Impact of Co2+ Substitution on Microstructure and Magnetic Properties of CoxZn1-xFe2O4 Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
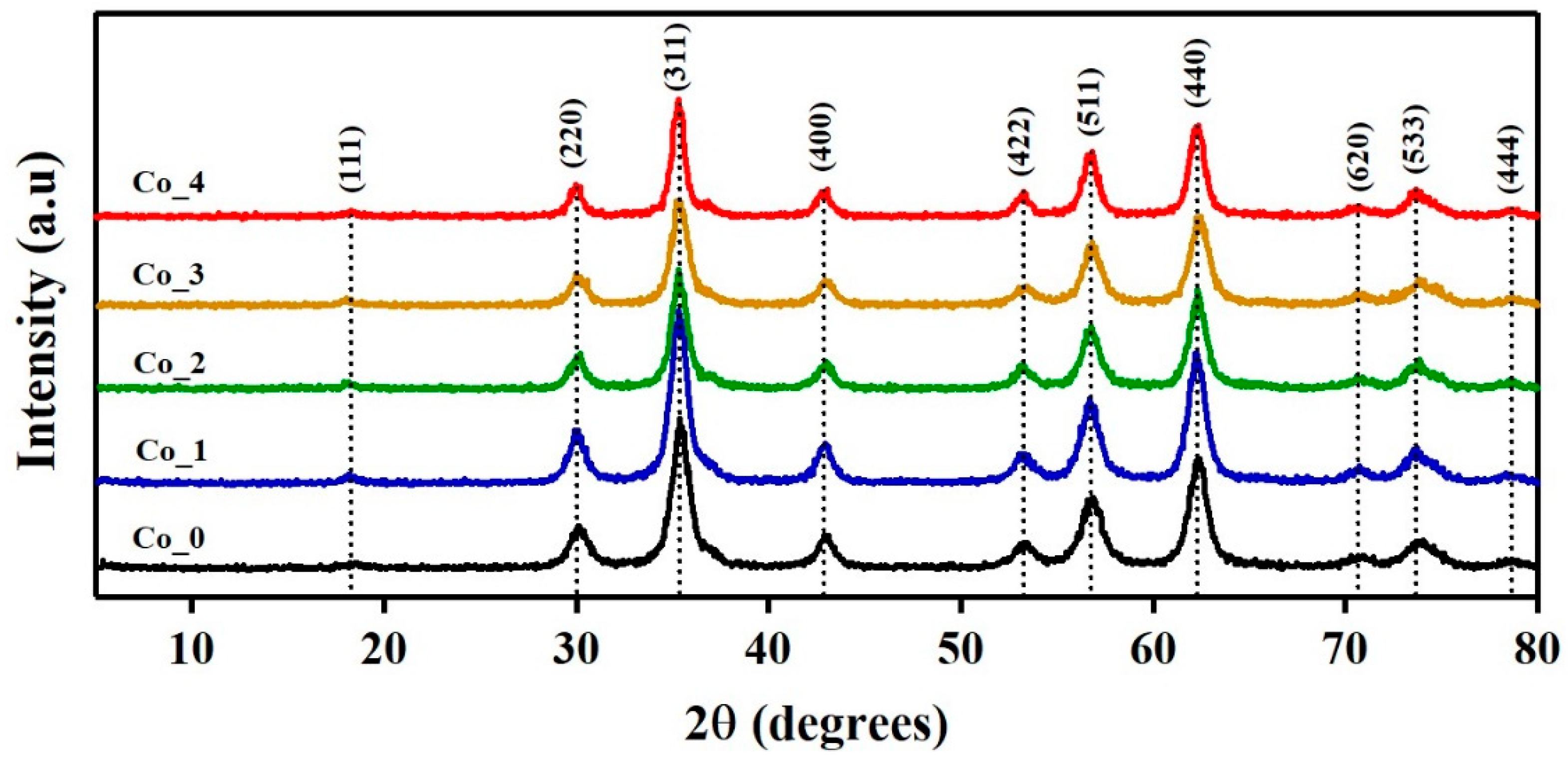
3.1. XRPD Analysis
3.1.1. Phase Identification
3.1.2. Microstructural Studies by Williamson–Hall (W–H) Method
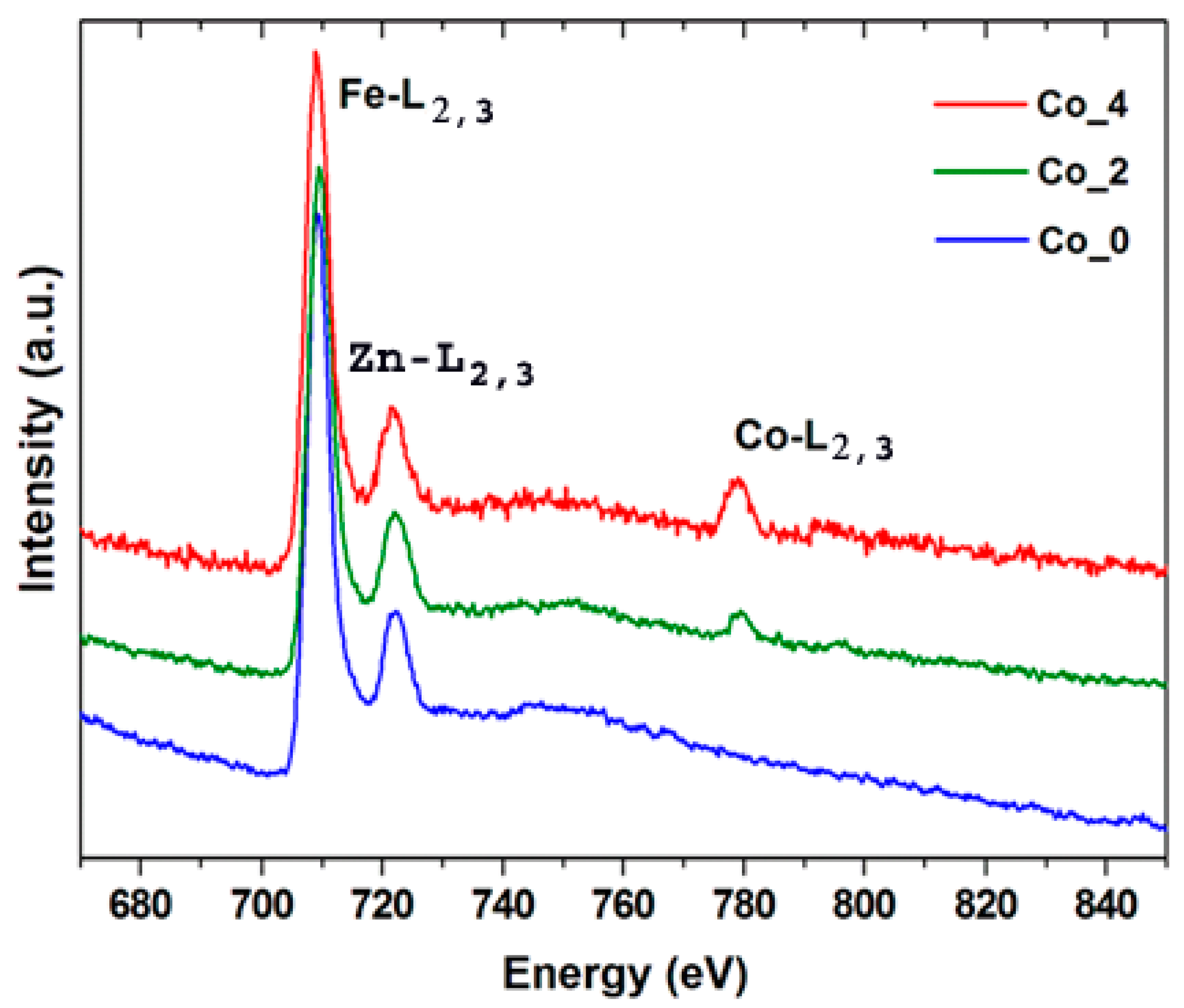
3.2. Electron Microscopy Analysis
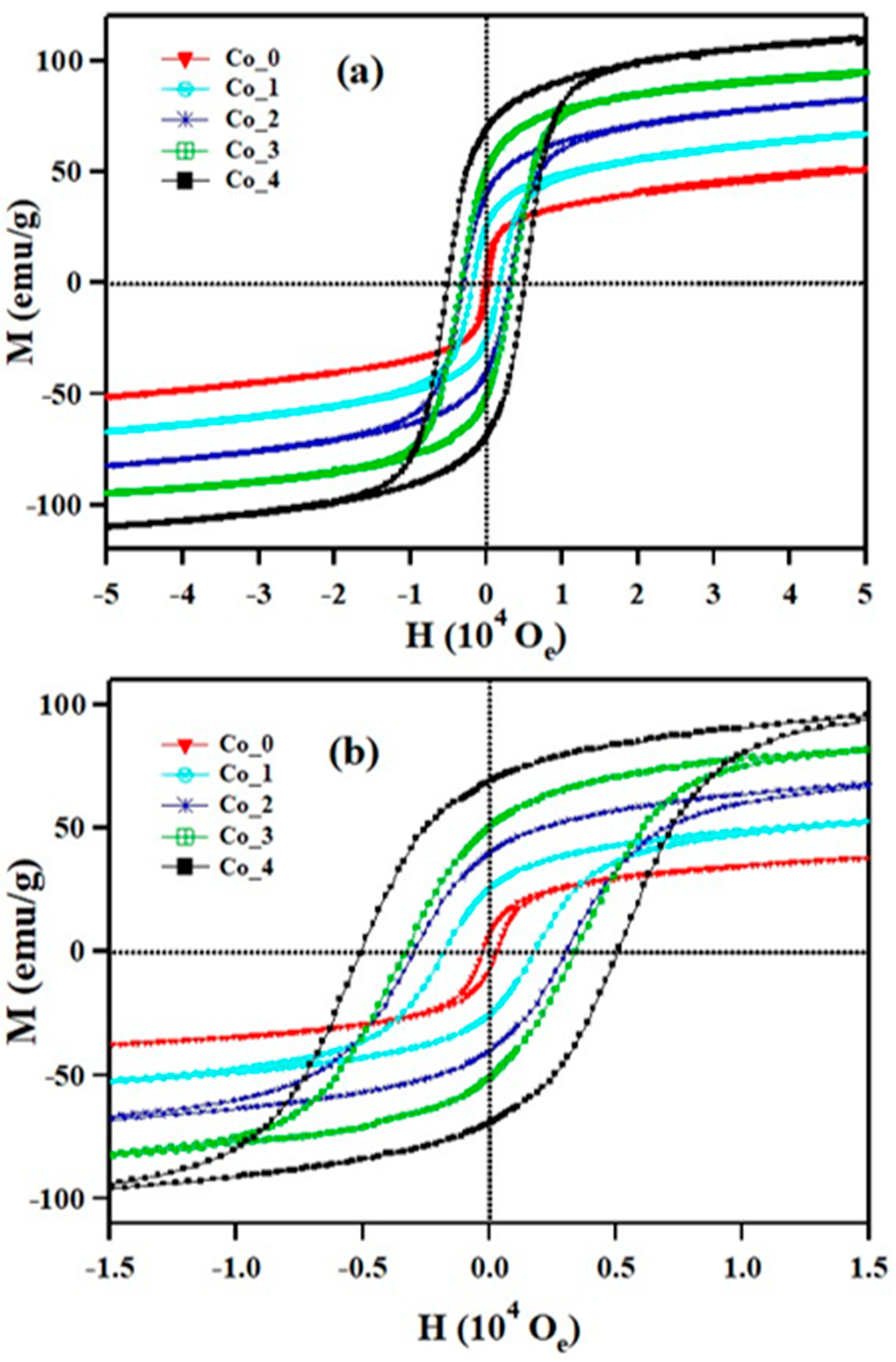
3.3. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aziz, H.S.; Khan, R.A.; Shah, F.; Ismail, B.J.; Nisar, S.M.; Shah, A.; Khan, A.R. Improved electrical, dielectric and magnetic properties of Al-Sm co-doped NiFe2O4 spinel ferrites nanoparticles. Mater. Sci. Eng. B 2019, 243, 47–53. [Google Scholar] [CrossRef]
- Yadav, R.S.; Havlica, J.; Masilko, J.; Kalina, L.; Wasserbauer, J.; Hajdúchová, M.; Enev, V.; Kuritka, I.; Kozáková, Z. Impact of Nd3+ in CoFe2O4 spinel ferrite nanoparticles on cation distribution, structural and magnetic properties. J. Magn. Magn. Mater. 2016, 399, 109–117. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Msagati, T.A.M.; Mamba, B.B. Ferrite nanoparticles: Synthesis, characterisation and applications in electronic device. Mater. Sci. Eng. B 2017, 215, 37–55. [Google Scholar] [CrossRef]
- Frajera, G.; Isnard, O.; Chazal, H.; Delette, G. Effect of cobalt addition on the magneto-crystalline anisotropy parameter of sintered NiZn ferrites evaluated from magnetization curves. J. Magn. Magn. Mater. 2019, 473, 92–98. [Google Scholar] [CrossRef]
- Tovstolytkin, A.I.; Kulyk, M.M.; Kalita, V.M.; Ryabchenko, S.M.; Zamorskyi, V.O.; Fedorchuk, O.P.; Solopan, S.O.; Belous, A.G. Nickel-zinc spinel nanoferrites: Magnetic characterization and prospects of the use in self-controlled magnetic hyperthermia. J. Magn. Magn. Mater. 2019, 473, 422–427. [Google Scholar] [CrossRef]
- Sutka, A.K.; Grossa, A. Spinel ferrite oxide semiconductor gas sensors. Sens. Actuators B 2016, 222, 95–105. [Google Scholar] [CrossRef]
