Evaluation of Solar-Driven Photocatalytic Activity of Thermal Treated TiO2 under Various Atmospheres
Abstract
1. Introduction
2. Materials and Methods
2.1. Instruments
2.2. Sample Preparation
2.3. Photocatalytic Degradation Experiments
3. Results and Discussion
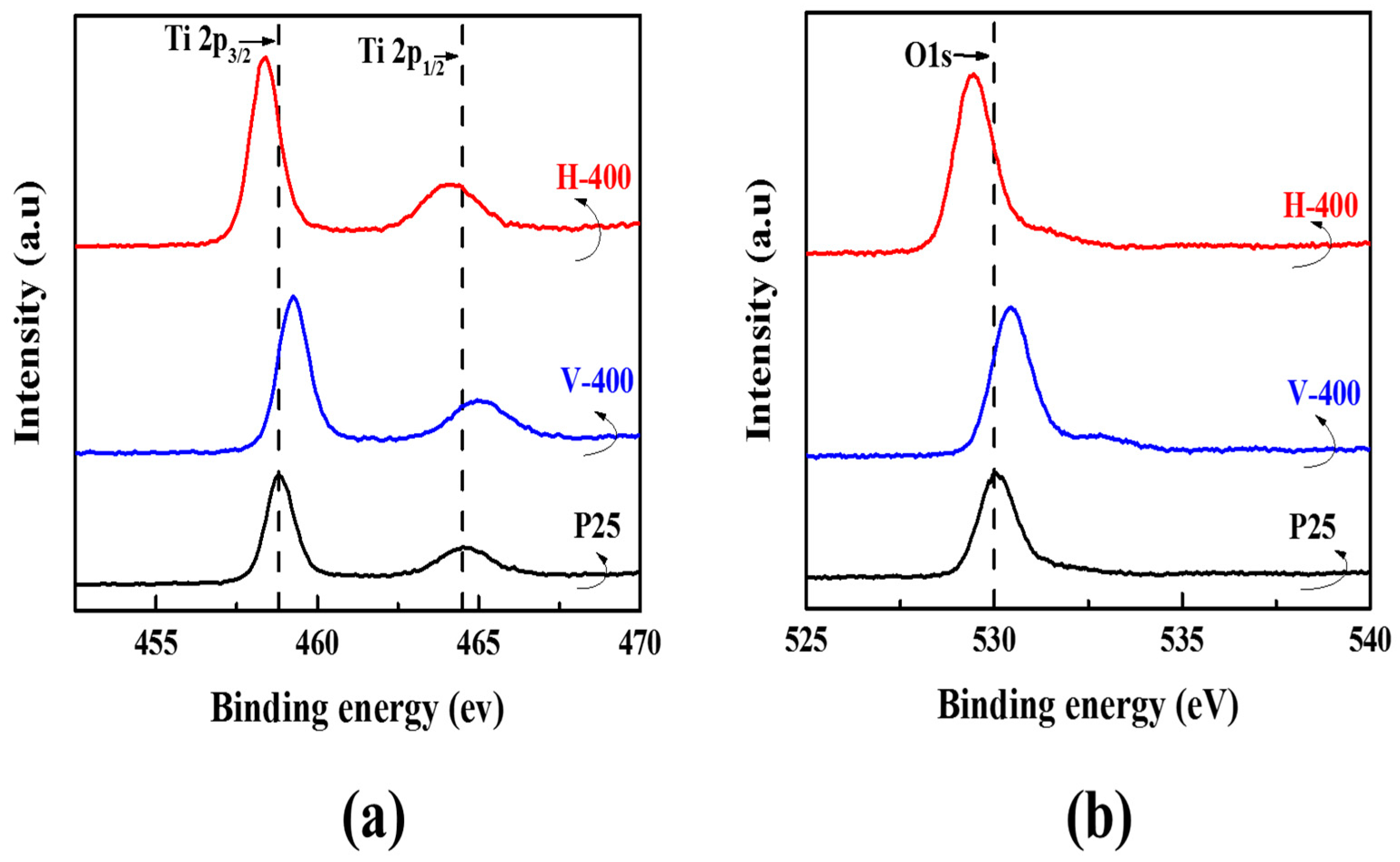
3.1. Physiochemical Characterization of Photocatalyst
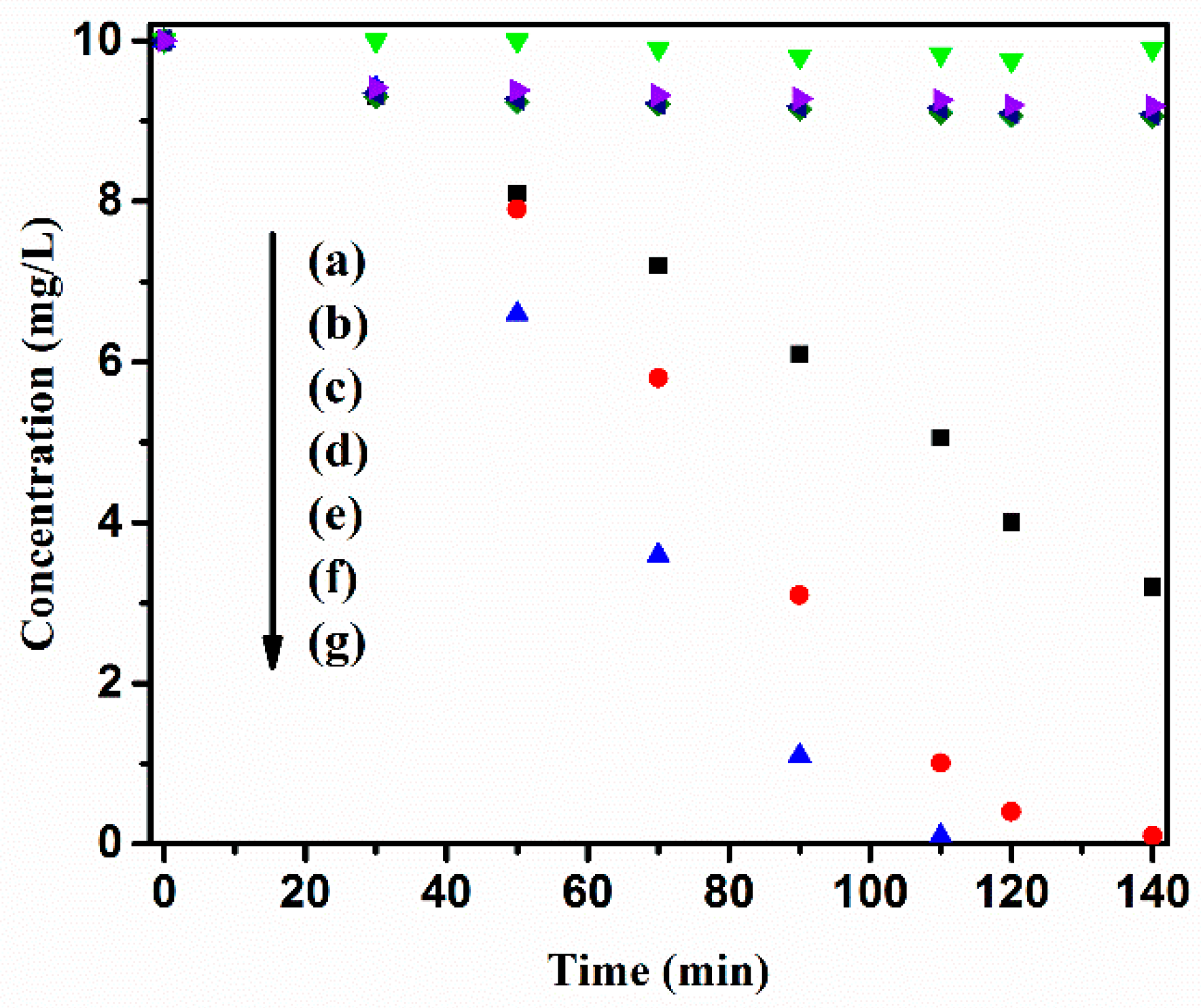
3.2. Solar light-driven Photocatalytic Degradation of Methylene Blue (MB) in Aqueous Solution
3.3. The Influence of Free Radicals on MB Photocatalytic Degradation and Mechanistic Studies
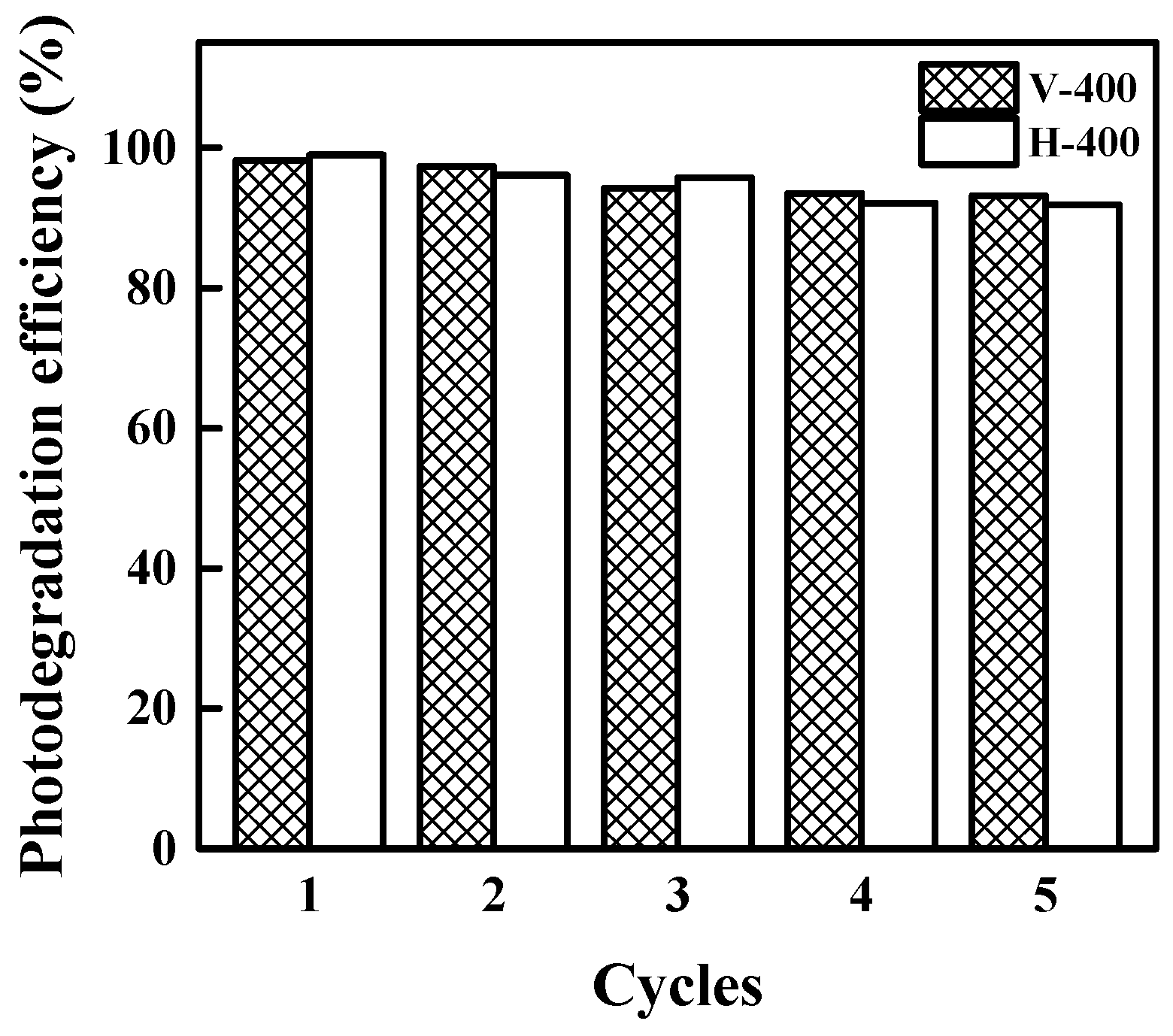
3.4. Stability and Recyclability of The Photocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Wang, Z.; Liu, C.; Tang, C.; Wang, X.; Ding, G.; Ding, Y.; Yang, L. TiO2 Nanotubes/Ag/MoS2 Meshy Photoelectrode with Excellent Photoelectrocatalytic Degradation Activity for Tetracycline Hydrochloride. Nanomaterials 2018, 8, 666. [Google Scholar] [CrossRef] [PubMed]
- Justicia, I.; Ordejón, P.; Canto, G.; Mozos, J.L.; Fraxedas, J.; Battiston, G.A.; Gerbasi, R.; Figueras, A. Designed Self-Doped Titanium Oxide Thin Films for Efficient Visible-Light Photocatalysis. Adv. Mater. 2002, 14, 1399–1402. [Google Scholar] [CrossRef]
- Gonell, F.; Puga, A.V.; López, B.J.; García, H.; Corma, A. Copper-doped titania photocatalysts for simultaneous reduction of CO2 and production of H2 from aqueous sulfide. Appl. Catal. B Environ. 2016, 180, 263–270. [Google Scholar] [CrossRef]
- Navas, J.; Sánchez-Coronilla, A.; Aguilar, T.; Hernández, N.C.; de los Santos, D.M.; Sánchez-Márquez, J.; Zorrilla, D.; Fernández-Lorenzo, C.; Alcántara, R.; Martín-Calleja, J. Experimental and theoretical study of the electronic properties of Cu-doped anatase TiO2. Phys. Chem. Chem. Phys. 2014, 16, 3835–3845. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; Zhang, J.; Chen, F.; Anpo, M. Synthesis and Characterization of Nitrogen-Doped TiO2 Nanophotocatalyst with High Visible Light Activity. J. Phys. Chem. C 2007, 111, 6976–6982. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-Light Photocatalysis in Nitrogen-Doped Titanium Oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef]
