Diagnosis in a Preclinical Model of Bladder Pain Syndrome Using a Au/ZnO Nanorod-based SERS Substrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Urine Sampling of IC/BPS Rat Model
2.1.1. IC/BPS Rat Model
2.1.2. Urine Extraction and Analysis of Voiding Pattern
2.2. Surface Enhanced Raman Measurements
2.2.1. ZnO Nanorod Based SERS Chip
2.2.2. Raman Spectra Acquisition and Analysis
3. Results
3.1. IC/BPS Rat Models and Sample Preparation
3.1.1. Voiding Frequency and Sample Drop
3.1.2. Analysis of Measurement Area
3.2. Raman Measurement and Statistical Analysis
3.2.1. Surface Enhancement Raman Measurement of Nanometric Biomarker
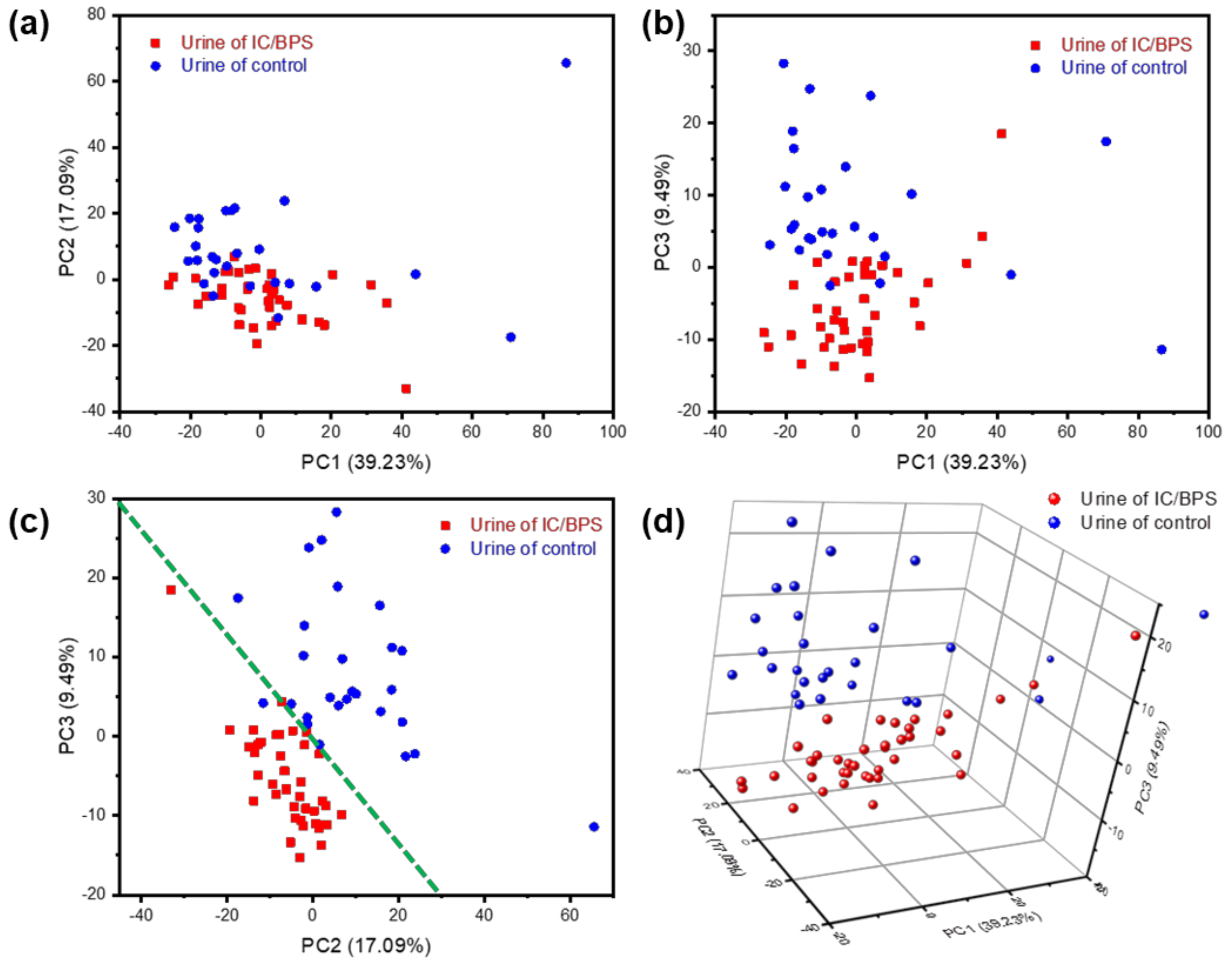
3.2.2. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ishigaki, M.; Maeda, Y.; Taketani, A.; Andriana, B.B.; Ishihara, R.; Wongravee, K.; Ozaki, Y.; Sato, H. Diagnosis of early-stage esophageal cancer by Raman spectroscopy and chemometric techniques. Analyst 2016, 141, 1027–1033. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Kawabata, T.; Okazaki, S.; Hiramatsu, Y.; Baba, M.; Ohta, M.; Kamiya, K.; Tanaka, T.; Konno, H. Near-infrared multichannel Raman spectroscopy with a 1064-nm excitation wavelength for ex vivo diagnosis of gastric cancer. Cancer Res. 2011, 71, e137–e143. [Google Scholar] [CrossRef]
- Carvalho, L.F.C.S.; Bonnier, F.; O’Callaghan, K.; O’Sullivan, J.; Flint, S.; Byrne, H.J.; Lyng, F.M. Raman micro-spectroscopy for rapid screening of oral squamous cell carcinoma. Exp. Mol. Pathol. 2015, 98, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, O.K.; Lee, S.; Kim, J.K. Local-dependency of morphological and optical properties between breast cancer cell lines. Spectrochim. Acta A 2018, 205, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Smith, R.; Wright, K.L.; Ashton, L. Raman spectroscopy: an evolving technique for live cell studies. Analyst 2016, 141, 3590–3600. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.Y.; Tsai, K.T.; Wang, H.H.; Chen, Y.; Chen, Y.H.; Chao, Y.C.; Chang, H.H.; Lin, C.H.; Wang, J.K.; Wang, Y.L. Functionalized arrays of Raman-enhancing nanoparticles for capture and culture-free analysis of bacteria in human blood. Nat. Commun. 2011, 2, 538. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.B.; Yang, D.T.; Ivleva, N.P.; Mircescu, N.E.; Niessner, R.; Haisch, C. SERS Detection of Bacteria in Water by in Situ Coating with Ag Nanoparticles. Anal. Chem. 2014, 86, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Bhat, A.; Sharma, S.; Sharma, A. Elucidating diversity of exosomes: biophysical and molecular characterization methods. Nanomedicine 2016, 11, 2359–2377. [Google Scholar] [CrossRef]
