Improving Photocatalytic Degradation Activity of Organic Pollutant by Sn4+ Doping of Anatase TiO2 Hierarchical Nanospheres with Dominant {001} Facets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
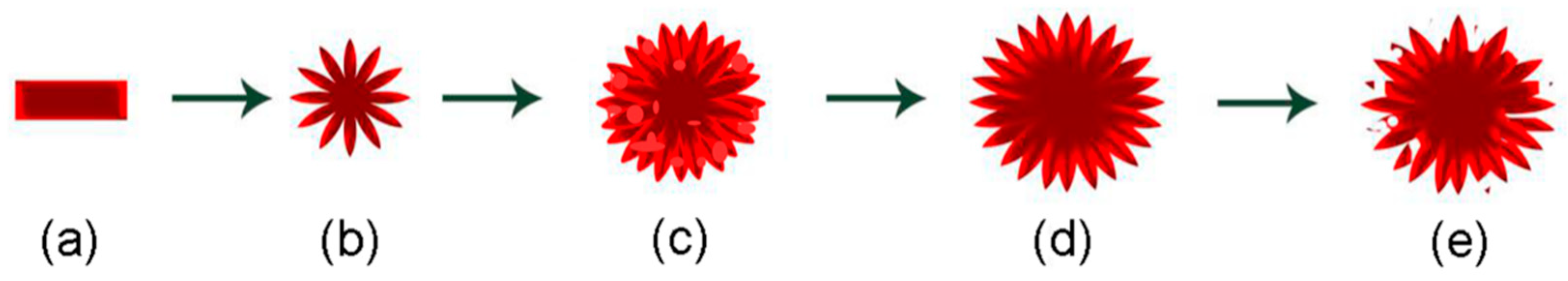
2.2. Preparation of TiO2 Hierarchical Nanospheres
2.3. Preparation of STO Hierarchical Nanospheres
2.4. Characterization
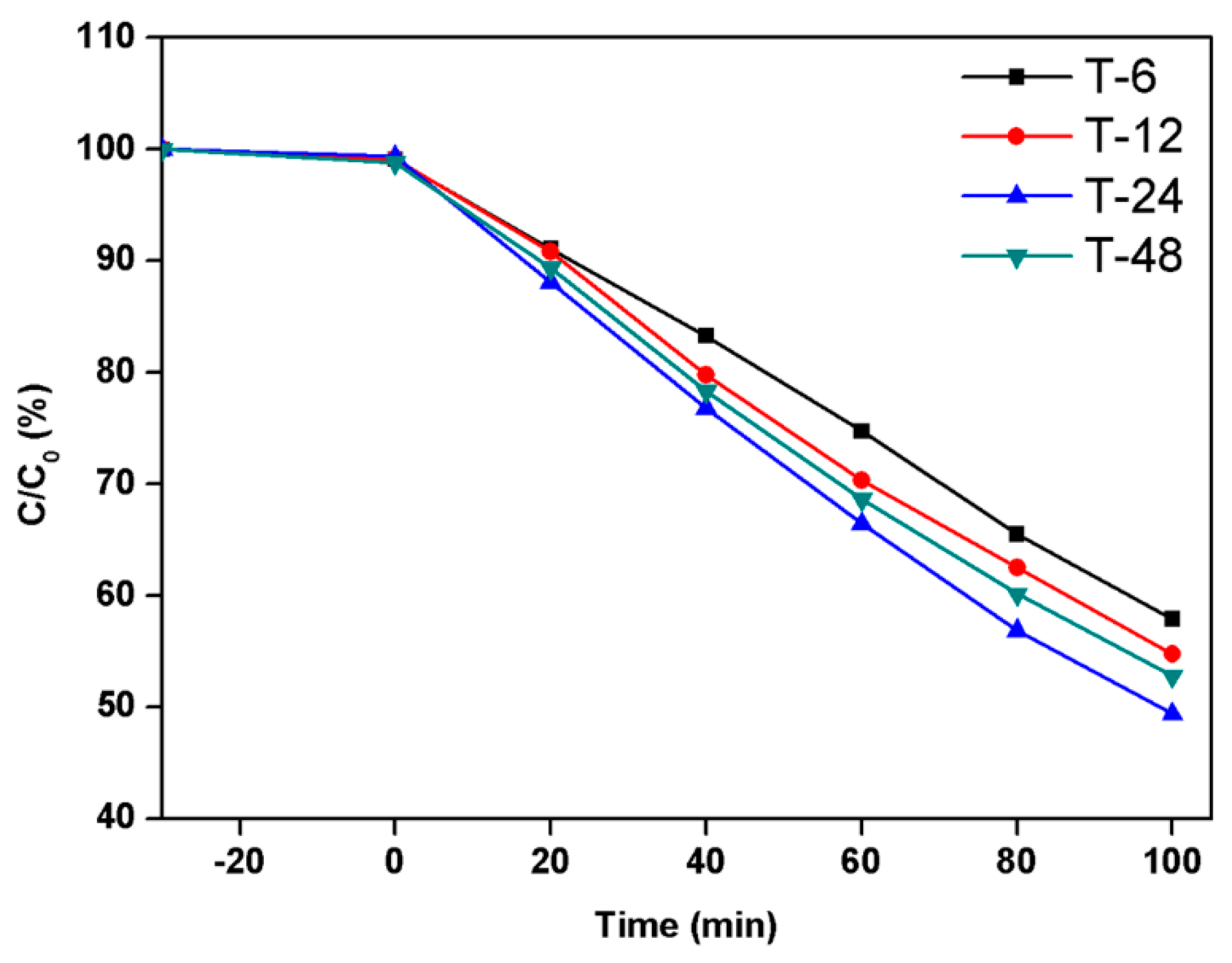
2.5. Photocatalytic Activity Tests
2.6. Radical Trapping Experiments
3. Results and Discussion
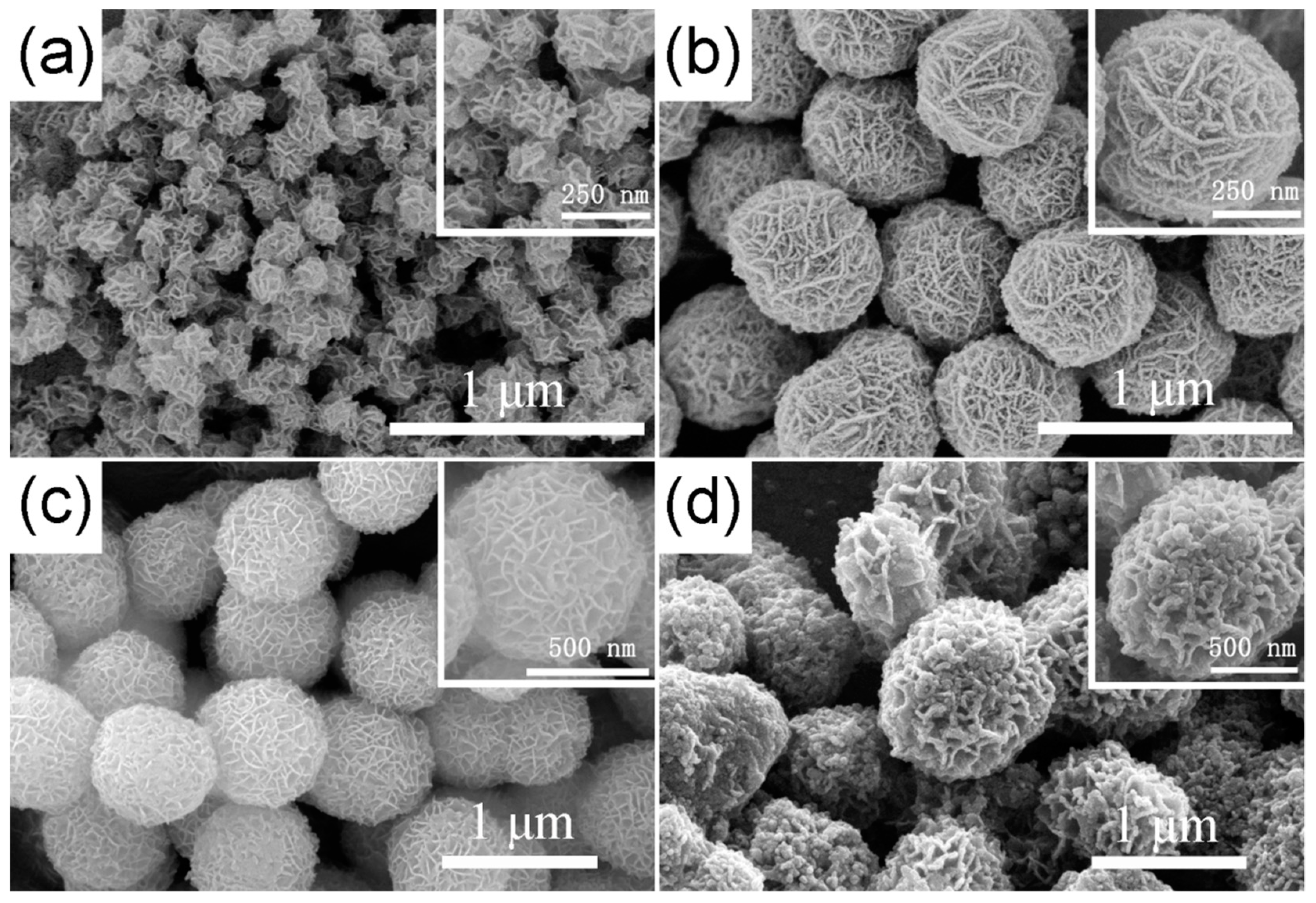
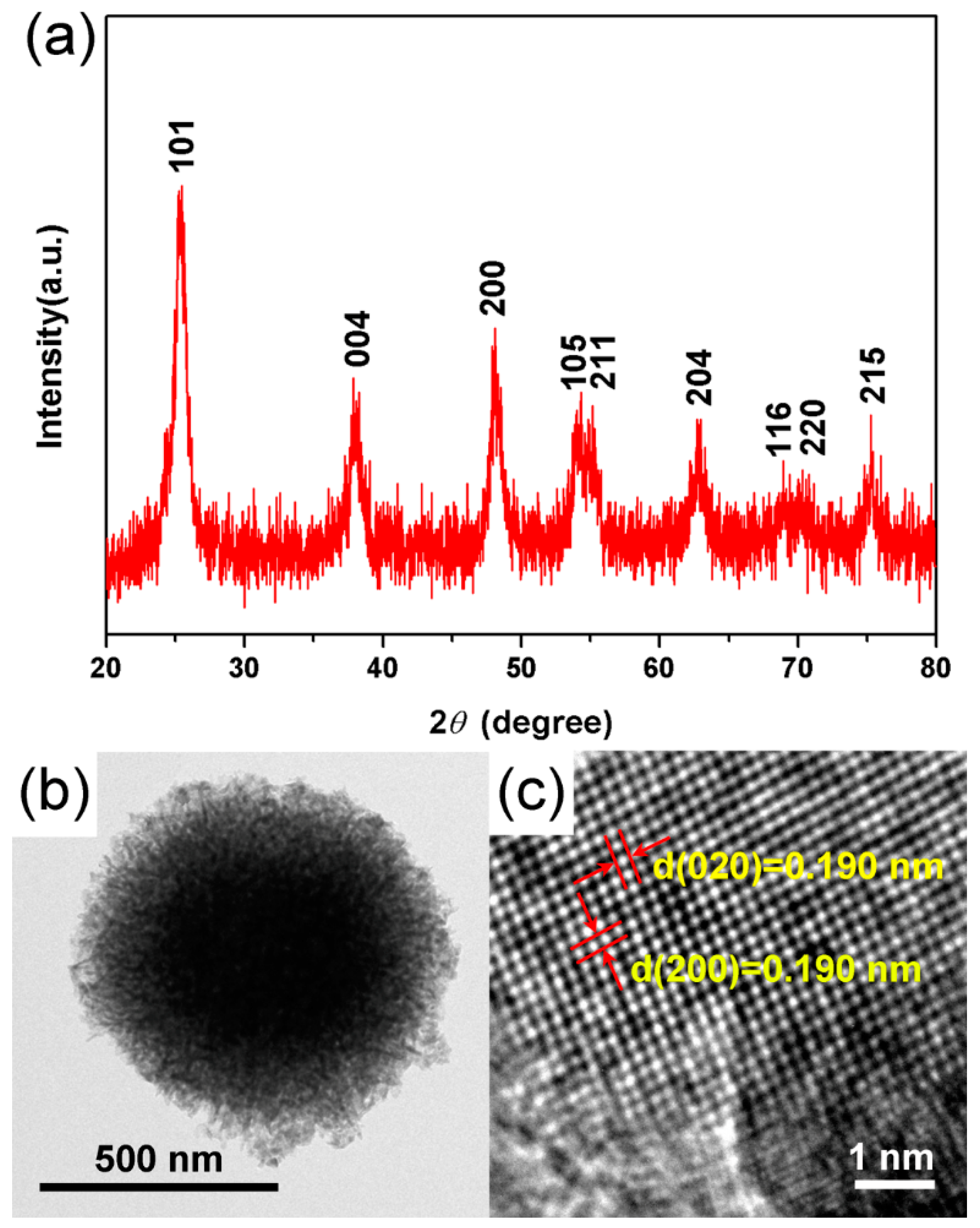
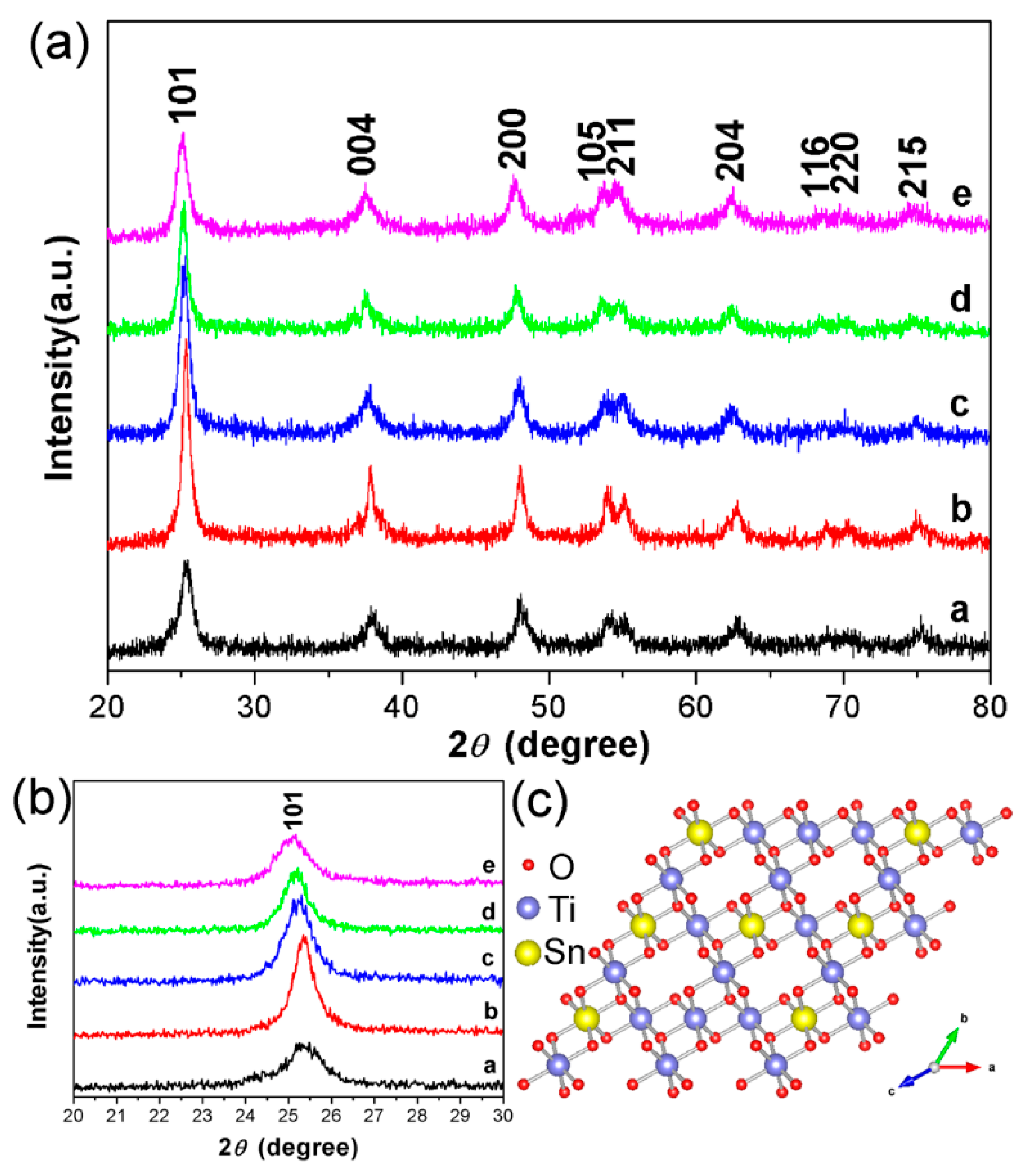
3.1. Characterizations of TiO2 Hierarchical Nanospheres
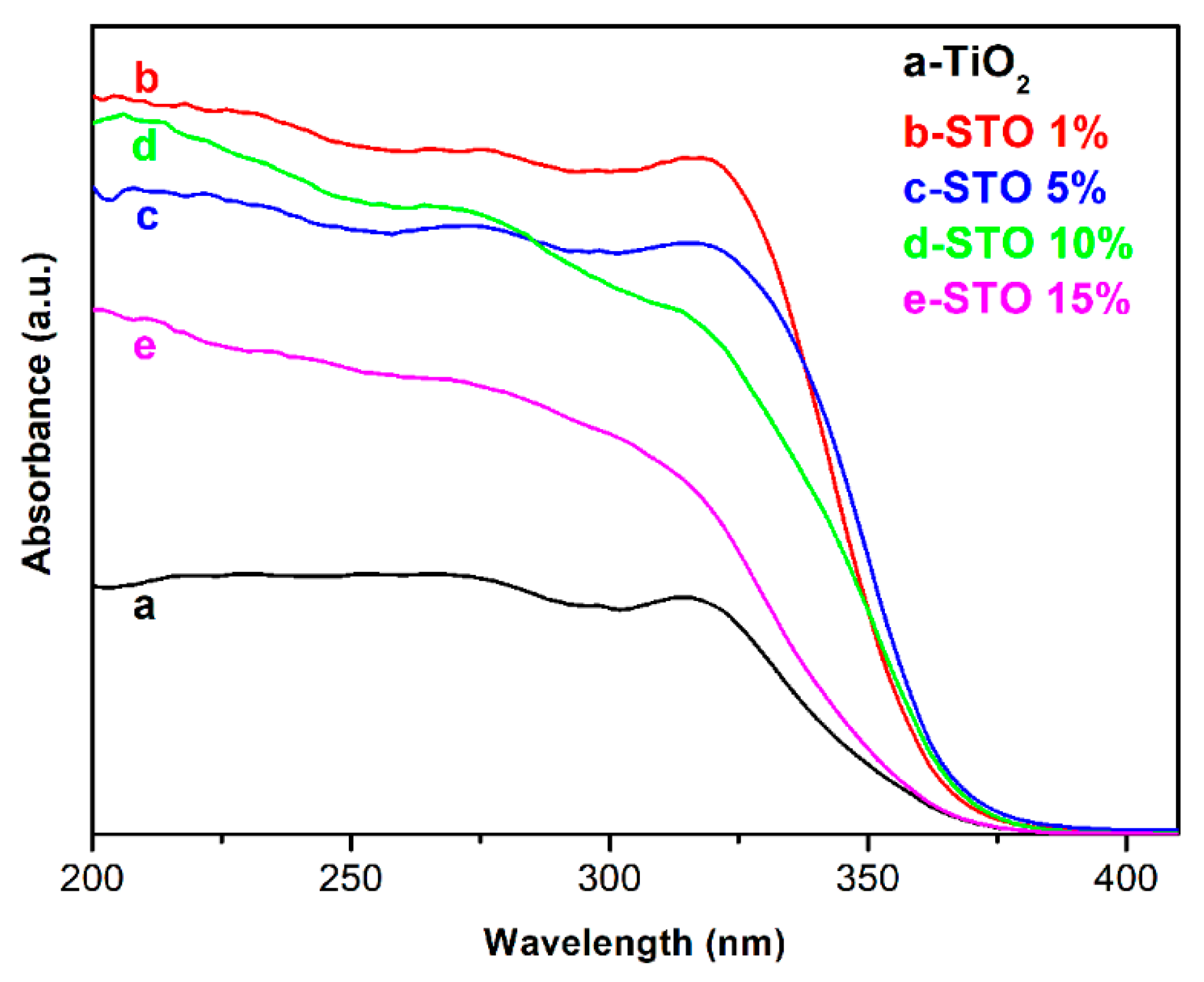
3.2. Characterizations of STO Hierarchical Nanospheres
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carey, J.H.; Lawrence, J.; Tosine, H.M. Photodechlorination of PCB’s in the presence of titanium dioxide in aqueous suspensions. Bull. Environ. Contam. Toxicol. 1976, 16, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Borràs-Ferrís, J.; Sánchez-Tovar, R.; Blasco-Tamarit, E.; Muñoz-Portero, M.J.; Fernández-Domene, R.M.; García-Antón, J. TiO2 Nanostructures for Photoelectrocatalytic Degradation of Acetaminophen. Nanomaterials 2019, 9, 583. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Cui, H.; Song, Q.; Zhang, L.; Song, X.; Wang, K.; Zhang, Y.; Li, J.; Wen, J.; Tian, J. Ag2O nanoparticle/TiO2 nanobelt heterostructures with remarkable photo-response and photocatalytic properties under UV, visible and near-infrared irradiation. Appl. Catal. B Environ. 2016, 198, 83–90. [Google Scholar] [CrossRef]
