Application of Polyphenol-Loaded Nanoparticles in Food Industry
Abstract
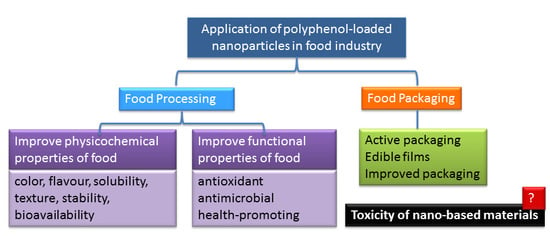
:1. Introduction

2. Use of Polyphenol-Loaded Nanoparticles in Food Processing
2.1. Polyphenol-Loaded Nanoparticles for the Enhancement of Physicochemical Properties of Food
2.2. Polyphenol-Loaded Nanoparticles for the Enhancement of Functional Properties of Food
2.2.1. Antioxidant Properties
2.2.2. Antimicrobial Properties
2.2.3. Health-Promoting Properties
3. Use of Polyphenol-Loaded Nanoparticles in Food Packaging
4. Nanomaterial and Polyphenol Toxicity
4.1. Nanomaterial Toxicity
| Active Compounds | Nanocarriers | Particle Size (nm) | Activity (Details of Research) | Reference |
|---|---|---|---|---|
| Catechin (CAT); Catechin-Zn complex (CAT-Zn) | β-chitosan NPs (β-CS NPs) | 208–591 nm | Both CAT and CAT-Zn complex-loaded β-CS NPs exhibited a strong antibacterial activity against E. coli and L. innocua, and they can be used as food supplements or for incorporation into food-packaging materials. | Zhang et al. [79] |
| Carvacrol | Starch and gelatinized starch | 495–529 nm | Carvacrol increased the flexibility, solubility, water vapor permeability, antioxidant, and antimicrobial activity of formed dispersion films, so they can be used as bioactive films. | Homayouni et al. [141] |
| Eugenol | SiO2-eugenol liposome | 315.7 ± 0.7 nm | SiO2-eugenol liposomes have stabile and pronounced antioxidant activity during 60 days of storage. SiO2-eugenol liposome-loaded electrospun nanofibrous membranes showed strong antioxidant activity on beef, and, in the future, they can be used for food preservation. | Cui et al. [142] |
| Cinnamaldehyde | Nanoliposomes (lipid bilayers of polydiacetylene-N-hydroxysuccini-mide) | 100–400 nm | Nanoencapsulated cinnamaldehyde immobilized on glass surfaces showed significant antimicrobial effect, and, in the future, it can be used as an active packaging material for preserving liquid foods. | Makwana et al. [134] |
| Cinnamaldehyde | Pectin/papaya puree nanoemulsion | 20–500 nm | Edible films for food packaging containing small droplets of polyphenol-loaded nanoemulsion had pronounced antimicrobial effect, because encapsulated cinnamaldehyde showed significant antimicrobial properties against food pathogens such as E. coli, S. enterica, L. monocytogenes, and S. aureus. | Otoni et al. [133] |
| Green tea extracts | Hydroxypropyl-methylcellulose (HPMC) containing polylactic acid (PLA) NPs | 47–244.4 nm | Films containing green-tea polyphenols showed a significant antioxidative capacity, and they can be used for protection of food containing a high percent of fats. | Wrona et al. [136] |
| Tea polyphenols | Gelatin | Not reported | CS NPs provided controlled-release of tea polyphenols, and this increased its antioxidant properties. EGCG-loaded nanocomplex can be used for protection of fatty foods. | Liu et al. [143] |
| Epigallocatechin gallate (EGCG) | Zein/chitosan NPs | 155.5–240.6 nm | EGCG-loaded zein/chitosan NPs possess high antioxidant activity and can be applied against degradation and oxidation of fatty foods; moreover, in the future, these nanocomplexes can be applied as active material for edible films in the food industry. | Liang et al. [135] |
| Gallic acid | Zein ultra-fine fibers | 327–387 nm | Gallic acid retained its antioxidant activity after incorporation into zein ultra-fine fibers, and thus this prepared ingredient can find application in packaging materials. | Neo et al. [8] |
| Rosemary (Rosmarinus officinalis) polyphenols | Polyvinyl alcohol (PVA) electrospun nanofibers | 307 ± 33 nm/282 ± 39 nm | PVA active mats successfully incorporated bioactive components from rosemary extract, showing an excellent antioxidant activity. This may find application for active food packaging, especially for hydrophilic and acid food products. | Estevez-Areco et al. [144] |
4.2. Polyphenol Toxicity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Acevedo-Fani, A.; Soliva-Fortuny, R.; Martín-Belloso, O. Nanostructured emulsions and nanolaminates for delivery of active ingredients: Improving food safety and functionality. Trends Food Sci. Technol. 2017, 60, 12–22. [Google Scholar] [CrossRef]
- Faridi Esfanjani, A.; Jafari, S.M. Biopolymer nano-particles and natural nano-carriers for nano-encapsulation of phenolic compounds. Colloids Surf. B Biointerfaces 2016, 146, 532–543. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yu, H.; Ru, Q. Bioavailability and delivery of nutraceuticals using nanotechnology. J. Food Sci. 2010, 75, R50–R57. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J.; Decker, E.A.; Park, Y.; Weiss, J. Structural design principles for delivery of bioactive components in nutraceuticals and functional foods. Crit. Rev. Food Sci. Nutr. 2009, 49, 577–606. [Google Scholar] [CrossRef]
- Ranjan, S.; Dasgupta, N.; Chakraborty, A.R.; Melvin Samuel, S.; Ramalingam, C.; Shanker, R.; Kumar, A. Nanoscience and nanotechnologies in food industries: Opportunities and research trends. J. Nanopart. Res. 2014, 16, 2464. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S. Nanotechnology in Food Sector. In An Introduction to Food Grade Nanoemulsions; Dasgupta, N., Ranjan, S., Eds.; Springer: Singapore, 2018; pp. 1–18. [Google Scholar] [CrossRef]
- Ummi, A.S.; Siddiquee, S. Chapter 15—Nanotechnology Applications in Food: Opportunities and Challenges in Food Industry. In Applications in Energy, Drug and Food; Siddiquee, S., Hong, M.G.J., Rahman, M.M., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 298–308. [Google Scholar] [CrossRef]
- Neo, Y.P.; Ray, S.; Jin, J.; Gizdavic-Nikolaidis, M.; Nieuwoudt, M.K.; Liu, D.; Quek, S.Y. Encapsulation of food grade antioxidant in natural biopolymer by electrospinning technique: A physicochemical study based on zein–gallic acid system. Food Chem. 2013, 136, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Sanguansri, P.; Augustin, M.A. Nanoscale materials development—A food industry perspective. Trends Food Sci. Technol. 2006, 17, 547–556. [Google Scholar] [CrossRef]
- Ahmad, S.; Munir, S.; Zeb, N.; Ullah, A.; Khan, B.; Ali, J.; Bilal, M.; Omer, M.; Alamzeb, M.; Salman, S.M.; et al. Green nanotechnology: A review on green synthesis of silver nanoparticles—An ecofriendly approach. Int. J. Nanomed. 2019, 14, 5087–5107. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Sajjadi, M.; Issaabadi, Z. Chapter 1—An Introduction to Nanotechnology. In An Introduction to Green Nanotechnology; Nasrollahzadeh, M., Sajadi, S.M., Sajjadi, M., Issaabadi, Z., Atarod, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 28, pp. 1–27. [Google Scholar]
- Sanjay, S.S. Chapter 2—Safe nano is green nano. In Green Synthesis, Characterization and Applications of Nanoparticles; Shukla, A.K., Iravani, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 27–36. [Google Scholar] [CrossRef]
- Ramachandraiah, K.; Han, S.G.; Chin, K.B. Nanotechnology in meat processing and packaging: Potential applications—A review. Asian-Australas. J. Anim. Sci. 2015, 28, 290–302. [Google Scholar] [CrossRef]
