Significant Enhancement of Hydrogen-Sensing Properties of ZnO Nanofibers through NiO Loading
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Solution for Electrospinning
2.2. Electrospinning
2.3. Material Characterization
2.4. Gas Sensing Measurements
3. Results and Discussion
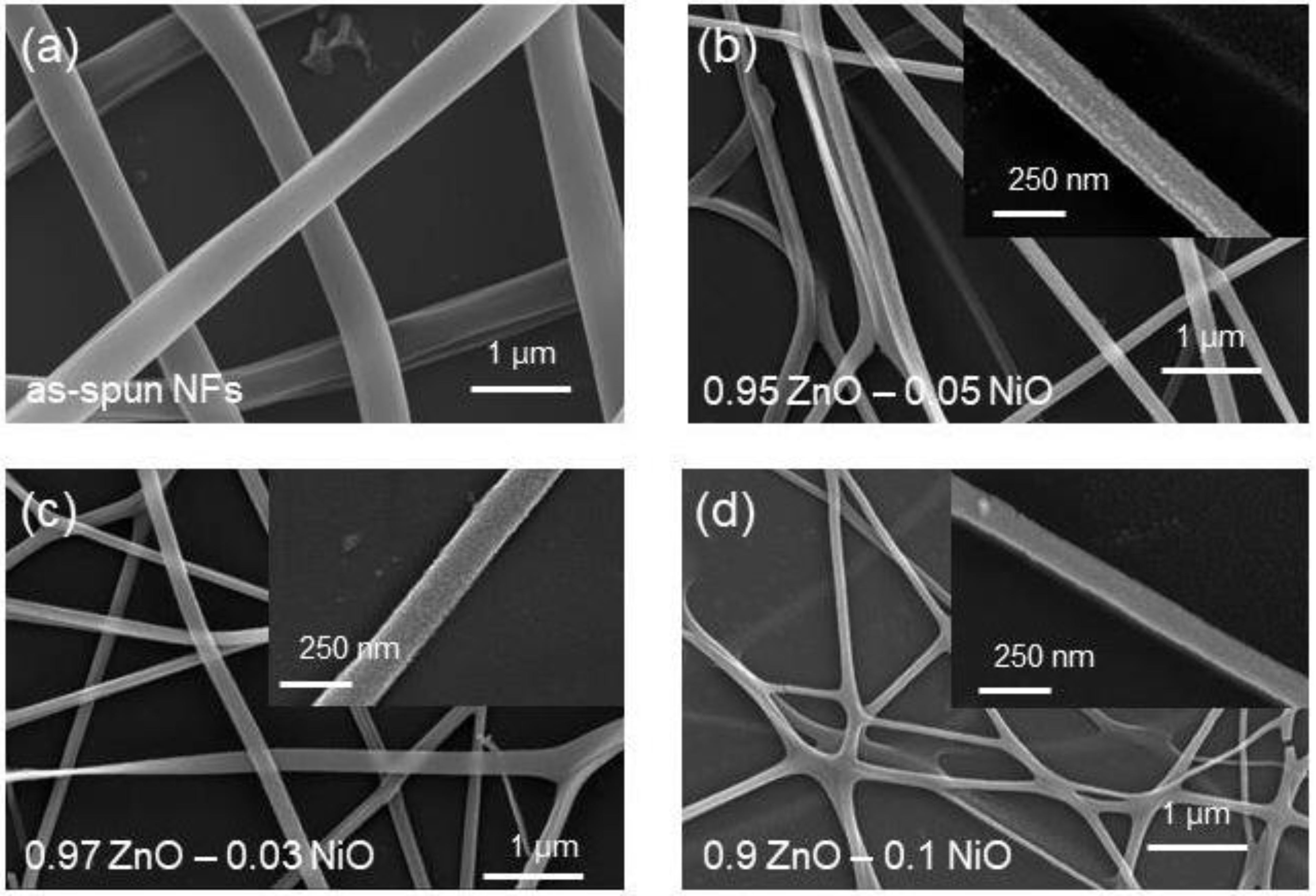
3.1. Morphological and Structural Analyses
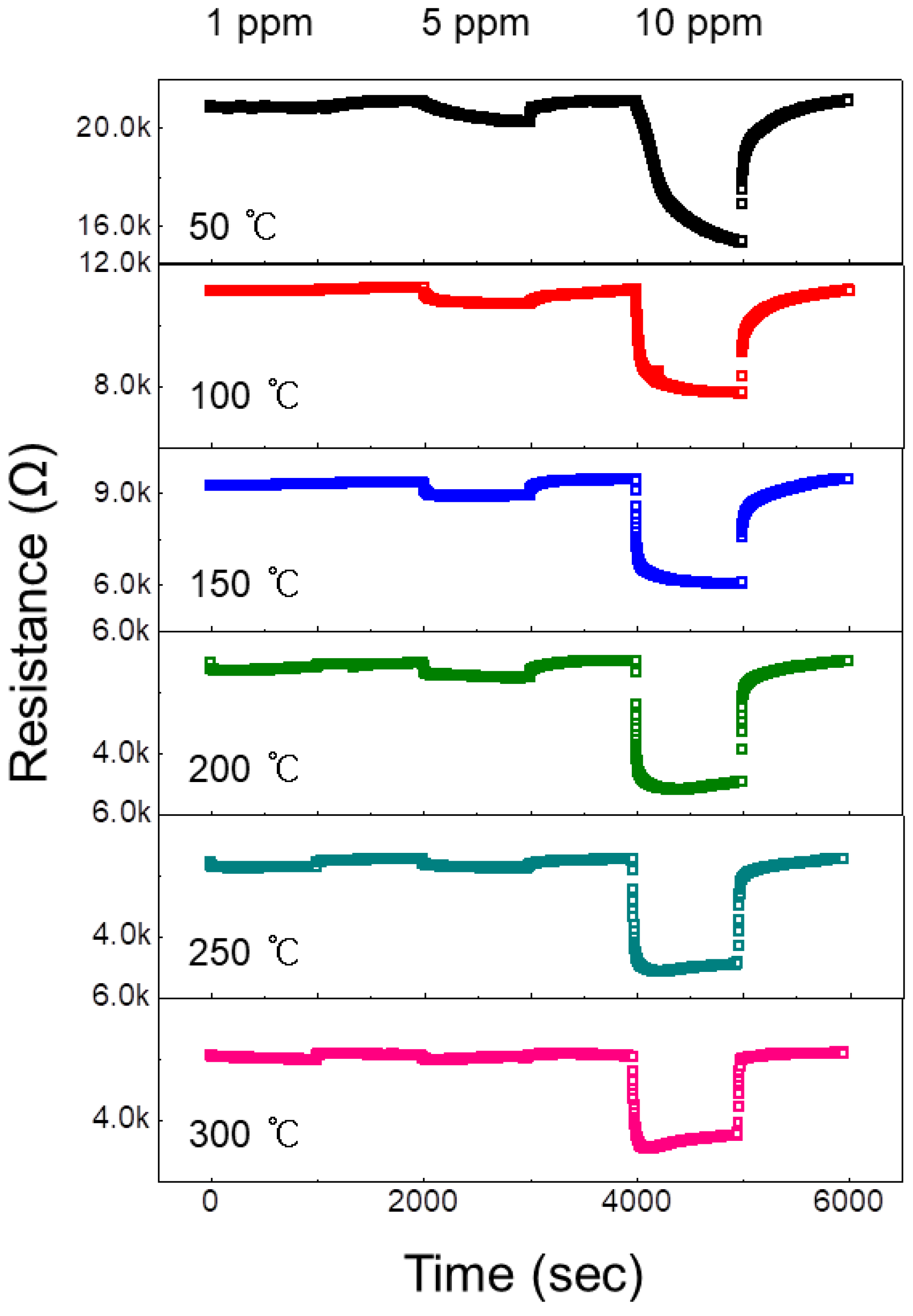
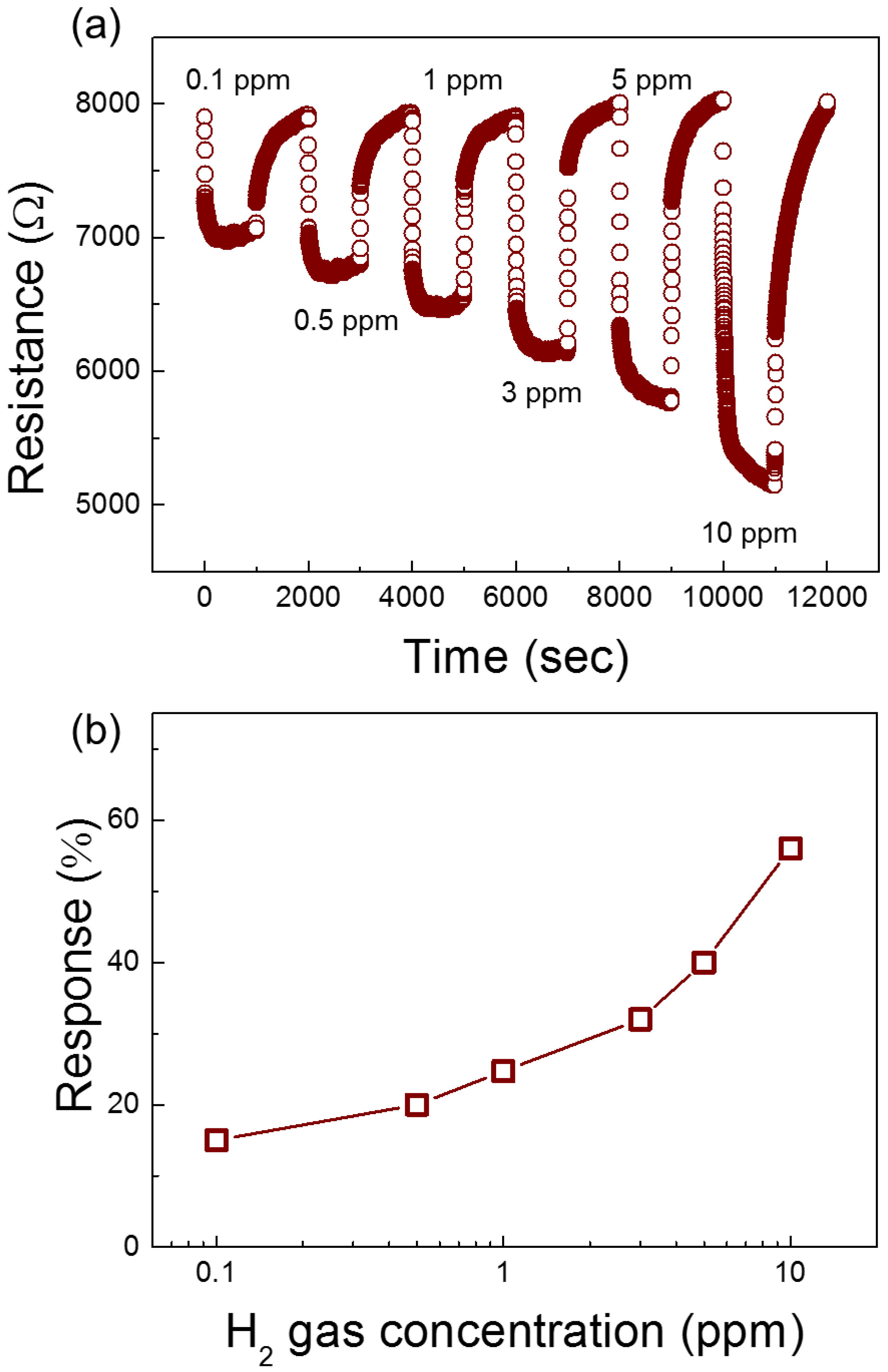
3.2. Gas Sensing Properties
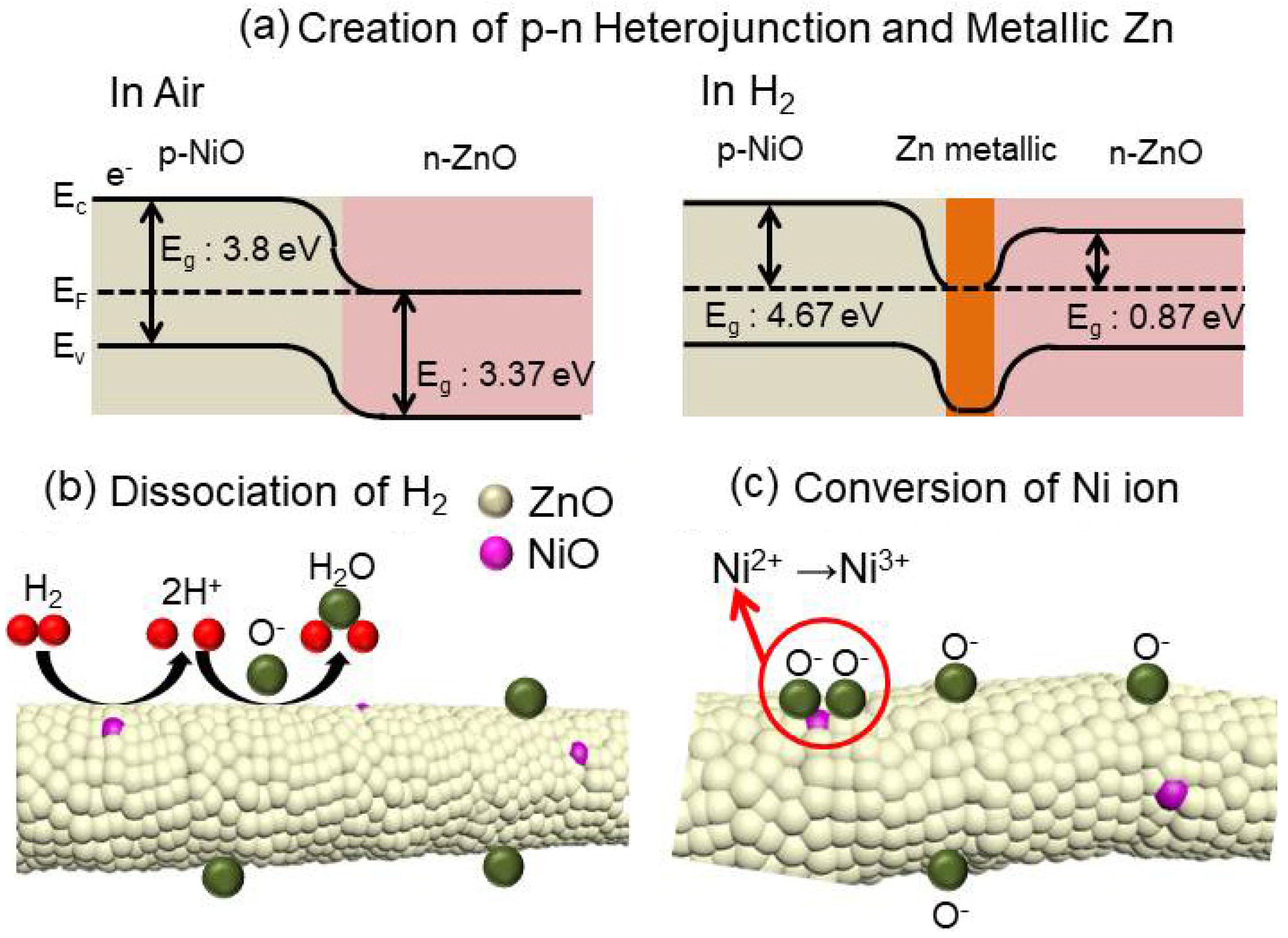
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Neri, G. Metal-core@metal oxide-shell nanomaterials for gas-sensing applications: A review. J. Nanopart. Res. 2015, 17, 371. [Google Scholar] [CrossRef]
- Choi, K.J.; Jang, H.W. One-dimensional oxide nanostructures as gas-sensing materials: Review and issues. Sensors 2010, 10, 4083–4099. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Katoch, A.; Choi, S.-W.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Extraordinary improvement of gas-sensing performances in SnO2 nanofibers due to creation of local p-n heterojunctions by loading reduced graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2015, 7, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Kang, S.Y.; Mirzaei, A.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Enhancement of gas sensing properties by the functionalization of ZnO-branched SnO2 nanowires with Cr2O3 nanoparticles. Sens. Actuators B Chem. 2017, 249, 656–666. [Google Scholar] [CrossRef]
