Atmospheric Pressure Plasma-Mediated Synthesis of Platinum Nanoparticles Stabilized by Poly(vinylpyrrolidone) with Application in Heat Management Systems for Internal Combustion Chambers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
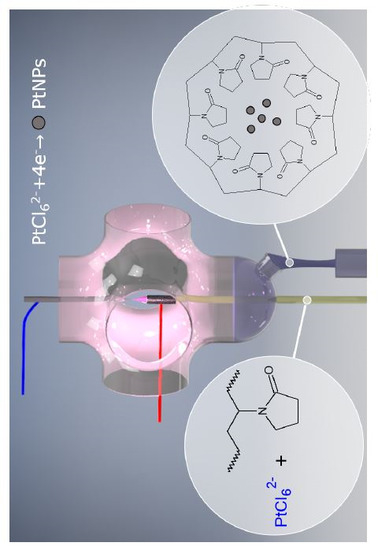
2.2. One-Step Synthesis of PVP-PtNPs
2.3. Characterization of PVP-PtNPs
2.4. Surface Functionalization of PtNPs by PVP
2.5. Application of PVP-PtNPs in the HMS
3. Results and Discussion
3.1. Application of dc-APGD for Synthesis of PVP-PtNPs
3.2. Characterization of PVP-PtNPs
3.3. Mechanism of PtNPs Formation
3.4. Enhanced HMS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- d’Agostino, R.; Favia, P.; Oehr, C.; Wertheimer, M.R. Low-temperature plasma processing of materials: past, present, and future. Plasma Process. Polym. 2005, 2, 7–15. [Google Scholar] [CrossRef]
- Weltmann, K.D.; von Woedtke, T. Plasma medicine—Current state of research and medical application. Plasma Phys. Control. Fusion 2017, 59, 014031. [Google Scholar] [CrossRef]
- Weltmann, K.D.; Kindel, E.; von Woedtke, T.; Hahnel, M.; Stieber, M.; Brandenburg, R. Atmospheric-pressure plasma sources: Prospective tools for plasma medicine. Pure Appl. Chem. 2010, 82, 1223–1237. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Xiong, Z.; Zhao, F.; Xian, Y.; Xiong, Q.; Gong, W.; Zou, C.; Jiang, Z.; Pan, Y. A simple atmospheric pressure room-temperature air plasma needle device for biomedical applications. Appl. Phys. Lett. 2009, 95, 181501. [Google Scholar] [CrossRef]
- Jamroz, P.; Greda, K.; Pohl, P. Development of direct-current, atmospheric-pressure, glow discharges generated in contact with flowing electrolyte solutions for elemental analysis by optical emission spectrometry. TrAC Trends Anal. Chem. 2012, 41, 105–121. [Google Scholar] [CrossRef]
- Zhou, Z.; Huang, Y.; Yang, S.; Chen, W. Introduction of a new atmospheric pressure plasma device and application on tomato seeds. J. Agric. Sci. 2011, 2, 23–27. [Google Scholar] [CrossRef]
- Oda, T. Non-thermal plasma processing for environmental protection: Decomposition of dilute vocs in air. J. Electrostat. 2003, 57, 293–311. [Google Scholar] [CrossRef]
- Cyganowski, P.; Dzimitrowicz, A.; Jamroz, P.; Jermakowicz-Bartkowiak, D.; Pohl, P. Polymerization-driven immobilization of dc-APGD synthesized gold nanoparticles into a quaternary ammonium-based hydrogel resulting in a polymeric nanocomposite with heat-transfer applications. Polymers 2018, 10, 377. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Bielawska-Pohl, A.; diCenzo, G.; Jamroz, P.; Macioszczyk, J.; Klimczak, A.; Pohl, P. Pulse-modulated radio-frequency alternating-current-driven atmospheric-pressure glow discharge for continuous-flow synthesis of silver nanoparticles and evaluation of their cytotoxicity toward human melanoma cells. Nanomaterials 2018, 8, 398. [Google Scholar] [CrossRef] [PubMed]
- Rioux, R.; Song, H.; Grass, M.; Habas, S.; Niesz, K.; Hoefelmeyer, J.; Yang, P.; Somorjai, G. Monodisperse platinum nanoparticles of well-defined shape: Synthesis, characterization, catalytic properties and future prospects. Top. Catal. 2006, 39, 167–174. [Google Scholar] [CrossRef]
- Choi, K.M.; Na, K.; Somorjai, G.A.; Yaghi, O.M. Chemical environment control and enhanced catalytic performance of platinum nanoparticles embedded in nanocrystalline metal–organic frameworks. J. Am. Chem. Soc. 2015, 137, 7810–7816. [Google Scholar] [CrossRef] [PubMed]
