Enhanced Anticancer Activity of 5’-DFUR-PCL-MPEG Polymeric Prodrug Micelles Encapsulating Chemotherapeutic Drugs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization Methods
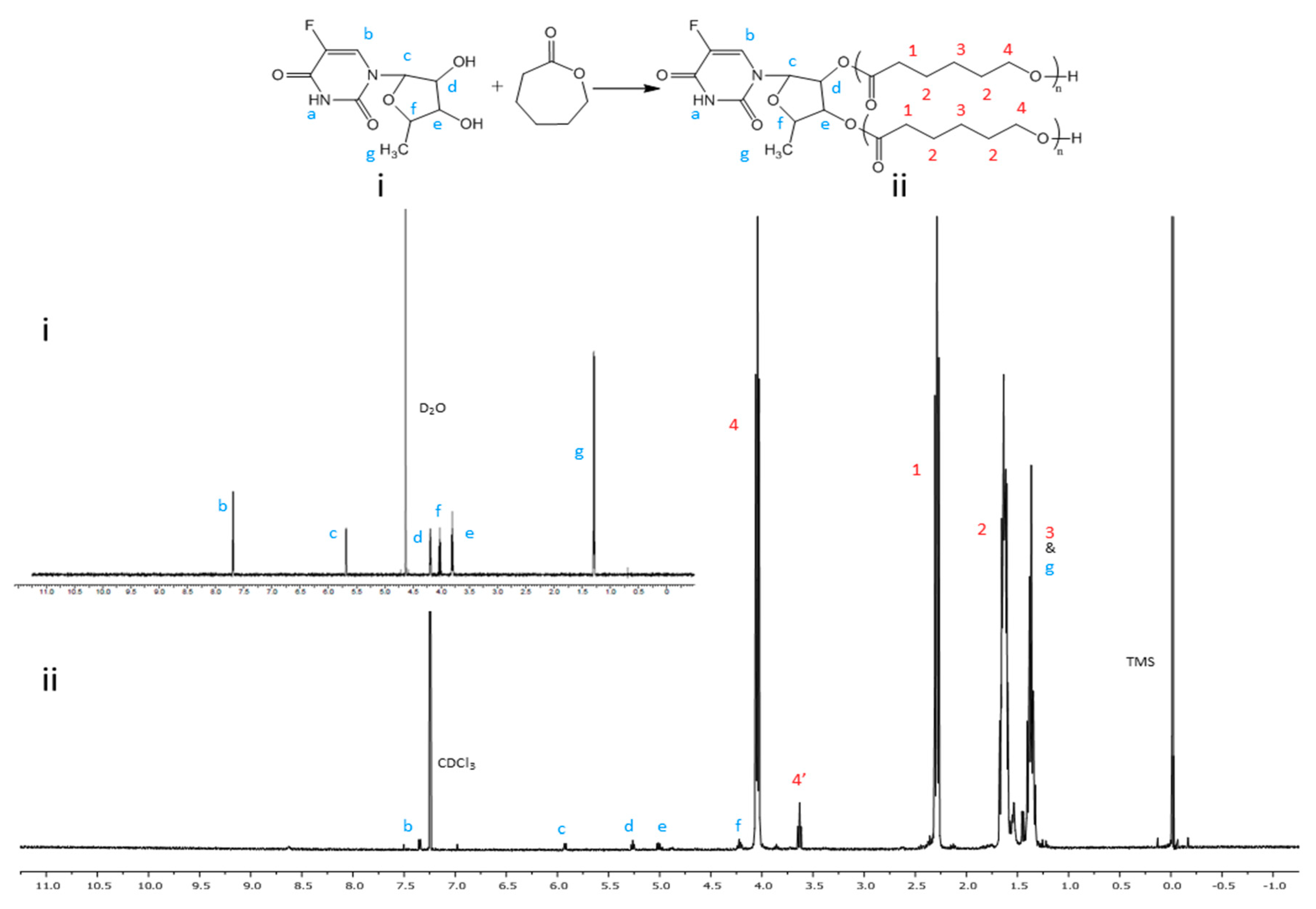
2.3. Synthesis of 5’-DFUR-Incorporated Amphiphilic Polymers
2.4. Preparation of Polymeric Prodrug Micelles
2.5. CMC Determination
2.6. Size and Morphology of 5’-DFUR-Incorporated Polymeric Micelles
2.7. Drug Loading Content and Entrapment Efficiency
2.8. Drug Release Profiles
2.9. TP Activity Assay
2.10. Cytotoxicity Studies
3. Results and Discussion
3.1. Synthesis and Characterization of Amphiphilic Prodrug Polymers
3.2. Formation and Characterization of 5’-DFUR-Incorporated Polymeric Micelles
3.3. Evaluation of Drug Loading Content and Entrapment Efficiency
3.4. TP Activity Assay
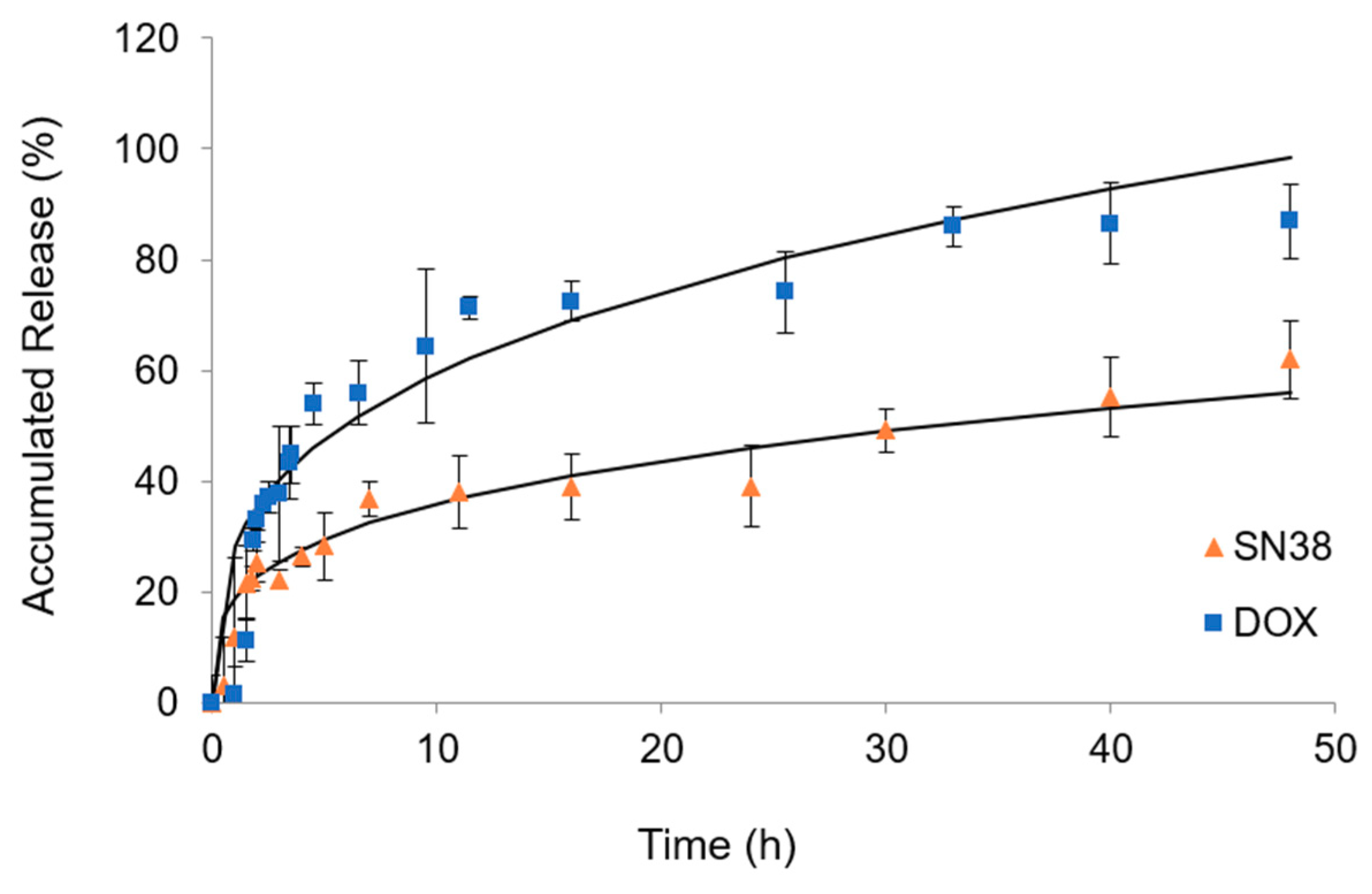
3.5. In Vitro Drug Release
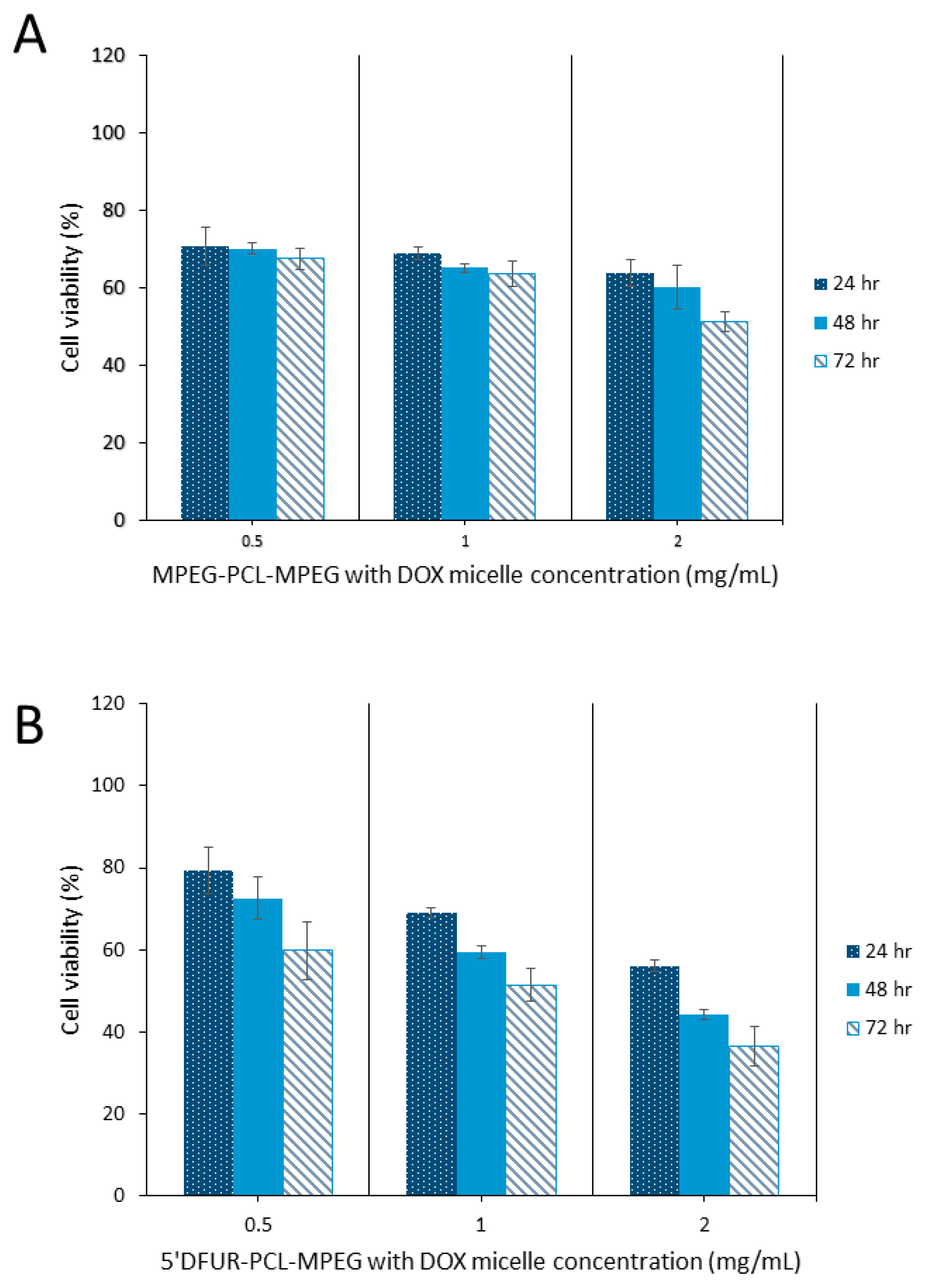
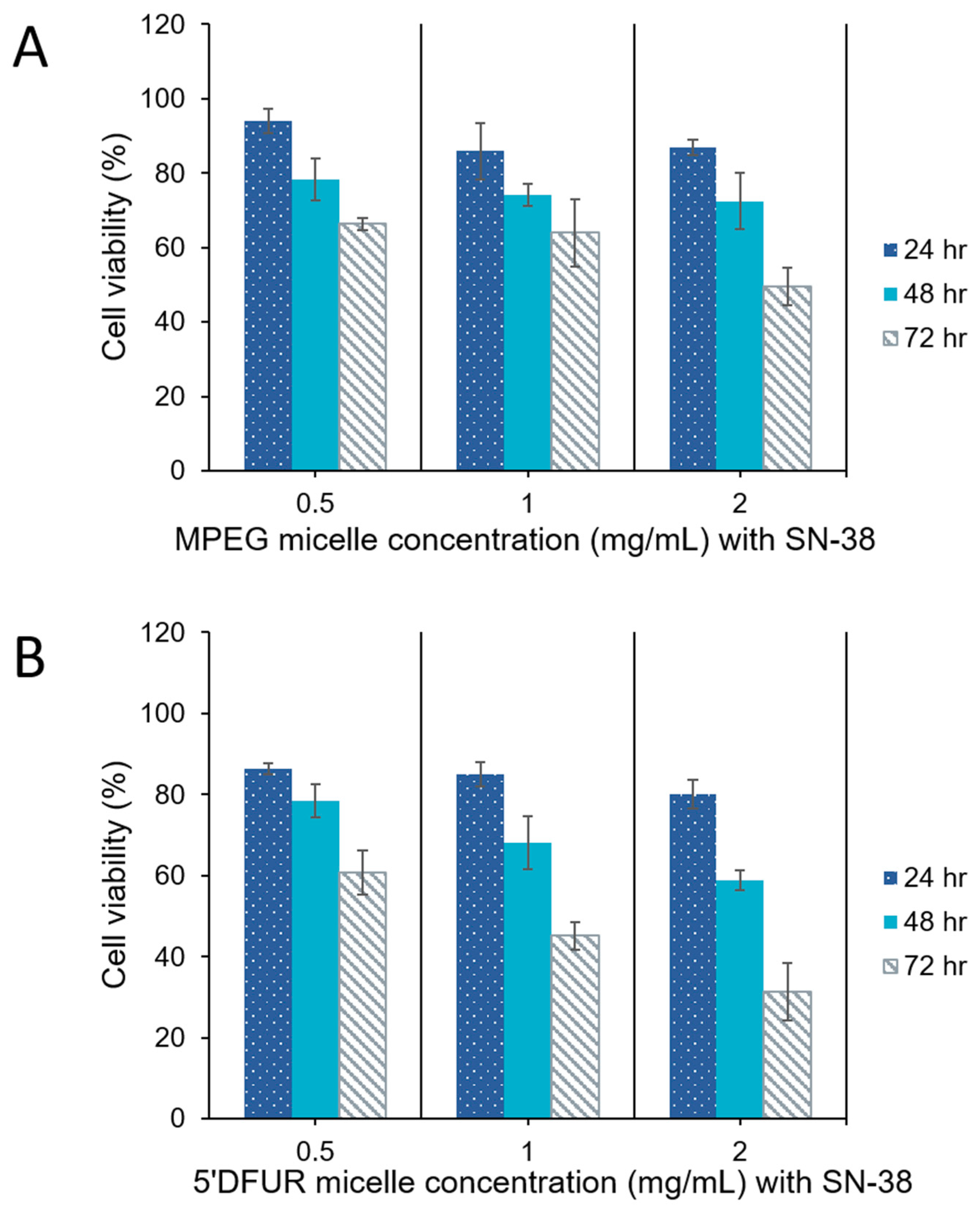
3.6. Cytotoxicity of Polymeric Prodrug Micelles Loaded with Chemotherapeutic Drugs
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Gramont, A.; Louvet, C.; André, T.; Tournigand, C.; Krulik, M. A review of GERCOD trials of bimonthly leucovorin plus 5-fluorouracil 48-h continuous infusion in advanced colorectal cancer: Evolution of a Regimen. Eur. J. Cancer 1998, 34, 619–626. [Google Scholar] [CrossRef]
- Baek, J.H.; Kim, J.G.; Kim, S.N.; Kim, D.H.; Sohn, S.K.; Hong, Y.J.; Lee, K.B. Unpredicted severe toxicity after 5-fluorouracil treatment due to dihydropyrimidine dehydrogenase deficiency. Korean J. Intern. Med. 2006, 21, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Lestuzzi, C.; Vaccher, E.; Talamini, R.; Lleshi, A.; Meneguzzo, N.; Viel, E.; Scalone, S.; Tartuferi, L.; Buonadonna, A.; Ejiofor, L.; et al. Effort myocardial ischemia during chemotherapy with 5-fluorouracil: An underestimated risk. Ann. Oncol. 