air-liquid interface with a self-contained and easy-to-use exposure system. J. Aerosol Sci. 2016, 96, 18. [Google Scholar] [CrossRef]
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Woodrow Wilson International Center for Scholars. The Project on Emerging Nanotechnologies (PEN). Available online: http://nanotechproject.org/cpi/ (accessed on 17 February 2017).
- Lee, Y.S.; Kim, D.W.; Lee, Y.H.; Oh, J.H.; Yoon, S.; Choi, M.S.; Lee, S.K.; Kim, J.W.; Lee, K.; Song, C.W. Silver nanoparticles induce apoptosis and G2/M arrest via PKCzeta-dependent signaling in A549 lung cells. Arch. Toxicol. 2011, 85, 1529–1540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Y.; Sun, J. A study on the bio-safety for nano-silver as anti-bacterial materials. Zhongguo Yi Liao Qi Xie Za Zhi 2007, 31, 36–38. [Google Scholar] [PubMed]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Edwards-Jones, V. The benefits of silver in hygiene, personal care and healthcare. Lett. Appl. Microbiol. 2009, 49, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Bok, S.; Lubguban, A.A.; Gao, Y.; Bhattacharya, S.; Korampally, V.; Hossain, M.; Gillis, K.D.; Gangopadhyay, S. Electrochemical Properties of Carbon Nanoparticles Entrapped in Silica Matrix. J. Electrochem. Soc. 2008, 155, K91–K95. [Google Scholar] [CrossRef] [PubMed]
- Ramanakumar, A.V.; Parent, M.E.; Latreille, B.; Siemiatycki, J. Risk of lung cancer following exposure to carbon black, titanium dioxide and talc: results from two case-control studies in Montreal. Int. J. Cancer 2008, 122, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, C.W.; Kleber, M.; Field, J.A. Quantitative analysis of fullerene nanomaterials in environmental systems: A critical review. Environ. Sci. Technol. 2009, 43, 6463–6474. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Toffoli, G.; Rizzolio, F. Fluorescent Carbon Nanoparticles in Medicine for Cancer Therapy. ACS Med. Chem. Lett. 2013, 4, 1012–1013. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.E.; Curran, M.A.; Gonzalez, M.A. An examination of existing data for the industrial manufacture and use of nanocomponents and their role in the life impact of nanoproducts. Environ. Sci. Technol. 2009, 43, 1256–1263. [Google Scholar] [CrossRef] [PubMed]
- Hagendorfer, H.; Lorenz, C.; Kaegi, R.; Sinnet, B.; Gehrig, R.; von Goetz, N. Size-fractionated characterization and quantification of nanoparticle release rates from a consumer spray product containing engineered nanoparticles. J. Nanopart. Res. 2010, 12, 2481–2494. [Google Scholar] [CrossRef]
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Rytting, E.; Nguyen, J.; Wang, X.; Kissel, T. Biodegradable polymeric nanocarriers for pulmonary drug delivery. Expert Opin. Drug Deliv. 2008, 5, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Hinds, W.C. Aerosol Technology; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Geiser, M.; Kreyling, W.G. Deposition and biokinetics of inhaled nanoparticles. Part. Fibre Toxicol. 