- Ozogul, Y.; Yuvka, İ.; Ucar, Y.; Durmus, M.; Kösker, A.R.; Öz, M.; Ozogul, F. Evaluation of effects of nanoemulsion based on herb essential oils (rosemary, laurel, thyme and sage) on sensory, chemical and microbiological quality of rainbow trout (Oncorhynchus mykiss) fillets during ice storage. LWT 2017, 75, 677–684. [Google Scholar] [CrossRef]
- Mangiacapra, P.; Gorrasi, G.; Sorrentino, A.; Vittoria, V. Biodegradable nanocomposites obtained by ball milling of pectin and montmorillonites. Carbohydr. Polym. 2006, 64, 516–523. [Google Scholar] [CrossRef]
- Giosafatto, C.V.L.; Sabbah, M.; Al-Asmar, A.; Esposito, M.; Sanchez, A.; Villalonga Santana, R.; Cammarota, M.; Mariniello, L.; Di Pierro, P.; Porta, R. Effect of Mesoporous Silica Nanoparticles on Glycerol-Plasticized Anionic and Cationic Polysaccharide Edible Films. Coatings 2019, 9, 172. [Google Scholar] [CrossRef]
- Handford, C.E.; Dean, M.; Spence, M.; Henchion, M.; Elliott, C.T.; Campbell, K. Awareness and attitudes towards the emerging use of nanotechnology in the agri-food sector. Food Control 2015, 57, 24–34. [Google Scholar] [CrossRef]
- Alrgei, H.O.S.; Dabić, D.Č.; Natić, M.M.; Rakonjac, V.S.; Milojković-Opsenica, D.; Tešić, Ž.L.; Fotirić Akšić, M.M. Chemical profile of major taste- and health-related compounds of Oblačinska sour cherry. J. Sci. Food Agric. 2016, 96, 1241–1251. [Google Scholar] [CrossRef]
- Stanisavljević, N.S.; Ilić, M.D.; Matić, I.Z.; Jovanović, Ž.S.; Čupić, T.; Dabić, D.Č.; Natić, M.M.; Tešić, Ž.L. Identification of Phenolic Compounds from Seed Coats of Differently Colored European Varieties of Pea (Pisum sativum L.) and Characterization of Their Antioxidant and In Vitro Anticancer Activities. Nutr. Cancer 2016, 68, 988–1000. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Gašić, U.; Tešić, Ž.; Zengin, G.; Zeković, Z.; Đurović, S. Isolation of apigenin from subcritical water extracts: Optimization of the process. J. Supercrit. Fluids 2017, 120, 32–42. [Google Scholar] [CrossRef]
- Fotirić Akšić, M.M.; Dabić, D.Č.; Gašić, U.M.; Zec, G.N.; Vulić, T.B.; Tešić, Ž.L.; Natić, M.M. Polyphenolic Profile of Pear Leaves with Different Resistance to Pear Psylla (Cacopsylla pyri). J. Agric. Food Chem. 2015, 63, 7476–7486. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Gašić, U.M.; Pešić, M.B.; Stanojević, S.P.; Barać, M.B.; Mačukanović-Jocić, M.P.; Avramov, S.N.; Tešić, Ž.L. Phytochemical Analysis and Total Antioxidant Capacity of Rhizome, Above-Ground Vegetative Parts and Flower of Three Iris Species. Chem. Biodivers. 2019, 16, e1800565. [Google Scholar] [CrossRef]
- Kostić, A.Ž. Polyphenolic profile and antioxidant properties of bee-collected pollen from sunflower (Helianthus annuus L.) plant. LWT Food Sci. Technol. 2019, 112, 108244. [Google Scholar] [CrossRef]
- Mišić, D.; Šiler, B.; Gašić, U.; Avramov, S.; Živković, S.; Nestorović Živković, J.; Milutinović, M.; Tešić, Ž. Simultaneous UHPLC/DAD/(+/−)HESI–MS/MS Analysis of Phenolic Acids and Nepetalactones in Methanol Extracts of Nepeta Species: A Possible Application in Chemotaxonomic Studies. Phytochem. Anal. 2015, 26, 72–85. [Google Scholar] [CrossRef]
- Natić, M.M.; Dabić, D.Č.; Papetti, A.; Fotirić Akšić, M.M.; Ognjanov, V.; Ljubojević, M.; Tešić, Ž.L. Analysis and characterisation of phytochemicals in mulberry (Morus alba L.) fruits grown in Vojvodina, North Serbia. Food Chem. 2015, 171, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Pantelić, M.M.; Dabić Zagorac, D.Č.; Davidović, S.M.; Todić, S.R.; Bešlić, Z.S.; Gašić, U.M.; Tešić, Ž.L.; Natić, M.M. Identification and quantification of phenolic compounds in berry skin, pulp, and seeds in 13 grapevine varieties grown in Serbia. Food Chem. 2016, 211, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Pavlović, A.V.; Papetti, A.; Dabić Zagorac, D.Č.; Gašić, U.M.; Mišić, D.M.; Tešić, Ž.L.; Natić, M.M. Phenolics composition of leaf extracts of raspberry and blackberry cultivars grown in Serbia. Ind. Crops Prod. 2016, 87, 304–314. [Google Scholar] [CrossRef]
- Ristivojević, P.; Trifković, J.; Gašić, U.; Andrić, F.; Nedić, N.; Tešić, Ž.; Milojković-Opsenica, D. Ultrahigh-performance Liquid Chromatography and Mass Spectrometry (UHPLC-LTQ/Orbitrap/MS/MS) study of phenolic profile of Serbian poplar type propolis. Phytochem. Anal. 2015, 26, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Stanisavljević, N.S.; Ilić, M.; Jovanović, Z.S.; Čupić, T.; Dabić, D.C.; Natić, M.M.; Tešić, Z.L.; Radović, S.S. Identification of seed coat phenolic compounds from differently colored pea varieties and characterization of their antioxidant activity. Arch. Biol. Sci. 2015, 67, 829–840. [Google Scholar] [CrossRef]
- Pešić, M.B.; Milinčić, D.D.; Kostić, A.Ž.; Stanisavljević, N.S.; Vukotić, G.N.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Popović, D.A.; et al. In vitro digestion of meat- and cereal-based food matrix enriched with grape extracts: How are polyphenol composition, bioaccessibility and antioxidant activity affected? Food Chem. 2019, 284, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Estaca, J.; Balaguer, M.; Gavara, R.; Hernandez-Munoz, P. Formation of zein nanoparticles by electrohydrodynamic atomization: Effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocoll. 2012, 28, 82–91. [Google Scholar] [CrossRef]
- Madureira, A.R.; Pereira, A.; Castro, P.M.; Pintado, M. Production of antimicrobial chitosan nanoparticles against food pathogens. J. Food Eng. 2015, 167, 210–216. [Google Scholar] [CrossRef]
- Aguirre, A.; Borneo, R. Chapter 4—Improving Bioavailability of Polyphenols Using Nanodelivery Systems Based on Food Polymers. In Polyphenols in Plants (Second Edition); Watson, R.R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 59–65. [Google Scholar] [CrossRef]
- Hu, B.; Liu, X.; Zhang, C.; Zeng, X. Food macromolecule based nanodelivery systems for enhancing the bioavailability of polyphenols. J. Food Drug Anal. 2017, 25, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Antoniou, J.; Li, Y.; Yi, J.; Yokoyama, W.; Ma, J.; Zhong, F. Preparation of gelatin films incorporated with tea polyphenol nanoparticles for enhancing controlled-release antioxidant properties. J. Agric. Food Chem. 2015, 63, 3987–3995. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Yu, S.-H.; Tsai, G.-J.; Tang, D.-W.; Mi, F.-L.; Peng, Y.-P. Novel Technology for the Preparation of Self-Assembled Catechin/Gelatin Nanoparticles and Their Characterization. J. Agric. Food Chem. 2010, 58, 6728–6734. [Google Scholar] [CrossRef] [PubMed]
- Prakash Upputuri, R.T.; Azad Mandal, A.K. Sustained Release of Green Tea Polyphenols from Liposomal Nanoparticles; Release Kinetics and Mathematical Modelling. Iran. J. Biotechnol. 2017, 15, 277–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, B.; Wang, Y.; Wang, T.; Mao, L.; Lu, Y.; Wang, H.; Feng, Z. Structure and Functional Properties of Antioxidant Nanoemulsions Prepared with Tea Polyphenols and Soybean Protein Isolate. J. Oleo Sci. 2019, 68, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Fu, Y.; Chen, G.; Shi, Y.; Li, X.; Zhang, H.; Shen, Y. Fabrication and characterization of carboxymethyl chitosan and tea polyphenols coating on zein nanoparticles to encapsulate β-carotene by anti-solvent precipitation method. Food Hydrocoll. 2018, 77, 577–587. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Puligundla, P.; Gao, X.; Zhou, Y.; Wan, X. Applications of chitosan nanoparticles to enhance absorption and bioavailability of tea polyphenols: A review. Food Hydrocoll. 2017, 69, 286–292. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Teixeira, M.C.; Fernandes, A.R.; Arraez-Roman, D.; Martinez-Ferez, A.; Segura-Carretero, A.; Souto, E.B. Lipid nanocarriers for the loading of polyphenols—A comprehensive review. Adv. Colloid Interface Sci. 2018, 260, 85–94. [Google Scholar] [CrossRef]