- Meng, A.; Zhang, L.; Cheng, B.; Yu, J. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Fu, W.; Yang, H.; Zhang, L.; Ma, J.; Zhao, H.; Sun, M.; Yang, L. Photoelectrochemical Performance of Multiple Semiconductors (CdS/CdSe/ZnS) Cosensitized TiO2 Photoelectrodes. J. Phys. Chem. C 2012, 116, 2615–2621. [Google Scholar] [CrossRef]
- Mi, Q.; Zhanaidarova, A.; Brunschwig, B.S.; Gray, H.B.; Lewis, N.S. A quantitative assessment of the competition between water and anion oxidation at WO3 photoanodes in acidic aqueous electrolytes. Energy Environ. Sci. 2012, 5, 5694–5700. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Paszkiewicz-Gawron, M.; Gołabiewska, A.; Pancielejko, A.; Lisowski, W.; Zwara, J.; Paszkiewicz, M.; Zaleska-Medynska, A.; Łuczak, J. Impact of Tetrazolium Ionic Liquid Thermal Decomposition in Solvothermal Reaction on the Remarkable Photocatalytic Properties of TiO2 Particles. Nanomaterials 2019, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Sun, M.; Zhao, G.; Yin, G.; Liu, B. Hierarchical Fe2O3 nanorods/TiO2 nanosheets heterostructure: Growth mechanism, enhanced visible-light photocatalytic and photoelectrochemical performances. Appl. Surf. Sci. 2019, 475, 380–388. [Google Scholar] [CrossRef]