- Spencer, M.J. Gas sensing applications of 1D-nanostructured zinc oxide: Insights from density functional theory calculations. Prog. Mater. Sci. 2012, 57, 437–486. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas-sensor applications: A review. Nano-Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Eranna, G.; Joshi, B.C.; Runthala, D.P.; Gupta, R.P. Oxide materials for development of integrated gas sensors—A comprehensive review. Crit. Rev. Solid State Mater. Sci. 2004, 29, 111–188. [Google Scholar] [CrossRef]
- Galstyan, V.; Comini, E.; Baratto, C.; Faglia, G.; Sberveglieri, G. Nanostructured ZnO chemical gas sensors. Ceram. Inter. 2015, 41, 14239–14244. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Maccato, C.; Sada, C.; Sberveglieri, G.; Tondello, E. Urchin-like ZnO nanorod arrays for gas sensing applications. CrystEngComm 2010, 12, 3419–3421. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Faglia, G.; Ferroni, M.; Sberveglieri, G. Single crystal ZnO nanowires as optical and conductometric chemical sensor. J. Physics D: Appl. Phys. 2007, 40, 7255. [Google Scholar] [CrossRef]
- Barreca, D.; Bekermann, D.; Comini, E.; Devi, A.; Fischer, R.A.; Gasparotto, A.; Maccato, C.; Sberveglieri, G.; Tondello, E. 1D ZnO nano-assemblies by Plasma-CVD as chemical sensors for flammable and toxic gases. Sens. Actuators B Chem. 2010, 149, 1–7. [Google Scholar] [CrossRef]
- Singh, N.; Ponzoni, A.; Gupta, R.K.; Lee, P.S.; Comini, E. Synthesis of In2O3-ZnO core–shell nanowires and their application in gas sensing. Sens. Actuators B Chem. 2011, 160, 1346–1351. [Google Scholar] [CrossRef]