- Balanč, B.; Trifkovic, K.; Pravilović, R.; Đorđević, V.; Levic, S.; Bugarski, B.; Nedovic, V. Chapter 16-Lipid Nanocarriers for Phytochemical Delivery in Foods. In Nanotechnology Applications in the Food Industry; Ravishankar Rai, V., Bai, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 357–384. [Google Scholar] [CrossRef]
- Chang, C.; Wang, T.; Hu, Q.; Luo, Y. Caseinate-zein-polysaccharide complex nanoparticles as potential oral delivery vehicles for curcumin: Effect of polysaccharide type and chemical cross-linking. Food Hydrocoll. 2017, 72, 254–262. [Google Scholar] [CrossRef]
- Kalušević, A.M.; Lević, S.M.; Čalija, B.R.; Milić, J.R.; Pavlović, V.B.; Bugarski, B.M.; Nedović, V.A. Effects of different carrier materials on physicochemical properties of microencapsulated grape skin extract. J. Food Sci. Technol. 2017, 54, 3411–3420. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Yang, H.J.; Kim, H.W.; Wan, X.; Lee, J.; Ko, S. Synthesis and controlled-release properties of chitosan/beta-Lactoglobulin nanoparticles as carriers for oral administration of epigallocatechin gallate. Food Sci. Biotechnol. 2016, 25, 1583–1590. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Recent progress in biopolymer nanoparticle and microparticle formation by heat-treating electrostatic protein-polysaccharide complexes. Adv. Colloid Interface Sci. 2011, 167, 49–62. [Google Scholar] [CrossRef]
- Turgeon, S.; Schmitt, C.; Sanchez, C. Protein-Polysaccharide complexes and coacervates. Curr. Opin. Colloid Interface Sci. 2007, 12, 166–178. [Google Scholar] [CrossRef]
- Veneranda, M.; Hu, Q.; Wang, T.; Luo, Y.; Castro, K.; Madariaga, J.M. Formation and characterization of zein-caseinate-pectin complex nanoparticles for encapsulation of eugenol. LWT 2018, 89, 596–603. [Google Scholar] [CrossRef]
- Luo, Y.; Pan, K.; Zhong, Q. Casein/pectin nanocomplexes as potential oral delivery vehicles. Int. J. Pharm. 2015, 486, 59–68. [Google Scholar] [CrossRef]
- Hu, B.; Ting, Y.; Zeng, X.; Huang, Q. Cellular uptake and cytotoxicity of chitosan-caseinophosphopeptides nanocomplexes loaded with epigallocatechin gallate. Carbohydr. Polym. 2012, 89, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Silva, H.D.; Cerqueira, M.Â.; Vicente, A.A. Erratum to: Nanoemulsions for Food Applications: Development and Characterization. Food Bioprocess Technol. 2014, 7, 306. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.; Wang, T.; Hu, Q.; Zhou, M.; Jingyi, X.; Luo, Y. Pectin coating improves physicochemical properties of caseinate/zein nanoparticles as oral delivery vehicles for curcumin. Food Hydrocoll. 2017, 70, 143–151. [Google Scholar] [CrossRef]
- Ramalingam, P.; Yoo, S.W.; Ko, Y.T. Nanodelivery systems based on mucoadhesive polymer coated solid lipid nanoparticles to improve the oral intake of food curcumin. Food Res. Int. 2016, 84, 113–119. [Google Scholar] [CrossRef]
- Tang, D.-W.; Yu, S.-H.; Ho, Y.-C.; Huang, B.-Q.; Tsai, G.-J.; Hsieh, H.-Y.; Sung, H.-W.; Mi, F.-L. Characterization of tea catechins-loaded nanoparticles prepared from chitosan and an edible polypeptide. Food Hydrocoll. 2013, 30, 33–41. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.A.; Larson, I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010, 41, 219–225. [Google Scholar] [CrossRef]
- Dudhani, A.R.; Kosaraju, S.L. Bioadhesive chitosan nanoparticles: Preparation and characterization. Carbohydr. Polym. 2010, 81, 243–251. [Google Scholar] [CrossRef]
- Liang, J.; Li, F.; Fang, Y.; Yang, W.; An, X.; Zhao, L.; Xin, Z.; Cao, L.; Hu, Q. Synthesis, characterization and cytotoxicity studies of chitosan-coated tea polyphenols nanoparticles. Colloids Surf. B Biointerfaces 2011, 82, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Aluani, D.; Tzankova, V.; Kondeva-Burdina, M.; Yordanov, Y.; Nikolova, E.; Odzhakov, F.; Apostolov, A.; Markova, T.; Yoncheva, K. Evaluation of biocompatibility and antioxidant efficiency of chitosan-alginate nanoparticles loaded with quercetin. Int. J. Biol. Macromol. 2017, 103, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Pakade, Y.B.; Singh, B.; Yadav, S.C. Development of biodegradable nanoparticles for delivery of quercetin. Colloids Surf. B Biointerfaces 2010, 80, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhao, X.; Ma, Y.; Zhai, G.; Li, L.; Lou, H. Enhancement of gastrointestinal absorption of quercetin by solid lipid nanoparticles. J. Control. Release 2009, 133, 238–244. [Google Scholar] [CrossRef]
- Dube, A.; Nicolazzo, J.; Larson, I. Chitosan nanoparticles enhance the plasma exposure of (-)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur. J. Pharm. Sci. 2011, 44, 422–426. [Google Scholar] [CrossRef]
- Moeiniafshari, A.-A.; Zarrabi, A.; Bordbar, A.-K. Exploring the interaction of naringenin with bovine beta-casein nanoparticles using spectroscopy. Food Hydrocoll. 2015, 51, 1–6. [Google Scholar] [CrossRef]
- Morsy, M.K.; Mekawi, E.; Elsabagh, R. Impact of pomegranate peel nanoparticles on quality attributes of meatballs during refrigerated storage. LWT 2018, 89, 489–495. [Google Scholar] [CrossRef]
- Pereira, M.C.; Oliveira, D.A.; Hill, L.E.; Zambiazi, R.C.; Borges, C.D.; Vizzotto, M.; Mertens-Talcott, S.; Talcott, S.; Gomes, C.L. Effect of nanoencapsulation using PLGA on antioxidant and antimicrobial activities of guabiroba fruit phenolic extract. Food Chem. 2018, 240, 396–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, Y.; Luo, Y.; Ge, M.; Yang, T.; Yu, L.; Wang, Q. Fabrication, characterization and antimicrobial activities of thymol-loaded zein nanoparticles stabilized by sodium caseinate–chitosan hydrochloride double layers. Food Chem. 2014, 142, 269–275. [Google Scholar] [CrossRef]
- Engel, J.B.; Heckler, C.; Tondo, E.C.; Daroit, D.J.; da Silva Malheiros, P. Antimicrobial activity of free and liposome-encapsulated thymol and carvacrol against Salmonella and Staphylococcus aureus adhered to stainless steel. Int. J. Food Microbiol. 2017, 252, 18–23. [Google Scholar] [CrossRef]
- da Rosa, C.G.; de Oliveira Brisola Maciel, M.V.; de Carvalho, S.M.; de Melo, A.P.Z.; Jummes, B.; da Silva, T.; Martelli, S.M.; Villetti, M.A.; Bertoldi, F.C.; Barreto, P.L.M. Characterization and evaluation of physicochemical and antimicrobial properties of zein nanoparticles loaded with phenolics monoterpenes. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 337–344. [Google Scholar] [CrossRef]