- Lee, C.; Carney, R.P.; Hazari, S.; Smith, Z.J.; Knudson, A.; Robertson, C.S.; Lam, K.S.; Wachsmann-Hogiu, S. 3D plasmonic nanobowl platform for the study of exosomes in solution. Nanoscale 2015, 7, 9290–9297. [Google Scholar] [CrossRef]
- Maiti, N.C.; Apetri, M.M.; Zagorski, M.G.; Carey, P.R.; Anderson, V.E. Raman spectroscopic characterization of secondary structure in natively unfolded proteins: alpha-synuclein. J. Am. Chem. Soc. 2004, 126, 2399–2408. [Google Scholar] [CrossRef]
- Rygula, A.; Majzner, K.; Marzec, K.M.; Kaczor, A.; Pilarczyk, M.; Baranska, M. Raman spectroscopy of proteins: a review. J. Raman Spectrosc. 2013, 44, 1061–1076. [Google Scholar] [CrossRef]
- Lee, S.; Lee, S.H.; Paulson, B.; Lee, J.C.; Kim, J.K. Enhancement of local surface plasmon resonance (LSPR) effect by biocompatible metal clustering based on ZnO nanorods in Raman measurements. Spectrochim. Acta A 2018, 204, 203–208. [Google Scholar] [CrossRef]
- Kim, W.; Lee, S.H.; Kim, S.H.; Lee, J.C.; Moon, S.W.; Yu, J.S.; Choi, S. Highly Reproducible Au-Decorated ZnO Nanorod Array on a Graphite Sensor for Classification of Human Aqueous Humors. ACS Appl. Mater. Inter. 2017, 9, 5891–5899. [Google Scholar] [CrossRef] [PubMed]
- Akgun, M.C.; Kalay, Y.E.; Unalan, H.E. Hydrothermal zinc oxide nanowire growth using zinc acetate dihydrate salt. J. Mater. Res. 2012, 27, 1445–1451. [Google Scholar] [CrossRef]
- Ibupoto, Z.H.; Khun, K.; Eriksson, M.; AlSalhi, M.; Atif, M.; Ansari, A.; Willander, M. Hydrothermal Growth of Vertically Aligned ZnO Nanorods Using a Biocomposite Seed Layer of ZnO Nanoparticles. Materials 2013, 6, 3584–3597. [Google Scholar] [CrossRef] [PubMed]
- Tripp, D.A.; Nickel, J.C.; Wong, J.; Pontari, M.; Moldwin, R.; Mayer, R.; Carr, L.K.; Doggweiler, R.; Yang, C.C.; Mishra, N.; et al. Mapping of Pain Phenotypes in Female Patients with Bladder Pain Syndrome/Interstitial Cystitis and Controls. Eur. Urol. 2012, 62, 1188–1194. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.; Yu, H.Y.; Lim, J.; Ryu, C.M.; Kim, Y.H.; Heo, J.; Han, J.Y.; Lee, S.; Bae, Y.S.; Kim, J.Y.; et al. Improved efficacy and in vivo cellular properties of human embryonic stem cell derivative in a preclinical model of bladder pain syndrome. Sci. Rep. 2017, 7, 8872. [Google Scholar] [CrossRef]
