Preparation of Pd/C by Atmospheric-Pressure Ethanol Cold Plasma and Its Preparation Mechanism
Abstract
:1. Introduction
2. Experimental
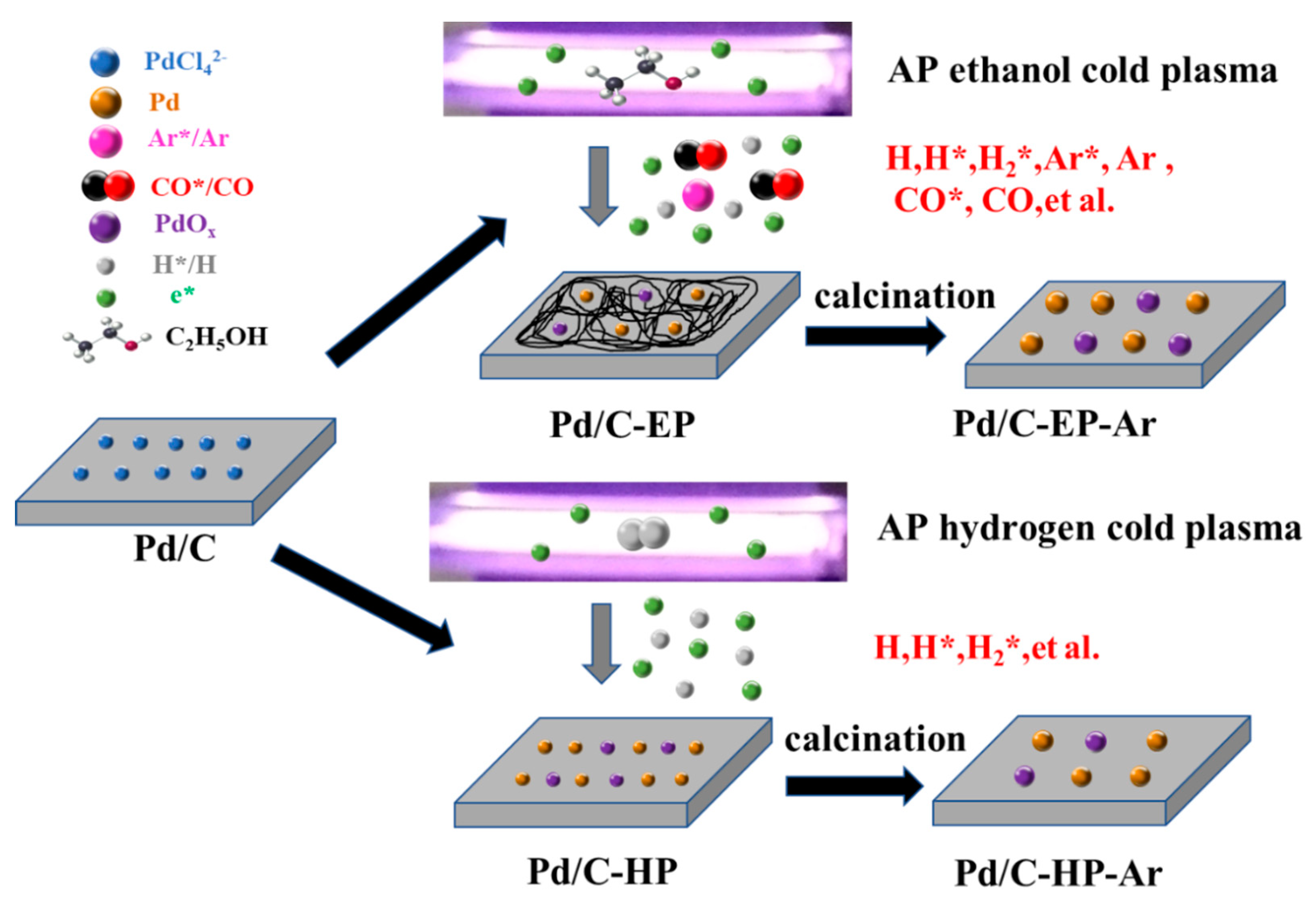
2.1. Catalysts Preparation
2.2. Catalysts Characterization and Activity Test
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fihri, A.; Cha, D.; Bouhrara, M.; Almana, N.; Polshettiwar, V. Fibrous nano-silica (KCC-1) -supported palladium catalyst: Suzuki coupling reactions under sustainable conditions. ChemSusChem 2012, 5, 85–89. [Google Scholar] [CrossRef]
- Zeng, M.F.; Zhang, X.; Shao, L.J.; Qi, C.Z.; Zhang, X.M. Highly porous chitosan microspheres supported palladium catalyst for coupling reactions in organic and aqueous solutions. J. Organomet. Chem. 2012, 704, 29–37. [Google Scholar] [CrossRef]
- Li, Y.Z.; Yu, Y.; Wang, J.G.; Song, J.; Li, Q.; Dong, M.D.; Liu, C.J. CO oxidation over graphene supported palladium catalyst. Appl. Catal. B Environ. 2012, 125, 189–196. [Google Scholar] [CrossRef]
- Monguchi, Y.; Wakayama, F.; Ueda, S.; Ito, R.; Takada, H.; Inoue, H.; Nakamura, A.; Sawama, Y.; Sajiki, H. Amphipathic monolith-supported palladium catalysts for chemoselective hydrogenation and cross-coupling reactions. RSC Adv. 2017, 7, 1833–1840. [Google Scholar] [CrossRef] [Green Version]
- Blaser, H.U.; Indolese, A.; Schnyder, A.; Steiner, H.; Studer, M. Supported palladium catalysts for fine chemicals synthesis. J. Mol. Catal. A Chem. 2001, 173, 3–18. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Bhanage, B.M.; Shirai, M.; Arai, M. Heck reactions of iodobenzene and methyl acrylate with conventional supported palladium catalysts in the presence of organic and/or inorganic bases without ligands. Chem. Eur. J. 2000, 6, 843–848. [Google Scholar] [CrossRef]
- Sekizawa, K.; Widjaja, H.; Maeda, S.; Ozawab, Y.; Eguchia, K. Low temperature oxidation of methane over Pd catalyst supported on metal oxides. Catal. Today 2000, 59, 69–74. [Google Scholar] [CrossRef]
