Colloidal Lignin Particles as Adhesives for Soft Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Proteins
2.3. Enzymes
2.4. Nanocellulose
2.5. Preparation of CLPs
2.6. Adsorption of Proteins on Lignin
2.7. Coating CLPs with Proteins
2.8. Stabilization of Protein Coated CLPs
2.9. Physicochemical Characterization of CLPs
2.10. SEC
2.11. FTIR
2.12. AFM
2.13. TEM
2.14. Sample Preparation
3. Results and Discussion
3.1. Tailoring CLP Surfaces with Proteins
3.1.1. β-Casein
3.1.2. Poly(l-glutamic acid)
3.2. Stability of β-Casein Coated CLP
3.2.1. Effect of Enzymatic Cross-Linking
3.2.2. Effect of pH
3.3. Adhesive Interactions
3.4. Applications
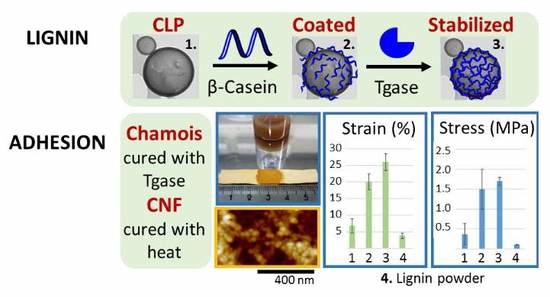
3.4.1. Agglutination of Chamois Specimens with CLPs
3.4.2. Grafting CLPs on CNF Surfaces
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fu, J.; Su, J.; Wang, P.; Yu, Y.; Wang, Q.; Cavaco-Paulo, A. Enzymatic processing of protein-based fibers. Appl. Microbiol. Biotechnol. 2015, 99, 10387–10397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, H.-T.; Bechtold, T. Surface modification of textile material through deposition of regenerated silk fibroin. J. Appl. Polym. Sci. 2017, 134, 45098–45109. [Google Scholar] [CrossRef]
- Hemmilä, V.; Adamopoulos, S.; Karlsson, O.; Kumar, A. Development of sustainable bio-adhesives for engineered wood panels—A Review. RSC Adv. 2017, 7, 38604–38630. [Google Scholar] [CrossRef]
- Esposito, D.; Antonietti, M. Redefining biorefinery: The search for unconventional building blocks for materials. Chem. Soc. Rev. 2015, 44, 5821–5835. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A new family of nature-based materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, S.J.; Dufresne, A.; Aranguren, M.; Marcovich, N.E.; Capadona, J.R.; Rowan, S.J.; Weder, C.; Thielemans, W.; Roman, M.; Renneckar, S.; et al. Review: Current international research into cellulose nanofibers and nanocomposites. J. Mater. Sci. 2010, 45, 1–33. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose nanocrystals vs. Cellulose nanofibrils: A comparative study on their microstructures and effects as polymer reinforcing agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef]
- Abe, K.; Iwamoto, S.; Yano, H. Obtaining cellulose nanofibers with a uniform width of 15 nm from wood. Biomacromolecules 2007, 8, 3276–3278. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Malinen, M.M.; Lauren, P.; Lou, Y.-R.; Kuisma, S.W.; Kanninen, L.; Lille, M.; Corlu, A.; Guen-Guillouzo, C.; Ikkala, O.; et al. Nanofibrillar cellulose hydrogel promotes three-dimensional liver cell culture. J. Control. Release 2012, 164, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Lou, Y.-R.; Kanninen, L.; Kuisma, T.; Niklander, J.; Noon, L.A.; Burks, D.; Urtti, A.; Yliperttula, M. The use of nanofibrillar cellulose hydrogel as a flexible three-dimensional model to culture human pluripotent stem cells. Stem Cells Dev. 2014, 23, 380–392. [Google Scholar] [CrossRef]