2014, 25, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Sara, J.D.; Kaur, J.; Khodadadi, R.; Rehman, M.; Lobo, R.; Chakrabarti, S.; Herrmann, J.; Lerman, A.; Grothey, A. 5-fluorouracil and cardiotoxicity: A review. Ther. Adv. Med. Oncol. 2018, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Kinouchi, M.; Ishida, K.; Fujibuchi, W.; Naitoh, T.; Ogawa, H.; Ando, T.; Yazaki, N.; Watanabe, K.; Haneda, S.; et al. 5-fu metabolism in cancer and orally-administrable 5-fu drugs. Cancers 2010, 2, 1717–1730. [Google Scholar] [CrossRef] [PubMed]
- Shimma, N.; Umeda, I.; Arasaki, M.; Murasaki, C.; Masubuchi, K.; Kohchi, Y.; Miwa, M.; Ura, M.; Sawada, N.; Tahara, H.; et al. The design and synthesis of a new tumor-selective fluoropyrimidine carbamate, Capecitabine. Bioorg. Med. Chem. 2000, 8, 1697–1706. [Google Scholar] [CrossRef]
- Bronckaers, A.; Gago, F.; Balzarini, J.; Liekens, S. The dual role of thymidine phosphorylase in cancer development and chemotherapy. Med. Res. Rev. 2009, 29, 903–953. [Google Scholar] [CrossRef] [PubMed]
- Friedkin, M.; Roberts, D. The enzymatic synthesis of nucleosides. I. Thymidine phosphorylase in mammalian tissue. J. Biol. Chem. 1954, 207, 245–256. [Google Scholar] [PubMed]
- Krenitsky, T.A.; Koszalka, G.W.; Tuttle, J.V. Purine nucleoside synthesis, an efficient method employing nucleoside phosphorylases. Biochemistry 1981, 20, 3615–3621. [Google Scholar] [CrossRef]
- Takebayashi, Y.; Yamada, K.; Miyadera, K.; Sumizawa, T.; Furukawa, T.; Kinoshita, F.; Aoki, D.; Okumura, H.; Yamada, Y.; Akiyama, S.; et al. The activity and expression of thymidine phosphorylase in human solid tumours. Eur. J. Cancer 1996, 32, 1227–1232. [Google Scholar] [CrossRef]
- Akiyama, S.; Furukawa, T.; Sumizawa, T.; Takebayashi, Y.; Nakajima, Y.; Shimaoka, S.; Haraguchi, M. The role of thymidine phosphorylase, an angiogenic enzyme, in tumor progression. Cancer Sci. 2004, 95, 851–857. [Google Scholar] [CrossRef] [Green Version]