2010, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Jeannet, N.; Fierz, M.; Schneider, S.; Künzi, L.; Schmid, N.; Salathe, M.; Burtscher, H.; Geiser, M. Acute toxicity of silver and carbon nanoaerosols on normal and cystic fibrosis human bronchial epithelial cells. Nanotoxicology 2016, 10, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Jeannet, N.; Fierz, M.; Kalberer, M.; Burtscher, H.; Geiser, M. Nano Aerosol Chamber for In Vitro Toxicity Studies. Nanotoxicology 2015, 9, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Morrow, P.E.; Mercer, T.T. A point-to-plane electrostatic precipitator for particle size sampling. Am. Ind. Hyg. Assoc. J. 1964, 25, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.Y.H.; Whitby, K.T.; Yu, H.H.S. Electrostatic aerosol sampler for light and electron microscopy. Rev. Sci. Instrum. 1967, 38, 100–102. [Google Scholar] [CrossRef] [PubMed]
- Dixkens, J.; Fissan, H. Development of an electrostatic precipitator for off-line particle analysis. Aerosol Sci. Technol. 1999, 30, 438–453. [Google Scholar] [CrossRef]
- Fierz, M.; Kaegi, R.; Burtscher, H. Theoretical and experimental evaluation of a portable electrostatic TEM sampler. J. Aerosol Sci. Technol. 2007, 41, 520–528. [Google Scholar] [CrossRef]
- Mertes, P.; Praplan, A.P.; Künzi, L.; Dommen, J.; Baltensperger, U.; Geiser, M.; Weingartner, E.; Ricka, J.; Fierz, M.; Kalberer, M. A compact and portable deposition chamber to study nanoparticles in air-exposed tissue. J. Aerosol Med. Pulm. Drug Deliv. 2013, 26, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Kägi, R.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Müller, E.; Vonbank, R.; Boller, M.; Burkhardt, M. Release of silver nanoparticles from outdoor facades. Environ. Pollut. 2010, 158, 2900–2905. [Google Scholar] [CrossRef] [PubMed]
- Weibel, E.R. Design and morphometry of the pulmonary gas exchanger. In The Lung: Scientific Foundations, 2nd ed.; Crystal, R.G., West, J.B., Weibel, E.R., Barnes, P.J., Eds.; Lippincott-Raven: Philadelphia, PA, USA, 1997; pp. 1147–1157. [Google Scholar]
- Whitsett, J.A.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.A.; Holgate, S.T. The airway epithelium: Structural and functional properties in health and disease. Respirology 2003, 8, 432–446. [Google Scholar] [CrossRef] [PubMed]
- Künzi, L.; Krapf, M.; Daher, N.; Dommen, J.; Jeannet, N.; Schneider, S.; Platt, S.; Slowik, J.G.; Baumlin, N.; Salathe, M.; et al. Toxicity of aged gasoline exhaust particles to normal and diseased airway epithelia. Nat. Sci. Rep. 2015, 5, 11801. [Google Scholar] [CrossRef] [PubMed]
- Künzi, L.; Mertes, P.; Schneider, S.; Jeannet, N.; Menzi, C.; Dommen, J.; Baltenperger, U.; Prévôt, A.S.H.; Salathe, M.; Kalberer, M.; et al. Responses of lung cells to realistic exposure of primary and aged carbonaceous aerosols. Atmos. Environ. 2013, 68, 143–150. [Google Scholar] [CrossRef]
- Kreyling, W.G.; Biswas, P.; Messing, M.E.; Gibson, N.; Geiser, M.; Wenk, A.; Sahu, M.; Deppert, K.; Cydzik, I.; Wigge, C.; et al. Generation and characterization of stable, highly concentrated titanium dioxide nanoparticle aerosols for rodent inhalation studies. J. Nanopart. Res. 2010, 13, 511–524. [Google Scholar] [CrossRef]
- Möller, W.; Gibson, N.; Geiser, M.; Pokhrel, S.; Wenk, A.; Takenaka, S.; Bulgheroni, A.; Simonelli, F.; Kozempel, J.; Holzwarth, U.; et al. Gold nanoparticle aerosols for rodent inhalation and translocation studies. J. Nanopart. Res. 2013, 15, 1574. [Google Scholar] [CrossRef]