- Petersen, N.; Gatenholm, P. Bacterial cellulose-based materials and medical devices: Current state and perspectives. Appl. Microbiol. Biotechnol. 2011, 91, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Forsman, N.; Lozhechnikova, A.; Khakalo, A.; Johansson, L.-S.; Vartiainen, J.; Österberg, M. Hydrophobic simple and sustainable coating of CNF films and cellulosic textiles based on layer-by-layer deposition of poly-L-lysine and natural wax particles. Carbohydr. Polym. 2017, 173, 392–402. [Google Scholar] [CrossRef]
- Oliveira, C.; Santos, C.; Bezerra, F.; Bezerra, M.; Rodrigues, L. Utilization of Cyanoacrylates Adhesives in Skin suture. Rev. Bras. Cir. Plást. 2010, 25, 573–576. [Google Scholar] [CrossRef]
- Peng, H.; Shek, P. Novel wound sealants: Biomaterials and applications. Expert Rev. Med. Devices 2010, 7, 639–659. [Google Scholar] [CrossRef] [PubMed]
- Meddahi-Pelle, A.; Legrand, A.; Marcellan, A.; Louedec, L.; Letourneur, D.; Leibler, L. Organ Repair, Hemostasis, and In Vivo Bonding of Medical Devices by Aqueous Solutions of Nanoparticles. Angew. Chem. Int. Ed. 2014, 53, 6369–6373. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, K.; Nio, N.; Kikuch, Y. Properties and applications of microbial transglutaminase. Appl. Microbiol. Biot. 2004, 64, 447–454. [Google Scholar] [CrossRef]
- Cortez, J.; Anghieri, A.; Bonner, P.; Griffin, M.; Freddi, G. Transglutaminase mediated grafting of silk proteins onto wool fabrics leading to improved physical and mechanical properties. Enzyme Microb. Technol. 2007, 40, 1698–1704. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Dionisopoulou, N.K. Irradiation of Food Commodities. Techniques, Applications, Detection, Legislation, Safety and Consumer Opinion, 1st ed.; Arvanitoyannis, I.S., Ed.; Academic Press: London, UK, 2010; pp. 609–634. ISBN 13 978-0128101919. [Google Scholar]
- Bonnaillie, L.; Aburto, L.; Tunick, M.; Mulherin, J.; Du, M.; Kwoczak, R.; Akkurt, S.; Tomasula, P. Advances in food packaging films from milk proteins. In Proceedings of the 252nd National Meeting & Exposition of ACS, AGFD: Division of Agricultural and Food Chemistry, Philadelphia, PA, USA, 21–25 August 2016; p. 327816. [Google Scholar]
- Bonnaillie, M.L.; Tomasula, P.M. Application of Humidity-Controlled Dynamic Mechanical Analysis (DMA-RH) to Moisture-Sensitive Edible Casein Films for Use in Food Packaging. Polymers 2015, 7, 91–114. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Ma, J.; Xu, Q.; Zhou, J.; Simion, D.; Carmen, G.; Wang, J.; Li, Y. Hollow Casein-Based Polymeric Nanospheres for Opaque Coatings. ACS Appl. Mater. Interfaces 2016, 8, 11739–11748. [Google Scholar] [CrossRef]
- Stewart, R.J.; Wang, C.S.; Shao, H. Complex coacervates as a foundation for synthetic underwater adhesives. Adv. Colloid Interface Sci. 2011, 167, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Rose, S.; Prevoteau, A.; Elziére, P.; Hourdet, D.; Marcellan, A.; Leibler, L. Nanoparticle solutions as adhesives for gels and biological tissues. Nature 2014, 505, 382–385. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2016, 18, 1416–1422. [Google Scholar] [CrossRef] [Green Version]
