Preparation, Characterization, and Performance Control of Nanographitic Films
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Morphology Characteristics of the NGFs
3.2. Structural and Photoelectric Properties of the NGFs
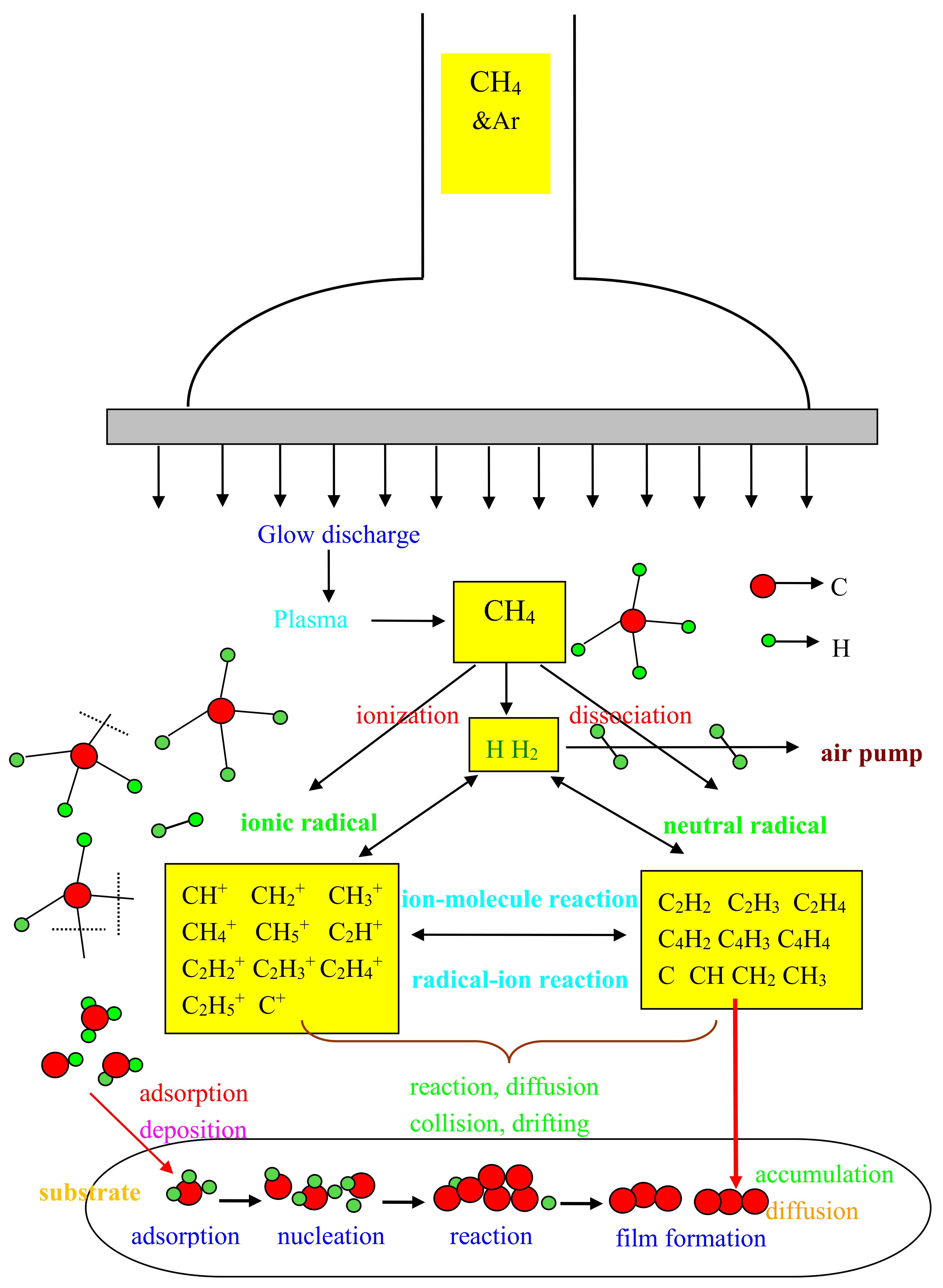
3.3. Growing Mechanism of NGFs on a Glass Substrate
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lee, J.; Kim, J.; Hyeon, T. Recent Progress in the Synthesis of Porous Carbon Materials. Adv. Mater. 2006, 18, 2073–2094. [Google Scholar] [CrossRef]
- Robertson, J. Diamond-like amorphous carbon. Mater. Sci. Eng. R Rep. 2002, 37, 129–281. [Google Scholar] [CrossRef]
- Babangida, A.Y.; Maniruzzaman Aziz, A.M.; Kassim, K.A.; Shahid, S.; Wan, A.W.; Bakar, A.; Sashruddin, T.N.; Farahiyah, A.R.; Ahamed, M.I. A review on green economy and development of green roads and highways using carbon neutral materials. Renew. Sustain. Energy Rev. 2019, 101, 600–613. [Google Scholar]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Falko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–201. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Sun, Z.; Hasan, T.; Ferrari, A.C. Graphene Photonics and Optoelectronics. Nat. Photon. 2010, 4, 611–622. [Google Scholar] [CrossRef]
- Debin, K.; Zhichang, X.; Yang, G.; Xinghao, Z.; Ruiying, G.; Xiaoxiong, H.; Xianglong, L.; Linjie, Z. Sp2-carbon dominant carbonaceous materials for energy conversion and storage. Mater. Sci. Eng. R Rep. 2019, 137, 1–37. [Google Scholar]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The Electronic Properties of Graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef]
- ChunXian, G.; GuanHong, G.; ChangMing, L. Graphene Based Materials: Enhancing Solar Energy Harvesting. Adv. Energy Mater. 2011, 1, 448–452. [Google Scholar]
- Ching-Fang, L.; Yu-Chien, L.; Tien-Yu, Y.; Chi-Chang, H. Carbon materials for high-voltage supercapacitors. Carbon 2019, 145, 529–548. [Google Scholar]
- Virendra, S.; Daeha, J.; Lei, Z.; Soumen, D.; Sudipta, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar]
- Xinming, L.; Dan, X.; Hyesung, P.; Tingying, H.Z.; Kunlin, W.; Jinquan, W.; Minlin, Z.; Dehai, W.; Jing, K.; Hongwei, Z. Anomalous Behaviors of Graphene Transparent Conductors in Graphene-Silicon Heterojunction Solar Cells. Adv. Energy Mater. 2013, 52, 1–6. [Google Scholar]
