Hierarchical PANI/NiCo-LDH Core-Shell Composite Networks on Carbon Cloth for High Performance Asymmetric Supercapacitor
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of PANI Nanofiber Network
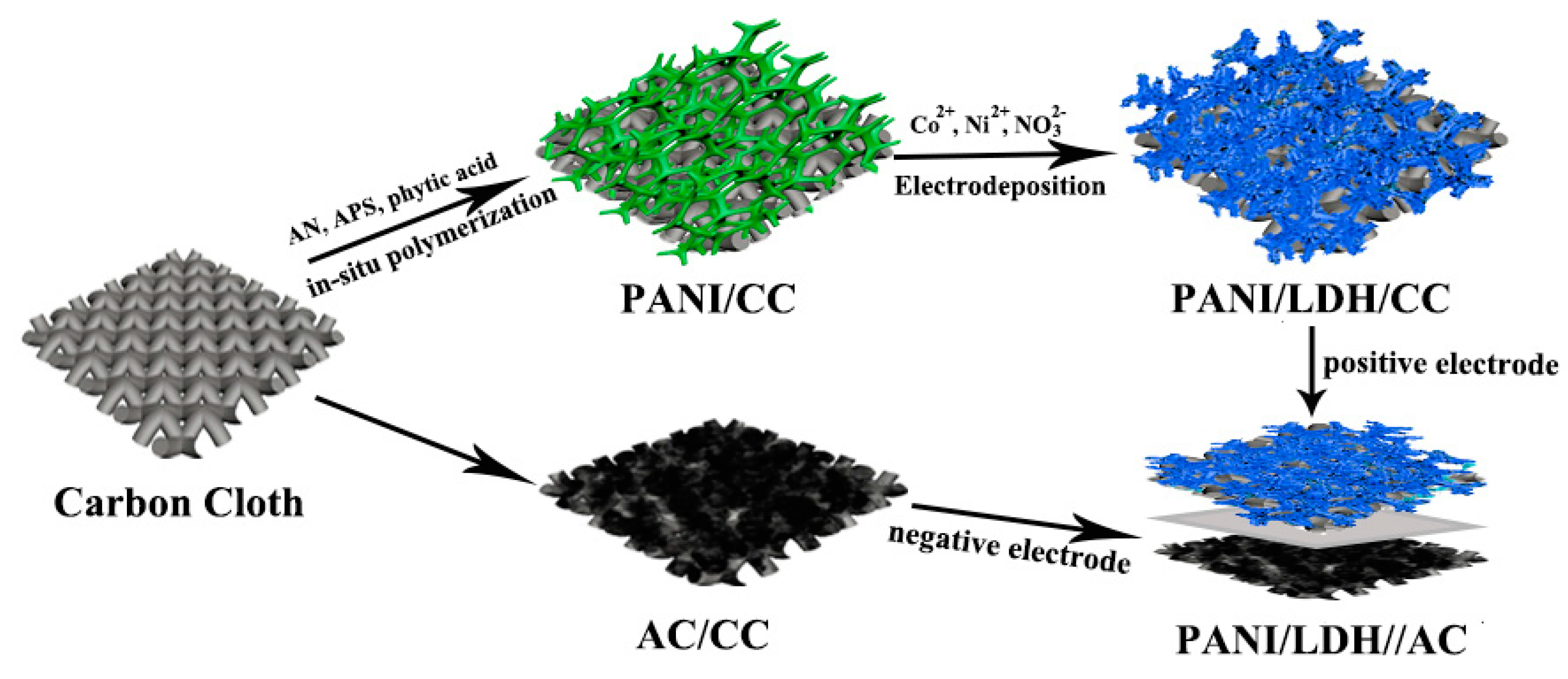
2.3. Preparation of Hierarchical PANI/LDH Core-Shell Nanostructures
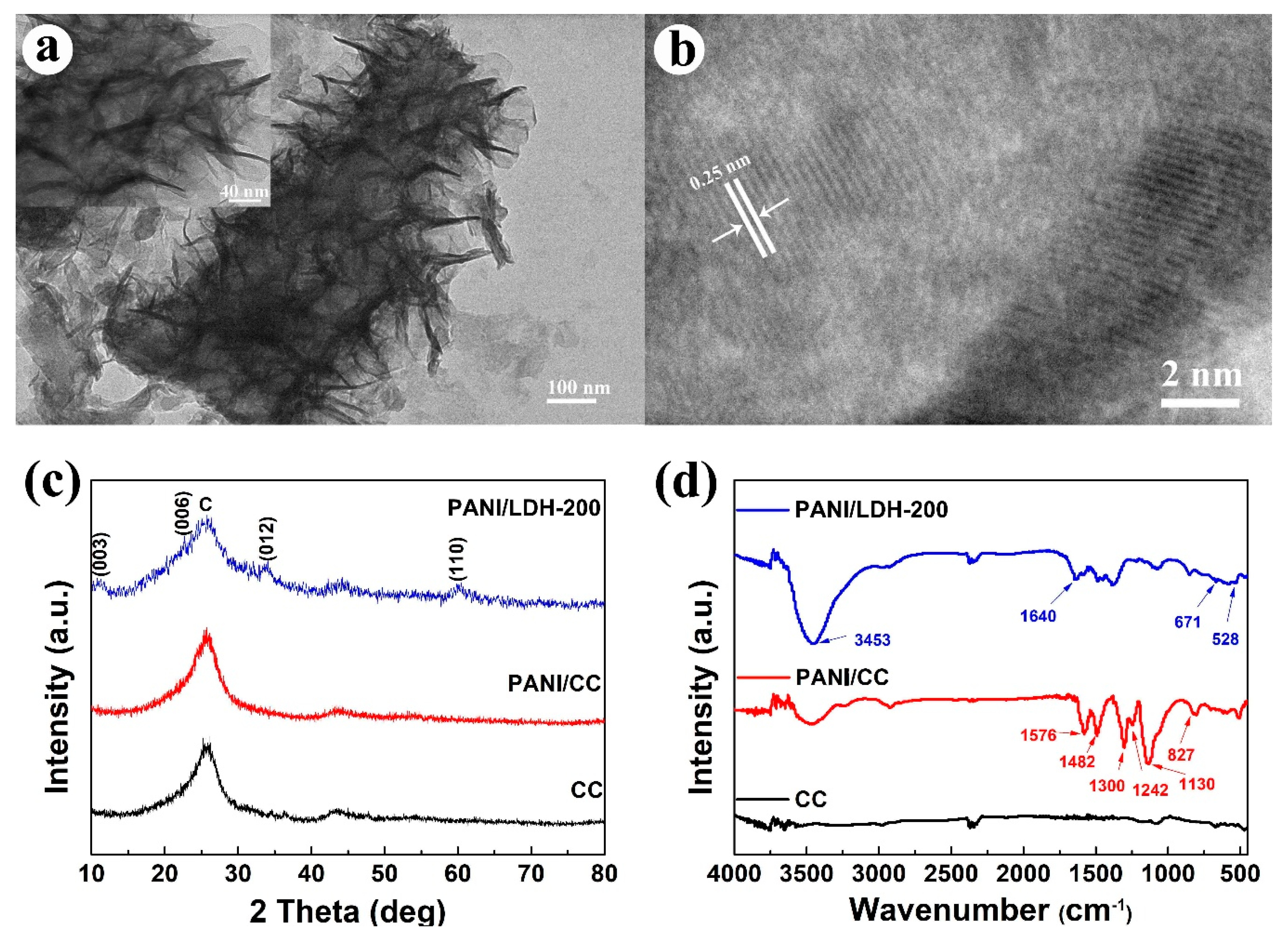
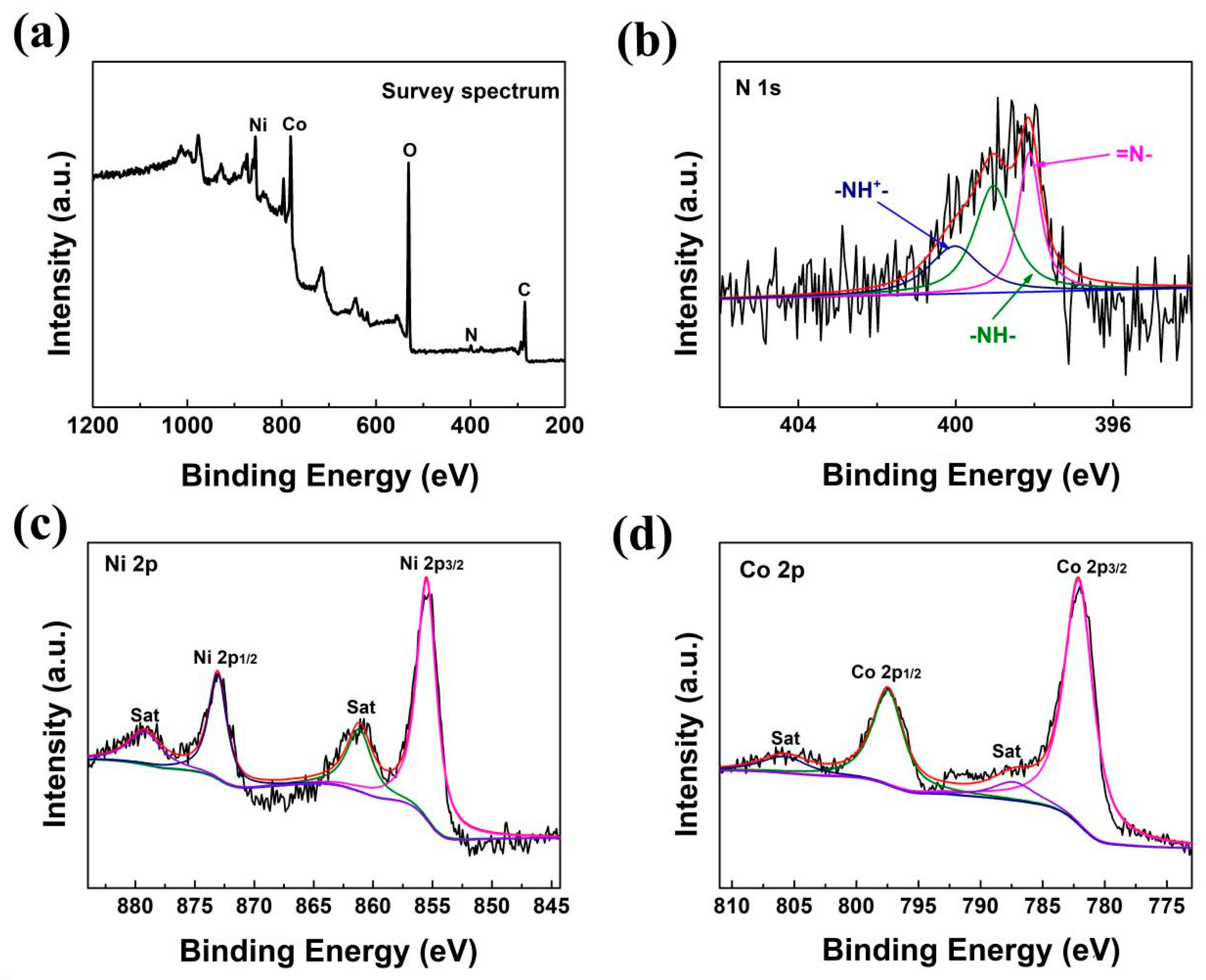
2.4. Material Characterization
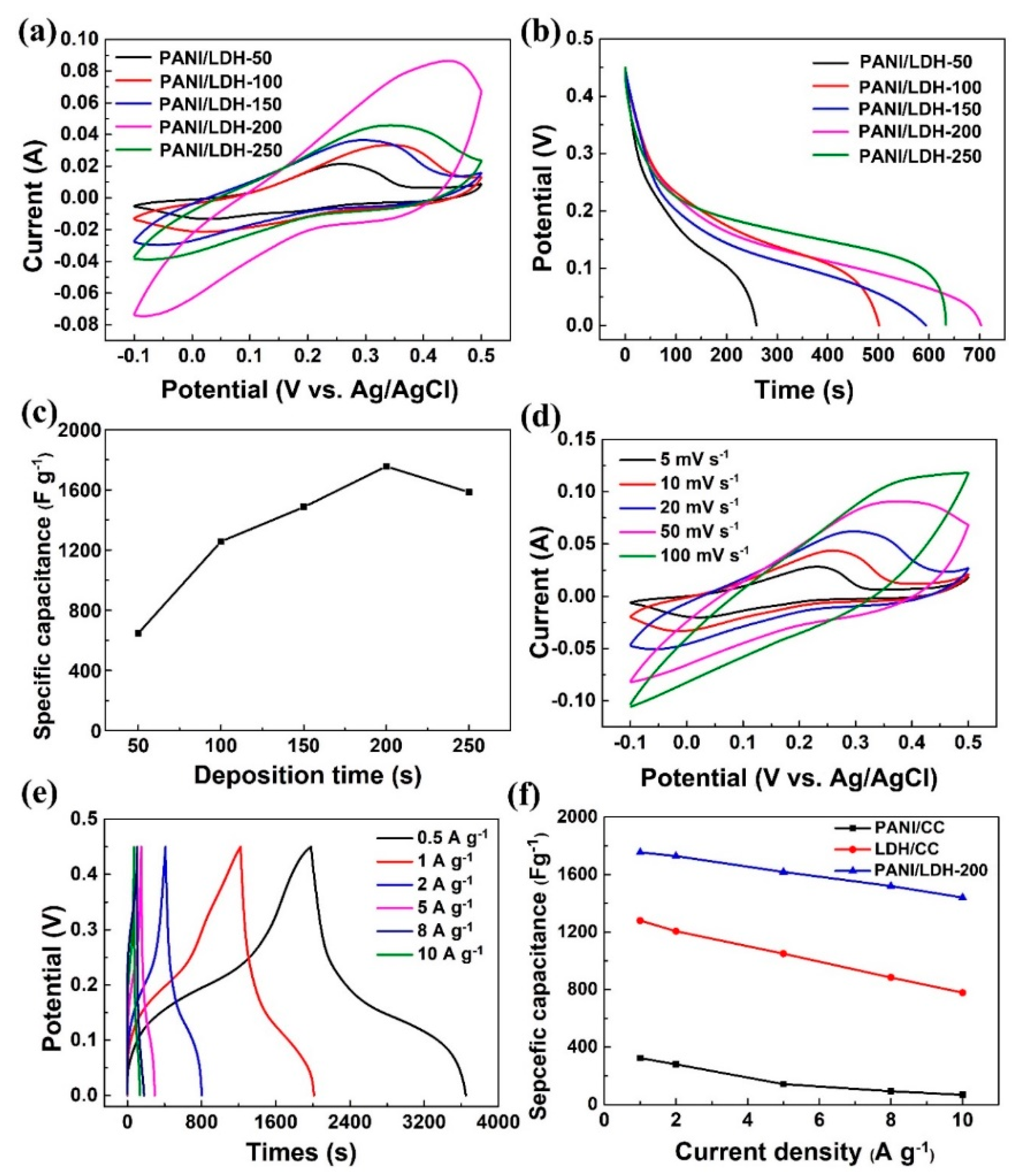
2.5. Electrochemical Measurements
2.6. Fabrication of Asymmetric Supercapacitor (ASC) Devices
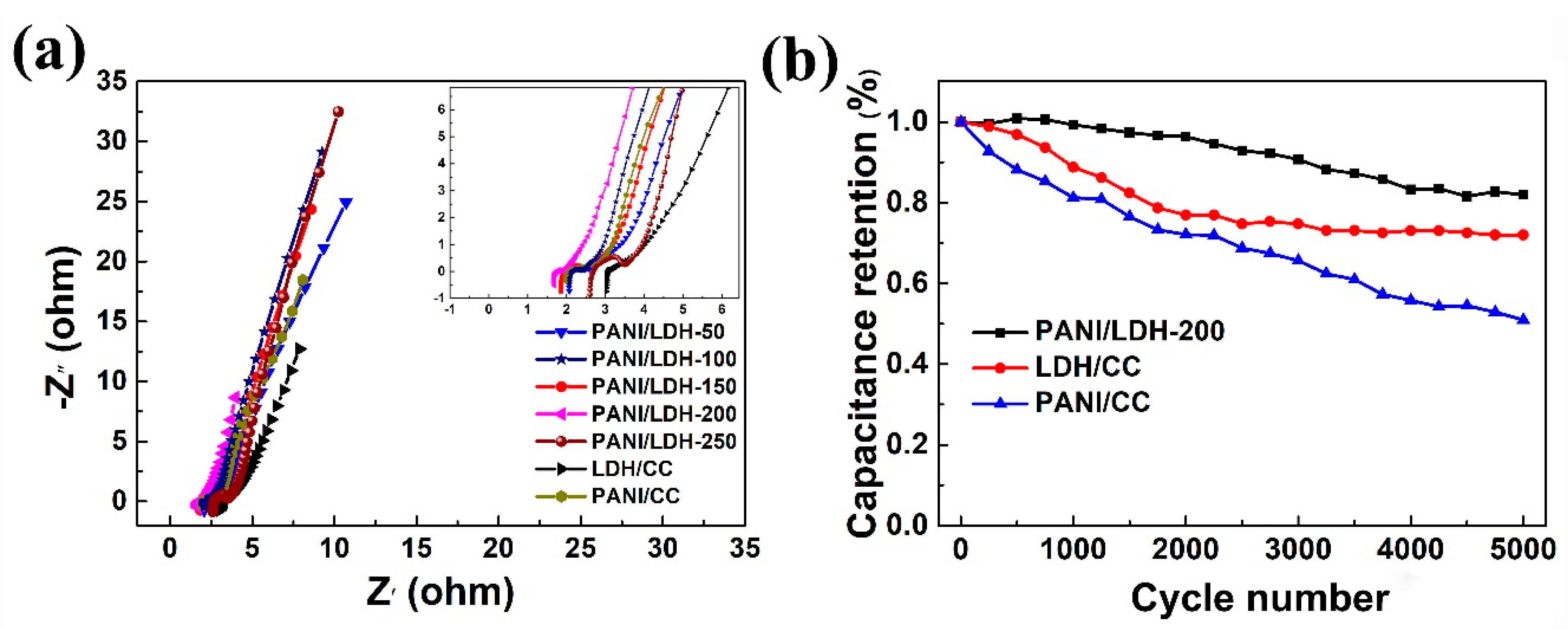
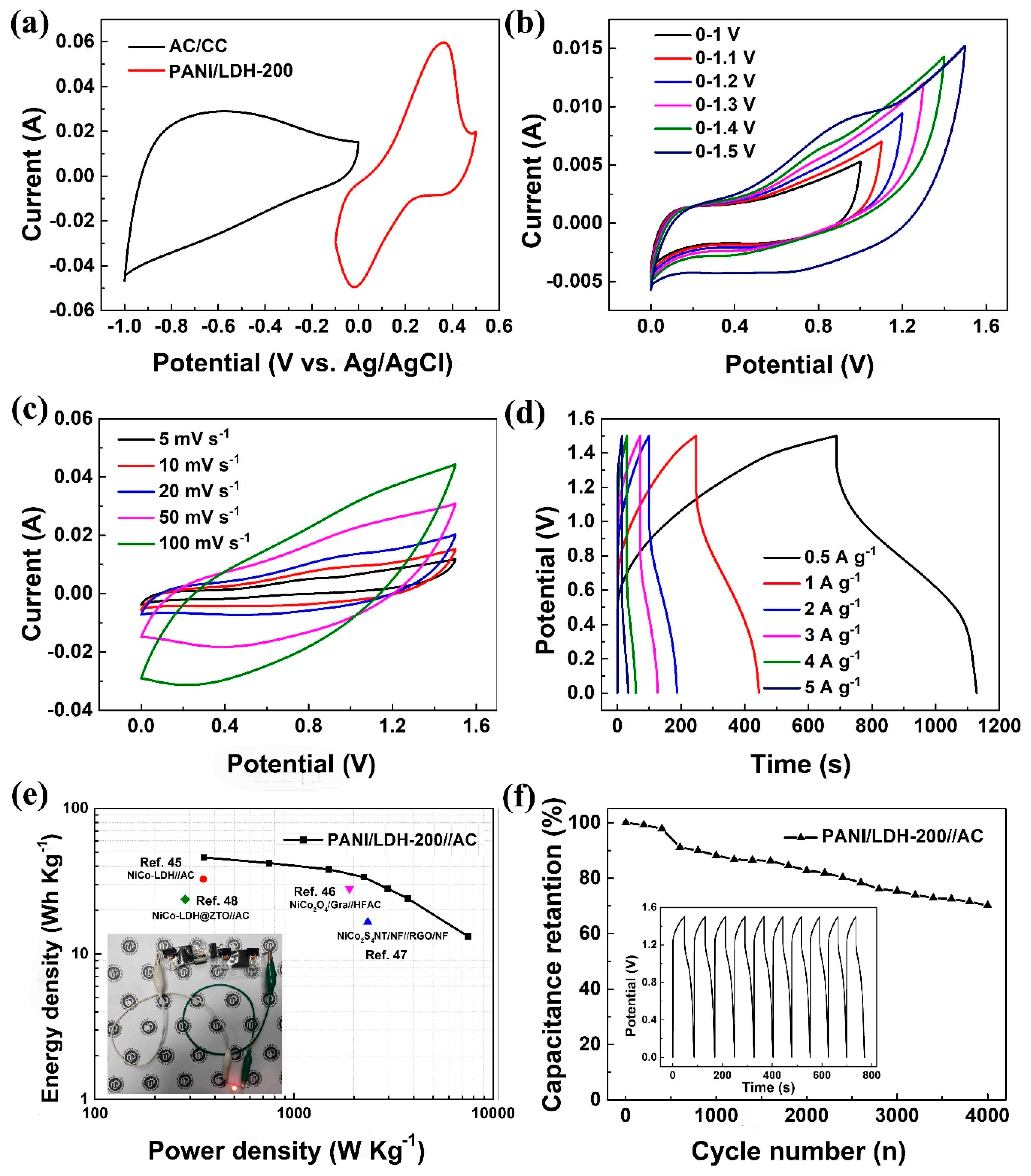
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Dong, L.; Xu, C.; Li, Y.; Huang, Z.H.; Kang, F.; Yang, Q.H.; Zhao, X. Flexible electrodes and supercapacitors for wearable energy storage: A review by category. J. Mater. Chem. A 2016, 4, 4659–4685. [Google Scholar] [CrossRef]
- Liu, Y.; Peng, X. Recent advances of supercapacitors based on two-dimensional materials. Appl. Mater. Today 2017, 7, 104–115. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Asymmetric supercapacitor electrodes and devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Cai, K.; Chen, Y.; Chen, L. Research progress on conducting polymer based supercapacitor electrode materials. Nano Energy 2017, 36, 268–285. [Google Scholar] [CrossRef]
- Liu, C.; Li, F.; Ma, L.P.; Cheng, H.M. Advanced materials for energy storage. Adv. Mater. 2010, 22, 28–62. [Google Scholar] [CrossRef]
- Strauss, V.; Marsh, K.; Kowal, M.D.; El-Kady, M.; Kaner, R.B. A Simple Route to porous graphene from carbon nanodots for supercapacitor applications. Adv. Mater. 2018, 30, 1704449. [Google Scholar] [CrossRef]