- Nishina, T.; Hyodo, I.; Miyaike, J.; Inaba, T.; Suzuki, S.; Shiratori, Y. The ratio of thymidine phosphorylase to dihydropyrimidine dehydrogenase in tumour tissues of patients with metastatic gastric cancer is predictive of the clinical response to 5′-deoxy-5-fluorouridine. Eur. J. Cancer 2004, 40, 1566–1571. [Google Scholar] [CrossRef] [Green Version]
- Patterson, A.V.; Zhang, H.; Moghaddam, A.; Bicknell, R.; Talbot, D.C.; Stratford, I.J.; Harris, A.L. Increased sensitivity to the prodrug 5′-deoxy-5-fluorouridine and modulation of 5-fluoro-2′-deoxyuridine sensitivity in MCF-7 cells transfected with thymidine phosphorylase. Br. J. Cancer 1995, 72, 669–675. [Google Scholar] [CrossRef]
- Haraguchi, M.; Furukawa, T.; Sumizawa, T.; Akiyama, S. Sensitivity of human KB cells expressing platelet-derived endothelial cell growth factor to pyrimidine antimetabolites. Cancer Res. 1993, 53, 5680–5682. [Google Scholar]
- Nakano, Y.; Ohota, J.; Fujita, M.; Taguchi, T. [Clinical effect of 5′-deoxy-5-fluorouridine (5′-DFUR)]. Gan No Rinsho 1985, 31, 746–750. [Google Scholar]
- Sumimoto, R.; Takahashi, M.; Etoh, T.; Ichiba, Y.; Emoto, K.; Imaoka, Y.; Ishizaki, Y.; Tokumoto, N. [Effectiveness of high-dose, intermittent 5′-DFUR therapy for advanced gastric cancer]. Gan To Kagaku Ryoho 2001, 28, 83–86. [Google Scholar]
- Hirano, M.; Kato, A.; Murakami, N.; Saito, H.; Watanabe, S.; Oosawa, T.; Kikkawa, H.; Kitagawa, K.; Masuda, S.; Mori, H. [A case of recurrent colon cancer treated markedly effective with 5′-DFUR]. Gan To Kagaku Ryoho 1993, 20, 1063–1066. [Google Scholar]
- Tagaya, N.; Kakihara, Y.; Hamada, K.; Sawada, T.; Kubota, K. [Docetaxel (TXT), epirubicin (EPI) and doxifluridine (5′-DFUR) combination neoadjuvant chemotherapy for outpatients with locally advanced breast cancer]. Gan To Kagaku Ryoho 2004, 31, 2155–2158. [Google Scholar]
