The Impact of TiO2 Nanoparticle Concentration Levels on Impulse Breakdown Performance of Mineral Oil-Based Nanofluids
Abstract
:1. Introduction
2. Experimental Descriptions
2.1. Sample Preparation
2.2. Breakdown Test Procedure
3. Experimental Results
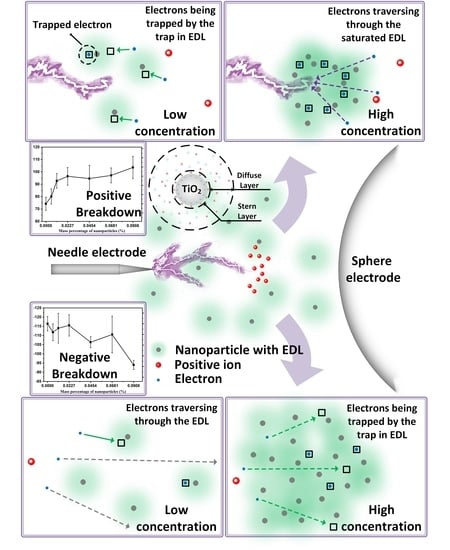
3.1. Breakdown Characteristics under Positive Polarity
3.2. Breakdown Characteristics under Negative Polarity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Choi, S.U.S. Enhancing Thermal Conductivity Offluids with Nanoparticles. In Proceedings of the 1995 ASME International Mechanical Engineering Congress and Exposition, San Francisco, CA, USA, 12–17 November 1995; pp. 99–105. [Google Scholar]
- Segal, V.; Hjortsberg, A.; Rabinovich, A.; Nattrass, D.; Raj, K. AC (60 Hz) and impulse breakdown strength of a colloidal fluid based on transformer oil and magnetite nanoparticles. In Proceedings of the 1998 IEEE International Symposium on Electrical Insulation, Arlington, VA, USA, 7–10 June 1998; pp. 619–622. [Google Scholar]
- Kaityar, A.; Dhar, P.; Nandi, T.; Das, S.K. Effects of nanostructure permittivity and dimensions on the increased dielectric strength of nano insulating oils. Colloid Surf. A-Physicochem. Eng. Asp. 2016, 509, 235–243. [Google Scholar] [CrossRef]
- Madavan, R.; Balaraman, S. Investigation on effects of different types of nanoparticles on critical parameters of nano-liquid insulation systems. J. Mol. Liq. 2017, 230, 437–444. [Google Scholar]
- Cavallini, A.; Karthik, R.; Negri, F. The effect of magnetite, graphene oxide and silicone oxide nanoparticles on dielectric withstand characteristics of mineral oil. IEEE Trans. Dielectr. Electr. Insul. 2015, 22, 2592–2600. [Google Scholar] [CrossRef]
- Jin, H.; Andritsch, T.; Tsekmes, I.A.; Kochetov, R.; Morshuis, P.H.F.; Smit, J.J. Properties of mineral oil based silica nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1100–1108. [Google Scholar]
- Liu, D.; Zhou, Y.; Yang, Y.; Zhang, L.; Jin, F. Characterization of high performance AIN nanoparticle-based transformer oil nanofluids. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2757–2767. [Google Scholar] [CrossRef]
- Ahmad, F.; Khan, A.A.; Khan, Q.; Hussain, M.R. State-of-Art in Nano-Based Dielectric Oil: A Review. IEEE Access 2019, 7, 13396–13410. [Google Scholar] [CrossRef]
- Lee, J.; Lee, W.; Lee, S.; Lee, S. Positive and negative effects of dielectric breakdown in transformer oil based magnetic fluids. Mater. Res. Bull. 2012, 47, 2984–2987. [Google Scholar] [CrossRef]
- Singh, I.; Birajdar, B. Effective La-Na Co-Doped TiO2 Nano-Particles for Dye Adsorption: Synthesis, Characterization and Study on Adsorption Kinetics. Nanomaterials 2019, 9, 400. [Google Scholar] [CrossRef]