- Negrín-Montecelo, Y.; Testa-Anta, M.; Marín-Caba, L.; Pérez-Lorenzo, M.; Salgueiriño, V.; Correa-Duarte, M.A.; Comesaña-Hermo, M. Titanate Nanowires as One-Dimensional Hot Spot Generators for Broadband Au–TiO2 Photocatalysis. Nanomaterials 2019, 9, 990. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.G.; Sun, C.H.; Qiao, S.Z.; Zou, J.; Liu, G.; Smith, S.C.; Cheng, H.M.; Lu, G.Q. Anatase TiO2 single crystals with a large percentage of reactive facets. Nature 2008, 453, 638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Sun, M.; Liu, X.; Xuan, J.; Kong, W.; Zhang, R.; Sun, Y.; Jia, F.; Yin, G.; Liu, B. Fabrication of CdS quantum dots sensitized ZnO nanorods/TiO2 nanosheets hierarchical heterostructure films for enhanced photoelectrochemical performance. Electrochim. Acta 2019, 204, 334–341. [Google Scholar] [CrossRef]
- Menga, A.; Zhang, J.; Xu, D.; Cheng, B.; Yu, J. Enhanced photocatalytic H2-production activity of anatase TiO2 nanosheet by selectively depositing dual-cocatalysts on {101} and {001} facets. Appl. Catal. B Environ. 2016, 198, 286–294. [Google Scholar] [CrossRef]
- Gan, W.; Fu, X.; Zhang, J. Ag@AgCl decorated graphene-like TiO2 nanosheets with nearly 100% exposed (001) facets for efficient solar light photocatalysis. Mater. Sci. Eng. B 2018, 229, 44–52. [Google Scholar] [CrossRef]
- Diak, M.; Grabowska, E.; Zaleska, A. Synthesis, characterization and photocatalytic activity of noble metal-modified TiO2 nanosheets with exposed {001} facets. Appl. Surf. Sci. 2015, 347, 275–285. [Google Scholar] [CrossRef]