- Kim, H.J. Update on the Pathology and Diagnosis of Interstitial Cystitis/Bladder Pain Syndrome: A Review. Int. Neurourol. J. 2016, 20, 13–17. [Google Scholar] [CrossRef]
- Evans, R.J.; Moldwin, R.M.; Cossons, N.; Darekar, A.; Mills, I.W.; Scholfield, D. Proof of Concept Trial of Tanezumab for the Treatment of Symptoms Associated With Interstitial Cystitis. J. Urol. 2011, 185, 1716–1721. [Google Scholar] [CrossRef]
- Hanno, P.M.; Erickson, D.; Moldwin, R.; Faraday, M.M. Diagnosis and Treatment of Interstitial Cystitis/Bladder Pain Syndrome: AUA Guideline Amendment. J. Urol. 2015, 193, 1545–1553. [Google Scholar] [CrossRef]
- Nickel, J.C.; Herschorn, S.; Whitmore, K.E.; Forrest, J.B.; Hu, P.; Friedman, A.J.; Baseman, A.S. Pentosan Polysulfate Sodium for Treatment of Interstitial Cystitis/Bladder Pain Syndrome: Insights from a Randomized, Double-Blind, Placebo Controlled Study. J. Urol. 2015, 193, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Etchegoin, P.G.; Le Ru, E.C. A perspective on single molecule SERS: current status and future challenges. Phys. Chem. Chem. Phys. 2008, 10, 6079–6089. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.L.; Irudayaraj, J. Surface-enhanced Raman spectroscopy at single-molecule scale and its implications in biology. Philos. T. R. Soc. B 2013, 368, 20120026. [Google Scholar] [CrossRef] [PubMed]
- Kirimoto, T.; Nakano, K.; Irimura, K.; Hayashi, Y.; Matsuura, N.; Kiniwa, M.; Oka, T.; Yoshimura, N. Beneficial effects of suplatast tosilate (IPD-1151T) in a rat cystitis model induced by intravesical hydrochloric acid. BJU Int. 2007, 100, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Birder, L.; Andersson, K.E. Animal Modelling of Interstitial Cystitis/Bladder Pain Syndrome. Int. Neurourol. J. 2018, 22, S3–S9. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Park, J.; Choo, M.S. Mesenchymal Stem-Cell Therapy Alleviates Interstitial Cystitis by Activating Wnt Signaling Pathway. J. Urol. 2015, 193, E217–E217. [Google Scholar] [CrossRef]