- Waclawik, E.R.; Chang, J.; Ponzoni, A.; Concina, I.; Zappa, D.; Comini, E.; Motta, N.; Faglia, G.; Sberveglieri, G. Functionalised zinc oxide nanowire gas sensors: Enhanced NO2 gas sensor response by chemical modification of nanowire surfaces. Beilstein J. Nanotech. 2012, 3, 368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comini, E.; Faglia, G.; Ferroni, M.; Sberveglieri, G. Gas sensing properties of zinc oxide nanostructures prepared by thermal evaporation. Appl. Phys. A 2007, 88, 45–48. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Zheng, Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Synthesis and selective sensing properties of rGO/metal-coloaded SnO2 nanofibers. J. Elec. Mater. 2017, 46, 3531–3541. [Google Scholar] [CrossRef]
- Abideen, Z.U.; Kim, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Sening behavior to ppm-level gases and synergistic sensing mechanism in metal-functionalized rGO-loaded ZnO nanofibers. Sens. Actuators B Chem. 2018, 252, 1884–1896. [Google Scholar] [CrossRef]
- Katoch, A.; Kim, J.-H.; Kwon, Y.J.; Kim, H.W.; Kim, S.S. Excellent gas detection of ZnO nanofibers by loading with reduced graphene oxide nanosheets. Sens. Actuators B Chem. 2015, 221, 1499–1507. [Google Scholar]
