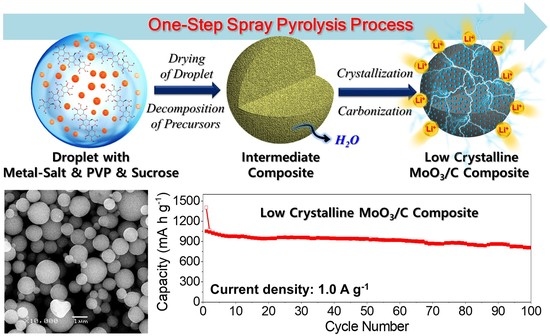
Large Scale Process for Low Crystalline MoO3-Carbon Composite Microspheres Prepared by One-Step Spray Pyrolysis for Anodes in Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization Techniques
2.3. Electrochemical Measurements
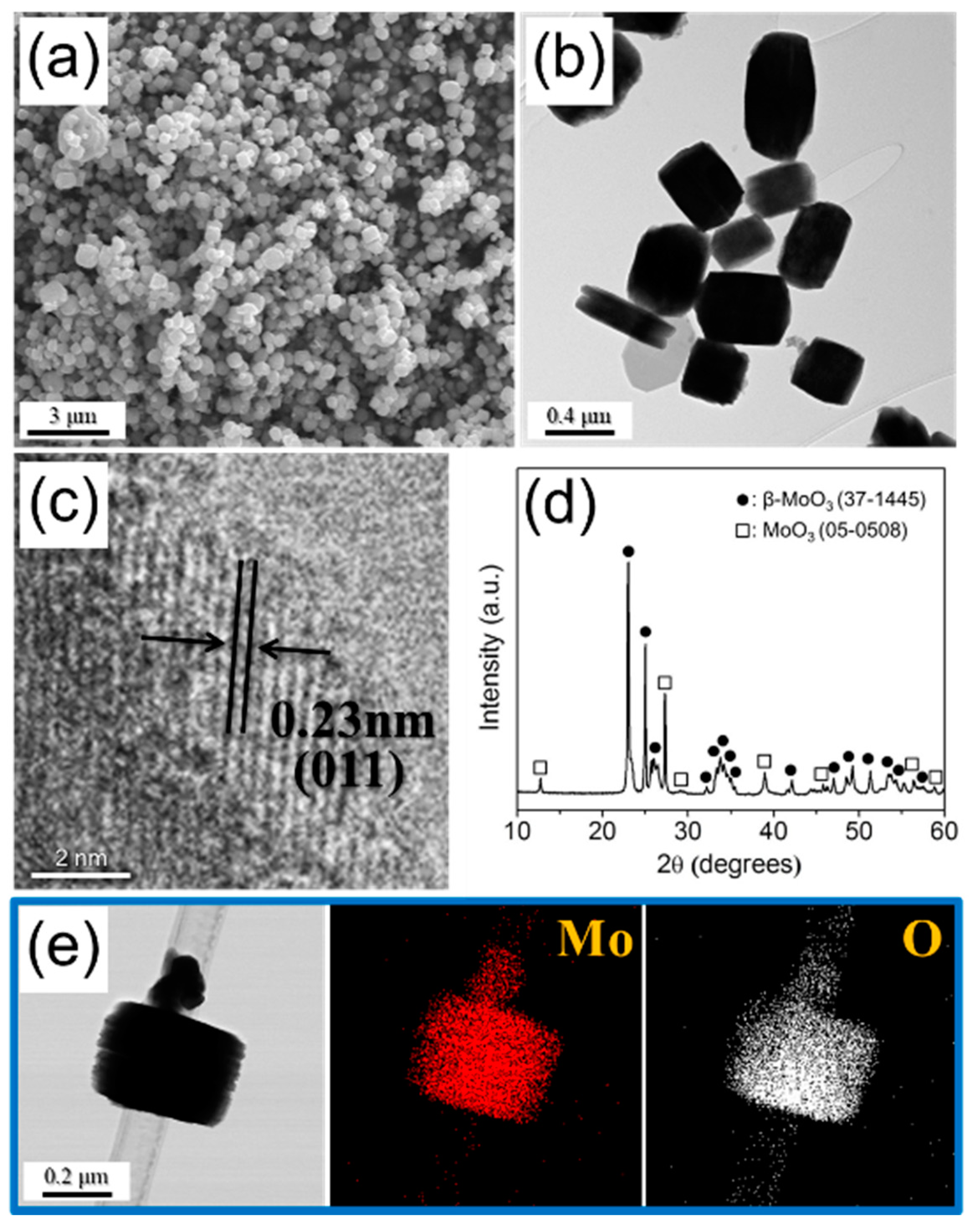
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Chen, X.; Wang, Z.; Zhang, R.; Xu, L.; Sun, D. A novel polyoxometalate-based hybrid containing a 2D [CoMo8O26]∞ structure as the anode for lithium-ion batteries. Chem. Commun. 2017, 53, 10560–10563. [Google Scholar] [CrossRef]
- Lin, H.B.; Rong, H.B.; Huang, W.Z.; Liao, Y.H.; Xing, L.D.; Xu, M.Q.; Li, X.P.; Li, W.S. Triple-shelled Mn2O3 hollow nanocubes: Force-induced synthesis and excellent performance as the anode in lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14189–14194. [Google Scholar] [CrossRef]
- Zhu, Z.; Wang, S.; Du, J.; Jin, Q.; Zhang, T.; Cheng, F.; Chen, J. Ultrasmall Sn nanoparticles embedded in nitrogen-doped porous carbon as high-performance anode for lithium-ion batteries. Nano Lett. 2013, 14, 153–157. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, Y.; Zhang, Y.; Guo, C.; Tang, B.; Sun, D. MOFs-derived porous Mn2O3 as high-performance anode material for Li-ion battery. J. Mater. Chem. A 2015, 3, 5266–5269. [Google Scholar] [CrossRef]
- Cui, L.; Shen, J.; Cheng, F.; Tao, Z.; Chen, J. SnO2 nanoparticles@ polypyrrole nanowires composite as anode materials for rechargeable lithium-ion batteries. J. Power Sources 2011, 196, 2195–2201. [Google Scholar] [CrossRef]
- Zhong, X.; Huan, H.; Liu, X.; Yu, Y. Facile synthesis of porous germanium-iron bimetal oxide nanowires as anode materials for lithium-ion batteries. Nano Res. 2018, 11, 3702–3709. [Google Scholar] [CrossRef]
- Jin, L.; Qiu, Y.; Deng, H.; Li, W.; Li, H.; Yang, S. Hollow CuFe2O4 spheres encapsulated in carbon shells as an anode material for rechargeable lithium-ion batteries. Electrochim. Acta 2011, 56, 9127–9132. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, J.; Wu, H.; Xu, M.; Peng, Z.; Zheng, G. Hierarchical SnO2–Fe2O3 heterostructures as lithium-ion battery anodes. J. Mater. Chem. 2012, 22, 21923–21927. [Google Scholar] [CrossRef]
- Han, F.; Li, D.; Li, W.-C.; Lei, C.; Sun, Q.; Lu, A.-H. Nanoengineered polypyrrole-coated Fe2O3@C multifunctional composites with an improved cycle stability as lithium-ion anodes. Adv. Funct. Mater. 2013, 23, 1692–1700. [Google Scholar] [CrossRef]
- Huang, X.H.; Tu, J.P.; Zhang, C.Q.; Chen, X.T.; Yuan, Y.F.; Wu, H.M. Spherical NiO-C composite for anode material of lithium ion batteries. Electrochim. Acta 2007, 52, 4177–4181. [Google Scholar] [CrossRef]
- Jo, M.S.; Ghosh, S.; Jeong, S.M.; Kang, Y.C.; Cho, J.S. Coral-like yolk–shell-structured nickel oxide/carbon composite microspheres for high-performance Li-ion storage anodes. Nano-Micro Lett. 2019, 11, 3. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.; Fu, W.; Li, F.; Wang, J.; Wang, W. α-Fe2O3@C nanorings as anode materials for high performance lithium ion batteries. J. Alloy. Compd. 2015, 647, 105–109. [Google Scholar] [CrossRef]