- Dong, S.; He, X.; Zhang, H.; Xie, X.; Yu, M.; Yu, C.; Xiao, N.; Qiu, J. Surface modification of biomass-derived hard carbon by grafting porous carbon nanosheets for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 15954–15960. [Google Scholar] [CrossRef]
- Zheng, W.; Lv, R.; Na, B.; Liu, H.; Jin, T.; Yuan, D. Nanocellulose-mediated hybrid polyaniline electrodes for high performance flexible supercapacitors. J. Mater. Chem. A 2017, 5, 12969–12976. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, X.; Shang, Y.; Hua, C.; Song, P.; Li, X.; Zhang, Y.; Cao, A. Highly flexible all-solid-state supercapacitors based on carbon nanotube/polypyrrole composite films and fibers. RSC Adv. 2016, 6, 62062–62070. [Google Scholar] [CrossRef]
- Xiong, D.; Li, X.; Bai, Z.; Li, J.; Shan, H.; Fan, L.; Long, C.; Li, D.; Lu, X. Rational design of hybrid Co3O4/graphene films: Free-standing flexible electrodes for high performance supercapacitors. Electrochim. Acta 2018, 259, 338–347. [Google Scholar] [CrossRef]
- Xing, L.; Dong, Y.; Hu, F.; Wu, X.; Umaret, A. Co3O4 nanowire@NiO nanosheet arrays for high performance asymmetric supercapacitors. Dalton Trans. 2018, 47, 5687–5694. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Song, N.; Li, X. Microstructural design of hybrid CoO@NiO and graphene nano-architectures for flexible high performance supercapacitors. J. Mater. Chem. A 2015, 3, 14833–14844. [Google Scholar] [CrossRef]
- Eskusson, J.; Rauwel, P.; Nerut, J.; Jänes, A. A hybrid capacitor based on Fe3O4-graphene nanocomposite/few-layer graphene in different aqueous electrolytes. J. Electrochem. Soc. 2016, 163, A2768–A2775. [Google Scholar] [CrossRef]