- Stone, N.; Stavroulaki, P.; Kendall, C.; Birchall, M.; Barr, H. Raman spectroscopy for early detection of laryngeal malignancy: Preliminary results. Laryngoscope 2000, 110, 1756–1763. [Google Scholar] [CrossRef]
- Synytsya, A.; Judexova, M.; Hoskovec, D.; Miskovicova, M.; Petruzelka, L. Raman spectroscopy at different excitation wavelengths (1064, 785 and 532 nm) as a tool for diagnosis of colon cancer. J. Raman Spectrosc. 2014, 45, 903–911. [Google Scholar] [CrossRef]
- Chan, J.W.; Taylor, D.S.; Zwerdling, T.; Lane, S.M.; Ihara, K.; Huser, T. Micro-Raman spectroscopy detects individual neoplastic and normal hematopoietic cells. Biophys. J. 2006, 90, 648–656. [Google Scholar] [CrossRef]
- Wang, H.; Huang, N.; Zhao, J.; Lui, H.; Korbelik, M.; Zeng, H. Depth-resolved in vivo micro-Raman spectroscopy of a murine skin tumor model reveals cancer-specific spectral biomarkers. J. Raman Spectrosc. 2011, 42, 160–166. [Google Scholar] [CrossRef]
- Huang, Z.W.; McWilliams, A.; Lui, H.; McLean, D.I.; Lam, S.; Zeng, H.S. Near-infrared Raman spectroscopy for optical diagnosis of lung cancer. Int. J. Cancer 2003, 107, 1047–1052. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.; Namgoong, J.-M.; Yu, H.Y.; Jue, M.; Kim, G.; Jeon, S.; Shin, D.-M.; Choo, M.-S.; Joo, J.; Pack, C.-G.; et al. Diagnosis in a Preclinical Model of Bladder Pain Syndrome Using a Au/ZnO Nanorod-based SERS Substrate. Nanomaterials 2019, 9, 224. https://doi.org/10.3390/nano9020224
Lee S, Namgoong J-M, Yu HY, Jue M, Kim G, Jeon S, Shin D-M, Choo M-S, Joo J, Pack C-G, et al. Diagnosis in a Preclinical Model of Bladder Pain Syndrome Using a Au/ZnO Nanorod-based SERS Substrate. Nanomaterials. 2019; 9(2):224. https://doi.org/10.3390/nano9020224
Chicago/Turabian StyleLee, Sanghwa, Jung-Man Namgoong, Hwan Yeul Yu, Miyeon Jue, Gwanho Kim, Sangmin Jeon, Dong-Myung Shin, Myung-Soo Choo, Jinmyoung Joo, Chan-Gi Pack, and et al. 2019. "Diagnosis in a Preclinical Model of Bladder Pain Syndrome Using a Au/ZnO Nanorod-based SERS Substrate" Nanomaterials 9, no. 2: 224. https://doi.org/10.3390/nano9020224
APA StyleLee, S., Namgoong, J.-M., Yu, H. Y., Jue, M., Kim, G., Jeon, S., Shin, D.-M., Choo, M.-S., Joo, J., Pack, C.-G., & Kim, J. K. (2019). Diagnosis in a Preclinical Model of Bladder Pain Syndrome Using a Au/ZnO Nanorod-based SERS Substrate. Nanomaterials, 9(2), 224. https://doi.org/10.3390/nano9020224