- Katoch, A.; Kim, J.-H.; Kwon, Y.J.; Kim, H.W.; Kim, S.S. Bifunctional sensing mechanism of SnO2-ZnO composite nanofibers for drastically enhancing the sensing behavior in H2 gas. ACS Appl. Mater. Interfaces 2015, 7, 11351–11358. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B Chem. 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Katoch, A.; Choi, S.-W.; Kim, J.-H.; Lee, J.H.; Lee, J.-S.; Kim, S.S. Importance of the nanograin size on the H2S-sensing properties of ZnO-CuO composite nanofibers. Sens. Actuators B Chem. 2015, 214, 111–116. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Deng, X.; Zhang, L.; Guo, J.; Chen, Q.; Ma, J. ZnO enhanced NiO-based gas sensors towards ethanol. Mater. Res. Bull. 2017, 90, 170–174. [Google Scholar] [CrossRef]
- Gao, H.; Wei, D.; Lin, P.; Liu, C.; Sun, P.; Shimanoe, K.; Yamazoe, N.; Lu, G. The design of excellent xylene gas sensor using Sn-doped nio hierarchical nanostructure. Sens. Actuators B Chem. 2017, 253, 1152–1162. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, L.; Wang, B.; Sun, P.; Wang, Q.; Gao, Y.; Liang, X.; Zhang, T.; Lu, G. Acetone gas sensor based on NiO/ZnO hollow spheres: Fast response and recovery, and low (ppb) detection limit. J. Colloid Interf. Sci. 2017, 495, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Zhang, J.; Li, J.; Zhang, N.; Hong, R.; Deng, X.; Tang, P.; Li, D. Hydrogen sensing properties of Pt-Au bimetallic nanoparticles loaded on ZnO nanorods. Sens. Actuators B Chem. 2017, 241, 895–903. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. SnO2 (n)-NiO (p) composite nanowebs: Gas sensing properties and sensing mechanisms. Sens. Actuators B Chem. 2018, 258, 204–214. [Google Scholar] [CrossRef]