- Jo, M.S.; Cho, J.S.; Wang, X.L.; Jin, E.M.; Jeong, S.M.; Kang, D. Improving of the Photovoltaic Characteristics of Dye-Sensitized Solar Cells Using a Photoelectrode with Electrospun Porous TiO2 Nanofibers. Nanomaterials 2019, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lv, Y.; Li, C.; Zhong, Y.; Chen, M.; Zhang, S.; Zhou, Y.; Chen, Z. Effect of water adsorption at nanoparticle–oil interface on charge transport in high humidity transformer oil-based nanofluid. Colloid Surf. A-Physicochem. Eng. Asp. 2012, 415, 153–158. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Li, C.; Chen, M.; Zhou, J.; Li, X.; Zhou, Y.; Tu, Y. Effect of electron shallow trap on breakdown performance of transformer oil-based nanofluids. J. Appl. Phys. 2011, 110, 104104. [Google Scholar] [CrossRef]
- Zhou, Y.; Sui, S.Y.; Li, J.; Wang, Z.Y.; Cui, W.; Lv, Y.Z.; Li, C.R. Statistical analysis of moisture’s effect on AC breakdown strength of TiO2 nanofluids. J. Mol. Liq. 2018, 249, 420–428. [Google Scholar] [CrossRef]
- Lv, Y.; Du, Y.; Li, C.; Qi, B.; Zhong, Y.; Chen, M. TiO2 nanoparticle induced space charge decay in thermal aged transformer oil. Appl. Phys. Lett. 2013, 102, 132902. [Google Scholar] [CrossRef]
- Kelley, E.F.; Hebner, R.E., Jr. Prebreakdown phenomena between sphere-sphere electrodes in transformer oil. Appl. Phys. Lett. 1981, 38, 231–233. [Google Scholar] [CrossRef]
- Danikas, M.G. Breakdown of transformer oil. IEEE Electr. Insul. Mag. 1990, 6, 27–34. [Google Scholar] [CrossRef]
- Beroual, A.; Zahn, M.; Badent, A.; Kist, K.; Schwabe, A.J.; Yamashita, H.; Yamazawa, K.; Danikas, M.; Chadband, W.D.; Torshin, Y. Propagation and structure of streamers in liquid dielectrics. IEEE Electr. Insul. Mag. 1998, 14, 6–17. [Google Scholar] [CrossRef]
- Wedin, P. Electrical breakdown in dielectric liquids-a short overview. IEEE Electr. Insul. Mag. 2014, 30, 20–25. [Google Scholar] [CrossRef]
- Sun, A.; Huo, C.; Zhuang, J. Formation mechanism of streamer discharges in liquids: A review. High Volt. 2016, 1, 74–80. [Google Scholar] [CrossRef]
- Devins, J.C.; Rzad, S.J.; Schwabe, R.J. Breakdown and prebreakdown phenomena in liquids. J. Appl. Phys. 1981, 52, 4531–4545. [Google Scholar] [CrossRef]
- Zhou, Y.; Sui, S.; Li, J.; Ouyang, Z.; Lv, Y.; Li, C.; Lu, W. The effects of shallow traps on the positive streamer electrodynamics in transformer oil based nanofluids. J. Phys. D-Appl. Phys. 2018, 51, 105304. [Google Scholar] [CrossRef] [Green Version]
- Bartnikas, R. Electrical Insulating Liquids; Astm International: Philadelphia, PA, USA, 1994. [Google Scholar]
- Hwang, J.G.; Zahn, M.; O’Sullivan, F.M.; Pettersson, L.A.A.; Hjortstam, O.; Liu, R.; Morais, P.C. Effects of nanoparticle charging on streamer development in transformer oil-based nanofluids. J. Appl. Phys. 2010, 107, 416–622. [Google Scholar] [CrossRef]
- Velasco, J.; Frascella, R.; Albarracín, R.; Burgos, J.C.; Dong, M.; Ren, M.; Yang, L. Comparison of Positive Streamers in Liquid Dielectrics with and without Nanoparticles Simulated with Finite-Element Software. Energies 2018, 11, 361. [Google Scholar] [CrossRef]