- Iba, T.; Kidokoro, A.; Fukunaga, M.; Sugiyama, K.; Aihara, N.; Suda, M. [The efficacy of combination chemotherapy of 5′-deoxy-5-fluorouridine (5′-DFUR), cyclophosphamide (CPA) and medroxyprogesterone acetate (MPA) for bone metastasis in breast cancer patients]. Gan To Kagaku Ryoho 2001, 28, 973–977. [Google Scholar]
- Takatsuka, Y.; Yayoi, E.; Miyauchi, K.; Aikawa, T.; Maeura, Y.; Hirai, T.; Kotsuma, Y. [A comparative study with 5′-DFUR alone or in combination with tamoxifen (TAM) or medroxyprogesterone acetate (MPA) for advanced or recurrent breast cancer]. Gan To Kagaku Ryoho 1992, 19, 631–636. [Google Scholar]
- Pierri, E.; Avgoustakis, K. Poly(lactide)-poly(ethylene glycol) micelles as a carrier for griseofulvin. J. Biomed. Mater. Res. A 2005, 75, 639–647. [Google Scholar] [CrossRef]
- Yue, J.; Liu, S.; Wang, R.; Hu, X.; Xie, Z.; Huang, Y.; Jing, X. Transferrin-Conjugated Micelles: Enhanced Accumulation and Antitumor Effect for Transferrin-Receptor-Overexpressing Cancer Models. Mol. Pharm. 2012, 7, 1919–1931. [Google Scholar] [CrossRef]
- Tabatabaei Rezaei, S.J.; Nabid, M.R.; Niknejad, H.; Entezami, A.A. Multifunctional and thermoresponsive unimolecular micelles for tumor-targeted delivery and site-specifically release of anticancer drugs. Polymer 2012, 53, 3485–3497. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kataoka, K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009, 100, 572–579. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, T.; Kato, K.; Yasui, H.; Morizane, C.; Ikeda, M.; Ueno, H.; Muro, K.; Yamada, Y.; Okusaka, T.; Shirao, K.; et al. A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br. J. Cancer 2007, 97, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Matsumura, Y.; Hamaguchi, T.; Ura, T.; Muro, K.; Yamada, Y.; Shimada, Y.; Shirao, K.; Okusaka, T.; Ueno, H.; Ikeda, M.; et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br. J. Cancer 2004, 91, 1775–1781. [Google Scholar] [CrossRef]