- Han, J.K.; Choi, H.J. Non-stoichiometric zinc-doped spinel ferrite nanoparticles with enhanced magnetic property and their magnetorheology. Colloid Polym. Sci. 2018, 296, 405–409. [Google Scholar] [CrossRef]
- Samavati, A.; Ismail, A.F. Antibacterial properties of copper-substituted cobalt ferrite nanoparticles synthesized by co-precipitation method. Particuology 2017, 30, 158–163. [Google Scholar] [CrossRef]
- Wang, M.; Huang, Y.; Chen, X.; Wang, K.; Wu, H.; Zhang, N.; Fu, H. Synthesis of nitrogen and sulfur co-doped graphene supported hollow ZnFe2O4 nanosphere composites for application in lithium-ion batteries. J. Alloys Compd. 2017, 691, 407–415. [Google Scholar] [CrossRef]
- Yue, H.; Du, T.; Wang, Q.; Shi, Z.; Dong, H.; Cao, Z.; Qiao, Y.; Yin, Y.; Xing, R.; Yang, S. Biomimetic Synthesis of Polydopamine Coated ZnFe2O4 Composites as Anode Materials for Lithium-Ion Batteries. ACS Omega 2018, 3, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Gao, S.; Wang, C.; Chai, B.; Li, J.; Song, G.; Chen, S. A facile electrospinning and direct annealing method for the fabrication of multi-porous ZnFe2O4 nanotubes with enhanced photocatalytic activity. Mater. Lett. 2016, 184, 43–46. [Google Scholar] [CrossRef]
- Petrova, E.; Kotsikau, D.; Pankov, V.; Fahmi, A. Influence of synthesis methods on structural and magnetic characteristics of Mg–Zn-ferrite nanopowders. J. Magn. Magn. Mater. 2019, 473, 85–91. [Google Scholar] [CrossRef]
- Harrisa, V.G.; Šepelák, V. Mechanochemically processed zinc ferrite nanoparticles: Evolution of structure and impact of induced cation inversion. J. Magn. Magn. Mater. 2018, 465, 603–610. [Google Scholar] [CrossRef]
- Li, S.; John, V.T.; O’Connor, C.; Harris, V.; Carpenter, E. Cobalt-ferrite nanoparticles: Structure, cation distributions, and magnetic properties. J. Appl. Phys. 2000, 87, 6223. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Khan, M.A. Effect of rare earth doping on the structural and magnetic features of nanocrystalline spinel ferrites prepared via sol gel route. J. Magn. Magn. Mater. 2018, 460, 268–277. [Google Scholar] [CrossRef]