- Kim, G.; Kang, J.; Choe, G.; Yim, S. Enhanced energy density of supercapacitors using hybrid electrodes based on Fe2O3 and MnO2 nanoparticles. Int. J. Electrochem. Sci. 2017, 12, 10015–10022. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, S.; Yan, X.; Yu, M.; Wang, L.; Bell, J.; Wang, H. 2-Methylimidazole-derived Ni-Co layered double hydroxide nanosheets as high rate capability and high energy density storage material in hybrid supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 15510–15524. [Google Scholar] [CrossRef]
- Gao, L.; Surjadi, J.U.; Cao, K.; Zhang, H.; Li, P.; Su, S.; Jiang, C.; Song, J.; Sun, D.; Lu, Y. Flexible fiber-shaped supercapacitor based on nickel-cobalt double hydroxide and pen ink electrodes on metallized carbon fiber. ACS Appl. Mater. Interfaces 2017, 9, 5409–5418. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Hu, Z.; Shao, X.; Cheng, P.; Li, S.; Yu, W.; Lin, W.; Yuan, D. Large scale synthesis of NiCo layered double hydroxides for superior asymmetric electrochemical capacitor. Sci. Rep. 2016, 6, 18737. [Google Scholar] [CrossRef]
- Li, T.; Li, G.; Li, L.; Liu, L.; Xu, Y.; Ding, H.; Zhang, T. Large-scale self-assembly of 3D flower-like hierarchical Ni/Co-LDHs microspheres for high-performance flexible asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 2562–2572. [Google Scholar] [CrossRef]
- Nagaraju, G.; Raju, G.S.; Co, Y.H.; Yu, J.S. Hierarchical Ni-Co layered double hydroxide nanosheets entrapped on conductive textile fibers: A cost-effective and flexible electrode for high-performance pseudocapacitors. Nanoscale 2016, 8, 812–825. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Dou, Y.; Zhao, J.; Wei, M.; Evans, D.G.; Duan, X. Flexible CoAl LDH@PEDOT core/shell nanoplatelet array for high-performance energy storage. Small 2013, 9, 98–106. [Google Scholar] [CrossRef]