- Ghosh, V.; Mukherjee, A.; Chandrasekaran, N. Eugenol-loaded antimicrobial nanoemulsion preserves fruit juice against, microbial spoilage. Colloids Surf. B Biointerfaces 2014, 114, 392–397. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Gerhard, H.; Upadhyaya, I.; Venkitanarayanan, K.; Luo, Y. Antimicrobial eugenol nanoemulsion prepared by gum arabic and lecithin and evaluation of drying technologies. Int. J. Biol. Macromol. 2016, 87, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Vieira, A.C.; Chaves, L.L.; Nunes, C.; Neves, A.R.; Pinheiro, M.; Reis, S. Folate-targeted nanostructured lipid carriers for enhanced oral delivery of epigallocatechin-3-gallate. Food Chem. 2017, 237, 803–810. [Google Scholar] [CrossRef]
- Dahiya, S.; Rani, R.; Dhingra, D.; Kumar, S.; Dilbaghi, N. Conjugation of epigallocatechin gallate and piperine into a zein nanocarrier: Implication on antioxidant and anticancer potential. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 035011. [Google Scholar] [CrossRef]
- Dobrucka, R.; Kaczmarek, M.; Dlugaszewska, J. Cytotoxic and antimicrobial effect of biosynthesized silver nanoparticles using the fruit extract of Ribes nigrum. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025015. [Google Scholar] [CrossRef]
- Ghayour, N.; Hosseini, S.M.H.; Eskandari, M.H.; Esteghlal, S.; Nekoei, A.-R.; Hashemi Gahruie, H.; Tatar, M.; Naghibalhossaini, F. Nanoencapsulation of quercetin and curcumin in casein-based delivery systems. Food Hydrocoll. 2019, 87, 394–403. [Google Scholar] [CrossRef]
- Khan, M.A.; Yue, C.; Fang, Z.; Hu, S.; Cheng, H.; Bakry, A.M.; Liang, L. Alginate/chitosan-coated zein nanoparticles for the delivery of resveratrol. J. Food Eng. 2019, 258, 45–53. [Google Scholar] [CrossRef]
- Chang, Y.; McClements, D.J. Optimization of Orange Oil Nanoemulsion Formation by Isothermal Low-Energy Methods: Influence of the Oil Phase, Surfactant, and Temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef]
- Chang, Y.; McLandsborough, L.; McClements, D.J. Physical Properties and Antimicrobial Efficacy of Thyme Oil Nanoemulsions: Influence of Ripening Inhibitors. J. Agric. Food Chem. 2012, 60, 12056–12063. [Google Scholar] [CrossRef]
- Schwarz, B.; Hofmann, T. Is there a direct relationship between oral astringency and human salivary protein binding. Eur. Food Res. Technol. 2008, 227, 1693–1698. [Google Scholar] [CrossRef]
- Jöbstl, E.; O’Connell, J.; Fairclough, J.P.A.; Williamson, M.P. Molecular Model for Astringency Produced by Polyphenol/Protein Interactions. Biomacromolecules 2004, 5, 942–949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jung, J.; Zhao, Y. Preparation, characterization and evaluation of antibacterial activity of catechins and catechins-Zn complex loaded beta-chitosan nanoparticles of different particle sizes. Carbohydr. Polym. 2016, 137, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Nedović, V.; Kalusević, A.; Manojlović, V.; Lević, S.; Bugarski, B. An overview of encapsulation technologies for food applications. Procedia Food Sci. 2011, 1, 1806–1815. [Google Scholar] [CrossRef] [Green Version]
- Popović, D.A.; Milinčić, D.D.; Pešić, M.B.; Kalušević, A.M.; Tešić, Ž.Lj.; Nedović, V.A. Chapter 12—Encapsulation technologies for polyphenol-loaded microparticles in food industry. In Green Food Processing Techniques: Preservation, Transformation and Extraction; Chemat, F., Eugene, V., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 335–368. [Google Scholar] [CrossRef]
- Sarkar, P.; Choudhary, R.; Panigrahi, S.; Syed, I.; Sivapratha, S.; Dhumal, C.V. Nano-inspired systems in food technology and packaging. Environ. Chem. Lett. 2017, 15, 607–622. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, S. Chapter 32: Nanoparticle-based methods for food safety evaluation. In Evaluation Technologies for Food Quality; Zhong, J., Wang, X., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 817–835. [Google Scholar] [CrossRef]
- Lević, S.; Djordjević, V.; Rajić, N.; Milivojević, M.; Bugarski, B.; Nedović, V. Entrapment of ethyl vanillin in calcium alginate and calcium alginate/poly(vinyl alcohol) beads. Chem. Pap. 2013, 67, 221–228. [Google Scholar] [CrossRef]
- Isailović, B.; Djordjević, V.; Lević, S.; Milanović, J.; Bugarski, B.; Nedović, V. Encapsulation of flavors and aromas: Controlled release. In Edible Films and Coatings: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2016; pp. 317–344. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, Z.; Lv, F.; Bie, X. Study on microencapsulation of curcumin pigments by spray drying. Eur. Food Res. Technol. 2009, 229, 391–396. [Google Scholar] [CrossRef]
- Tonnesen, H.H. Solubility, chemical and photochemical stability of curcumin in surfactant solutions. Studies of curcumin and curcuminoids, XXVIII. Die Pharm. 2002, 57, 820–824. [Google Scholar]
- Serpa Guerra, A.M.; Gómez Hoyos, C.; Velásquez-Cock, J.A.; Vélez Acosta, L.; Gañán Rojo, P.; Velásquez Giraldo, A.M.; Zuluaga Gallego, R. The nanotech potential of turmeric (Curcuma longa L.) in food technology: A review. Crit. Rev. Food Sci. Nutr. 2019, 24, 1–13. [Google Scholar] [CrossRef]
- Dalgleish, D.G. Chapter 3—The Basis of Structure in Dairy-Based Foods: Casein Micelles and their Properties. In Food Structures, Digestion and Health; Boland, M., Golding, M., Singh, H., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 83–105. [Google Scholar] [CrossRef]
- Pesić, M.B.; Barać, M.B.; Stanojević, S.P.; Ristić, N.M.; Macej, O.D.; Vrvić, M.M. Heat induced casein-whey protein interactions at natural pH of milk: A comparison between caprine and bovine milk. Small Rumin. Res. 2012, 108, 77–86. [Google Scholar] [CrossRef]
- Pesić, M.B.; Barać, M.B.; Stanojević, S.P.; Vrvić, M.M. Effect of pH on heat-induced casein-whey protein interactions: A comparison between caprine milk and bovine milk. Int. Dairy J. 2014, 39, 178–183. [Google Scholar] [CrossRef]
- Pesić, M.B.; Barać, M.B.; Stanojević, S.P.; Vrvić, M.M. Chapter 9-Heat-Induced Casein–Whey Protein Interactions in Caprine Milk: Whether Are Similar to Bovine Milk? In Emerging and Traditional Technologies for Safe, Healthy and Quality Food; Nedovic, V., Raspor, P., Levic, J., Tumbas, V., Barbosa-Cánovas, G., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 163–175. [Google Scholar] [CrossRef]
- Moeller, H.; Martin, D.; Schrader, K.; Hoffmann, W.; Lorenzen, P.C. Native casein micelles as nanocarriers for β-carotene: pH-and temperature-induced opening of the micellar structure. Int. J. Food Sci. Technol. 2017, 52, 1122–1130. [Google Scholar] [CrossRef]
- Moeller, H.; Martin, D.; Schrader, K.; Hoffmann, W.; Lorenzen, P.C. Spray- or freeze-drying of casein micelles loaded with Vitamin D2: Studies on storage stability and in vitro digestibility. LWT 2018, 97, 87–93. [Google Scholar] [CrossRef]
- Haratifar, S.; Corredig, M. Interactions between tea catechins and casein micelles and their impact on renneting functionality. Food Chem. 2014, 143, 27–32. [Google Scholar] [CrossRef]
- O’Connell, J.E.; Fox, P.D.; Tan-Kintia, R.; Fox, P.F. Effects of Tea, Coffee and Cocoa Extracts on the Colloidal Stability of Milk and Concentrated Milk. Int. Dairy J. 1998, 8, 689–693. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Select Committee on GRAS Substances (SCOGS) Opinion: Calcium Caseinate; Casein; Sodium Caseinate. 2015. Available online: http://wayback.archive-it.org/7993/20171031061247/https:/www.fda.gov/Food/IngredientsPackagingLabeling/GRAS/SCOGS/ucm20171031260879.htm (accessed on 19 September 2019).