- Chabner, B.A.; Longo, D.L. Cancer Chemotherapy and Biotherapy: Principles and Practice; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2011. [Google Scholar]
- latter, J.G.; Schaaf, L.J.; Sams, J.P.; Feenstra, K.L.; Johnson, M.G.; Bombardt, P.A.; Cathcart, K.S.; Verburg, M.T.; Pearson, L.K.; Compton, L.D. Pharmacokinetics, metabolism, and excretion of irinotecan (CPT-11) following iv infusion of [14C] CPT-11 in cancer patients. Drug Metab. Dispos. 2000, 28, 423–433. [Google Scholar]
- Rothenberg, M.L.; Kuhn, J.G.; Burris, H.; Nelson, J.; Eckardt, J.R.; Tristan-Morales, M.; Hilsenbeck, S.G.; Weiss, G.R.; Smith, L.S.; Rodriguez, G.I. Phase I and pharmacokinetic trial of weekly CPT-11. J. Clin. Oncol. 1993, 11, 2194–2204. [Google Scholar] [CrossRef]
- Chang, K.-Y.; Lee, Y.-D. Ring-opening polymerization of ε-caprolactone initiated by the antitumor agent doxifluridine. Acta Biomater. 2009, 5, 1075–1081. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef]
- Cerrai, P.; Tricoli, M. Block copolymers from L-lactide and poly(ethylene glycol) through a non-catalyzed route. Makromol. Chem. Rapid Comm. 1993, 14, 529–538. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhou, Y.; Wang, X.; Fan, Y.; Huang, Y.; Liu, Y. Preparation and Evaluation of Poly(Ethylene Glycol)-Poly(Lactide) Micelles as Nanocarriers for Oral Delivery of Cyclosporine A. Nanoscale Res. Lett. 2010, 5, 917–925. [Google Scholar] [CrossRef]
- Xue, B.; Wang, Y.; Tang, X.; Xie, P.; Luo, F.; Wu, C.; Qian, Z. Biodegradable self-assembled MPEG-PCL micelles for hydrophobic oridonin delivery in vitro. J. Biomed. Nanotechnol. 2012, 8, 80–89. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2012, 56, 1649–1659. [Google Scholar] [CrossRef]
- Wilhelm, M.; Zhao, C.L.; Wang, Y.; Xu, R.; Winnik, M.A.; Mura, J.L.; Riess, G.; Croucher, M.D. Poly(styrene-ethylene oxide) block copolymer micelle formation in water: A fluorescence probe study. Macromolecules 1991, 24, 1033–1040. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Thomas, J.K. Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J. Am. Chem. Soc. 1977, 99, 2039–2044. [Google Scholar] [CrossRef]