- Xiao, L.H.; Sun, K.P.; Xu, X.L.; Li, X.N. Low-temperature catalytic combustion of methane over Pd/CeO2 prepared by deposition-precipitation method. Catal. Commun. 2005, 6, 796–801. [Google Scholar] [CrossRef]
- Vichaphund, S.; Aht-ong, D.; Sricharoenchaikul, V.; Atong, D. Production of aromatic compounds from catalytic fast pyrolysis of Jatropha residues using metal/HZSM-5 prepared by ion-exchange and impregnation methods. Renew. Energy 2015, 79, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Scudiero, L.; Ha, S. Electronic effect on oxidation of formic acid on supported Pd–Cu bimetallic surface. Electrochim. Acta 2012, 83, 354–358. [Google Scholar] [CrossRef]
- Yang, M.M.; Wang, Z.Y.; Wang, W.; Liu, C.J. Synthesis of AuPd alloyed nanoparticles via room-temperature electron reduction with argon glow discharge as electron source. Nanoscale Res. Lett. 2014, 9, 405. [Google Scholar] [CrossRef]
- Ouyang, L.Z.; Cao, Z.J.; Wang, H.; Hu, R.Z.; Zhu, M. Application of dielectric barrier discharge plasma-assisted milling in energy storage materials-A review. J. Alloys Compd. 2017, 691, 422–435. [Google Scholar] [CrossRef]
- Deng, X.Q.; Zhu, B.; Li, X.S.; Liu, J.L.; Zhu, X.B.; Zhu, A.M. Visible-light photocatalytic oxidation of CO over plasmonic Au/TiO2: Unusual features of oxygen plasma activation. Appl. Catal. B Environ. 2016, 188, 48–55. [Google Scholar] [CrossRef]
- Di, L.B.; Xu, W.J.; Zhan, Z.B.; Zhang, X.L. Synthesis of alumina supported Pd–Cu alloy nanoparticles for CO oxidation via a fast and facile method. RSC Adv. 2015, 5, 71854–71858. [Google Scholar] [CrossRef]
- Gallon, H.J.; Tu, X.; Twigg, M.V.; Whitehead, J.C. Plasma-assisted methane reduction of a NiO catalyst low temperature activation of methane and formation of carbon nanofibres. Appl. Catal. B Environ. 2011, 106, 616–620. [Google Scholar] [CrossRef]
- Zhang, J.S.; Di, L.B.; Yu, F.; Duan, D.Z.; Zhang, X.L. Atmospheric-pressure cold plasma activating Au/P25 for CO oxidation: Effect of working gas. Nanomaterials 2018, 8, 742. [Google Scholar] [CrossRef]