- Hu, B.; Pan, C.; Sun, Y.; Hou, Z.; Ye, H.; Zeng, X. Optimization of fabrication parameters to produce chitosan-tripolyphosphate nanoparticles for delivery of tea catechins. J. Agric. Food Chem. 2008, 56, 7451–7458. [Google Scholar] [CrossRef]
- Peng, G.; Wargovich, M.J.; Dixon, D.A. Anti-proliferative effects of green tea polyphenol EGCG on Ha-Ras-induced transformation of intestinal epithelial cells. Cancer Lett. 2006, 238, 260–270. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Ko, S.; Liang, J.; Recharla, N. Nanotechnological approaches to enhance the bioavailability and therapeutic efficacy of green tea polyphenols. J. Funct. Foods 2017, 34, 139–151. [Google Scholar] [CrossRef]
- Lante, A.; Friso, D. Oxidative stability and rheological properties of nanoemulsion with ultrasonic extracted green tea infusion. Food Res. Int. 2013, 54, 269–276. [Google Scholar] [CrossRef]
- Zou, L.; Peng, S.; Liu, W.; Chen, X.; Liu, C. A novel delivery system dextran sulfate coated amphiphilic chitosan derivatives-based nanoliposome: Capacity to improve in vitro digestion stability of (−)-epigallocatechin gallate. Food Res. Int. 2015, 69, 114–120. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Z.; Kong, Q.; Zhang, R.; Yang, X. Enhancing the antitumor activity of tea polyphenols encapsulated in biodegradable nanogels by macromolecular self-assembly. RSC Adv. 2019, 9, 10004–10016. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Wang, Y.; Yang, H.; Liu, X.; Lu, Z. Preparation and biological activity studies of resveratrol loaded ionically cross-linked chitosan-TPP nanoparticles. Carbohydr. Polym. 2017, 175, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Natesan, S.; Pandian, S.; Ponnusamy, C.; Palanichamy, R.; Muthusamy, S.; Kandasamy, R. Co-encapsulated resveratrol and quercetin in chitosan and peg modified chitosan nanoparticles: For efficient intra ocular pressure reduction. Int. J. Biol. Macromol. 2017, 104, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sangwan, P.; Lather, V.; Pandita, D. Biocompatible PLGA-oil hybrid nanoparticles for high loading and controlled delivery of resveratrol. J. Drug Deliv. Sci. Technol. 2015, 30, 54–62. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef]
- Fonseca, D.P.; Khalil, N.M.; Mainardes, R.M. Bovine serum albumin-based nanoparticles containing resveratrol: Characterization and antioxidant activity. J. Drug Deliv. Sci. Technol. 2017, 39, 147–155. [Google Scholar] [CrossRef]
- Jung, K.-H.; Lee, J.H.; Park, J.W.; Quach, C.H.T.; Moon, S.-H.; Cho, Y.S.; Lee, K.-H. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int. J. Pharm. 2015, 478, 251–257. [Google Scholar] [CrossRef]
- Musazzi, U.M.; Youm, I.; Murowchick, J.B.; Ezoulin, M.J.; Youan, B.-B.C. Resveratrol-loaded nanocarriers: Formulation, optimization, characterization and in vitro toxicity on cochlear cells. Colloids Surf. B Biointerfaces 2014, 118, 234–242. [Google Scholar] [CrossRef]
- Cosco, D.; Paolino, D.; Maiuolo, J.; Marzio, L.D.; Carafa, M.; Ventura, C.A.; Fresta, M. Ultradeformable liposomes as multidrug carrier of resveratrol and 5-fluorouracil for their topical delivery. Int. J. Pharm. 2015, 489, 1–10. [Google Scholar] [CrossRef]
- Chauhan, P.; Mahajan, S.; Prasad, G.B.K.S. Preparation and characterization of CS-ZnO-NC nanoparticles for imparting anti-diabetic activities in experimental diabetes. J. Drug Deliv. Sci. Technol. 2019, 52, 738–747. [Google Scholar] [CrossRef]
- Granata, G.; Consoli, G.M.L.; Lo Nigro, R.; Geraci, C. Hydroxycinnamic acids loaded in lipid-core nanocapsules. Food Chem. 2018, 245, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Silva, S.; Veiga, M.; Costa, E.M.; Oliveira, A.L.S.; Madureira, A.R.; Pintado, M. Nanoencapsulation of Polyphenols towards Dairy Beverage Incorporation. Beverages 2018, 4, 61. [Google Scholar] [CrossRef] [Green Version]
- Kuswandi, B. Environmental friendly food nano-packaging. Environ. Chem. Lett. 2017, 15, 205–221. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ofethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Wattanasatcha, A.; Rengpipat, S.; Wanichwecharungruang, S. Thymol nanospheres as an effective anti-bacterial agent. Int. J. Pharm. 2012, 434, 360–365. [Google Scholar] [CrossRef]
- Tatli Seven, P.; Seven, I.; Gul Baykalir, B.; Iflazoglu Mutlu, S.; Salem, A.Z.M. Nanotechnology and nano-propolis in animal production and health: An overview. Ital. J. Anim. Sci. 2018, 17, 921–930. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, Z.; Mohammadinejad, R.; Ashrafizadeh, M. Drug delivery systems for resveratrol, a non-flavonoid polyphenol: Emerging evidence in last decades. J. Drug Deliv. Sci. Technol. 2019, 51, 591–604. [Google Scholar] [CrossRef]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Collado-Gonzalez, M.; Magalhaes, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanotechnology-based formulations for resveratrol delivery: Effects on resveratrol in vivo bioavailability and bioactivity. Colloids Surf. B Biointerfaces 2019, 180, 127–140. [Google Scholar] [CrossRef]
- Sivasami, P.; Hemalatha, T. Augmentation of therapeutic potential of curcumin using nanotechnology: Current perspectives. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Di Costanzo, A.; Angelico, R. Formulation Strategies for Enhancing the Bioavailability of Silymarin: The State of the Art. Molecules 2019, 24, 2155. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; McClements, D.J.; Wei, Z.; Wang, G.; Liu, X.; Liu, F. Delivery of synergistic polyphenol combinations using biopolymer-based systems: Advances in physicochemical properties, stability and bioavailability. Crit. Rev. Food Sci. Nutr. 2019, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Prakash, M.; Basavaraj, B.V.; Chidambara Murthy, K.N. Biological functions of epicatechin: Plant cell to human cell health. Ournal Funct. Foods 2019, 52, 14–24. [Google Scholar] [CrossRef]
- Ye, J.-H.; Augustin, M.A. Nano- and micro-particles for delivery of catechins: Physical and biological performance. Crit. Rev. Food Sci. Nutr. 2019, 59, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Agency Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of health claims related to Camellia sinensis (L.) Kuntze (tea), including catechins in green tea and tannins in black tea, and protection of DNA, proteins and lipids from oxidative damage (ID 1103, 1276, 1311, 1708, 2664), reduction of acid production in dental plaque (ID 1105, 1111), maintenance of bone (ID 1109), decreasing potentially pathogenic intestinal microorganisms (ID 1116), maintenance of vision (ID 1280), maintenance of normal blood pressure (ID 1546) and maintenance of normal blood cholesterol concentrations (ID 1113, 1114) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2010, 8, 1463. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Agency Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of a health claim related to polyphenols in olive and maintenance of normal blood HDL cholesterol concentrations (ID 1639, further assessment) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2012, 10, 2848. [Google Scholar] [CrossRef] [Green Version]