- Tatarchuk, T.R.; Bououdina, M.; Paliychuk, N.D.; Yaremiy, I.P.; Moklyak, V.V. Structural characterization and antistructure modeling of cobalt-substituted zinc ferrites. J. Alloys Compd. 2017, 694, 777–791. [Google Scholar] [CrossRef]
- Mallick, A.; Mahapatra, A.S.; Mitra, A.; Greneche, J.M.; Ningthoujam, R.S.; Chakrabarti, P.K. Magnetic properties and bio-medical applications in hyperthermia of lithium zinc ferrite nanoparticles integrated with reduced graphene oxide. J. Appl. Phys. 2018, 123, 055103. [Google Scholar] [CrossRef]
- Kefeni, K.K.; Mamba, B.B.; Msagati, T.A.M. Application of spinel ferrite nanoparticles in water and wastewater treatment: A review. Sep. Purif. Technol. 2017, 188, 399–422. [Google Scholar] [CrossRef]
- Sagayaraj, R.; Aravazhi, S.; Praveen, P.; Chandrasekaran, G. Structural, morphological and magnetic characters of PVP coated ZnFe2O4 nanoparticles. J. Mater. Sci. Mater. Electron. 2018, 29, 2151–2158. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, S.; Li, X.; Huang, Y.; Wang, Z.; Han, Y.; Wu, J.; Meng, H.; Zhang, X. Synthesis, characterization, and photocatalytic degradation properties of ZnO/ZnFe2O4 magnetic heterostructures. New J. Chem. 2017, 41, 15433–15438. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Abdelbaky, M.S.M.; Martínez-Blanco, D.; Amghouz, Z.; García-Granda, S. Effect of chromium substitution on the structural and magnetic properties of nanocrystalline zinc ferrite. Mater. Chem. Phys. 2016, 174, 164–171. [Google Scholar]
- Feng, J.; Xiong, R.; Liu, Y.; Su, F.; Zhang, X. Preparation of cobalt substituted zinc ferrite nanopowders via auto-combustion route: An investigation to their structural and magnetic properties. J. Mater. Sci. Mater. Electron. 2018, 29, 18358–18371. [Google Scholar] [CrossRef]