- Choi, S.W.; Park, J.Y.; Kim, S.S. Synthesis of SnO2-ZnO core-shell nanofibers via a novel two-step process and their gas sensing properties. Nanotechnology 2009, 20, 465603. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Zang, Y.; Mirzaei, A.; Kim, S.S. Excellent carbon monoxide sensing performance of Au-decorated SnO2 nanofibers. Korean J. Mater. Res. 2016, 26, 741–750. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Liu, T.; Sun, P.; Gao, Y.; Liu, F.; Lu, G. Facile synthesis and gas sensing properties of the flower-like NiO-decorated ZnO microstructures. Sens. Actuators B Chem. 2016, 235, 294–301. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Sun, J.; Zhang, H.; Wang, W.; Zheng, W.; Wang, C. Improved hydrogen monitoring properties based on p-NiO/n-SnO2 heterojunction composite nanofibers. J. Phys. Chem. C 2010, 114, 6100–6105. [Google Scholar] [CrossRef]
- Bierer, B.; Kneer, J.; Wöllenstein, J.; Palzer, S. MEMS based metal oxide sensor for simultaneous measurement of gas induced changes of the heating power and the sensing resistance. Microsys. Tech. 2016, 22, 1855–1863. [Google Scholar] [CrossRef]
- Kim, H.W.; Kwon, Y.J.; Mirzaei, A.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S. Synthesis of zinc oxide semiconductors-graphene nanocomposites by microwave irradiation for application to gas sensors. Sens. Actuators B Chem. 2017, 249, 590–601. [Google Scholar] [CrossRef]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. A novel gas sensor based on Ag/Fe2O3 core-shell nanocomposites. Ceram. Inter. 2016, 42, 18974–18982. [Google Scholar] [CrossRef]