- Cheng, N.; Banis, M.N.; Liu, J.; Riese, A.; Li, X.; Li, R.; Ye, S.; Knights, S.; Sun, X. Extremely stable platinum nanoparticles encapsulatedin a zirconia nanocage by area-selective atomic layer deposition for the oxygen reduction reaction. Adv. Mater. 2015, 27, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Yadav, O.; Palmqvist, A.; Cruise, N.; Holmberg, K. Synthesis of platinum nanoparticles in microemulsions and their catalytic activity for the oxidation of carbon monoxide. Colloids Surf. A Physicochem. Eng. Asp. 2003, 221, 131–134. [Google Scholar] [CrossRef]
- Nagao, H.; Ichiji, M.; Hirasawa, I. Synthesis of platinum nanoparticles by reductive crystallization using polyethyleneimine. Chem. Eng. Technol. 2017, 40, 1242–1246. [Google Scholar] [CrossRef]
- Koo, I.G.; Lee, M.S.; Shim, J.H.; Ahn, J.H.; Lee, W.M. Platinum nanoparticles prepared by a plasma-chemical reduction method. J. Mater. Chem. 2005, 15, 4125–4128. [Google Scholar] [CrossRef]
- Shim, J.; Joung, K.J.; Ahn, J.H.; Lee, W.M. Carbon-supported platinum nanoparticles synthesized by plasma-chemical reduction method for fuel cell applications. J. Electrochem. Soc. 2007, 154, B165–B169. [Google Scholar] [CrossRef]
- Hu, X.; Takai, O.; Saito, N. Simple synthesis of platinum nanoparticles by plasma sputtering in water. Jpn. J. Appl. Phys. 2013, 52, 01AN05. [Google Scholar] [CrossRef]
- Sato, S.; Mori, K.; Ariyada, O.; Atsushi, H.; Yonezawa, T. Synthesis of nanoparticles of silver and platinum by microwave-induced plasma in liquid. Surf. Coat. Technol. 2011, 206, 955–958. [Google Scholar] [CrossRef]
- Ichin, Y.; Mitamura, K.; Saito, N.; Takai, O. Characterization of platinum catalyst supported on carbon nanoballs prepared by solution plasma processing. J. Vac. Sci. Technol. A. 2009, 27, 826–830. [Google Scholar] [CrossRef]
- Dao, V.-D.; Tran, C.Q.; Ko, S.-H.; Choi, H.-S. Dry plasma reduction to synthesize supported platinum nanoparticles for flexible dye-sensitized solar cells. J. Mater. Chem. A 2013, 1, 4436–4443. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Greda, K.; Lesniewicz, T.; Jamroz, P.; Nyk, M.; Pohl, P. Size-controlled synthesis of gold nanoparticles by a novel atmospheric pressure glow discharge system with a metallic pin electrode and a flowing liquid electrode. RSC Adv. 2016, 6, 80773–80783. [Google Scholar] [CrossRef] [Green Version]
- Dzimitrowicz, A.; Jamroz, P.; Pogoda, D.; Nyk, M.; Pohl, P. Direct current atmospheric pressure glow discharge generated between a pin-type solid cathode and a flowing liquid anode as a new tool for silver nanoparticles production. Plasma Process. Polym. 2017, 14, 1600251. [Google Scholar] [CrossRef]
- Dzimitrowicz, A.; Motyka, A.; Jamroz, P.; Lojkowska, E.; Babinska, W.; Terefinko, D.; Pohl, P.; Sledz, W. Application of silver nanostructures synthesized by cold atmospheric pressure plasma for inactivation of bacterial phytopathogens from the genera Dickeya and Pectobacterium. Materials 2018, 11, 331. [Google Scholar] [CrossRef] [PubMed]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles–a new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef]
- Das, S.K.; Choi, S.U.S.; Patel, H.E. Heat transfer in nanofluids—A review. Heat Transfer Eng. 2006, 27, 3–19. [Google Scholar] [CrossRef]