- Xinming, L.; Hongwei, Z.; Kunlin, W.; Anyuan, C.; Jinquan, W.; Chunyan, L.; Yi, J.; Zhen, L.; Xiao, L.; Dehai, W. Graphene-On-Silicon Schottky Junction Solar Cells. Adv. Mater. 2010, 22, 2743–2748. [Google Scholar]
- Ramuz, M.P.; Vosgueritchian, M.; Wei, P.; Wang, C.; Gao, Y.; Wu, Y.; Chen, Y.; Bao, Z. Evaluation of Solution-Processable Carbon-Based Electrodes for All-Carbon Solar Cells. ACS Nano 2012, 6, 10384–10395. [Google Scholar] [CrossRef] [PubMed]
- Shumin, C.; Ming, G.; Xiaohong, F.; Zhongquan, M. Modifications and multiple roles of graphene in SIS structural solar cells. Sol. Energy 2015, 122C, 658–666. [Google Scholar]
- Yunzhou, X.; Bin, W.; Lang, J.; Yunlong, G.; Liping, H.; Jianyi, C.; Jiahui, T.; Dechao, G.; Birong, L.; Wenping, H.; et al. Low Temperature Growth of Highly Nitrogen-Doped Single Crystal Graphene Arrays by Chemical Vapor Deposition. J. Am. Chem. Soc. 2012, 134, 11060–11063. [Google Scholar]
- Guermoune, A.; Chari, T.; Popescu, F.; Sabri, S.S.; Guillemette, J.; Skulason, H.S.; Szkopek, T.; Siaj, M. Chemical Vapor Deposition Synthesis of Graphene on Copper with Methanol, Ethanol, and Propanol Precursors. Carbon 2011, 49, 4204–4210. [Google Scholar] [CrossRef]
- YuFen, L.; ShunTsung, L.; JhengCyuan, L.; Wenjing, Z.; JingYu, L.; FanHung, L.; ChuanMing, T.; YiHsien, L.; ChiTe, L.; LainJong, L. Nitrogen-Doped Graphene Sheets Grown by Chemical Vapor Deposition: Synthesis and Influence of Nitrogen Impurities on Carrier Transport. ACS Nano 2013, 7, 6522–6532. [Google Scholar]
- Dalkeun, P.; Young, H.K.; Joong, K.L. Synthesis of carbon nanotubes on metallic substrates by a sequential combination of PECVD and thermal CVD. Carbon 2003, 41, 1025–1029. [Google Scholar]
- Chen, S.M.; Gao, M.; Cao, R.N.; Yang, J.; Du, H.W.; Zhao, L.; Ma, Z.Q. Hydrogen-free synthesis of graphene-graphitic films directly on Si substrate by plasma enhanced chemical vapor deposition. J. Mater. Sci. Mater. Electr. 2015, 26, 1485–1493. [Google Scholar] [CrossRef]