- Li, X.Y.; Brown, D.; Smith, S.; Macnee, W.; Donaldson, K. Short-term inflammatory responses following intratracheal instillation of fine and ultrafine carbon black in rats. Inhal. Toxicol. 1999, 11, 709–931. [Google Scholar] [PubMed]
- Bitterle, E.; Karg, E.; Schroeppel, A.; Kreyling, W.G.; Tippe, A.; Ferron, G.A.; Schmid, O.; Heyder, J.; Maier, K.L.; Hofer, T. Dose-controlled exposure of A549 epithelial cells at the air-liquid interface to airborne ultrafine carbonaceous particles. Chemosphere 2006, 65, 1784–1790. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.H.; Jung, J.H.; Kim, S.S.; Yoon, J.U.; Park, J.D.; Choi, B.S.; Chung, Y.H.; Kwon, I.H.; Jeong, J.; Han, B.S.; et al. Twenty-eight-day inhalation toxicity study of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2007, 19, 857–871. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.S.; Lee, B.S.; Ryu, H.Y.; Sung, J.H.; Chung, K.H.; Yu, I.J. Effects of repeated silver nanoparticles exposure on the histological structure and mucins of nasal respiratory mucosa in rats. Toxicol. Lett. 2008, 182, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Kang, M.G.; Cho, H.W.; Han, J.H.; Chung, Y.H.; Rim, K.T.; Yang, J.S.; Kim, H.; Lee, M.Y. Effect of Nano-sized Carbon Black Particles on Lung and Circulatory System by Inhalation Exposure in Rats. Saf. Health Work 2011, 2, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Barlow, P.G.; Clouter-Baker, A.; Donaldson, K.; Maccallum, J.; Stone, V. Carbon black nanoparticles induce type II epithelial cells to release chemotaxins for alveolar macrophages. Part. Fibre Toxicol. 2005, 2, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.S.; Sung, J.H.; Ji, J.H.; Song, K.S.; Lee, J.H.; Kang, C.S.; Yu, I.J. In vivo Genotoxicity of Silver Nanoparticles after 90-day Silver Nanoparticle Inhalation Exposure. Saf. Health Work 2011, 2, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Asharani, P.V.; Low Kah Mun, G.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Totlandsdal, A.I.; Refsnes, M.; Lag, M. Mechanisms involved in ultrafine carbon black-induced release of IL-6 from primary rat epithelial lung cells. Toxicol. In Vitro 2010, 24, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Park, H.K.; Lee, Y.M.; Kim, K.; Park, S.B. A practical procedure for producing silver nanocoated fabric and its antibacterial evaluation for biomedical applications. Chem. Commun. 2007, 28, 2959–2961. [Google Scholar] [CrossRef] [PubMed]
- Suliman, Y.A.; Ali, D.; Alarifi, S.; Harrath, A.H.; Mansour, L.; Alwasel, S.H. Evaluation of cytotoxic, oxidative stress, proinflammatory and genotoxic effect of silver nanoparticles in human lung epithelial cells. Environ. Toxicol. 2013, 30, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Seagrave, J.; Mcdonald, J.D.; Mauderly, J.L. In vitro versus in vivo exposure to combustion emissions. Exp. Toxicol. Pathol. 2005, 57, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Sayes, C.M.; Reed, K.L.; Warheit, D.B. Assessing toxicity of fine and nanoparticles: Comparing in vitro measurements to in vivo pulmonary toxicity profiles. Toxicol. Sci. 2007, 97, 163–180. [Google Scholar] [CrossRef] [PubMed]