- Wang, W.; Wang, D.; Qu, W.; Lu, L.; Xu, A. Large Ultrathin Anatase TiO2 Nanosheets with Exposed {001} Facets on Graphene for Enhanced Visible Light Photocatalytic Activity. J. Phys. Chem. C 2012, 116, 19893–19901. [Google Scholar] [CrossRef]
- Cottineau, T.; Béalu, N.; Gross, P.A.; Pronkin, S.N.; Keller, N.; Savinova, E.R.; Keller, V. One step synthesis of niobium doped titania nanotube arrays to form (N,Nb) co-doped TiO2 with high visible light photoelectrochemical activity. J. Mater. Chem. A 2013, 1, 2151–2160. [Google Scholar] [CrossRef]
- Hoang, S.; Guo, S.; Mullins, C.B. Coincorporation of N and Ta into TiO2 Nanowires for Visible Light Driven Photoelectrochemical Water Oxidation. J. Phys. Chem. C 2012, 116, 23283–23290. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, G.; Wang, X.; Yuan, X.; Lin, T.; Huang, F. Progress in black titania: A new material for advanced photocatalysis. Adv. Energy Mater. 2016, 6, 1600452. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, C.; Feng, Y.; Zhang, T.; Chen, Q.; Chi, Q.; Liu, L.; Wang, X.; Lei, Q. Energy storage enhancement of P(VDF-TrFE-CFE)-based composites with double-shell structured BZCT nanofibers of parallel and orthogonal configurations. Nano Energy 2019. [Google Scholar] [CrossRef]
- Feng, Y.; Zhou, Y.; Zhang, T.; Zhang, C.; Zhang, Y.; Zhang, Y.; Chen, Q.; Chi, Q. Ultrahigh discharge efficiency and excellent energy density in oriented core-shell nanofiber-polyetherimide composites. Energy Storage Mater. 2019. [Google Scholar] [CrossRef]