- Ovanesyan, R.A.; Hausmann, D.M.; Agarwal, S. Low-temperature conformal atomic layer deposition of SiNx films using Si2Cl6 and NH3 plasma. ACS Appl. Mater. Interfaces 2015, 7, 10806–10813. [Google Scholar] [CrossRef]
- Guo, Z.F.; Yi, Y.H.; Wang, L.; Yang, J.H.; Guo, H.C. Pt/TS-1 catalyst promoted C–N coupling reaction in CH4–NH3 plasma for HCN synthesis at low temperature. ACS Catal. 2018, 8, 10219–10224. [Google Scholar] [CrossRef]
- Du, C.M.; Ma, D.Y.; Wu, J.; Lin, Y.C.; Xiao, W.; Ruan, J.J.; Huang, D.W. Plasma-catalysis reforming for H2 production from ethanol. Int. J. Hydrog. Energy 2015, 40, 15398–15410. [Google Scholar] [CrossRef]
- Xu, W.Q.; Liu, Z.Y.; Johnston-Peck, A.C.; Senanayake, S.D.; Zhou, G.; Stacchiola, D.; Stach, E.A.; Rodriguez, J.A. Steam reforming of ethanol on Ni/CeO2: Reaction pathway and interaction between Ni and the CeO2 support. ACS Catal. 2013, 3, 975–984. [Google Scholar] [CrossRef]
- Hou, T.F.; Yu, B.; Zhang, S.Y.; Xu, T.K.; Wang, D.Z.; Cai, W.J. Hydrogen production from ethanol steam reforming over Rh/CeO2 catalyst. Catal. Commun. 2015, 58, 137–140. [Google Scholar] [CrossRef]
- Wang, B.; Sun, B.; Zhu, X.M.; Yan, Z.Y.; Liu, Y.J.; Liu, H.; Liu, Q. Hydrogen production from alcohol solution by microwave discharge in liquid. Int. J. Hydrog. Energy 2016, 41, 7280–7291. [Google Scholar] [CrossRef]
- Wu, Y.W.; Chung, W.C.; Chang, M.B. Modification of Ni/γ-Al2O3 catalyst with plasma for steam reforming of ethanol to generate hydrogen. Int. J. Hydrog. Energy 2015, 40, 8071–8080. [Google Scholar] [CrossRef]
- Di, L.B.; Li, Z.; Zhang, X.L.; Wang, H.Y.; Fan, Z.Y. Reduction of supported metal ions by a safe atmospheric pressure alcohol cold plasma method. Catal. Today 2019, 337, 55–62. [Google Scholar] [CrossRef]