- Han, B.; Cheng, G.; Zhang, E.; Zhang, L.; Wang, X. Three dimensional hierarchically porous ZIF-8 derived carbon/LDH core-shell composite for high performance supercapacitors. Electrochim. Acta 2018, 263, 391–399. [Google Scholar] [CrossRef]
- Shakir, I.; Shahid, M.; Rana, U.A.; Nashef, I.M.A.; Hussain, R. Nickel–Cobalt layered double hydroxide anchored zinc oxide nanowires grown on carbon fiber cloth for high-performance flexible pseudocapacitive energy storage devices. Electrochim. Acta 2014, 129, 28–32. [Google Scholar] [CrossRef]
- Chen, H.; Cai, F.; Kang, Y.; Zeng, S.; Chen, M.; Li, Q. Facile assembly of Ni-Co hydroxide nanoflakes on carbon nanotube network with highly electrochemical capacitive performance. ACS Appl. Mater. Interfaces 2014, 6, 19630–19637. [Google Scholar] [CrossRef]
- Li, X.; Shen, J.; Sun, W.; Hong, X.; Wang, R.; Zhao, X.; Yan, X. A super-high energy density asymmetric supercapacitor based on 3D core–shell structured NiCo-layered double hydroxide@carbon nanotube and activated polyaniline-derived carbon electrodes with commercial level mass loading. J. Mater. Chem. A 2015, 3, 13244–13253. [Google Scholar] [CrossRef]
- Liang, H.; Lin, J.; Jia, L.H.; Chen, S.; Qi, J.; Cao, J.; Lin, T.; Fei, W.; Feng, J. Hierarchical NiCo-LDH@NiOOH core-shell heterostructure on carbon fiber cloth as battery-like electrode for supercapacitor. J. Power Sources 2018, 378, 248–254. [Google Scholar] [CrossRef]
- Wang, X.; Xu, M.; Fu, Y.; Wang, S.; Yang, T.; Jiao, K. A highly conductive and hierarchical PANI micro/nanostructure and its supercapacitor application. Electrochim. Acta 2016, 222, 701–708. [Google Scholar] [CrossRef]