- Avisar, D.; Horovitz, I.; Lozzi, L.; Ruggieri, F.; Baker, M.; Abel, M.-L.; Mamane, H. Impact of water quality on removal of carbamazepine in natural waters by N-doped TiO2 photo-catalytic thin film surfaces. J. Hazard. Mater. 2013, 244–245, 463–471. [Google Scholar] [CrossRef]
- Yu, X.; Lin, D.; Li, P.; Su, Z. Recent advances in the synthesis and energy applications of TiO2-graphene nanohybrids. Sol. Energy Mater. Sol. Cells 2017, 172, 252–269. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, X.; Wang, X.; Zhang, Y.; Wei, W.; Sun, Y.; Antonietti, M.; Titirici, M.-M. One-Step Solvothermal Synthesis of a Carbon@TiO2 Dyade Structure Effectively Promoting Visible-Light Photocatalysis. Adv. Mater. 2010, 22, 3317–3321. [Google Scholar] [CrossRef]
- Xu, P.; Xu, T.; Lu, J.; Gao, S.; Hosmane, N.S.; Huang, B.; Dai, Y.; Wang, Y. Visible-light-driven photocatalytic S- and C-codoped meso/nanoporous TiO2. Energy Environ. Sci. 2010, 3, 1128. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, Y.; Xing, M.; Leghari, S.A.K.; Sajjad, S. Development of modified N doped TiO2 photocatalyst with metals, non-metals and metal oxides. Energy Environ. Sci. 2010, 3, 715. [Google Scholar] [CrossRef]
- Takeuchi, M.; Matsuoka, M.; Anpo, M. Ion engineering techniques for the preparation of the highly effective TiO2 photocatalysts operating under visible light irradiation. Res. Chem. Intermed. 2012, 38, 1261–1277. [Google Scholar] [CrossRef]
- Xing, M.; Wu, Y.; Zhang, J.; Chen, F. Effect of synergy on the visible light activity of B, N and Fe co-doped TiO2 for the degradation of MO. Nanoscale 2010, 2, 1233. [Google Scholar] [CrossRef] [PubMed]
- Dashora, A.; Patel, N.; Kothari, D.C.; Ahuja, B.L.; Miotello, A. Formation of an intermediate band in the energy gap of TiO2 by Cu–N-codoping: First principles study and experimental evidence. Sol. Energy Mater. Sol. Cells 2014, 125, 120–126. [Google Scholar] [CrossRef]
- Fang, W.; Xing, M.; Zhang, J. A new approach to prepare Ti3+ self-doped TiO2 via NaBH4 reduction and hydrochloric acid treatment. Appl. Catal. B Environ. 2014, 160–161, 240–246. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nanocrystals. Science 2011, 331, 746–750. [Google Scholar] [CrossRef]