- Baisch, B.L.; Corson, N.M.; Wade-Mercer, P.; Gelein, R.; Kennell, A.J.; Oberdorster, G.; Elder, A. Equivalent titanium dioxide nanoparticle deposition by intratracheal instillation and whole body inhalation: The effect of dose rate on acute respiratory tract inflammation. Part. Fibre Toxicol. 2014, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- FHNW. Gute Nanoteilchen—Schädliche Nanoteilchen? Hochschule für Technik FHNW, Forschungseinblicke FHNW: Windsch, Switzerland, 2015; pp. 36–41. [Google Scholar]



| Parameter | PSL, 200 nm | AgNP, 20 nm | Cell Type |
|---|---|---|---|
| Aerosol conditioning | |||
| Relative humidity * (%) | 85–95 | 85–95 | |
| Temperature * (°C) | 37 | 37 | |
| CO2 * (%) | 5 | 5 | |
| Aerosol flow per insert (mL/min) | 25 | 25 | |
| Particle distribution on Transwell® inserts | Even, singlets | Even, singlets | No cells |
| Deposition efficiency (%) | 15 | 40 | No cells |
| Particle-cell contact | |||
| CLSM | p-uptake | n.d. | Macs, BEAS-2B |
| ICP-MS | n.d. | 2/3 assoc. with cells | HBE, BEAS-2B |
| Cytotoxicity # (%) | |||
| Particles pipetted | <0.5 | n.d. | BEAS-2B |
| P-free air | <0.5 | n.d. | BEAS-2B |
| Exposed to aerosol | <0.5 | n.d. | BEAS-2B |
| Parameter | NACIVT [38] | Cultex® RFS/RFS compact [7,13] | VITROCELL® [19,20] |
|---|---|---|---|
| Cell exposure | |||
| Number of cell cultures | 24 | 3/6 in radial order around system inlet | 6/12/24/48 |
| Diameter of inserts (mm) | 6.5 | 6.5/12/24/35 (Petri dish) | 6.5/12/24/35 (Petri dish) |
| special adapters | special adapters | ||
| Cell cultures separated from each other | Yes | Yes | Yes |
| Duration of exposures (h) | °24 | °24 | °24 |
| Aerosol flow per insert (mL/min) | 25, adjustable | 5, 30, adjustable, separately for each chamber | 2, 5, 100, adjustable, separately for each chamber |
| Temperature and control | On-line, temperature sensors within the chamber, adjustable from computer via LabVIEW | 37 °C by temperature-controlled water flow (RFS) | 37 °C by temperature-controlled water flow. Automatic temperature control by sensors |
| On-line, temperature sensors within one chamber, adjustable (RFS Compact) | |||
| Particle deposition | |||
| Thermophoresis | No | No | Per extension kit |
| Electrostatic deposition | Switchable, bipolar, or unipolar charger | Can be added, unipolar charger | None |
| Electrical field | Up to 2 kV/insert, adjustable, both polarities DC or AC | 40–450 kV/m, adjustable | ±1.500 V, adjustable |
| Deposited dose | Aerosol electrometer, online | Gravimetric (precision balance) | Microbalance sensor, online photometer |
| Particle-free air control | Particle filter in-line before aerosol enters chamber | Parallel exposure of three inserts to test substance and three inserts to particle free air within one system | Independent clean air control modules or clean air positions in exposure module. |
| Concept of chamber | All-in-one | Modular | All-in-one or modular, automated exposure stations |
| Connectability to aerosol sources/generators | No restriction | No restriction | No restriction |