- Di, L.B.; Li, Z.; Lee, B.; Park, D.W. An alternative atmospheric-pressure cold plasma method for synthesizing Pd/P25 catalysts with the assistance of ethanol. Int. J. Hydrog. Energy 2017, 42, 11372–11378. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.L.; Zhang, Y.Z.; Duan, D.Z.; Di, L.B. Hydrogen cold plasma for synthesizing Pd/C catalysts: The effect of support-metal ion interaction. Plasma Sci. Technol. 2018, 20, 014016. [Google Scholar] [CrossRef]
- Qi, B.; Di, L.D.; Xu, W.J.; Zhang, X.L. Dry plasma reduction to prepare a high performance Pd/C catalyst at atmospheric pressure for CO oxidation. J. Mater. Chem. A 2014, 2, 11885–11890. [Google Scholar] [CrossRef]
- Lazzarini, A.; Piovano, A.; Pellegrini, R.; Leofanti, G.; Agostini, G.; Rudić, S.; Chierotti, M.R.; Gobetto, R.; Battiato, A.; Spoto, G.; et al. A comprehensive approach to investigate the structural and surface properties of activated carbons and related Pd-based catalysts. Catal. Sci. Technol. 2016, 6, 4910–4922. [Google Scholar] [CrossRef] [Green Version]
- Di, L.B.; Duan, D.Z.; Park, D.W.; Ahn, W.S.; Lee, B.J.; Zhang, X.L. Cold plasma for synthesizing high performance bimetallic PdCu catalysts: Effect of reduction sequence and Pd/Cu atomic ratios. Top. Catal. 2017, 12–14, 925–933. [Google Scholar] [CrossRef]
- Di, L.B.; Zhang, J.S.; Zhang, X.L. A review on the recent progress, challenges, and perspectives of atmospheric-pressure cold plasma for preparation of supported metal catalysts. Plasma Processes Polym. 2018, 15, 1700234. [Google Scholar] [CrossRef]
- Fang, G.D.; Dionysiou, D.D.; Wang, Y.; Al-Abed, S.R.; Zhou, D.M. Sulfate radical-based degradation of polychlorinated biphenyls: Effects of chloride ion and reaction kinetics. J. Hazard. Mater. 2012, 227–228, 394–401. [Google Scholar] [CrossRef]
- Bao, L.S.; Zhang, X.F.; Yu, L.; Zhu, G.S. Study on the influence of chloride ion content on pavement performance of base material with fly-ash-flushed-by-seawater. Adv. Mater. Res. 2011, 194–196, 993–1000. [Google Scholar] [CrossRef]