- European Food Safety Agency Panel on Dietetic Products, Nutrition and Allergies. Scientific Opinion on the substantiation of health claims related to: Flavonoids and ascorbic acid in fruit juices, including berry juices (ID 1186); flavonoids from citrus (ID 1471); flavonoids from Citrus paradisi Macfad. (ID 3324, 3325); flavonoids (ID 1470, 1693, 1920); flavonoids in cranberry juice (ID 1804); carotenoids (ID 1496, 1621, 1622, 1796); polyphenols (ID 1636, 1637, 1640, 1641, 1642, 1643); rye bread (ID 1179); protein hydrolysate (ID 1646); carbohydrates with a low/reduced glycaemic load (ID 476, 477, 478, 479, 602) and carbohydrates which induce a low/reduced glycaemic response (ID 727, 1122, 1171); alfalfa (ID 1361, 2585, 2722, 2793); caffeinated carbohydrate-containing energy drinks (ID 1272); and soups (ID 1132, 1133) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2082. [Google Scholar] [CrossRef]
- Dasgupta, N.; Ranjan, S.; Mundekkad, D.; Chidambaram, R.; Shanker, R.; Kumar, A. Nanotechnology in agro-food: From field to plate. Food Res. Int. 2015, 69, 381–400. [Google Scholar] [CrossRef]
- Mlalila, N.; Kadam, D.M.; Swai, H.; Hilonga, A. Transformation of food packaging from passive to innovative via nanotechnology: Concepts and critiques. J. Food Sci. Technol. 2016, 53, 3395–3407. [Google Scholar] [CrossRef] [Green Version]
- Salgado, P.R.; Di Giorgio, L.; Musso, Y.S.; Mauri, A.N. Chapter 9—Bioactive Packaging: Combining Nanotechnologies With Packaging for Improved Food Functionality. In Nanomaterials for Food Applications; López Rubio, A., Fabra Rovira, M.J., Martínez Sanz, M., Gómez-Mascaraque, L.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 233–270. [Google Scholar] [CrossRef]
- Enescu, D.; Cerqueira, M.A.; Fucinos, P.; Pastrana, L.M. Recent advances and challenges on applications of nanotechnology in food packaging. A literature review. Food Chem. Toxicol. 2019, 134, 110814. [Google Scholar] [CrossRef]
- Otoni, C.G.; Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de Soares, N.F.F.; Mattoso, L.H.C. Antimicrobial and physical-mechanical properties of pectin/papaya puree/cinnamaldehyde nanoemulsion edible composite films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Makwana, S.; Choudhary, R.; Dogra, N.; Kohli, P.; Haddock, J. Nanoencapsulation and immobilization of cinnamaldehyde for developing antimicrobial food packaging material. LWT Food Sci. Technol. 2014, 57, 470–476. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Wang, X.; Zhou, Y.; Gao, X.; Puligundla, P.; Wan, X. Encapsulation of epigallocatechin gallate in zein/chitosan nanoparticles for controlled applications in food systems. Food Chem. 2017, 231, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Wrona, M.; Cran, M.J.; Nerín, C.; Bigger, S.W. Development and characterisation of HPMC films containing PLA nanoparticles loaded with green tea extract for food packaging applications. Carbohydr. Polym. 2017, 156, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Salević, A.; Prieto, C.; Cabedo, L.; Nedović, V.; Lagaron, J.M. Physicochemical, Antioxidant and Antimicrobial Properties of Electrospun Poly(ε-caprolactone) Films Containing a Solid Dispersion of Sage (Salvia officinalis L.) Extract. Nanomaterials 2019, 9, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Manghani, C.; Kohli, S.; Nigam, D.; Rani, V. Tea and human health: The dark shadows. Toxicol. Lett. 2013, 220, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, K.; Stone, V.; Tran, C.L.; Kreyling, W.; Borm, P.J.A. Nanotoxicology. Occup. Environ. Med. 2004, 61, 727. [Google Scholar] [CrossRef] [PubMed]
- Warheit, D.B.; Sayes, C.M.; Reed, K.L.; Swain, K.A. Health effects related to nanoparticle exposures: Environmental, health and safety considerations for assessing hazards and risks. Pharmacol. Ther. 2008, 120, 35–42. [Google Scholar] [CrossRef]
- Homayouni, H.; Kavoosi, G.; Nassiri, S.M. Physicochemical, antioxidant and antibacterial properties of dispersion made from tapioca and gelatinized tapioca starch incorporated with carvacrol. LWT Food Sci. Technol. 2017, 77, 503–509. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Li, W.; Lin, L. Antioxidant property of SiO2-eugenol liposome loaded nanofibrous membranes on beef. Food Packag. Shelf Life 2017, 11, 49–57. [Google Scholar] [CrossRef]
- Liu, F.; Avena-Bustillos, R.J.; Chiou, B.-S.; Li, Y.; Ma, Y.; Williams, T.G.; Wood, D.F.; McHugh, T.H.; Zhong, F. Controlled-release of tea polyphenol from gelatin films incorporated with different ratios of free/nanoencapsulated tea polyphenols into fatty food simulants. Food Hydrocoll. 2017, 62, 212–221. [Google Scholar] [CrossRef]
- Estevez-Areco, S.; Guz, L.; Candal, R.; Goyanes, S. Release kinetics of rosemary (Rosmarinus officinalis) polyphenols from polyvinyl alcohol (PVA) electrospun nanofibers in several food simulants. Food Packag. Shelf Life 2018, 18, 42–50. [Google Scholar] [CrossRef]
- Carlson, C.; Hussain, S.M.; Schrand, A.M.; Braydich-Stolle, L.K.; Hess, K.L.; Jones, R.L.; Schlager, J.J. Unique cellular interaction of silver nanoparticles: Size-dependent generation of reactive oxygen species. J. Phys. Chem. B 2008, 112, 13608–13619. [Google Scholar] [CrossRef] [PubMed]
- Smolkova, B.; El Yamani, N.; Collins, A.R.; Gutleb, A.C.; Dusinska, M. Nanoparticles in food. Epigenetic changes induced by nanomaterials and possible impact on health. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 77, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Bhabra, G.; Sood, A.; Fisher, B.; Cartwright, L.; Saunders, M.; Evans, W.H.; Surprenant, A.; Lopez-Castejon, G.; Mann, S.; Davis, S.A.; et al. Nanoparticles can cause DNA damage across a cellular barrier. Nat. Nanotechnol. 2009, 4, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-dependent cytotoxicity of gold nanoparticles. Small (Weinh. Der Bergstr. Ger.) 2007, 3, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, M.; Park, H.S.; Shin, U.S.; Gong, M.S.; Kim, H.W. Size-dependent cellular toxicity of silver nanoparticles. J. Biomed. Mater. Res. Part A 2012, 100, 1033–1043. [Google Scholar] [CrossRef]
- Manickam, V.; Velusamy, R.K.; Lochana, R.; Amiti, A.; Rajendran, B.; Tamizhselvi, R. Applications and genotoxicity of nanomaterials in the food industry. Environ. Chem. Lett. 2017, 15, 399–412. [Google Scholar] [CrossRef]
- Amenta, V.; Aschberger, K.; Arena, M.; Bouwmeester, H.; Botelho Moniz, F.; Brandhoff, P.; Gottardo, S.; Marvin, H.J.P.; Mech, A.; Quiros Pesudo, L.; et al. Regulatory aspects of nanotechnology in the agri/feed/food sector in EU and non-EU countries. Regul. Toxicol. Pharmacol. 2015, 73, 463–476. [Google Scholar] [CrossRef]
- Cushen, M.; Kerry, J.; Morris, M.; Cruz-Romero, M.; Cummins, E. Nanotechnologies in the food industry—Recent developments, risks and regulation. Trends Food Sci. Technol. 2012, 24, 30–46. [Google Scholar] [CrossRef]
- EFSA Scientific Committee; Hardy, A.B.D.; Halldorsson, T.; Jeger, M.J.; Knutsen, H.K.; More, S.; Naegeli, H.; Noteborn, H.; Ockleford, C.; Ricci, A.; et al. Guidance on risk assessment of the application of nanoscience and nanotechnologies in the food and feed chain: Part 1, human and animal health. EFSA J. 2018, 16, 5327–5395. [Google Scholar]
- The European Regulation on Registration, Evaluation and Authorisation and Restriction of Chemicals (REACH). Commission recommendation of 18 October 2011 on the definition of nanomaterial (2011/696/EU). Off. J. Eur. Union 2011, L275/238–L275/240. [Google Scholar]
- Kessler, R. Engineered nanoparticles in consumer products: Understanding a new ingredient. Environ. Health Perspect. 2011, 119, a120–a125. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A. Phenolics: Prooxidants or Antioxidants? Nutr. Rev. 1997, 55, 396–398. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; et al. Scientific opinion on the safety of green tea catechins. EFSA J. 2018, 16, e05239. [Google Scholar] [CrossRef] [Green Version]