- Phan, T.L.; Tran, N.; Kim, D.H.; Dang, N.T.; Manh, D.H.; Bach, T.N.; Liu, C.L.; Lee, B.W. Magnetic and Magnetocaloric Properties of Zn1−x Co xFe2O4 Nanoparticles. J. Electron. Mater. 2017, 46, 4214–4226. [Google Scholar] [CrossRef]
- Sharifi, I.; Shokrollahi, H. Nanostructural, magnetic and Mössbauer studies of nanosized Co1−xZnxFe2O4 synthesized by co-precipitation. J. Magn. Magn. Mater. 2012, 324, 2397–2403. [Google Scholar] [CrossRef]
- Köseoǧlu, Y.; Baykal, A.; Gözüak, F.; Kavas, H. Structural and magnetic properties of CoxZn1−xFe2O4 nanocrystals synthesized by microwave method. Polyhedron 2009, 28, 2887–2892. [Google Scholar] [CrossRef]
- Singh, C.; Jauhar, S.; Kumar, V.; Singh, J.; Singhal, S. Synthesis of zinc substituted cobalt ferrites via reverse micelle technique involving in situ template formation: A study on their structural, magnetic, optical and catalytic properties. Mater. Chem. Phys. 2015, 156, 188–197. [Google Scholar] [CrossRef]
- Shoba, M.; Kaleemulla, S. Structural, optical and dielectric studies of Er substituted zinc ferrite nanospheres. J. Phys. Chem. Solids 2017, 111, 447–457. [Google Scholar] [CrossRef]
- Malik, H.; Mahmood, A.; Mahmood, K.; Lodhi, M.Y.; Warsi, M.F.; Shakir, I.; Wahab, H.; Asghar, M.; Khan, M.A. Influence of cobalt substitution on the magnetic properties of zinc nanocrystals synthesized via micro-emulsion route. Ceram. Int. 2014, 40, 9439–9444. [Google Scholar] [CrossRef]
- Gómez-Polo, C.; Recarte, V.; Cervera, L.; Beato-López, J.J.; López-García, J.; Rodríguez-Velamazán, J.A.; Ugarte, M.D.; Mendonça, E.C.; Duque, J.G.S. Tailoring the structural and magnetic properties of Co-Zn nanosized ferrites for hyperthermia applications. J. Magn. Magn. Mater. 2018, 465, 211–219. [Google Scholar] [CrossRef]