- Park, G.D.; Kim, J.H.; Park, S.-K.; Kang, Y.C. MoSe2 embedded CNT-reduced graphene oxide composite microsphere with superior sodium ion storage and electrocatalytic hydrogen evolution performances. ACS Appl. Mater. Interfaces 2017, 9, 10673–10683. [Google Scholar] [CrossRef]
- Cho, J.S.; Won, J.M.; Lee, J.-K.; Kang, Y.C. Design and synthesis of multiroom-structured metal compounds–carbon hybrid microspheres as anode materials for rechargeable batteries. Nano Energy 2016, 26, 466–478. [Google Scholar] [CrossRef]
- Zhang, X.; Suresh Kumar, P.; Aravindan, V.; Liu, H.H.; Sundaramurthy, J.; Mhaisalkar, S.G.; Duong, H.M.; Ramakrishna, S.; Madhavi, S. Electrospun TiO2–graphene composite nanofibers as a highly durable insertion anode for lithium ion batteries. J. Phys. Chem. C 2012, 116, 14780–14788. [Google Scholar] [CrossRef]
- Bhaskar, A.; Deepa, M.; Narasinga Rao, T. MoO2/multiwalled carbon nanotubes (MWCNT) hybrid for use as a Li-ion battery anode. ACS Appl. Mater. Interfaces 2013, 5, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Hong, Y.J.; Kang, Y.C. Design and synthesis of bubble-nanorod-structured Fe2O3–carbon nanofibers as advanced anode material for Li-ion batteries. ACS Nano 2015, 9, 4026–4035. [Google Scholar] [CrossRef]
- Park, S.-K.; Park, G.D.; Kang, Y.C. Three-dimensional porous microspheres comprising hollow Fe2O3 nanorods/CNT building blocks with superior electrochemical performance for lithium ion batteries. Nanoscale 2018, 10, 11150–11157. [Google Scholar] [CrossRef]
- Whittingham, M.S. The role of ternary phased in cathode reactions. Electrochem. Soc. 1976, 123, 315–320. [Google Scholar] [CrossRef]
- Choi, S.H.; Kang, Y.C. Crumpled graphene–molybdenum oxide composite powders: Preparation and application in lithium-ion batteries. ChemSusChem 2014, 7, 523–528. [Google Scholar] [CrossRef]
- Xue, X.Y.; Chen, Z.H.; Xing, L.L.; Yuan, S.; Chen, Y.J. SnO2/a-MoO3 core-shell nanobelts and their extraordinarily high reversible capacity as lithium-ion battery anodes. Chem. Commun. 2011, 47, 5205–5207. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, Y.H.; Deshpande, R.; Parilla, P.A.; Whitney, E.; Gillaspie, D.T.; Kim, M.J.; Mahan, A.H.; Zhang, S.B.; Dillon, A.C. Reversible lithium-ion insertion in molybdenum oxide nanoparticles. Adv. Mater. 2008, 20, 3627–3632. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, M.; Hu, C. Thermal oxidation synthesis hollow MoO3 microspheres and their applications in lithium storage and gas-sensing. Mater. Res. Bull. 2013, 48, 2289–2295. [Google Scholar] [CrossRef]
- Mohan, V.M.; Bin, H.; Chen, W. Enhancement of electrochemical properties of MoO3 nanobelts electrode using PEG as surfactant for lithium battery. J. Solid State Electrochem. 2010, 14, 1769–1775. [Google Scholar] [CrossRef]