- Schwartz, E.L.; Baptiste, N.; Wadler, S.; Makower, D. Thymidine phosphorylase mediates the sensitivity of human colon carcinoma cells to 5-fluorouracil. J. Biol. Chem. 1995, 270, 19073–19077. [Google Scholar] [CrossRef]
- Deng, J.; Lei, W.; Fu, J.-C.; Zhang, L.; Li, J.-H.; Xiong, J.-P. Targeting miR-21 enhances the sensitivity of human colon cancer HT-29 cells to chemoradiotherapy in vitro. Biochem. Biophys. Res. Commun. 2014, 443, 789–795. [Google Scholar] [CrossRef]
- Schwartz, E.L.; Baptiste, N.; O′Connor, C.J.; Wadler, S.; Otter, B.A. Potentiation of the antitumor activity of 5-fluorouracil in colon carcinoma cells by the combination of interferon and deoxyribonucleosides results from complementary effects on thymidine phosphorylase. Cancer Res. 1994, 54, 1472–1478. [Google Scholar]
- Puppin, C.; Puglisi, F.; Pandolfi, M.; Di Loreto, C.; Damante, G. Histone deacetylase inhibitors induce thymidine phosphorylase expression in cultured breast cancer cell lines. Oncol. Rep. 2011, 26, 309–314. [Google Scholar] [CrossRef]
- Lee, S.J.; Yeo, J.S.; Lee, H.J.; Lee, E.J.; Kim, S.Y.; Jang, S.J.; Lee, J.J.; Ryu, J.S.; Moon, D.H. Thymidine phosphorylase influences [(18)F]fluorothymidine uptake in cancer cells and patients with non-small cell lung cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1327–1335. [Google Scholar] [CrossRef]
- Takebayashi, Y.; Akiyama, S.-I.; Akiba, S.; Yamada, K.; Miyadera, K.; Sumizawa, T.; Yamada, Y.; Murata, F.; Aikou, T. Clinicopathologic and Prognostic Significance of an Angiogenic Factor, Thymidine Phosphorylase, in Human Colorectal Carcinoma. J. Natl. Cancer Inst. 1996, 88, 1110–1117. [Google Scholar] [CrossRef] [Green Version]