- Kwon, Y.J.; Mirzaei, A.; Kang, S.Y.; Choi, M.S.; Bang, J.H.; Kim, S.S.; Kim, H.W. Synthesis, characterization and gas sensing properties of ZnO-decorated MWCNTs. Appl. Surf. Sci. 2017, 413, 242–252. [Google Scholar] [CrossRef]
- Yamazoe, N.; Sakai, G.; Shimanoe, K. Oxide semiconductor gas sensors. Catal. Sur. Asia 2003, 7, 63–75. [Google Scholar] [CrossRef]
- Barsan, N.; Weimar, U. Conduction model of metal oxide gas sensors. J. Electroceram. 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Mirzaei, A.; Leonardi, S.G.; Neri, G. Detection of hazardous volatile organic compounds (VOCs) by metal oxide nanostructures-based gas sensors: A review. Ceram. Inter. 2016, 42, 15119–15141. [Google Scholar] [CrossRef]
- Falsafi, F.; Hashemi, B.; Mirzaei, A.; Fazio, E.; Neri, F.; Donato, N.; Leonardi, S.G.; Neri, G. Sm-doped cobalt ferrite nanoparticles: A novel sensing material for conductometric hydrogen leak sensor. Ceram. Inter. 2017, 43, 1029–1037. [Google Scholar] [CrossRef]
- Takeguchi, T.; Furukawa, S.-N.; Inoue, M. Hydrogen spillover from nio to the large surface area CeO2-ZrO2 solid solutions and activity of the NiO/CeO2-ZrO2 catalysts for partial oxidation of methane. J. Catal. 2001, 202, 14–24. [Google Scholar] [CrossRef]
- Sanger, A.; Kumar, A.; Kumar, A.; Chandra, R. Highly sensitive and selective hydrogen gas sensor using sputtered grown pd decorated MnO2 nanowalls. Sens. Actuators B Chem. 2016, 234, 8–14. [Google Scholar] [CrossRef]