- Patel, H.E.; Das, S.K.; Sundararajan, T.; Sreekumaran Nair, A.; George, B.; Pradeep, T. Thermal conductivities of naked and monolayer protected metal nanoparticle based nanofluids: Manifestation of anomalous enhancement and chemical effects. Appl. Phys. Lett. 2003, 83, 2931–2933. [Google Scholar] [CrossRef]
- Wang, P.; Lv, J.; Bai, M.; Li, G.; Zeng, K. The reciprocating motion characteristics of nanofluid inside the piston cooling gallery. Powder Technol. 2015, 274, 402–417. [Google Scholar] [CrossRef]
- Kajiwara, H.; Fujioka, Y.; Negishi, H. Prediction of temperatures on pistons with cooling gallery in diesel engines using CFD tool. SAE Tech. Pap. 2003, 1, 0986. [Google Scholar]
- Hatami, M.; Ganji, D.; Gorji-Bandpy, M. A review of different heat exchangers designs for increasing the diesel exhaust waste heat recovery. Renew. Sustain. Energy Rev. 2014, 37, 168–181. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q. Heat transfer enhancement of nanofluids. Int. J. Heat Fluid Flow 2000, 21, 58–64. [Google Scholar] [CrossRef]
- Kakac, S.; Pramuanjaroenkij, A. Review of convective heat transfer enhancement with nanofluids. Int. J. Heat Mass Transf. 2009, 52, 3187–3196. [Google Scholar] [CrossRef]
- Moghaieb, H.S.; Abdel-Hamid, H.M.; Shedid, M.H.; Helali, A.B. Engine cooling using Al2O3/water nanofluids. Appl. Therm. Eng. 2017, 115, 152–159. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Chien, H.T.; Ding, P.P.; Chan, B.; Luh, T.Y.; Chen, P.H. Effect of structural character of gold nanoparticles in nanofluid on heat pipe thermal performance. Mater. Lett. 2004, 58, 1461–1465. [Google Scholar] [CrossRef]
- Greda, K.; Swiderski, K.; Jamroz, P.; Pohl, P. Flowing liquid anode atmospheric pressure glow discharge as an excitation source for optical emission spectrometry with the improved detectability of Ag, Cd, Hg, Pb, Tl, and Zn. Anal. Chem. 2016, 88, 8812–8820. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, L.C. Solutions manual. In Convective Heat Transfer; John Wiley & Sons, Incorporated: New York, NY, USA, 1993. [Google Scholar]
- Wang, C.; Daimon, H.; Onodera, T.; Koda, T.; Sun, S. A general approach to the size-and shape-controlled synthesis of platinum nanoparticles and their catalytic reduction of oxygen. Angew. Chem. Int. Ed. 2008, 47, 3588–3591. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Ma, Y.; Tang, J.; Yang, X. “Green synthesis” of monodisperse Pt nanoparticles and their catalytic properties. Colloids Surf. A Physicochem. Eng. Asp. 2007, 302, 628–633. [Google Scholar] [CrossRef]
- Chytil, S.; Glomm, W.R.; Vollebekk, E.; Bergem, H.; Walmsley, J.; Sjoblom, J.; Blekkan, E.A. Platinum nanoparticles encapsulated in mesoporous silica: Preparation, characterisation and catalytic activity in toluene hydrogenation. Microporous Mesoporous Mater. 2005, 86, 198–206. [Google Scholar] [CrossRef]
- Abdelghany, A.; Mekhail, M.S.; Abdelrazek, E.; Aboud, M. Combined DFT/FTIR structural studies of monodispersed PVP/Gold and silver nano particles. J. Alloys Compd. 2015, 646, 326–332. [Google Scholar] [CrossRef]
- Song, Y.-J.; Wang, M.; Zhang, X.-Y.; Wu, J.-Y.; Zhang, T. Investigation on the role of the molecular weight of polyvinyl pyrrolidone in the shape control of high-yield silver nanospheres and nanowires. Nanoscale Res. Lett. 2014, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Long, D.A. Infrared and Raman characteristic group frequencies. Tables and charts George Socrates John Wiley and sons, ltd, chichester, third edition, 2001. J. Raman Spectrosc. 2004, 35, 905. [Google Scholar] [CrossRef]