- Bijnsdorp, I.V.; Capriotti, F.; Kruyt, F.A.E.; Losekoot, N.; Fukushima, M.; Griffioen, A.W.; Thijssen, V.L.; Peters, G.J. Thymidine phosphorylase in cancer cells stimulates human endothelial cell migration and invasion by the secretion of angiogenic factors. Br. J. Cancer 2011, 104, 1185. [Google Scholar] [CrossRef]
- Liewald, F.; Demmel, N.; Wirsching, R.; Kahle, H.; Valet, G. Intracellular pH, esterase activity, and DNA measurements of human lung carcinomas by flow cytometry. Cytometry 1990, 11, 341–348. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, M.; Kunugi, S. Kinetic Studies of Wheat Carboxypeptidase-Catalyzed Reaction: Differences in Pressure and Temperature Dependence of Peptidase and Esterase Activities. J. Biochem. 1987, 101, 233–240. [Google Scholar] [CrossRef]
- Guichard, S.; Terret, C.; Hennebelle, I.; Lochon, I.; Chevreau, P.; Fretigny, E.; Selves, J.; Chatelut, E.; Bugat, R.; Canal, P. CPT-11 converting carboxylesterase and topoisomerase activities in tumour and normal colon and liver tissues. Br. J. Cancer 1999, 80, 364–370. [Google Scholar] [CrossRef]
- Sawdon, A.J.; Peng, C.-A. Polymeric micelles for acyclovir drug delivery. Colloids Surf. B 2014, 122, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Siepmann, J.; Siepmann, F. Mathematical modeling of drug delivery. Int. J. Pharm. 2008, 364, 328–343. [Google Scholar] [CrossRef]
- Sawdon, A.J.; Peng, C.-A. Ring-opening polymerization of ε-caprolactone initiated by ganciclovir (GCV) for the preparation of GCV-tagged polymeric micelles. Molecules 2015, 20, 2857–2867. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Chen, L.; Gao, W.; Zeng, F.; Kong, L.X. Targeted delivery of 5-fluorouracil to HT-29 cells using high efficient folic acid-conjugated nanoparticles. Drug Deliv. 2015, 22, 191–198. [Google Scholar] [CrossRef]
- Niemirowicz, K.; Prokop, I.; Wilczewska, A.Z.; Wnorowska, U.; Piktel, E.; Wątek, M.; Savage, P.B.; Bucki, R. Magnetic nanoparticles enhance the anticancer activity of cathelicidin LL-37 peptide against colon cancer cells. Int. J. Nanomed. 2015, 10, 3843–3853. [Google Scholar] [CrossRef]