- Borowska, S.; Brzóska, M.M.; Tomczyk, M. Complexation of bioelements and toxic metals by polyphenolic compounds—Implications for health. Curr. Drug Targets 2018, 19, 1612–1638. [Google Scholar] [CrossRef]
- Martirosyan, A.; Grintzalis, K.; Polet, M.; Laloux, L.; Schneider, Y.-J. Tuning the inflammatory response to silver nanoparticles via quercetin in Caco-2 (co-)cultures as model of the human intestinal mucosa. Toxicol. Lett. 2016, 253, 36–45. [Google Scholar] [CrossRef]
| Active Compounds | Nanocarriers | Particle Size (nm) | Activity (Details of Research) | Reference |
|---|---|---|---|---|
| Polyphenol-Loaded Nanoparticles for Enhancement of Physicochemical Properties of Food | ||||
| Curcumin | Zein-nanoparticles (NPs) | 175–900 nm | The nanoparticles showed good dispersion and coloring capacity in semi-skimmed milk compared to commercial curcumin. The nanoparticles thus enable the use of curcumin as a coloring agent in aqueous food products. | Gomez-Estaca et al. [31] |
| Curcumin | Nanomicelles | 30 nm | Nanomicelles (natural colorants) allowed better intestinal resorption of active compounds and enhanced their stability. | Ranjan et al. [5] Silva et al. [51] |
| Herb essential oils (containing high percent of phenolic terpenes) | Nanoemulsion based on herb essential oils | 59.48–112.82 nm | Essential oil nanoemulsions enhanced organoleptic quality of rainbow trout and effectively affected the reduction of bacterial growth. | Ozogul et al. [14] |
| Polyphenol-Loaded Nanoparticles for Enhancement of Functional Properties of Food | ||||
| Curcumin | Pectin-coated sodium caseinate/zein NPs | 250–600 nm | Curcumin-loaded nanoparticles significantly enhanced curcumin antioxidant activity and prolonged release capabilities in simulated gastric and intestinal fluids. | Chang et al. [43] |
| Curcumin | Caseinate-zein-polysaccharide nanocomplex | 160–210 nm | Nanocarriers exhibited good physicochemical properties and possibility for future applications as oral delivery vehicles for lipophilic nutrients. | Chang et al. [52] |
| Curcumin | Chitosan-coated solid-lipid NPs | 451.8 ± 19.62 nm | Chitosan-coated solid-lipid nanoparticles as carriers for curcumin contributed to increased oral bioavailability and affected the wider application of curcumin nanostructures in food. | Ramalingam et al. [53] |
| Catechin | chitosan/poly-γ-glutamic acid NPs | Not reported | Chitosan/poly-γ-glutamic acid nanoparticles enhanced the oral delivery of catechins and improved antioxidant activity of catechins. | Tang et al. [54] |
| Catechin | Gelatin NPs | Around 200 nm | Catechin–gelatin nanoparticles can be a useful antioxidant carrier because catechin and gelatin are mutually protected from oxidation and enzymatic degradation. | Chen et al. [36] |
| (+)-catechin (−)-epigallocatechin | Chitosan nanoparticles (CS NPs) | ˂500 nm | Encapsulation of catechins in CS NPs enhanced catechins’ intestinal absorption and their bioavailability. | Dube et al. [55] |
| Catechin | Bioadhesive CS NPs | 110–130 nm | Encapsulation of catechins in CS NPs leads to enhanced oral bioavailability of catechin. | Dudhani & Kosaraju [56] |
| Tea polyphenols (TP) | CS NPs (using carboxymethyl chitosan and chitosan hydrochloride) | 407 ± 50 nm | CS-TP NPs showed significant antitumor activities. | Liang et al. [57] |
| Quercetin | Chitosan/alginate NPs | Not reported (˃100 nm) | Chitosan/alginate nanoparticles can be good carriers for quercetin because of their safe and improved protection of the encapsulated antioxidant. | Aluani et al. [58] |
| Quercetin | Poly-D,L-lactide (PLA) NPs | 130 ± 30 nm | Encapsulation of quercetin in poly-D,L-lactide (PLA) nanoparticles affected the increased solubility and stability of the quercetin. | Kumari et al. [59] Esfanjani & Jafari [2] |
| Quercetin | Solid-lipid nanoparticles (SLNs) | 155.3 nm | SLNs can be considered appropriate oral delivery carriers for poorly water-soluble quercetin because they enhanced their absorption. | Li et al. [60] |
| (−)-epigallocatechin gallate (EGCG) | Chitosan-tripolyphosphate nanoparticles (CS NPs) | 440 ± 37 nm | CS NPs can be useful carriers, providing better oral delivery and stability of EGCG. | Dube et al. [61] |
| (−)-epigallocatechin gallate (EGCG) | Chitosan/β-lactoblobulin (β-Lg) NPs | 100–500 nm | The prolonged release capabilities of EGCG-loaded chitosan/β-Lg nanoparticles affected the increase of effective absorption of EGCG in the human intestine. | Liang et al. [45] |
| (−)-epigallocatechin gallate (EGCG) | Chitosan-caseinophospho-peptide nanocomplexes (CS-CPP) | 150 ± 4.3 nm | CS-CPP nanocarriers influenced the enhancement of intestinal permeability of EGCG. | Hu et al. [50] |
| Rutin | Casein/pectin nanocomplex | Not reported | Sodium caseinate-pectin nanoparticles have high potential for oral delivery nutrients. They showed limited release of rutin in simulated intestinal conditions. | Luo et al. [49] |
| Naringenin | β-casein NPs | ˂100 nm | The research results suggested that naringenin binds with β-casein over Van der Waals forces, hydrogen bonds, and hydrophobic interactions, which improved individual functional characteristics of naringenins, primarily by enhancing their solubility. | Moeiniafshari et al. [62] |
| Phenolics of pomegranate peel | Lyophilized pomegranate peel-nanoparticles | Not reported | Lyophilized pomegranate peel-nanoparticles demonstrated effective antioxidant and antimicrobial properties, improving cooking characteristics of meatballs and prolonged quality of meatballs during storage. | Morsy et al. [63] |
| Guabiroba fruit phenolic extracts | Poly(D,L-lactic-co-glycolic)acid NPs(PLGA) | 202.5–243.8 nm | PLGA can be used as a nanocarrier for phenolic compounds. Loaded-nanoparticles have showed inhibitory effect on Listeria innocua and good antioxidant activity. | Pereira et al. [64] |
| Rosmaric acid, protocatechuic acid, 2,5-dihydroxybenzoic acid | CS NPs | ˃300 nm | Polyphenol-loaded chitosan nanoparticles showed effect against food pathogens. Better antimicrobial activity was obtained against Escherichia coli O157:H7 and Bacillus cereus, while the effect against Salmonella typhimurium was less pronounced. | Madureira et al. [32] |
| Thymol | Zein NPs-stabilized with sodium caseinate and chitosan hydrochloride | 204.75 nm | Thymol-loaded nanoparticles had strong activity against S. aureus and other Gram-positive bacterium under the experimental conditions and can be used as nanocarriers for antimicrobial agents in food. | Zhang et al. [65] |
| Thymol/carvacrol | Thymol/carvacrol liposomes (TCL) | 270.2 nm | TLC can be used to suppress biofilm formation in the early stages of bacterial attachment to food-contact surfaces and it showed antimicrobial activity against S. aureus and Salmonella. Application of TLC presents a good perspective for food quality and safety improvement. | Engel et al. [66] |
| Thymol/carvacrol | Zein NPs | 108–122 nm | Phenolic monoterpenes give a strong interaction with wall of zein. This phenolic-loaded NPs showed higher antimicrobial activity and phenolics remained stable during storage and food processing. | Da Rosa et al. [67] |
| Eugenol | Zein/sodium caseinate/pectin complex NPs | 140 nm | Eugenol-loaded colloidal nanoparticles can find application in the food industry as a dry powder formulation with antimicrobial properties. | Veneranda et al. [48] |