- Zuo, F.; Wang, L.; Wu, T.; Zhang, Z.; Borchardt, D.; Feng, P. Self-Doped Ti3+ Enhanced Photocatalyst for Hydrogen Production under Visible Light. J. Am. Chem. Soc. 2010, 132, 11856–11857. [Google Scholar] [CrossRef]
- Chen, C.S.; Chen, T.C.; Chen, C.C.; Lai, Y.T.; You, J.H.; Chou, T.M.; Chen, C.H.; Lee, J.-F. Effect of Ti3+ on TiO2-Supported Cu Catalysts Used for CO Oxidation. Langmuir 2012, 28, 9996–10006. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Liu, Z.; Marcus, M.A.; Wang, W.-C.; Oyler, N.A.; Grass, M.E.; Mao, B.; Glans, P.-A.; Yu, P.Y.; et al. Properties of Disorder-Engineered Black Titanium Dioxide Nanoparticles through Hydrogenation. Sci. Rep. 2013, 3, 1510. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Meng, X.; Wang, J.; Wang, S.; Lou, Z.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y. Metallic zinc-assisted synthesis of Ti3+ self-doped TiO2 with tunable phase composition and visible-light photocatalytic activity. Chem. Commun. 2013, 49, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Gao, S.; Xu, H.; Lou, Z.; Wang, W.; Huang, B.; Dai, Y. Green synthetic approach for Ti3+ self-doped TiO2−x nanoparticles with efficient visible light photocatalytic activity. Nanoscale 2013, 5, 1870. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Linsebigler, A.; Yates, J.T. Ti3+ Defect Sites on TiO2(110): Production and Chemical Detection of Active Sites. J. Phys. Chem. 1994, 98, 11733–11738. [Google Scholar] [CrossRef]
- Hoang, S.; Berglund, S.P.; Hahn, N.T.; Bard, A.J.; Mullins, C.B. Enhancing Visible Light Photo-oxidation of Water with TiO2 Nanowire Arrays via Cotreatment with H2 and NH3: Synergistic Effects between Ti3+ and N. J. Am. Chem. Soc. 2012, 134, 3659–3662. [Google Scholar] [CrossRef]
- Mezhenny, S.; Maksymovych, P.; Thompson, T.L.; Diwald, O.; Stahl, D.; Walck, S.D.; Yates, J.T. STM studies of defect production on the TiO2(110)-(1×1) and TiO2(110)-(1×2) surfaces induced by UV irradiation. Chem. Phys. Lett. 2003, 369, 152–158. [Google Scholar] [CrossRef]
- Thompson, T.L.; Yates, J.T. Surface Science Studies of the Photoactivation of TiO2 New Photochemical Processes. Chem. Rev. 2006, 106, 4428–4453. [Google Scholar] [CrossRef]