| Sample | Mw (Da) | Mn (Da) | Polydispersity (Mw/Mn) |
|---|---|---|---|
| 5’-DFUR-PCL | 18,796 | 15,158 | 1.24 |
| 5’-DFUR-PCL-MPEG | 33,927 | 28,510 | 1.19 |
| MPEG350-PCL | 24,600 | 17,053 | 1.44 |
| MPEG350-PCL-MPEG | 19,415 | 21,624 | 1.36 |
| Sample | Size (nm) | Zeta (mV) | Drug Loading Content (%) | Entrapment Efficiency (%) |
|---|---|---|---|---|
| 5’-DFUR-PCL-MPEG | 220.5 | 1.23 | -- | -- |
| 5’-DFUR-PCL-MPEG (DOX) | 167.5 | −0.11 | 10.8 | 68.8 |
| 5’-DFUR-PCL-MPEG (SN-38) | 267.5 | 1.01 | 3.4 | 86.3 |
| MPEG350-PCL-MPEG | 202.5 | 0.74 | -- | -- |
| MPEG350-PCL-MPEG (DOX) | 222 | 2.15 | 10.4 | 65.6 |
| MPEG350-PCL-MPEG (SN-38) | 148 | 1.21 | 3.9 | 97.6 |
| Cell Line | Thymine Released (µmol/mg protein/h) | 5-FU Released (µmol/mg protein/h) |
|---|---|---|
| HT-29 | 2.89 ± 0.27 | 2.21 ± 0.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sawdon, A.J.; Zhang, J.; Wang, X.; Peng, C.-A. Enhanced Anticancer Activity of 5’-DFUR-PCL-MPEG Polymeric Prodrug Micelles Encapsulating Chemotherapeutic Drugs. Nanomaterials 2018, 8, 1041. https://doi.org/10.3390/nano8121041
Sawdon AJ, Zhang J, Wang X, Peng C-A. Enhanced Anticancer Activity of 5’-DFUR-PCL-MPEG Polymeric Prodrug Micelles Encapsulating Chemotherapeutic Drugs. Nanomaterials. 2018; 8(12):1041. https://doi.org/10.3390/nano8121041
Chicago/Turabian StyleSawdon, Alicia J., Jun Zhang, Xutu Wang, and Ching-An Peng. 2018. "Enhanced Anticancer Activity of 5’-DFUR-PCL-MPEG Polymeric Prodrug Micelles Encapsulating Chemotherapeutic Drugs" Nanomaterials 8, no. 12: 1041. https://doi.org/10.3390/nano8121041
APA StyleSawdon, A. J., Zhang, J., Wang, X., & Peng, C.-A. (2018). Enhanced Anticancer Activity of 5’-DFUR-PCL-MPEG Polymeric Prodrug Micelles Encapsulating Chemotherapeutic Drugs. Nanomaterials, 8(12), 1041. https://doi.org/10.3390/nano8121041