- Bemana, H.; Nadimi, S.R. Effect of sulfur doping on photoelectrochemical performance of hematite. Electrochim. Acta 2017, 229, 396–403. [Google Scholar] [CrossRef]
- Hoang, S.; Guo, S.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Visible Light Driven Photoelectrochemical Water Oxidation on Nitrogen-Modified TiO2 Nanowires. Nano Lett. 2012, 12, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Mitsui, T.; Matsumura, M. Photocatalytic Activity of S-doped TiO2 Photocatalyst under Visible Light. Chem. Lett. 2003, 32, 364–365. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Liu, X.; Zhao, G.; Kong, W.; Xuan, J.; Tan, S.; Sun, Y.; Wei, S.; Ren, J.; Yin, G. Sn4+ doping combined with hydrogen treatment for CdS/TiO2 photoelectrodes: An efficient strategy to improve quantum dots loading and charge transport for high photoelectrochemical performance. J. Power Sources 2019, 430, 80–89. [Google Scholar] [CrossRef]
- Liu, X.; Kong, W.; Zhao, G.; Xuan, J.; Zhao, Y.; Zhou, T.; Xiu, J.; Wang, J.; Sun, M.; Yin, G. Enhanced CdS quantum dots loading density and charge transport by Sn4+ doping improve the photoelectrochemical performance of TiO2 nanosheets with highly exposed {001} facets. Appl. Surf. Sci. 2019, 486, 28–36. [Google Scholar] [CrossRef]