- Gözüak, F.; Köseoğlu, Y.; Baykal, A.; Kavas, H. Synthesis and characterization of CoxZn1−xFe2O4 magnetic nanoparticles via a PEG-assisted route. J. Magn. Magn. Mater. 2009, 321, 2170–2177. [Google Scholar] [CrossRef]
- Atif, M.; Asghar, M.W.; Nadeem, M.; Khalid, W.; Ali, Z.; Badshah, S. Synthesis and investigation of structural, magnetic and dielectric properties of zinc substituted cobalt ferrites. J. Phys. Chem. Solids 2018, 123, 36–42. [Google Scholar] [CrossRef]
- Hassanien, A.S.; Ak, A.A.; Saaedi, A.H. Synthesis, crystallography, microstructure, crystal defects, and morphology of BixZn1−xO nanoparticles prepared by sol–gel technique. CrystEngComm 2018, 20, 1716–1730. [Google Scholar] [CrossRef]
- Akl, A.A.; Mahmoud, S.A.; AL-Shomar, S.M.; Hassanien, A.S. Improving microstructural properties and minimizing crystal imperfections of nanocrystalline Cu2O thin films of different solution molarities for solar cell applications. Mater. Sci. Semicond. Process. 2018, 74C, 183–192. [Google Scholar] [CrossRef]
- Yogamalar, R.; Srinivasan, R.; Vinu, A.; Ariga, K.; Bose, A.C. X-ray peak broadening analysis in ZnO nanoparticles. Solid State Commun. 2009, 149, 1919–1923. [Google Scholar] [CrossRef]
- Mohamed, W.S.; Abu-Dief, A.M. Synthesis, characterization and photocatalysis enhancement of Eu2O3-ZnO mixed oxide nanoparticles. J. Phys. Chem. Solids. 2018, 116, 375–385. [Google Scholar] [CrossRef]
- Abu-Dief, A.M.; Mohamed, W.S. α-Bi2O3 nanorods: Synthesis, characterization and UV-photocatalytic activity. Mater. Res. Express 2017, 4, 035039. [Google Scholar] [CrossRef]
- Yadav, R.S.; Havlica, J.; Hnatko, M.; Šajgalík, P.; Alexander, C.; Palou, M.; Bartoníčková, E.; Boháč, M.; Frajkorová, F.; Masilko, J.; et al. Magnetic properties of Co1−xZnxFe2O4 spinel ferrite nanoparticles synthesized by starch-assisted sol–gel autocombustion method and its ball milling. J. Magn. Magn. Mater. 2015, 378, 190–199. [Google Scholar] [CrossRef]