- Zhang, F.; Jin, S.; Mao, Y.; Zheng, Z.; Chen, Y.; Liu, X. Surface characterization of titanium oxide films synthesized by ion beam enhanced deposition. Thin Solid Films 1997, 310, 29–33. [Google Scholar] [CrossRef]
- Nakamura, I.; Negishi, N.; Kutsuna, S.; Ihara, T.; Sugihara, S.; Takeuchi, K. Role of oxygen vacancy in the plasma-treated TiO2 photocatalyst with visible light activity for NO removal. J. Mol. Catal. A Chem. 2000, 161, 205–212. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhou, W.; Chi, L.; Zhang, X.; Hu, W.; Jiang, B.; Pan, K.; Tian, G.; Jiang, Z. Black N/H-TiO2 Nanoplates with a Flower-Like Hierarchical Architecture for Photocatalytic Hydrogen Evolution. ChemSusChem 2016, 9, 2841–2848. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, C.; Li, Z.; Xie, Y. Vacancy Engineering for Tuning Electron and Phonon Structures of Two-Dimensional Materials. Adv. Energy Mater. 2016, 6, 1600436. [Google Scholar] [CrossRef]
- Kong, M.; Li, Y.; Chen, X.; Tian, T.; Fang, P.; Zheng, F.; Zhao, X. Tuning the Relative Concentration Ratio of Bulk Defects to Surface Defects in TiO2 Nanocrystals Leads to High Photocatalytic Efficiency. J. Am. Chem. Soc. 2011, 133, 16414–16417. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Wu, G.; Guan, N.; Li, L.; Li, Z.; Cao, X. Understanding the effect of surface/bulk defects on the photocatalytic activity of TiO2: Anatase versus rutile. Phys. Chem. Chem. Phys. 2013, 15, 10978. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, W.; Zhang, X.; Sun, B.; Wang, L.; Pan, K.; Jiang, B.; Tian, G.; Fu, H. Self-floating amphiphilic black TiO2 foams with 3D macro-mesoporous architectures as efficient solar-driven photocatalysts. Appl. Catal. B Environ. 2017, 206, 336–343. [Google Scholar] [CrossRef]
- Xing, M.; Zhang, J.; Chen, F.; Tian, B. An economic method to prepare vacuum activated photocatalysts with high photo-activities and photosensitivities. Chem. Commun. 2011, 47, 4947–4949. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Weng, S.; Liu, P. Enhanced photocatalytic activity by bulk trapping and spatial separation of charge carriers: A case study of defect and facet mediated TiO2. Appl. Catal. B Environ. 2016, 180, 463–470. [Google Scholar] [CrossRef]