- Tan, E.Z.; Yin, P.G.; Guo, L. SERS-activity of immobilized silver nanoparticles: Effect of chloride ion. J. Light Scatt. 2010, 22, 305–308. [Google Scholar] [CrossRef]
- Hsieh, Y.C.; Senanayake, S.D.; Zhang, Y.; Xu, W.Q.; Polyansky, D.E. Effect of chloride anions on the synthesis and enhanced catalytic activity of silver nanocoral electrodes for CO2 electroreduction. ACS Catal. 2015, 46, 2584–2592. [Google Scholar] [CrossRef]
- Di, L.B.; Zhang, X.L.; Lee, B.; Lu, P.; Ahn, W.S.; Park, D.W. Feasibility of atmospheric-pressure CO cold plasma for reduction of supported metal ions. Plasma Chem. Plasma Process. 2017, 37, 1535–1549. [Google Scholar] [CrossRef]


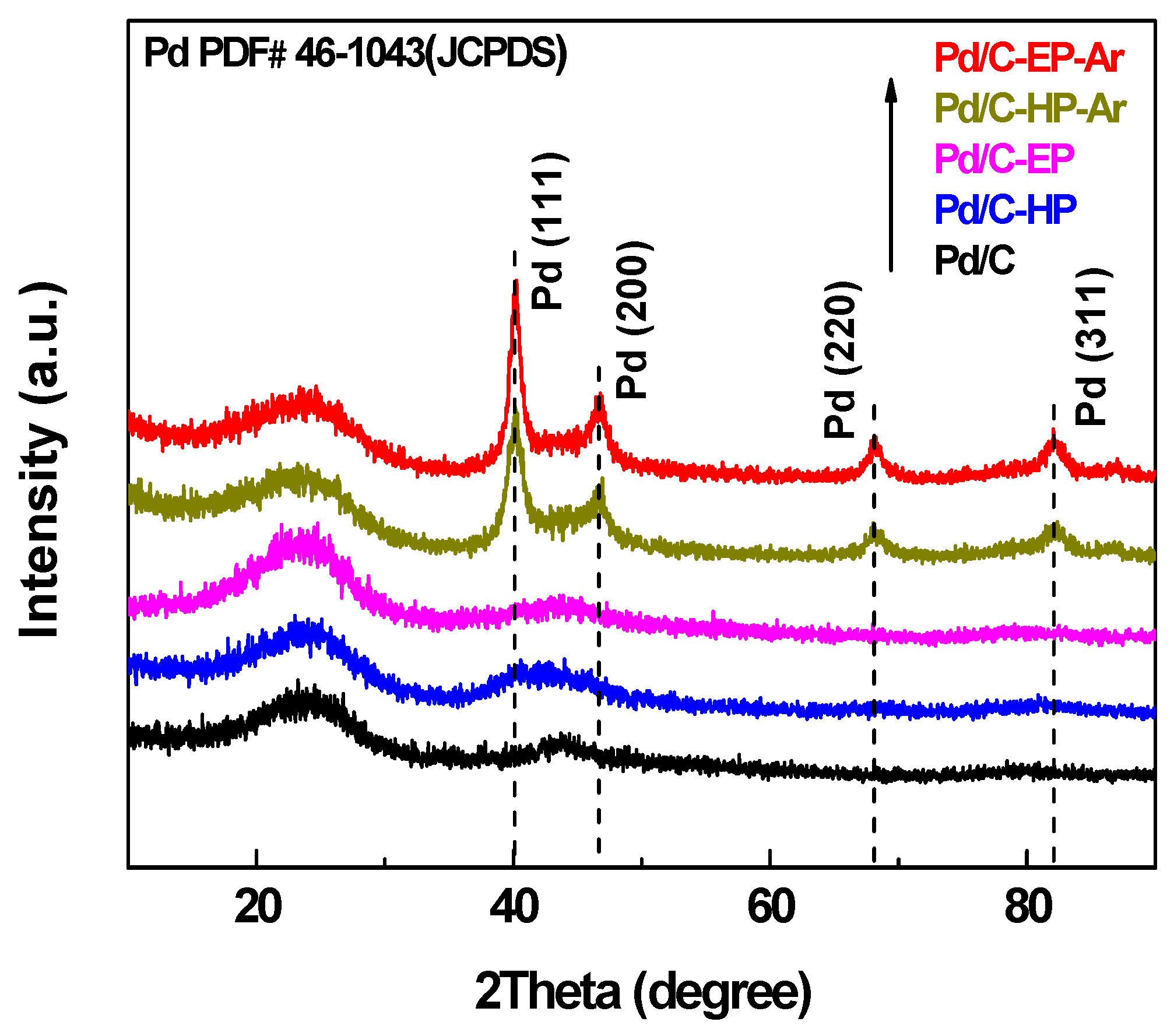



| Samples | Pore Diameter (nm) | Pore Volume (cm3·g−1) | Specific Surface Area (m2·g−1) |
|---|---|---|---|
| Pd/C-EP-Ar | 3.82 | 0.50 | 874 |
| Pd/C-HP-Ar | 3.82 | 0.47 | 871 |
| Pd/C-EP | 3.83 | 0.41 | 703 |
| Pd/C-HP | 3.83 | 0.41 | 736 |
| Pd/C | 3.84 | 0.39 | 690 |
| Catalyst | Pd Composition (%) | Pd/C Atomic Ratio | ||
|---|---|---|---|---|
| Pd0 | Pd2+ | Pd4+ | ||
| Pd/C-EP-Ar | 42.6 | 36.2 | 21.2 | 0.023 |
| Pd/C-HP-Ar | 58.7 | 19.8 | 21.5 | 0.010 |
| Pd/C-EP | 52.1 | 21.4 | 26.5 | 0.013 |
| Pd/C-HP | 61.4 | 31.1 | 7.5 | 0.026 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Zhang, J.; Wang, H.; Li, Z.; Zhang, X.; Di, L. Preparation of Pd/C by Atmospheric-Pressure Ethanol Cold Plasma and Its Preparation Mechanism. Nanomaterials 2019, 9, 1437. https://doi.org/10.3390/nano9101437
Li Z, Zhang J, Wang H, Li Z, Zhang X, Di L. Preparation of Pd/C by Atmospheric-Pressure Ethanol Cold Plasma and Its Preparation Mechanism. Nanomaterials. 2019; 9(10):1437. https://doi.org/10.3390/nano9101437
Chicago/Turabian StyleLi, Zhuang, Jingsen Zhang, Hongyang Wang, Zhihui Li, Xiuling Zhang, and Lanbo Di. 2019. "Preparation of Pd/C by Atmospheric-Pressure Ethanol Cold Plasma and Its Preparation Mechanism" Nanomaterials 9, no. 10: 1437. https://doi.org/10.3390/nano9101437