| Eugenol | Sesame oil blended eugenol-loaded nanoemulsion | 13–191 nm | Nanoemulsion exhibited activity against S. aureus and affected the reduction of heterotrophic bacteria in orange juice, and it can be used for food preservation (against microbial spoilage). | Ghosh et al. [68] |
| Eugenol | Nanoemulsions (using gum arabic and lecithin) | 103.6 ± 7.5 nm | Eugenol-loaded nanoemulsion possesses powerful antimicrobial properties and can be applied in the food industry as a food preservative. | Hu et al. [69] |
| EGCG | Nanostructured lipid carriers (NLC) functionalized with folic acid | 234–359 nm | The developed formulation of nanoencapsulated EGCG was suitable for the oral delivery and has potential for applications in the food industry. | Granja et al. [70] |
| EGCG and EGCG + piperine | Zein | 118.3 and 184.2 nm | Optimization of nanoformulation of EGCG alone and along with piperine into a protein nanocarrier and the study of their effect on in vitro antioxidant, hemolytic, and anticancer activities. | Dahiya et al. [71] |
| The fruit extract of Ribes nigrum | Silver nanoparticles (Ag-NPs) | 5–10 nm | Efficiency of nanoencapsulation, characterization and bactericidal, fungicidal, and anticancer activities of nanoparticles synthesized using the fruit extract of Ribes nigrum. | Dobrucka et al. [72] |
| Curcumin and quercetin | Re-assembled casein micelles (r-CM) and casein nanoparticles (CNPs) | 186.9, 66.2, 72.8, and 186.5 nm | Both CNP and r-CM significantly improved the chemical stability of phenolic compounds, and the aqueous solubility was higher than that of free molecules. | Ghayour et al. [73] |
| Resveratrol | Zein and zein + alginate/chitosan complex coating | 72 nm and 160.9 nm | Alginate/chitosan-complex coating improved the photostability, sustained release and bioaccessibility of resveratrol and could be suitable delivery system. | Khan et al. [74] |
| Orange oil nanoemulsions | Orange oil, carrier oil, nonionic surfactant | 25–100 nm | Orange oil as a lipophilic functional agent was successfully incorporated into nanoemulsions; the influence of surfactant, oil composition, temperature, and storage stability were evaluated. | Chang & McClements [75] |
| Thyme oil nanoemulsions | Thyme oil-in-water nanoemulsions | 120 and 1300 nm | Thyme oil was used as a core for preparation of antimicrobial system tested against acid-resistant spoilage yeast, Zygosaccharomyces bailii. | Chang et al. [76] |
| Antioxidant/Cytotoxic (Cell line/Animal Model) Assays | Active Compounds | Nanocarrier | Reference |
|---|---|---|---|
| ABTS radical scavenging activity | Curcumin | Pectin-coated sodium caseinate/zein NPs | Chang et al. [52] |
| Tea polyphenols | Lysozyme-carboxymethyl cellulose nanogels | Liu et al. [103] | |
| Catechin | Chitosan/poly-γ-glutamic acid NPs | Tang et al. [54] | |
| Curcumin | Caseinate-zein-polysaccharide nanocomplex | Chang et al. [43] | |
| DPPH radical scavenging activity | Resveratrol | Chitosan-TPP (sodium tripolyphosphate) NPs | Wu et al. [104] |
| Resveratrol/quercetin | Chitosan NPs/polyethylene glycol modified chitosan NPs | Natesan et al. [105] | |
| Resveratrol | PLGA [poly(lactic-co-glycolic acid)] -oil hybrid NPs | Kumar et al. [106] | |
| Resveratrol/quercetin | Liposome | Caddeo et al. [107] | |
| Tea polyphenols | Lysozyme-carboxymethyl cellulose nanogels | Liu et al. [103] | |
| Catechin | Chitosan/poly-γ-glutamic acid NPs | Tang et al. [54] | |
| Hypochlorous acid (HOCl) scavenging assay | Resveratrol | Bovine serum albumin-based NPs | Fonseca et al. [108] |
| Ferric-reducing ability (FRP) | Resveratrol/quercetin | Chitosan NPs/polyethylene glycol modified chitosan NPs | Natesan et al. [105] |
| Hydrogen peroxide scavenging assay | Resveratrol | PLGA [poly(lactic-co-glycolic acid)] -oil hybrid NPs | Kumar et al. [106] |
| TEER measurements and transport studies (Caco-2 cell) | Catechin | Chitosan/poly-γ-glutamic acid NPs | Tang et al. [54] |
| Cell viability (Hepatocellular carcinoma cells SMMC7721 and hepatocyte LO2 cells) | Resveratrol | Chitosan-TPP (sodium tripolyphosphate) NPs | Wu et al. [104] |
| Monkey kidney (Vero) cell lines-sulforhodamine B assay | Resveratrol | PLGA [poly(lactic-co-glycolic acid)] -oil hybrid NPs | Kumar et al. [106] |
| Antitumor effect in vitro assays (CT26 mouse colon cancer cells) | Resveratrol | Polyethylene glycol-polylactic acid polymer NPs | Jung et al. [109] |
| In vitro cell culture study (Cochlear cell lines (HEI-OC1 and SVK-1) | Resveratrol | Polymeric NPs | Musazzi et al. [110] |
| In vitro hemolytic/anticancer assay (human cancer cell lines i.e., leukemia cancer (HL60), oral cancer (SCC40), breast cancer (MCF7), cervix cancer (HeLa) and colon cancer (Colo205)- sulforhodamine B assay) | EGCG/EGCG + piperine | Zein | Dahiya et al. [71] |
| In vitro cytotoxicity assay (SK-MEL-28 and Colo-38 cells) | Resveratrol | Ultradeformable liposomes | Cosco et al. [111] |
| In vitro assays in cells from different origin (cultivated HepG2 cells, isolated primary rat hepatocytes, isolated murine spleen lymphocytes and macrophages) | Quercetin | Chitosan/alginate NPs | Aluani et al. [58] |
| In vitro cytotoxicity assay (Human hepatoma HepG2 cells) | Tea polyphenols (TP) | CS NPs (using carboxymethyl chitosan and chitosan hydrochloride) | Liang et al. [57] |
| In vitro assay (human hepatoblastoma cancer cell line HepG2) | Tea polyphenols | Lysozyme-carboxymethyl cellulose nanogels | Liu et al. [103] |
| Evaluation of cell proliferative activity (nonmalignant line of fibroblasts CCD-39Lu-isolated from lungs and adherent epithelial non-small cell lung cancer cell line A549) | The fruit extract of Ribes nigrum | Silver nanoparticles (Ag-NPs) | Dobrucka et al. [72] |
| In vitro assay (Human dermal fibroblasts) | Resveratrol/quercetin | Liposome | Caddeo et al. [107] |
| Antitumor effect in vivo assays (CT26 mouse colon cancer cells) | Resveratrol | Polyethylene glycol-polylactic acid polymer NPs | Jung et al. [109] |
| IOP reducing efficiency (normotensive rabbits) | Resveratrol/quercetin | Chitosan NPs/polyethylene glycol modified chitosan NPs | Natesan et al. [105] |
| In vivo study of antidiabetic activities (Wistar rats) | Curcumin | Chitosan CS-ZnO-NC NPs | Chauhan et al. [112] |
| In vivo toxicological evaluation (Male Wistar albino rats) | Quercetin | Chitosan/alginate NPs | Aluani et al. [58] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milinčić, D.D.; Popović, D.A.; Lević, S.M.; Kostić, A.Ž.; Tešić, Ž.L.; Nedović, V.A.; Pešić, M.B. Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomaterials 2019, 9, 1629. https://doi.org/10.3390/nano9111629
Milinčić DD, Popović DA, Lević SM, Kostić AŽ, Tešić ŽL, Nedović VA, Pešić MB. Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomaterials. 2019; 9(11):1629. https://doi.org/10.3390/nano9111629
Chicago/Turabian StyleMilinčić, Danijel D., Dušanka A. Popović, Steva M. Lević, Aleksandar Ž. Kostić, Živoslav Lj. Tešić, Viktor A. Nedović, and Mirjana B. Pešić. 2019. "Application of Polyphenol-Loaded Nanoparticles in Food Industry" Nanomaterials 9, no. 11: 1629. https://doi.org/10.3390/nano9111629
APA StyleMilinčić, D. D., Popović, D. A., Lević, S. M., Kostić, A. Ž., Tešić, Ž. L., Nedović, V. A., & Pešić, M. B. (2019). Application of Polyphenol-Loaded Nanoparticles in Food Industry. Nanomaterials, 9(11), 1629. https://doi.org/10.3390/nano9111629