- Frangville, C.; Rutkevičius, M.; Richter, A.P.; Velev, O.D.; Stoyanov, S.D.; Paunov, V.N. Fabrication of environmentally biodegradable lignin nanoparticles. ChemPhysChem 2012, 13, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Deng, Y.; Qiu, X.; Li, H.; Yang, D. Formation of uniform colloidal spheres from lignin, a renewable resource recovered from pulping spent liquor. Green Chem. 2014, 16, 2156–2163. [Google Scholar] [CrossRef]
- Qian, Y.; Zhang, Q.; Qiu, X.; Zhu, S. CO2-responsive diethylaminoethyl-modified lignin nanoparticles and their application as surfactants for CO2/N2-switchable Pickering emulsions. Green Chem. 2014, 16, 4963–4968. [Google Scholar] [CrossRef]
- Yiamsawas, D.; Baier, G.; Thines, E.; Landfester, K.; Wurm, F.R. Biodegradable lignin nanocontainers. RSC Adv. 2014, 4, 11661–11663. [Google Scholar] [CrossRef] [Green Version]
- Gilca, I.A.; Popa, V.I.; Crestini, C. Obtaining lignin nanoparticles by sonication. Ultrason. Sonochem. 2015, 23, 369–375. [Google Scholar] [CrossRef] [Green Version]
- Tortora, M.; Cavalieri, F.; Mosesso, P.; Ciaffardini, F.; Melone, F.; Crestini, C. Ultrasound driven assembly of lignin into microcapsules for storage and delivery of hydrophobic molecules. Biomacromolecules 2014, 15, 1634–1643. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.S.; Sharma, S.; Pu, Y.; Sun, Q.; Pan, S.; Zhu, J.Y.; Deng, Y.; Ragauskas, A.J. High shear homogenization of lignin to nanolignin and thermal stability of nanolignin-polyvinyl alcohol blends. ChemSusChem 2014, 7, 3513–3520. [Google Scholar] [CrossRef]
- Yearla, S.R.; Padmasree, K. Preparation and characterisation of lignin nanoparticles: Evaluation of their potential as antioxidants and UV protectants. J. Exp. Nanosci. 2016, 11, 289–302. [Google Scholar] [CrossRef]
- Richter, A.P.; Bharti, B.; Armstrong, H.B.; Brown, J.S.; Plemmons, D.A.; Paunov, V.N.; Stoyanov, S.D.; Velev, O.D. Synthesis and characterization of biodegradable lignin nanoparticles with tunable surface properties. Langmuir 2016, 32, 6468–6477. [Google Scholar] [CrossRef] [PubMed]
- Ago, M.; Huan, S.; Borghei, M.; Raula, J.; Kauppinen, E.; Rojas, O. High-throughput synthesis of lignin particles (∼30 nm to ∼2 μm) via aerosol flow reactor: Size fractionation and utilization in Pickering emulsions. ACS Appl. Mater. Interfaces 2016, 8, 23302–23310. [Google Scholar] [CrossRef]
- Beisl, S.; Miltner, A.; Friedl, A. Lignin from Micro- to Nanosize: Production Methods. Int. J. Mol. Sci. 2017, 18, 1244. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Spiess, A.C. Laccases for biorefinery applications: A critical review on challenges and perspectives. Bioproc. Biosyst. Eng. 2015, 38, 2285–2313. [Google Scholar] [CrossRef] [PubMed]
- Munk, L.; Sitarz, A.K.; Kalyani, D.C.; Mikkelsen, J.D.; Meyer, A.S. Can laccases catalyze bond cleavage in lignin? Biotechnol. Adv. 2015, 33, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Mattinen, M.-L.; Valle-Delgado, J.J.; Leskinen, T.; Anttila, T.; Riviere, G.; Sipponen, M.; Paananen, A.; Lintinen, K.; Kostiainen, K.; Österberg, M. Enzymatically and chemically oxidized lignin nanoparticles for biomaterial applications. Enzyme Microb. Technol. 2018, 111, 48–56. [Google Scholar] [CrossRef]
- Mohammadinejad, R.; Karimi, S.; Iravani, S.; Varma, R.S. Plant-derived nanostructures: Types and applications. Green Chem. 2016, 18, 20–52. [Google Scholar] [CrossRef]