- Wang, W.; Huang, W.; Ni, Y.; Lu, C.; Xu, Z. Different Upconversion Properties of β-NaYF4:Yb3+, Tm3+/Er3+ in Affecting the Near-Infrared-Driven Photocatalytic Activity of High-Reactive TiO2. ACS Appl. Mater. Interfaces 2014, 6, 340–348. [Google Scholar] [CrossRef]
- Zuo, F.; Bozhilov, K.; Dillon, R.J.; Wang, L.; Smith, P.; Zhao, X.; Bardeen, C.; Feng, P. Active Facets on Titanium(III)-Doped TiO2: An Effective Strategy to Improve the Visible-Light Photocatalytic Activity. Angew. Chem. Int. Ed. 2012, 124, 6327–6330. [Google Scholar] [CrossRef]
- Zheng, Z.; Huang, B.; Lu, J.; Wang, Z.; Qin, X.; Zhang, X.; Dai, Y.; Whangbo, M.-H. Hydrogenated titania: Synergy of surface modification and morphology improvement for enhanced photocatalytic activity. Chem. Commun. 2012, 48, 5733. [Google Scholar] [CrossRef] [PubMed]
- Andreev, A.S.; Kuznetsov, V.N.; Chizhov, Y.V. Atomic Hydrogen Activated TiO2 Nanocluster: DFT Calculations. J. Phys. Chem. C 2012, 116, 18139–18145. [Google Scholar] [CrossRef]
- Ren, R.; Wen, Z.; Cui, S.; Hou, Y.; Guo, X.; Chen, J. Controllable Synthesis and Tunable Photocatalytic Properties of Ti3+-doped TiO2. Sci. Rep. 2015, 5, 10714. [Google Scholar] [CrossRef] [PubMed]
- An, H.-R.; Park, S.Y.; Kim, H.; Lee, C.Y.; Choi, S.; Lee, S.C.; Seo, S.; Park, E.C.; Oh, Y.-K.; Song, C.-G.; et al. Advanced nanoporous TiO2 photocatalysts by hydrogen plasma for efficient solar-light photocatalytic application. Sci. Rep. 2016, 6, 29683. [Google Scholar] [CrossRef]
- Li, Y.; Hwang, D.-S.; Lee, N.H.; Kim, S.-J. Synthesis and characterization of carbon-doped titania as an artificial solar light sensitive photocatalyst. Chem. Phys. Lett. 2005, 404, 25–29. [Google Scholar] [CrossRef]
- Chatzisymeon, E.; Stypas, E.; Bousios, S.; Xekoukoulotakis, N.P.; Mantzavinos, D. Photocatalytic treatment of black table olive processing wastewater. J. Hazard. Mater. 2008, 154, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Yang, Y.; Na, Y.; Fan, R.; Li, L.; Wei, L.; Yang, B.; Cao, W. An Insight into the Role of Oxygen Vacancy in Hydrogenated TiO2 Nanocrystals in the Performance of Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2015, 7, 3754–3763. [Google Scholar] [CrossRef]