- Wei, C.; Cong, W.; Xiaohong, F.; Liyou, Y.; Xiaoyuan, C. Synthesis and characterization of nitrogen-doped graphene films using C5NCl5. Appl. Phys. Lett. 2015, 106, 253105. [Google Scholar]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Knight, D.S.; White, W.B. Characterization of diamond films by Raman spectroscopy. J. Mater. Res. 1989, 4, 385–393. [Google Scholar] [CrossRef]
- Shroder, R.E.; Nemanich, R.J. Analysis of the composite structures in diamond thin films by Raman spectroscopy. Phys. Rev. B 1990, 41, 3738–3745. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Katagiri, G.; Ishida, H.; Ishitani, A.; Ono, M. Characterization of crystalline quality of diamond films by Raman spectroscopy. Appl. Phys. Lett. 1989, 55, 2608–2610. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman Spectrum of Graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron–phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Venables, J.A.; Spiller, G.D.T.; Hanbucken, M. Nucleation and growth of thin films. Rep. Prog. Phys 1984, 47, 399–459. [Google Scholar] [CrossRef]
- Zhenyu, Z.; Max, G.L. Atomistic Processes in the Early Stages of Thin-Film Growth. Science 1997, 276, 377–383. [Google Scholar]
- Mingyao, Z.; Jianjun, W.; Brian, C.; Holloway, R.A.; Outlaw, X.Z.; Kun, H.; Shutthanandan, V.; Manos, D.M. A mechanism for carbon nanosheet formation. Carbon 2007, 45, 2229–2234. [Google Scholar]



| Vacuum Pressure/Pa | Substrate Temperature/°C | RF Power/W | Gas Flow/Sccm CH4:Ar | Deposition Time/Min | Plate Spacing/Cm |
|---|---|---|---|---|---|
| 50 | 300 | 200 | 2:50 | 60 | 5 |
| Points | 1(Red Arrow) | 2 (Purple Arrow) | 3(Blue Arrow) |
|---|---|---|---|
| FWHM of D/cm−1 | 164 | 126 | 134 |
| FWHM of G/cm−1 | 105 | 80 | 162 |
| I2D/IG | 0.30 | 0.32 | 0.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.; Jiang, Q.; Chen, Y.; Feng, L.; Wu, D. Preparation, Characterization, and Performance Control of Nanographitic Films. Nanomaterials 2019, 9, 628. https://doi.org/10.3390/nano9040628
Chen S, Jiang Q, Chen Y, Feng L, Wu D. Preparation, Characterization, and Performance Control of Nanographitic Films. Nanomaterials. 2019; 9(4):628. https://doi.org/10.3390/nano9040628
Chicago/Turabian StyleChen, Shumin, Qiang Jiang, Yong Chen, Lulu Feng, and Di Wu. 2019. "Preparation, Characterization, and Performance Control of Nanographitic Films" Nanomaterials 9, no. 4: 628. https://doi.org/10.3390/nano9040628