- Kim, J.H.; Min, B.R.; Kim, C.K.; Won, J.; Kang, Y.S. Spectroscopic interpretation of silver ion complexation with propylene in silver polymer electrolytes. J. Phys. Chem. B 2002, 106, 2786–2790. [Google Scholar] [CrossRef]
- Cyganowski, P.; Lesniewicz, A.; Polowczyk, I.; Checmanowski, J.; Kozlecki, T.; Pohl, P.; Jermakowicz-Bartkowiak, D. Surface-activated anion exchange resins for synthesis and immobilization of gold and palladium nano- and microstructures. React. Funct. Polym. 2018, 124, 90–103. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.S.; Li, Y.F. A review of plasma-liquid interactions for nanomaterials synthesis. J. Phys. D Appl. Phys. 2015, 48, 424005. [Google Scholar] [CrossRef]
- Bruggeman, P.J.; Kushner, M.J.; Locke, B.R.; Gardeniers, J.G.E.; Graham, W.G.; Graves, D.B.; Hofman-Caris, R.C.H.M; Maric, D.; Reid, J.P.; et al. Plasma-liquid interactions: A review and roadmap. Plasma Sources Sci. Technol. 2016, 25, 053002. [Google Scholar] [CrossRef]
- Hofft, O.; Endres, F. Plasma electrochemistry in ionic liquids: An alternative route to generate nanoparticles. Phys. Chem. Chem. Phys. 2011, 13, 13472–13478. [Google Scholar] [CrossRef] [PubMed]
- Borodko, Y.; Habas, S.E.; Koebel, M.; Yang, P.; Frei, H.; Somorjai, G.A. Probing the interaction of poly(vinylpyrrolidone) with platinum nanocrystals by UV-Raman and FTIR. J. Phys. Chem. B 2006, 110, 23052–23059. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.; Liu, F.; Zhao, L.Z.; Yang, W.S.; Yao, J.N. Evidence of a unique electron donor-acceptor property for platinum nanoparticles as studied by XPS. Langmuir 2006, 22, 4480–4482. [Google Scholar] [CrossRef] [PubMed]







| Medium of the Conductive Layer | Rate Constants [×10−3 s−1] | |
|---|---|---|
| kh | kc | |
| Water | 4.88 | 2.55 |
| PVP-PtNPs | 2.70 | 3.41 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzimitrowicz, A.; Cyganowski, P.; Pohl, P.; Jermakowicz-Bartkowiak, D.; Terefinko, D.; Jamroz, P. Atmospheric Pressure Plasma-Mediated Synthesis of Platinum Nanoparticles Stabilized by Poly(vinylpyrrolidone) with Application in Heat Management Systems for Internal Combustion Chambers. Nanomaterials 2018, 8, 619. https://doi.org/10.3390/nano8080619
Dzimitrowicz A, Cyganowski P, Pohl P, Jermakowicz-Bartkowiak D, Terefinko D, Jamroz P. Atmospheric Pressure Plasma-Mediated Synthesis of Platinum Nanoparticles Stabilized by Poly(vinylpyrrolidone) with Application in Heat Management Systems for Internal Combustion Chambers. Nanomaterials. 2018; 8(8):619. https://doi.org/10.3390/nano8080619
Chicago/Turabian StyleDzimitrowicz, Anna, Piotr Cyganowski, Pawel Pohl, Dorota Jermakowicz-Bartkowiak, Dominik Terefinko, and Piotr Jamroz. 2018. "Atmospheric Pressure Plasma-Mediated Synthesis of Platinum Nanoparticles Stabilized by Poly(vinylpyrrolidone) with Application in Heat Management Systems for Internal Combustion Chambers" Nanomaterials 8, no. 8: 619. https://doi.org/10.3390/nano8080619
APA StyleDzimitrowicz, A., Cyganowski, P., Pohl, P., Jermakowicz-Bartkowiak, D., Terefinko, D., & Jamroz, P. (2018). Atmospheric Pressure Plasma-Mediated Synthesis of Platinum Nanoparticles Stabilized by Poly(vinylpyrrolidone) with Application in Heat Management Systems for Internal Combustion Chambers. Nanomaterials, 8(8), 619. https://doi.org/10.3390/nano8080619