- Sartorattoa, P.P.C.; Neto, A.V.S.; Lima, E.C.D.; de Sá, A.L.C.R.; Morais, P.C. Preparation and electrical properties of oil-based magnetic fluids. J. Appl. Phys. 2005, 97, 10Q917. [Google Scholar] [CrossRef]
- Du, B.; Li, J.; Wang, F.; Yao, W.; Yao, S. Influence of monodisperse Fe3O4 nanoparticle size on electrical properties of vegetable oil-based nanofluids. J. Nanomater. 2015, 2015, 3. [Google Scholar] [CrossRef]
- Primo, V.A.; García, B.; Albarracín, R. Improvement of Transformer Liquid Insulation Using Nanodielectric Fluids: A Review. IEEE Electr. Insul. Mag. 2018, 34, 13–26. [Google Scholar] [CrossRef]
- Lewis, T.J. Nanometric Dielectrics. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 812–825. [Google Scholar] [CrossRef]
- Lewis, T.J. Interfaces are the dominant feature of dielectrics at the nanometric level. IEEE Trans. Dielectr. Electr. Insul. 2004, 11, 739–753. [Google Scholar] [CrossRef]
- Dong, M.; Dai, J.; Li, Y.; Xie, J.; Ren, M.; Dang, Z. Insight into the dielectric response of transformer oil-based nanofluids. AIP Adv. 2017, 7, 025307. [Google Scholar] [CrossRef] [Green Version]
- Lewis, T.J. Basic electrical processes in dielectric liquids. IEEE Trans. Dielectr. Electr. Insul. 1994, 1, 630–643. [Google Scholar] [CrossRef]
- Tian, F.; Bu, W.; Shi, L.; Yang, C.; Wang, Y.; Lei, Q. Theory of modified thermally stimulated current and direct determination of trap level distribution. J. Electrost. 2011, 69, 7–10. [Google Scholar] [CrossRef]
- Tanaka, T.; Kozako, M.; Fuse, N.; Ohki, Y. Proposal of a multi-core model for polymer nanocomposite dielectrics. IEEE Trans. Dielectr. Electr. Insul. 2005, 12, 669–681. [Google Scholar] [CrossRef]
- Fehse, M.; Ventosa, E. Is TiO2(B) the Future of Titanium-Based Battery Materials? ChemPlusChem 2015, 80, 785–795. [Google Scholar] [CrossRef]
- Shin, J.-Y.; Joo, J.H.; Samuelis, D.; Maier, J. Oxygen-Deficient TiO2-δ Nanoparticles via Hydrogen Reduction for High Rate Capability Lithium Batteries. Chem. Mat. 2012, 24, 543–551. [Google Scholar] [CrossRef]










| Concentration (wt%) | Interparticle Distance (nm) |
|---|---|
| 0.0057% | 386 |
| 0.0114% | 304 |
| 0.0227% | 239 |
| 0.0454% | 188 |
| 0.0681% | 160 |
| 0.0908% | 147 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhou, Y.; Lu, W.; Peng, N.; Chen, W. The Impact of TiO2 Nanoparticle Concentration Levels on Impulse Breakdown Performance of Mineral Oil-Based Nanofluids. Nanomaterials 2019, 9, 627. https://doi.org/10.3390/nano9040627
Wang Z, Zhou Y, Lu W, Peng N, Chen W. The Impact of TiO2 Nanoparticle Concentration Levels on Impulse Breakdown Performance of Mineral Oil-Based Nanofluids. Nanomaterials. 2019; 9(4):627. https://doi.org/10.3390/nano9040627
Chicago/Turabian StyleWang, Ziyi, You Zhou, Wu Lu, Neng Peng, and Weijie Chen. 2019. "The Impact of TiO2 Nanoparticle Concentration Levels on Impulse Breakdown Performance of Mineral Oil-Based Nanofluids" Nanomaterials 9, no. 4: 627. https://doi.org/10.3390/nano9040627