- Yadav, R.S.; Kuritka, I.; Havlica, J.; Hnatko, M.; Alexander, C.; Masilko, J.; Kalina, L.; Hajdúchová, M.; Rusnak, J.; Enev, V. Structural, magnetic, elastic, dielectric and electrical properties of hot-press sintered Co1−xZnxFe2O4 (x = 0.0, 0.5) spinel ferrite nanoparticles. J. Magn. Magn. Mater. 2018, 477, 48–57. [Google Scholar] [CrossRef]
- Tatarchuk, T.R.; Paliychuk, N.D.; Bououdina, M.; Al-Najar, B.; Pacia, M.; Macyk, W.; Shyichuk, A. Effect of cobalt substitution on structural, elastic, magnetic and optical properties of zinc ferrite nanoparticles. J. Alloys Compd. 2018, 731, 1256–1266. [Google Scholar] [CrossRef]
- Milanovic, M.; Moshopoulou, E.G.; Stamopoulos, D.; Devlin, E.; Giannakopoulos, K.P.; Kontos, A.G.; Eleftheriadis, K.; Gini, M.I.; Nikolic, L.M. Structure and magnetic properties of Zn1−xInxFe2O4 and ZnYxFe2−xO4 nanoparticles prepared by coprecipitation. Ceram. Int. 2013, 39, 3235–3242. [Google Scholar] [CrossRef]
- Houshiar, M.; Zebhi, F.; Razi, Z.J.; Alidoust, A.; Askari, Z. Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, coprecipitation, and precipitation methods: A comparison study of size, structural, and magnetic properties. J. Magn. Magn. Mater. 2014, 371, 43–48. [Google Scholar] [CrossRef]
- Rao, K.S.; Choudary, G.; Rao, K.H.; Sujatha, C. Structural and Magnetic Properties of Ultrafine CoFe2O4 Nanoparticles. Procedia Mater. Sci. 2015, 10, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Thakur, P.; Kumar, M.; Thakur, N.; Negi, N.S.; Sharma, P.; Sharma, V. Improvement in magnetic behaviour of cobalt doped magnesium zinc nano-ferrites via co-precipitation route. J. Alloys Compd. 2016, 684, 569–581. [Google Scholar] [CrossRef]
- Petrova, E.; Kotsikau, D.; Pankov, V. Structural characterization and magnetic properties of sol–gel derived ZnxFe3–xO4 nanoparticles. J. Magn. Magn. Mater. 2015, 378, 429–435. [Google Scholar] [CrossRef]