- Pan, K.; Zhong, Q. Organic Nanoparticles in Foods: Fabrication, Characterization and Utilization. Annu. Rev. Food Sci. Technol. 2016, 7, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Leskinen, T.; Witos, J.; Valle-Delgado, J.J.; Lintinen, K.; Kostiainen, M.A.; Wiedmer, S.K.; Österberg, M.; Mattinen, M.-L. Adsorption of Proteins on Colloidal Lignin Particles for Advanced Biomaterials. Biomacromolecules 2017, 18, 2767–2776. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.P.; Brown, J.S.; Bharti, B.; Wang, A.; Gangwal, S.; Houck, K.; Cohen, E.; Hubal, A.; Paunov, V.N.; Stoyanov, S.D.; et al. An environmentally benign antimicrobial nanoparticle based on a silver-infused lignin core. Nat. Nanotechnol. 2015, 10, 817–823. [Google Scholar] [CrossRef]
- Goffin, A.J.; Rajadas, J.; Fuller, G.G. Interfacial Flow Processing of Collagen. Langmuir 2010, 26, 3514–3521. [Google Scholar] [CrossRef]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef]
- Niku-Paavola, M.; Karhunen, E.; Salola, P.; Raunio, V. Ligninolytic enzymes of the white-rot-fungus Phlebia radiate. Biochem. J. 1988, 254, 877–884. [Google Scholar] [CrossRef]
- Rittstieg, K.; Suurnäkki, A.; Suortti, T.; Kruus, K.; Guebitz, G.; Buchert, J. Investigations on the laccase-catalyzed polymerization of lignin model compounds using size-exclusion HPLC. Enzyme Microb. Technol. 2002, 31, 403–410. [Google Scholar] [CrossRef]
- Lantto, R.; Puolanne, E.; Kalkkinen, N.; Buchert, J.; Autio, K. Enzyme-aided modification of chicken-breast myofibril proteins: Effect of laccase and transglutaminase on gelation and thermal stability. J. Agric. Food Chem. 2005, 53, 9231–9237. [Google Scholar] [CrossRef] [PubMed]
- Valle-Delgado, J.J.; Johansson, L.-S.; Österberg, M. Bioinspired lubricating films of cellulose nanofibrils and hyaluronic acid. Colloids Surf. B Biointerfaces 2016, 138, 86–93. [Google Scholar] [CrossRef] [Green Version]
- Salas, C.; Rojas, O.J.; Lucia, L.A.; Hubbe, M.A.; Genzer, J. On the surface interactions of proteins with lignin. ACS Appl. Mater. Interfaces 2013, 5, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Johannsmann, D.; Mathauer, K.; Wegner, G.; Knoll, W. Viscoelastic properties of thin films probed with a quartz-crystal resonator. Phys. Rev. B 1992, 46, 7808–7815. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Pihlajaniemi, V.; Sipponen, S.; Pastinen, O.; Laakso, S. Autohydrolysis and aqueous ammonia extraction of wheat straw: Effect of treatment severity on yield and structure of hemicellulose and lignin. RSC Adv. 2014, 4, 23177–23184. [Google Scholar] [CrossRef]
- Sader, J.E.; Chon, J.W.; Mulvaney, P. Calibration of rectangular atomic force microscope cantilevers. Rev. Sci. Instrum. 1999, 70, 3967–3969. [Google Scholar] [CrossRef] [Green Version]
- Nugroho, R.W.N.; Harjumäki, R.; Zhang, X.; Lou, Y.-R.; Yliperttula, M.; Valle-Delgado, J.J.; Österberg, M. Quantifying the interactions between biomimetic biomaterials—Collagen I, collagen IV, laminin 521 and cellulose nanofibrils—By colloidal probe microscopy. Colloids Surf. B Biointerfaces 2019, 173, 571–580. [Google Scholar] [CrossRef]