- Meduri, P.; Clark, E.; Kim, J.H.; Dayalan, E.; Sumanasekera, G.U.; Sunkara, M.K. MoO3–x nanowire arrays as stable and high-capacity anodes for lithium ion batteries. Nano Lett. 2012, 12, 1784–1788. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.N.; Park, S.B.; Jung, K.Y.; Kang, Y.C. One-pot facile synthesis of ant-cave- structured metal oxide-carbon microballs by continuous process for use as anode materials in Li-ion batteries. Nano Lett. 2013, 13, 5462–5466. [Google Scholar] [CrossRef]
- Xie, F.; Choy, W.C.H.; Wang, C.; Li, X.; Zhang, S.; Hou, J. Low-temperature solution-processed hydrogen molybdenum and vanadium bronzes for an efficient hole-transport layer in organic electronics. Adv. Mater. 2013, 25, 2051–2055. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, Z.; Cui, W.; Wu, Z.; Sun, Q.; Wang, S.; Kang, Z.; Sun, B. A cost-effective commercial soluble oxide cluster for highly efficient and stable organic solar cells. J. Mater. Chem. A 2014, 2, 1436–1442. [Google Scholar] [CrossRef]
- Kim, J.H.; Oh, Y.J.; Kang, Y.C. Design and synthesis of macroporous (Mn1/3Co2/3)O-carbon nanotubes composite microspheres as efficient catalysts for rechargeable Li-O2 batteries. Carbon 2018, 128, 125–133. [Google Scholar] [CrossRef]
- Park, G.D.; Lee, J.-K.; Kang, Y.C. Three-dimensional macroporous CNTs microspheres highly loaded with NiCo2O4 hollow nanospheres showing excellent lithium-ion storage performances. Carbon 2018, 128, 191–200. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Xie, J.; Pan, Q.; Wu, C.-Y.; Cao, G.-S.; Zhu, T.-J.; Zhao, X.-B. Graphene anchored with nanocrystal Fe2O3 with improved electrochemical Li-storage properties. Int. J. Electrochem. Sci. 2012, 7, 354–362. [Google Scholar]
- Cho, J.S.; Lee, S.Y.; Kang, Y.C. First introduction of NiSe2 to anode material for sodium-ion batteries: A hybrid of graphene-wrapped NiSe2/C porous nanofiber. Sci. Rep. 2016, 6, 23338. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Gogoi, B.; Buragohain, A.K.; Deb, P. Fe2O3/C nanocomposites having distinctive antioxidant activity and hemolysis prevention efficiency. Mater. Sci. Eng. C 2014, 42, 595–600. [Google Scholar] [CrossRef]
- Shakir, I.; Shahid, M.; Kang, D.J. MoO3 and Cu0.33MoO3 nanorods for unprecedented UV/visible light photocatalysis. Chem. Commun. 2010, 46, 4324–4326. [Google Scholar] [CrossRef] [PubMed]
- Sen, U.K.; Mitra, S. Synthesis of molybdenum oxides and their electrochemical properties against Li. Energy Procedia 2014, 54, 740–747. [Google Scholar] [CrossRef]
- Yu, Z.; Jiang, H.; Gu, D.; Li, J.; Wang, L.; Shen, L. A new way to prepare MoO3/C as anode of lithium ion battery for enhancing the electrochemical performance at room temperature. J. Electrochem. Sci. Technol. 2016, 7, 170–178. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, N.; Wang, L.; Zhang, K.; Zhu, Y.; Qian, Y. Synthesis of hexagonal MoO3 nanorods and a study of their electrochemical performance as anode materials for lithium-ion batteries. J. Mater. Chem. A 2015, 3, 7463–7468. [Google Scholar] [CrossRef]