- Chang, Y.; Shi, J.; Tang, Y.; Zhang, H.; Yue, Z.; Yao, W.; Bai, Y.; Cao, J. Investigation of significant magnetic transformation for hydrogenated ZnFe2O4 nanoparticles. J. Mater. Sci. 2019, 1–11. [Google Scholar] [CrossRef]
- Akhtar, M.N.; Khan, M.A.; Ahmad, M.; Nazir, M.S.; Imran, M.; Ali, A.; Sattar, A.; Murtaza, G. Evaluation of structural, morphological and magnetic properties of CuZnNi (CuxZn0.5−xNi0.5Fe2O4) nanocrystalline ferrites for core, switching and MLCI’s applications. J. Magn. Magn. Mater. 2017, 421, 260–268. [Google Scholar] [CrossRef]
- Jesus, J.R.; Lima, R.J.S.; Moura, K.O.; Duque, J.G.S.; Meneses, C.T. Anisotropic growth of α -Fe2O3 nanostructures. Ceram. Int. 2018, 44, 3585–3589. [Google Scholar] [CrossRef]
- Ebbing, A.; Hellwig, O.; Agudo, L.; Eggeler, G.; Petracic, O. Tuning the magnetic properties of Co nanoparticles by Pt capping. Phys. Rev. B 2011, 84, 012405. [Google Scholar] [CrossRef] [Green Version]
- Zysler, R.D.; Fiorani, D.; Testa, A.M. Investigation of magnetic properties of interacting Fe2O3 nanoparticles. J. Magn. Magn. Mater. 2001, 224, 5–11. [Google Scholar] [CrossRef]
- Peixoto, E.B.; Carvalho, M.H.; Meneses, C.T.; Sarmento, V.H.V.; Coelho, A.A.; Zucolotto, B.; Duque, J.G.S. Analysis of zero field and field cooled magnetization curves of CoFe2O4 nanoparticles with a T-dependence on the saturation magnetization. J. Alloys Compd. 2017, 721, 525–530. [Google Scholar] [CrossRef]
- Luchini, A.; Heenan, R.K.; Paduano, L.; Vitiello, G. Functionalized SPIONs: The surfactant nature modulates the self-assembly and cluster formation. Phys. Chem. Chem. Phys. 2016, 18, 18441–18449. [Google Scholar] [CrossRef] [PubMed]
- Bini, M.; Tondo, C.; Capsoni, D.; Mozzati, M.C.; Albini, B.; Galinetto, P. Superparamagnetic ZnFe2O4 nanoparticles: The effect of Ca and Gd doping. Mater. Chem. Phys. 2018, 204, 72–82. [Google Scholar] [CrossRef]
- Demirci, Ç.E.; Manna, P.K.; Wroczynskyj, Y.; Aktürk, S.; van Lierop, J. A comparison of the magnetism of cobalt-, manganese-, and nickel-ferrite nanoparticles. J. Phys. Appl. Phys. 2017, 51, 025003. [Google Scholar] [CrossRef]