- Butt, H.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef] [Green Version]
- Verwey, E. Theory of the stability of lyophobic colloids. J. Phys. Chem. 1947, 51, 631–636. [Google Scholar] [CrossRef]
- Assemi, S.; Nalaskowski, J.; Johnson, W.P. Direct force measurements between carboxylate-modified latex microspheres and glass using atomic force microscopy. Colloid Surf. A Physicochem. Eng. Asp. 2006, 286, 70–77. [Google Scholar] [CrossRef]
- Bowen, W.R.; Hilal, N.; Lovitt, R.W.; Wright, C.J. Direct measurement of interactions between adsorbed protein layers using an atomic force microscope. J. Colloid Interface Sci. 1998, 197, 348–352. [Google Scholar] [CrossRef]
- Schmidt, S.; Madaboosi, N.; Uhlig, K.; Köhler, D.; Skirtach, A.; Duschl, C.; Möhwald, H.; Volodkin, D.V. Control of Cell Adhesion by Mechanical Reinforcement of Soft Polyelectrolyte Films with Nanoparticles. Langmuir 2012, 28, 7249–7257. [Google Scholar] [CrossRef]
- Bye, C.N. Casein and mixed protein adhesives. In Handbook of Adhesives; Skeist, I., Ed.; Van Nostrand Reinold: New York, NY, USA, 1990; pp. 135–152. ISBN 978-1-4613-0671-9. [Google Scholar]
- Mazia, D. Adhesion of cells to surfaces coated with polylysine. Applications to electron microscopy. J. Cell Biol. 1975, 66, 198–200. [Google Scholar] [Green Version]
- Gu, Z.; Yang, Z.; Wang, L.; Zhou, H.; Jimenez-Cruz, C.A.; Zhou, R. The role of basic residues in the adsorption of blood proteins onto the graphene surface. Sci. Rep. 2015, 5, 10873–10884. [Google Scholar] [CrossRef]
- Van de Pas, D.; Hickson, A.; Donaldson, L.; Lloyd-Jones, G.; Tamminen, T.; Fernyhough, A.; Mattinen, M.-L. Characterization of fractionated lignins polymerized by fungal laccases. BioResources 2011, 6, 1105–1121. [Google Scholar]
- Figueiredo, P.; Lintinen, K.; Kiriazis, A.; Hynninen, V.; Liu, V.; Ramos, T.B.; Rahikkala, A.; Correia, A.; Kohout, T.; Sarmento, B.; et al. In vitro evaluation of biodegradable lignin-based nanoparticles for drug delivery and enhanced antiproliferation effect in cancer cells. Biomaterials 2017, 121, 97–108. [Google Scholar] [CrossRef]
- Monogioudi, E. Enzymatic Cross-Linking of β-Casein and Its Impact on Digestibility and Allergenicity; University of Helsinki: Helsinki, Finland, 18 January 2011; ISBN 978-951-38-7421-6. [Google Scholar]
- Berglin, M.; Delage, L.; Potin, P.; Vilter, H.; Elwing, H. Enzymatic Cross-Linking of a Phenolic Polymer Extracted from the Marine Alga Fucus serratus. Biomacromolecules 2004, 5, 2376–2383. [Google Scholar] [CrossRef]
- Boström, M.; Williams, D.; Ninham, B. Specific ion effects: Why DLVO theory fails for biology and colloid systems. Phys. Rev. Lett. 2001, 87, 168103–168107. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.; Choi, Y.; Lee, D.S.; Kim, J.; Yi, G.-R. Colloidal Mesoporous Silica Nanoparticles as Strong Adhesives for Hydrogels and Biological Tissues. ACS Appl. Mater. Interfaces 2017, 9, 31469–31477. [Google Scholar] [CrossRef]
- Shin, K.; Choi, J.W.; Ko, G.; Baik, S.; Kim, D.; Park, O.K.; Lee, K.; Cho, H.R.; Han, S.I.; Lee, S.H.; et al. Multifunctional nanoparticles as a tissue adhesive and an injectable marker for image-guided procedures. Nat. Commun. 2017, 8, 15807–15819. [Google Scholar] [CrossRef]