- Cronemeyer, D.C. Infrared Absorption of Reduced Rutile TiO2 Single Crystals. Phys. Rev. 1959, 113, 1222–1226. [Google Scholar] [CrossRef]
- Zhen, S.; Jan, H.; Osgood, R. Formation of TiO2 Nanoparticles by Reactive-Layer-Assisted Deposition and Characterization by XPS and STM. Nano Lett. 2005, 5, 1327–1332. [Google Scholar]
- Kim, K.-W.; Lee, E.-H.; Kim, Y.-J.; Lee, M.-H.; Kim, K.-H.; Shin, D.-W. A relation between the non-stoichiometry and hydroxyl radical generated at photocatalytic TiO2 on 4CP decomposition. J. Photochem. Photobiol. A 2003, 159, 301–310. [Google Scholar] [CrossRef]
- Belet, A.; Wolfs, C.; Mahy, J.G.; Poelman, D.; Vreuls, C.; Gillard, N.; Lambert, S.D. Sol-gel Syntheses of Photocatalysts for the Removal of Pharmaceutical Products in Water. Nanomaterials 2019, 9, 126. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, P.; Fu, X.; Li, Z.; Han, W.; Wang, X. Relationship between Oxygen Defects and the Photocatalytic Property of ZnO Nanocrystals in Nafion Membranes. Langmuir 2009, 25, 1218–1223. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Zhao, Z.; Niu, M.; Mao, C.; Cao, D.; Cheng, D.; Feng, P.; Sun, Z. A facile and versatile method for preparation of colored TiO2 with enhanced solar-driven photocatalytic activity. Nanoscale 2014, 6, 10216–10223. [Google Scholar] [CrossRef] [PubMed]
- Su, R.; Tiruvalam, R.; He, Q.; Dimitratos, N.; Kesavan, L.; Hammond, C.; Lopez-Sanchez, J.A.; Bechstein, R.; Kiely, C.J.; Hutchings, G.J.; et al. Promotion of Phenol Photodecomposition over TiO2 Using Au, Pd and Au–Pd Nanoparticles. ACS Nano 2012, 6, 6284–6292. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, Y.J.; Kim, J.H.; Jang, J.-W.; Choi, S.H.; Lee, J.S. Defective ZnFe2O4 nanorods with oxygen vacancy for photoelectrochemical water splitting. Nanoscale 2015, 7, 19144–19151. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, D.; Venkataswamy, P.; Devaiah, D.; Rangaswamy, A.; Reddy, B.M. Crucial role of titanium dioxide support in soot oxidation catalysis of manganese doped ceria. Catal. Sci. Technol. 2017, 7, 3045–3055. [Google Scholar] [CrossRef]
- Feng, X.; Wang, P.; Hou, J.; Qian, J.; Ao, Y.; Wang, C. Significantly Enhanced Visible Light Photocatalytic Efficiency of Phosphorus doped TiO2 with surface oxygen vacancies for Ciprofloxacin Degradation: Synergistic Effect and Intermediates Analysis. J. Hazard. Mater. 2018, 351, 196–205. [Google Scholar] [CrossRef]