- Cao, Y.; He, T.; Zhao, L.; Wang, E.; Yang, W.; Cao, Y. Structure and Phase Transition Behavior of Sn4+-Doped TiO2 Nanoparticles. J. Phys. Chem. C 2009, 113, 18121–18124. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Q.H.; Wang, W.H.; Ma, S.B.; Zhang, M.; Lv, J.G.; He, G.; Sun, Z.Q. Tuning the photoelectronic and photocatalytic properties of single-crystalline TiO2 nanosheet array films with dominant {001} facets by controlling the hydrochloric acid concentration. J. Mater. Sci. 2016, 51, 950–957. [Google Scholar] [CrossRef]
- Li, J.; Zeng, H.C. Hollowing Sn-Doped TiO2 Nanospheres via Ostwald Ripening. J. Am. Chem. Soc. 2007, 129, 15839–15847. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, L. Selective Nonaqueous Synthesis of C-Cl-Codoped TiO2 with Visible-Light Photocatalytic Activity. J. Phys. Chem. C 2010, 114, 11534–11541. [Google Scholar] [CrossRef]
- Bullock, E.L.; Patthey, L.; Steinemann, S.G. Clean and hydroxylated rutile TiO2 (110) surfaces studied by X-ray photoelectron spectroscopy. Surf. Sci. 1996, 352, 504–510. [Google Scholar] [CrossRef]
- Cao, Y.; Yang, W.; Zhang, W.; Liu, G.; Yue, P. Improved photocatalytic activity of Sn4+ doped TiO2 nanoparticulate films prepared by plasma-enhanced chemical vapor deposition. New J. Chem. 2004, 28, 218–222. [Google Scholar] [CrossRef]
- Jung, H.J.; Koutavarapu, R.; Lee, S.; Kim, J.H.; Choi, H.C.; Choi, M.Y. Enhanced photocatalytic degradation of lindane using metal-semiconductor Zn@ZnO and ZnO/Ag nanostructures. J. Environ. Sci. 2018, 74, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Babu, B.; Koutavarapu, R.; Harish, V.V.N.; Shim, J.; Yoo, K. Novel in-situ synthesis of Au/SnO2 quantum dots for enhanced visible-lightdriven photocatalytic applications. Ceram. Int. 2019, 45, 5743–5750. [Google Scholar] [CrossRef]