- Pradyawong, S.; Qi, G.; Li, N.; Sun, X.S.; Wang, D. Adhesion properties of soy protein adhesives enhanced by biomass lignin. Int. J. Adhes. Adhes. 2017, 75, 66–73. [Google Scholar] [CrossRef]
- Luo, J.; Yuan, C.; Zhang, W.; Li, J.; Gao, Q.; Chen, H. An eco-friendly wood adhesive from soy protein and lignin: Performance properties. RSC Adv. 2015, 5, 100849–100855. [Google Scholar] [CrossRef]
- Aracri, E.; Blanco, C.D.; Tzanov, T. An enzymatic approach to develop a lignin-based adhesive for wool floor coverings. Green Chem. 2014, 16, 2597–2603. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Didaskalou, C.; Buyuktiryaki, S.; Kecili, R.; Fonte, C.P.; Szekely, G. Valorisation of agricultural waste with an adsorption/nanofiltration hybrid process: From materials to sustainable process design. Green Chem. 2017, 19, 3116–3125. [Google Scholar] [CrossRef]
- Ashok, R.P.B.; Oinas, P.; Lintinen, K.; Sarwar, G.; Kostiainen, M.A.; Österberg, M. Techno-economic assessment for the large-scale production of colloidal lignin particles. Green Chem. 2018, 20, 4911–4919. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin valorization: Lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10, 192–203. [Google Scholar] [CrossRef]
- García, J.L.; Pans, G.; Phanopoulos, C. Use of lignin in polyurethane-based structural wood adhesives. J. Adhes. 2018, 94, 814–828. [Google Scholar] [CrossRef]
- Kalami, S.; Arefmanesh, M.; Master, E.; Nejad, M. Replacing 100% of phenol in phenolic adhesive formulations with lignin. J. Appl. Polym. Sci. 2017, 134, 45124–45133. [Google Scholar] [CrossRef]










| Sample | Average Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|
| pH 6.0 | |||
| Reference | 131 ± 1 | −30 ± 1 | 0.27 |
| MaL-treatment | 75 ± 1 | −25 ± 1 | 0.40 |
| ThL-treatment | 82 ± 2 | −33 ± 1 | 0.40 |
| pH 8.0 | |||
| Reference | 125 ± 1 | −22 ± 1 | 0.25 |
| MaL-treatment | 65 ± 1 | −30 ± 1 | 0.25 |
| ThL-treatment | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattinen, M.-L.; Riviere, G.; Henn, A.; Nugroho, R.W.N.; Leskinen, T.; Nivala, O.; Valle-Delgado, J.J.; Kostiainen, M.A.; Österberg, M. Colloidal Lignin Particles as Adhesives for Soft Materials. Nanomaterials 2018, 8, 1001. https://doi.org/10.3390/nano8121001
Mattinen M-L, Riviere G, Henn A, Nugroho RWN, Leskinen T, Nivala O, Valle-Delgado JJ, Kostiainen MA, Österberg M. Colloidal Lignin Particles as Adhesives for Soft Materials. Nanomaterials. 2018; 8(12):1001. https://doi.org/10.3390/nano8121001
Chicago/Turabian StyleMattinen, Maija-Liisa, Guillaume Riviere, Alexander Henn, Robertus Wahyu N. Nugroho, Timo Leskinen, Outi Nivala, Juan José Valle-Delgado, Mauri A. Kostiainen, and Monika Österberg. 2018. "Colloidal Lignin Particles as Adhesives for Soft Materials" Nanomaterials 8, no. 12: 1001. https://doi.org/10.3390/nano8121001
APA StyleMattinen, M. -L., Riviere, G., Henn, A., Nugroho, R. W. N., Leskinen, T., Nivala, O., Valle-Delgado, J. J., Kostiainen, M. A., & Österberg, M. (2018). Colloidal Lignin Particles as Adhesives for Soft Materials. Nanomaterials, 8(12), 1001. https://doi.org/10.3390/nano8121001