- Kneer, J.; Knobelspies, S.; Bierer, B.; Wöllenstein, J.; Palzer, S. New method to selectively determine hydrogen sulfide concentrations using CuO layers. Sens. Actuators B Chem. 2016, 222, 625–631. [Google Scholar] [CrossRef]
- Kneer, J.; Wollenstein, J.; Palzer, S. Specific, trace gas induced phase transition in copper(II)oxide for highly selective gas sensing. Appl. Phys. Lett. 2014, 105, 073509. [Google Scholar] [CrossRef]








| Reference (Synthetic Air) | Target Gas | Final Concentration | |||
|---|---|---|---|---|---|
| O2 | N2 | O2 | N2 | H2 (Concentration 100 ppm in N2) | |
| 20 mL/min | 80 mL/min | 20 mL/min | 79 mL/min | 1 mL/min | 1 ppm |
| 20 mL/min | 80 mL/min | 20 mL/min | 75 mL/min | 5mL/min | 5 ppm |
| 20 mL/min | 80 mL/min | 20 mL/min | 70 mL/min | 10 mL/min | 10 ppm |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Kim, J.-Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Significant Enhancement of Hydrogen-Sensing Properties of ZnO Nanofibers through NiO Loading. Nanomaterials 2018, 8, 902. https://doi.org/10.3390/nano8110902
Lee J-H, Kim J-Y, Mirzaei A, Kim HW, Kim SS. Significant Enhancement of Hydrogen-Sensing Properties of ZnO Nanofibers through NiO Loading. Nanomaterials. 2018; 8(11):902. https://doi.org/10.3390/nano8110902
Chicago/Turabian StyleLee, Jae-Hyoung, Jin-Young Kim, Ali Mirzaei, Hyoun Woo Kim, and Sang Sub Kim. 2018. "Significant Enhancement of Hydrogen-Sensing Properties of ZnO Nanofibers through NiO Loading" Nanomaterials 8, no. 11: 902. https://doi.org/10.3390/nano8110902