- Zhou, K.; He, Y.; Xu, Q.; Zhang, Q.; Zhou, A.; Lu, Z.; Yang, L.; Jiang, Y.; Ge, D.; Liu, X.Y.; et al. A hydrogel of ultrathin pure polyaniline nanofibers: Oxidant-templating preparation and supercapacitor application. ACS Nano 2018, 12, 5888–5894. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Dai, Y.; Zhang, H.; Wang, W.; Huang, Q.; Chen, Y.; Pu, L. Synthesis and electrochemical measurement of three dimensional carbon nanofibers/Co3O4-polyaniline composites as supercapacitor electrode materials in neutral electrolyte. Int. J. Electrochem. Sci. 2016, 11, 6279–6286. [Google Scholar] [CrossRef]
- Hai, Z.; Gao, L.; Zhang, Q.; Xu, H.; Cui, D.; Zhang, Z.; Tsoukalas, D.; Tang, J.; Yan, S.; Xue, C. Facile synthesis of core-shell structured PANI-Co3O4 nanocomposites with superior electrochemical performance in supercapacitors. Appl. Surf. Sci. 2016, 361, 57–62. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Zhai, D.; Lee, H.R.; Zhao, W.; Liu, N.; Wang, H.; Tee, B.; Shi, Y.; Cui, Y.; et al. Hierarchical nanostructured conducting polymer hydrogel with high electrochemical activity. Proc. Natl. Acad. Sci. USA 2012, 109, 9287–9292. [Google Scholar] [CrossRef]
- Shao, M.; Li, Z.; Zhang, R.; Ning, F.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical conducting polymer@clay core-shell arrays for flexible all-solid-state supercapacitor devices. Small 2015, 11, 3530–3538. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; Zhang, T.; He, Y.; Jia, C.; Saha, P.; Cheng, Q. Co3O4@CoS core-shell nanosheets on carbon cloth for high performance supercapacitor electrodes. Materials 2017, 10, 608. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, H.; Gu, Y.; Sun, Z.; Hu, J.; Cao, L.; Lv, F.; Li, M.; Wang, W.; Wang, Z.; et al. Facile electrodeposition of 3D concentration-gradient Ni-Co hydroxide nanostructures on nickel foam as high performance electrodes for asymmetric supercapacitors. Nano Res. 2015, 8, 2744–2754. [Google Scholar] [CrossRef]
- Jin, L.; Jiang, Y.; Zhang, M.; Li, H.; Xiao, L.; Li, M.; Ao, Y. Oriented polyaniline nanowire arrays grown on dendrimer (PAMAM) functionalized multiwalled carbon nanotubes as supercapacitor electrode materials. Sci. Rep. 2018, 8, 6268. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Shen, X.; Ma, L.; Ji, Z.; Xu, C.; Yuan, A. Solvothermal synthesis of NiCo-layered double hydroxide nanosheets decorated on RGO sheets for high performance supercapacitor. Chem. Eng. J. 2015, 268, 251–259. [Google Scholar] [CrossRef]
- Xie, L.; Hu, Z.; Lv, C.; Sun, G.; Wang, J.; Li, Y.; He, H.; Wang, J.; Li, K. CoxNi1−x double hydroxide nanoparticles with ultrahigh specific capacitances as supercapacitor electrode materials. Electrochim. Acta 2012, 78, 205–211. [Google Scholar] [CrossRef]
- Liu, X.B.; Wu, Z.; Yin, Y. Hierarchical NiCo2S4@PANI core/shell nanowires grown on carbon fiber with enhanced electrochemical performance for hybrid supercapacitors. Chem. Eng. J. 2017, 323, 330–339. [Google Scholar] [CrossRef]
- Brousse, T.; Bélanger, D.; Long, J.W. To be or not to be pseudocapacitive. J. Electrochem. Soc. 2015, 162, 5185–5189. [Google Scholar] [CrossRef]
- Cao, J.; Li, L.; Xi, Y.; Li, J.; Pan, X.; Chen, D.; Han, W. Core-shell structural PANI-derived carbon@Co-Ni LDH electrode for high-performance asymmetric supercapacitors. Sustain. Energy Fuels 2018, 2, 1350–1355. [Google Scholar] [CrossRef]