- Ji, L.; Lin, Z.; Guo, B.; Medford, A.J.; Zhang, X. Assembly of carbon–SnO2 core–sheath composite nanofibers for superior lithium storage. Chem.-Eur. J. 2010, 16, 11543–11548. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, J.K.; Kang, Y.C.; Cho, J.S. Three-dimensionally ordered mesoporous multicomponent (Ni, Mo) metal oxide/N-doped carbon composite with superior Li-ion storage performance. Nanoscale 2018, 10, 18734–18741. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Shen, R.; Yang, R.; Ji, W.; Jiang, M.; Ding, W.; Peng, L. Mixed molybdenum oxides with superior performances as an advanced anode material for lithium-ion batteries. Sci. Rep. 2017, 7, 44697. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhang, N.; Sun, K. Electrochemical preparation of porous MoO3 film with a high rate performance as anode for lithium ion batteries. J. Mater. Chem. A 2013, 1, 221–224. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, H.; Du, Z.; Zeng, Z.; Gao, C.; Zhang, Z.; Du, X.; Kulka, A.; Świerczek, K. Facile synthesis of MoO3/carbon nanobelts as high-performance anode material for lithium ion batteries. Electrochim. Acta 2015, 180, 947–956. [Google Scholar] [CrossRef]
- Lee, J.H.; Oh, S.H.; Jeong, S.Y.; Kang, Y.C.; Cho, J.S. Rattle-type porous Sn/C composite fibers with uniformly distributed nanovoids containing metallic Sn nanoparticles for high-performance anode materials in lithium-ion batteries. Nanoscale 2018, 10, 21483–21491. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.-H.; Feng, X.-Y.; Song, C.; Zou, B.-K.; Ding, C.-X.; Yu, Y.; Chen, C.-H. Facile synthesis of flower-like and yarn-like α-Fe2O3 spherical clusters as anode materials for lithium-ion batteries. Electrochim. Acta 2013, 93, 131–136. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, M.; Peng, Z.; Zheng, G. Direct growth of mesoporous Sn-doped TiO2 thin films on conducting substrates for lithium-ion battery anodes. J. Mater. Chem. A 2013, 1, 13222–13226. [Google Scholar] [CrossRef]







© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, J.S. Large Scale Process for Low Crystalline MoO3-Carbon Composite Microspheres Prepared by One-Step Spray Pyrolysis for Anodes in Lithium-Ion Batteries. Nanomaterials 2019, 9, 539. https://doi.org/10.3390/nano9040539
Cho JS. Large Scale Process for Low Crystalline MoO3-Carbon Composite Microspheres Prepared by One-Step Spray Pyrolysis for Anodes in Lithium-Ion Batteries. Nanomaterials. 2019; 9(4):539. https://doi.org/10.3390/nano9040539
Chicago/Turabian StyleCho, Jung Sang. 2019. "Large Scale Process for Low Crystalline MoO3-Carbon Composite Microspheres Prepared by One-Step Spray Pyrolysis for Anodes in Lithium-Ion Batteries" Nanomaterials 9, no. 4: 539. https://doi.org/10.3390/nano9040539
APA StyleCho, J. S. (2019). Large Scale Process for Low Crystalline MoO3-Carbon Composite Microspheres Prepared by One-Step Spray Pyrolysis for Anodes in Lithium-Ion Batteries. Nanomaterials, 9(4), 539. https://doi.org/10.3390/nano9040539