- Yin, G.; Sun, M.; Liu, Y.; Sun, Y.; Zhou, T.; Liu, B. Performance improvement in three-dimensional heterojunction solar cells by embedding CdS nanorod arrays in CdTe absorbing layers. Sol. Energy Mater. Sol. Cells 2017, 159, 418–426. [Google Scholar] [CrossRef]
- Sun, M.; Fu, W.; Li, Q.; Yin, G.; Chi, K.; Zhou, X.; Ma, J.; Yang, L.; Mu, Y.; Chen, Y.; et al. Facile fabrication of CdS nanorod arrays on the transparent conducting substrates and their photoelectrochemical properties. J. Cryst. Growth 2013, 377, 112–117. [Google Scholar] [CrossRef]
- Pan, J.; Hühne, S.M.; Shen, H.; Xiao, L.S.; Born, P.; Mader, W.; Mathur, S. SnO2-TiO2 Core-Shell Nanowire Structures: Investigations on Solid State Reactivity and Photocatalytic Behavior. J. Phys. Chem. C 2011, 115, 17265–17269. [Google Scholar] [CrossRef]
- Zhang, Q.; An, Q.; Luan, X.; Huang, H.; Li, X.; Meng, Z.; Tong, W.; Chen, X.; Chu, P.K.; Zhang, Y. Achieving significantly enhanced visible-light photocatalytic efficiency using a polyelectrolyte: The composites of exfoliated titania nanosheets, graphene, and poly(diallyl-dimethyl-ammonium chloride). Nanoscale 2015, 7, 14002–14009. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.C.; Yu, J.; Zhao, J. Enhanced photocatalytic activity of mesoporous and ordinary TiO2 thin films by sulfuric acid treatment. Appl. Catal. B Environ. 