- Pan, X.; Yang, M.-Q.; Fu, X.; Zhang, N.; Xu, Y.-J. Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 2013, 5, 3601–3614. [Google Scholar] [CrossRef]
- Priya, R.; Baiju, K.V.; Shukla, S.; Biju, S.; Reddy, M.L.P.; Patil, K.; Warrier, K.G.K. Comparing Ultraviolet and Chemical Reduction Techniques for Enhancing Photocatalytic Activity of Silver Oxide/Silver Deposited Nanocrystalline Anatase Titania. J. Phys. Chem. C 2009, 113, 6243–6255. [Google Scholar] [CrossRef]
- Pugazhenthiran, N.; Murugesan, S.; Anandan, S. High surface area Ag-TiO2 nanotubes for solar/visible-light photocatalytic degradation of ceftiofur sodium. J. Hazard. Mater. 2013, 263, 541–549. [Google Scholar] [CrossRef]
- De Haart, L.G.J.; Blasse, G. The observation of exciton emission from rutile single crystals. J. Solid State Chem. 1986, 61, 135–136. [Google Scholar] [CrossRef]
- Zhou, C.; Yan, J.; Chen, B.; Li, P.; Dong, X.; Xi, F.; Liu, J. Synthesis and application of ternary photocatalyst with a gradient band structure from two-dimensional nanosheets as precursors. RSC Adv. 2016, 6, 108955–108963. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Lin, Y.; Wang, P.; Chen, W.; Fu, X.; Shao, Y. Evidence for the Active Species Involved in the Photodegradation Process of Methyl Orange on TiO2. J. Phys. Chem. C 2012, 116, 3552–3560. [Google Scholar] [CrossRef]
- Choudhury, B.; Giri, P.K. Isotype heterostructure of bulk and nanosheets of graphitic carbon nitride for efficient visible light photodegradation of methylene blue. RSC Adv. 2016, 6, 24976–24984. [Google Scholar] [CrossRef]
- Rajender, G.; Kumar, J.; Giri, P.K. Interfacial charge transfer in oxygen deficient TiO2-graphene quantum dot hybrid and its influence on the enhanced visible light photocatalysis. Appl. Catal. B Environ. 2018, 224, 960–972. [Google Scholar] [CrossRef]
- Houas, A.; Lachheb, H.; Ksibi, M.; Elaloui, E.; Guillard, C.; Herrmann, J.-M. Photocatalytic degradation pathway of methylene blue in water. Appl. Catal. B Environ. 2001, 31, 145–147. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H. Heterogeneous Photocatalytic Degradation of Organic Contaminants over Titanium Dioxide: A Review of Fundamentals, Progress and Problems. J. Photochem. Photobiol. 2008, 9, 1–12. [Google Scholar] [CrossRef]