- Yu, C.; Yang, J.; Zhao, C.; Fan, X.; Wang, G.; Qiu, J. Nanohybrids from NiCoAl-LDH coupled with carbon for pseudocapacitors: Understanding the role of nano-structured carbon. Nanoscale 2014, 6, 3097–3104. [Google Scholar] [CrossRef]
- Bai, X.; Liu, Q.; Lu, Z.; Liu, J.; Chen, R.; Li, R.; Song, D.; Jing, X.; Liu, P.; Wang, J. Rational design of sandwiched Ni-Co layered double hydroxides hollow nanocages/graphene derived from metal–organic framework for sustainable energy storage. ACS Sustain. Chem. Eng. 2017, 5, 9923–9934. [Google Scholar] [CrossRef]
- Li, H.; Musharavati, F.; Zalenezhad, E.; Chen, X.; Hui, K.N.; Hui, K.S. Electrodeposited NiCo layered double hydroxides on titanium carbide as a binder-free electrode for supercapacitors. Electrochim. Acta 2018, 261, 178–187. [Google Scholar] [CrossRef]
- Chen, Y.; Pang, W.K.; Bai, H.; Zhou, T.; Liu, Y.; Li, S.; Guo, Z. Enhanced structural stability of nickel-cobalt hydroxide via intrinsic pillar effect of metaborate for high-power and long-life supercapacitor electrodes. Nano Lett. 2016, 17, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Teng, H. Influence of carbon nanotube grafting on the impedance behavior of activated carbon capacitors. J. Electrochem. Soc. 2008, 155, 739–744. [Google Scholar] [CrossRef]
- Liu, Y.; Teng, X.; Mi, Y.; Chen, Z. A new architecture design of Ni-Co LDH-based pseudocapacitors. J. Mater. Chem. A 2017, 5, 24407–24415. [Google Scholar] [CrossRef]
- Li, Z.; Xu, Z.; Wang, H.; Ding, J.; Zahiri, B.; Holt, C.; Tan, X.; Mitlin, D. Colossal pseudocapacitance in a high functionality–high surface area carbon anode doubles the energy of an asymmetric supercapacitor. Energy Environ. Sci. 2014, 7, 1708–1718. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, J.; Zhang, L.; Xia, D.; Zhao, Y.; Guo, D.; Qi, T.; Wan, H. In situ growth of NiCo2S4 nanotube arrays on Ni foam for supercapacitors: Maximizing utilization efficiency at high mass loading to achieve ultrahigh areal pseudocapacitance. J. Power Sources 2014, 254, 249–257. [Google Scholar] [CrossRef]
- Wang, X.; Sumboja, A.; Lin, M.; Yan, J.; Lee, P.S. Enhancing electrochemical reaction sites in nickel-cobalt layered double hydroxides on zinc tin oxide nanowires: A hybrid material for an asymmetric supercapacitor device. Nanoscale 2012, 4, 7266–7272. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, X.; He, Y.; Plachy, T.; Kazantseva, N.; Saha, P.; Cheng, Q. Hierarchical PANI/NiCo-LDH Core-Shell Composite Networks on Carbon Cloth for High Performance Asymmetric Supercapacitor. Nanomaterials 2019, 9, 527. https://doi.org/10.3390/nano9040527
Ge X, He Y, Plachy T, Kazantseva N, Saha P, Cheng Q. Hierarchical PANI/NiCo-LDH Core-Shell Composite Networks on Carbon Cloth for High Performance Asymmetric Supercapacitor. Nanomaterials. 2019; 9(4):527. https://doi.org/10.3390/nano9040527
Chicago/Turabian StyleGe, Xinjin, Ying He, Tomas Plachy, Natalia Kazantseva, Petr Saha, and Qilin Cheng. 2019. "Hierarchical PANI/NiCo-LDH Core-Shell Composite Networks on Carbon Cloth for High Performance Asymmetric Supercapacitor" Nanomaterials 9, no. 4: 527. https://doi.org/10.3390/nano9040527
APA StyleGe, X., He, Y., Plachy, T., Kazantseva, N., Saha, P., & Cheng, Q. (2019). Hierarchical PANI/NiCo-LDH Core-Shell Composite Networks on Carbon Cloth for High Performance Asymmetric Supercapacitor. Nanomaterials, 9(4), 527. https://doi.org/10.3390/nano9040527