2002, 36, 31–43. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, M.; Kong, W.; Zhao, Y.; Liu, X.; Xuan, J.; Liu, Y.; Jia, F.; Yin, G.; Wang, J.; Zhang, J. Improving Photocatalytic Degradation Activity of Organic Pollutant by Sn4+ Doping of Anatase TiO2 Hierarchical Nanospheres with Dominant {001} Facets. Nanomaterials 2019, 9, 1603. https://doi.org/10.3390/nano9111603
Sun M, Kong W, Zhao Y, Liu X, Xuan J, Liu Y, Jia F, Yin G, Wang J, Zhang J. Improving Photocatalytic Degradation Activity of Organic Pollutant by Sn4+ Doping of Anatase TiO2 Hierarchical Nanospheres with Dominant {001} Facets. Nanomaterials. 2019; 9(11):1603. https://doi.org/10.3390/nano9111603
Chicago/Turabian StyleSun, Meiling, Weichong Kong, Yunlong Zhao, Xiaolin Liu, Jingyue Xuan, Yunyan Liu, Fuchao Jia, Guangchao Yin, Jun Wang, and Junkai Zhang. 2019. "Improving Photocatalytic Degradation Activity of Organic Pollutant by Sn4+ Doping of Anatase TiO2 Hierarchical Nanospheres with Dominant {001} Facets" Nanomaterials 9, no. 11: 1603. https://doi.org/10.3390/nano9111603
APA StyleSun, M., Kong, W., Zhao, Y., Liu, X., Xuan, J., Liu, Y., Jia, F., Yin, G., Wang, J., & Zhang, J. (2019). Improving Photocatalytic Degradation Activity of Organic Pollutant by Sn4+ Doping of Anatase TiO2 Hierarchical Nanospheres with Dominant {001} Facets. Nanomaterials, 9(11), 1603. https://doi.org/10.3390/nano9111603