- Zhu, G.; Lin, T.; Lü, X.; Zhao, W.; Yang, C.; Wang, Z.; Yin, H.; Liu, Z.; Huang, F.; Lin, J. Black brookite titania with high solar absorption and excellent photocatalytic performance. J. Mater. Chem. A 2013, 1, 9650–9653. [Google Scholar] [CrossRef]
- Katal, R.; Salehi, M.; Farahani, M.H.D.A.; Masudy-Panah, S.; Ong, S.L.; Hu, J. Preparation of a new type of black TiO2 under vacuum atmosphere for sunlight photocatalysis. ACS Appl. Mater. Interfaces 2018, 10, 35316–35326. [Google Scholar] [CrossRef]
- Katal, R.; Masudy Panah, S.; Zarinejad, M.; Salehi, M.; Hu, J. Synthesis of Self-Gravity Settling Faceted-Anatase TiO2 with Dominant {010} Facets for the Photocatalytic Degradation of Acetaminophen and Study of the Type of Generated Oxygen Vacancy in Faceted-TiO2. Water 2018, 10, 1462. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katal, R.; Kholghi Eshkalak, S.; Masudy-panah, S.; Kosari, M.; Saeedikhani, M.; Zarinejad, M.; Ramakrishna, S. Evaluation of Solar-Driven Photocatalytic Activity of Thermal Treated TiO2 under Various Atmospheres. Nanomaterials 2019, 9, 163. https://doi.org/10.3390/nano9020163
Katal R, Kholghi Eshkalak S, Masudy-panah S, Kosari M, Saeedikhani M, Zarinejad M, Ramakrishna S. Evaluation of Solar-Driven Photocatalytic Activity of Thermal Treated TiO2 under Various Atmospheres. Nanomaterials. 2019; 9(2):163. https://doi.org/10.3390/nano9020163
Chicago/Turabian StyleKatal, Reza, Saeideh Kholghi Eshkalak, Saeid Masudy-panah, Mohammadreza Kosari, Mohsen Saeedikhani, Mehrdad Zarinejad, and Seeram Ramakrishna. 2019. "Evaluation of Solar-Driven Photocatalytic Activity of Thermal Treated TiO2 under Various Atmospheres" Nanomaterials 9, no. 2: 163. https://doi.org/10.3390/nano9020163
APA StyleKatal, R., Kholghi Eshkalak, S., Masudy-panah, S., Kosari, M., Saeedikhani, M., Zarinejad, M., & Ramakrishna, S. (2019). Evaluation of Solar-Driven Photocatalytic Activity of Thermal Treated TiO2 under Various Atmospheres. Nanomaterials, 9(2), 163. https://doi.org/10.3390/nano9020163