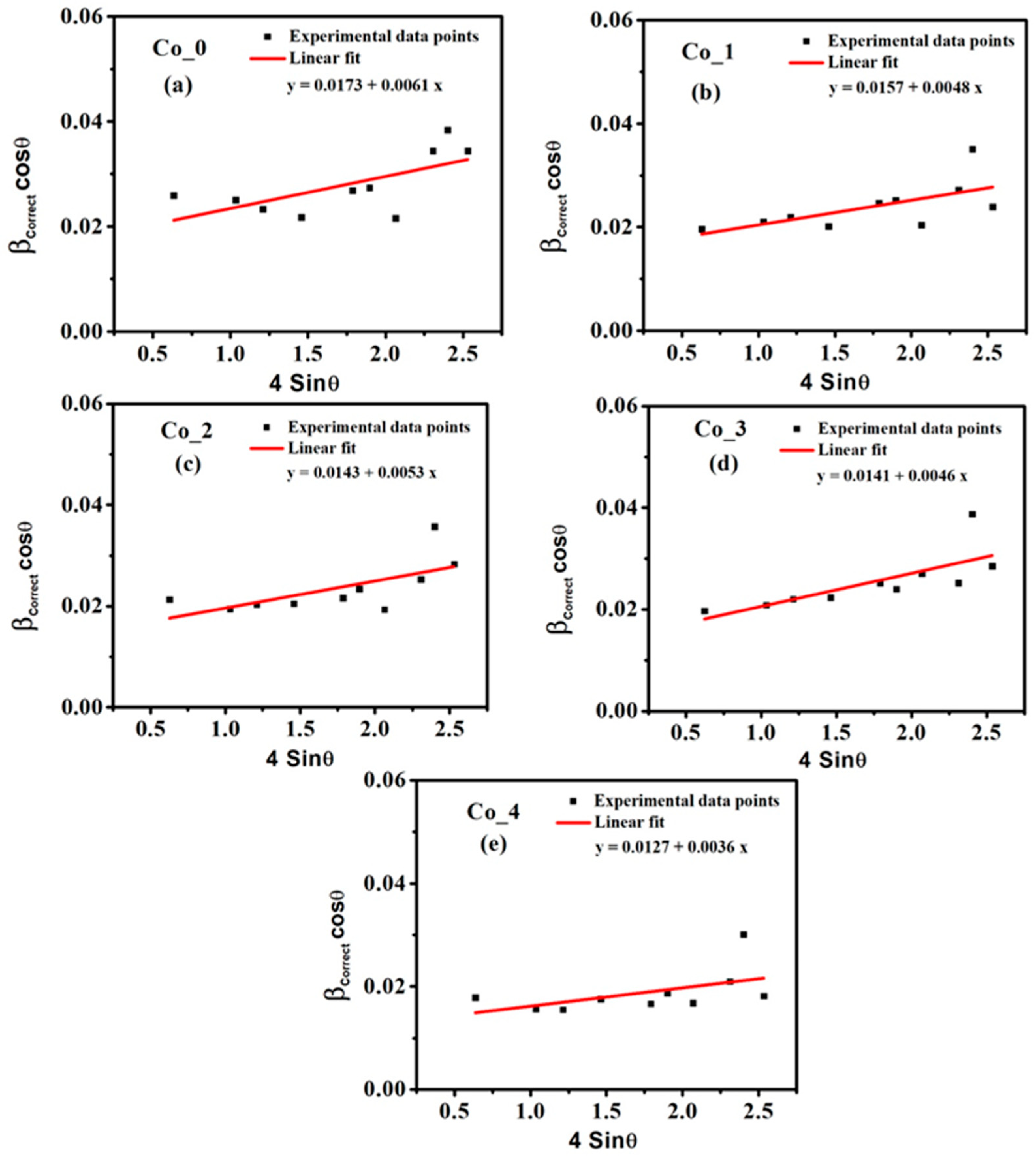




| Sample | DL (nm) | εL × 10−3 | δL × 10−3 Line per cm−2 | a (Å) |
|---|---|---|---|---|
| Co_0 | 8.378 | 6.1 | 14 | 8.454 |
| Co_1 | 9.231 | 4.8 | 11.7 | 8.447 |
| Co_2 | 10.135 | 5.3 | 9.7 | 8.446 |
| Co_3 | 10.279 | 4.6 | 9.4 | 8.440 |
| Co_4 | 11.412 | 3.6 | 7.6 | 8.431 |
| Sample | Element | Theoretical | STEM-EDS | EELS |
|---|---|---|---|---|
| Co_2 | Fe Zn | 66.7 26.7 | 65.6 27.1 | 66.5 - |
| Co | 6.6 | 7.3 | 6.8 | |
| Co_4 | Fe Zn | 66.7 20.0 | 66.2 18.5 | 65.8 - |
| Co | 13.3 | 15.3 | 14.2 |
| Sample | HC (Oe) | Mr (emu/g) | MS (emu/g) | Mr/MS | k (103 erg/g) | TB (K) | Tirr (K) |
|---|---|---|---|---|---|---|---|
| Co_0 | 300 | 7.3 | 40 | 0.18 | 12.5 | 25 | 24.6 |
| Co_1 | 1900 | 25.5 | 55 | 0.46 | 108.8 | 62 | 73 |
| Co_2 | 3100 | 40 | 71 | 0.56 | 229.3 | 114 | 133 |
| Co_3 | 3400 | 51 | 84 | 0.61 | 297.5 | 170 | 209 |
| Co_4 | 5100 | 69 | 98 | 0.70 | 520.6 | 240 | 284 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamed, W.S.; Alzaid, M.; S. M. Abdelbaky, M.; Amghouz, Z.; García-Granda, S.; M. Abu-Dief, A. Impact of Co2+ Substitution on Microstructure and Magnetic Properties of CoxZn1-xFe2O4 Nanoparticles. Nanomaterials 2019, 9, 1602. https://doi.org/10.3390/nano9111602
Mohamed WS, Alzaid M, S. M. Abdelbaky M, Amghouz Z, García-Granda S, M. Abu-Dief A. Impact of Co2+ Substitution on Microstructure and Magnetic Properties of CoxZn1-xFe2O4 Nanoparticles. Nanomaterials. 2019; 9(11):1602. https://doi.org/10.3390/nano9111602
Chicago/Turabian StyleMohamed, W. S., Meshal Alzaid, Mohammed S. M. Abdelbaky, Zakariae Amghouz, Santiago García-Granda, and Ahmed M. Abu-Dief. 2019. "Impact of Co2+ Substitution on Microstructure and Magnetic Properties of CoxZn1-xFe2O4 Nanoparticles" Nanomaterials 9, no. 11: 1602. https://doi.org/10.3390/nano9111602