| Portable | Yes | Yes | Turnkey setups which can be moved to various locations |
| NP Type | Cell Model | LDH | Caspase-3 | IL-6 | IL-8 | MCP-1 | Epithelial Integrity |
|---|---|---|---|---|---|---|---|
| Ag | CF HBE | + | = | = | + | − | = |
| Normal HBE | (−) | = | = | (+) | = | = | |
| BEAS-2B | − | = | = | = | = | = | |
| C | CF HBE | + | = | = | = | = | = |
| Normal HBE | = | = | + | + | (+) | = | |
| BEAS-2B | + * | = | + | + | + | = |
| NP Type | Cell Model | LDH % | Caspase-3 rfu/μg | IL-6 pg/mL | IL-8 pg/mL | MCP-1 pg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ctrl | D-1 | D-2 | D-3 | Ctrl | D-1 | D-2 | D-3 | Ctrl | D-1 | D-2 | D-3 | Ctrl | D-1 | D-2 | D-3 | Ctrl | D-1 | D-2 | D-3 | ||
| Ag | CF HBE | 8.7 4.7 | 23.1 # 7.7 | 18.2 # 5.2 | 14.4 # 6.3 | 46.9 18.7 | 85.0 # 14.2 | 34.1 4.4 | 54.4 # 3.8 | 134.3 66.8 | 118.4 28.0 | 155.4 54.8 | 189.3 104.9 | 4923 1752 | 8550 # 3232 | 7640 1404 | 9967 # 5582 | 19.6 6.3 | 16.0 5.4 | 11.8 4.4 | 11.6 # 6.4 |
| Normal HBE | 17.6 4.1 | 14.1 3.7 | 14.9 3.9 | 11.7 # 3.6 | 20.7 8.2 | 39.6 21.7 | 18.7 1.6 | 13.1 4.1 | 29.2 23.2 | 36.1 15.7 | 29.5 12.4 | 50.9 23.0 | 4593 2263 | 6955 1773 | 5504 1665 | 7339 2397 | 4.5 3.0 | 2.4 0.8 | 2.9 1.6 | 2.0 1.9 | |
| BEAS-2B | 12.8 4.6 | 8.9 # 1.1 | 12.3 0.6 | 8.4# 1.2 | 162.3 117.7 | 84.8 6.1 | 133.5 18.0 | 199.5 0.4 | 63.4 41.3 | 64.1 18.8 | 76.3 4.4 | 57.5 13.5 | 169.1 43.3 | 242.7 # 88.8 | 146.0 27.5 | 162.6 21.5 | 1298 301 | 1101 338 | 1573 78 | 1665 # 224 | |
| C | CF HBE | 8.7 4.7 | 15.6 # 3.9 | 16.8 # 2.4 | 14.8 # 3.0 | 46.9 18.7 | 67.6 29.7 | 53.5 5.7 | 40.8 4.7 | 134.3 66.8 | 117.4 48.5 | 156.0 40.4 | 134.3 81.3 | 4923 1752 | 5651 1046 | 7394 2051 | 5017 1814 | 19.6 6.3 | 16.8 1.7 | 20.8 7.7 | 22.5 3.9 |
| Normal HBE | 17.6 4.1 | 15.8 5.6 | 19.9 5.3 | 16.5 5.2 | 20.7 8.2 | 29.6 6.2 | 34.8 26.5 | 22.0 14.8 | 29.2 23.2 | 99.4 147.4 | 79.4 56.2 | 96.4 54.3 | 4593 2263 | 8571 9099 | 9992 # 3725 | 7697 1884 | 4.5 3.0 | 5.1 3.5 | 5.4 0.7 | 13.1 # 5.8 | |
| BEAS-2B | 12.8 4.6 | 12.0 1.1 | 12.0 1.4 | 25.7 # 4.8 | 162.3 117.7 | 127.1 31.3 | 90.0 22.8 | 393.4 # 29.4 | 63.4 41.3 | 159.5 # 25.9 | 129.8 # 37.3 | 151.9 # 8.7 | 169.1 43.3 | 246.4 # 66.2 | 221.3 # 10.5 | 225.7 # 23.4 | 1298 301 | 1791 # 123 | 1799 # 171 | 2005 # 226 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Geiser, M.; Jeannet, N.; Fierz, M.; Burtscher, H. Evaluating Adverse Effects of Inhaled Nanoparticles by Realistic In Vitro Technology. Nanomaterials 2017, 7, 49. https://doi.org/10.3390/nano7020049
Geiser M, Jeannet N, Fierz M, Burtscher H. Evaluating Adverse Effects of Inhaled Nanoparticles by Realistic In Vitro Technology. Nanomaterials. 2017; 7(2):49. https://doi.org/10.3390/nano7020049
Chicago/Turabian StyleGeiser, Marianne, Natalie Jeannet, Martin Fierz, and Heinz Burtscher. 2017. "Evaluating Adverse Effects of Inhaled Nanoparticles by Realistic In Vitro Technology" Nanomaterials 7, no. 2: 49. https://doi.org/10.3390/nano7020049
APA StyleGeiser, M., Jeannet, N., Fierz, M., & Burtscher, H. (2017). Evaluating Adverse Effects of Inhaled Nanoparticles by Realistic In Vitro Technology. Nanomaterials, 7(2), 49. https://doi.org/10.3390/nano7020049






