Graphene Oxide Nanoribbons in Chitosan for Simultaneous Electrochemical Detection of Guanine, Adenine, Thymine and Cytosine
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Instrumentation
2.3. Synthesis of Graphene Oxide Nanoribbons (GONRs)
2.4. Preparation of Nanocomposite-Modified GCE
2.5. Preparation of DNA Base Stock Solutions
3. Results and Discussion
3.1. Characterization of GONRs
3.2. Characterization of GCE and Stepwise GCE Modification
3.3. Electrochemical Characterization of GCE/GONRs-CH
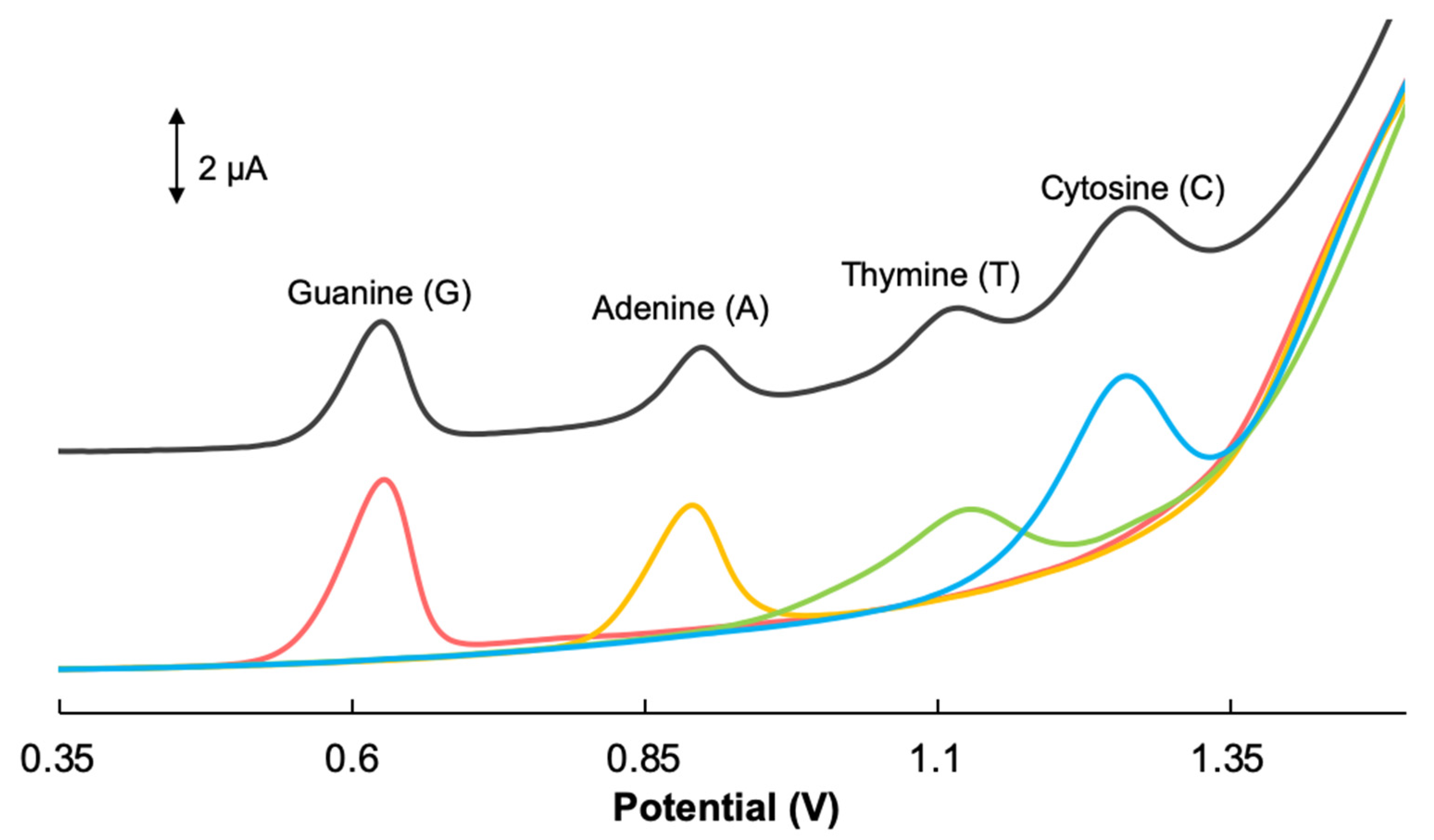
3.4. Simultaneous Detection of Guanine, Adenine, Thymine and Cytosine
3.5. pH Effect
3.6. Calibration Curves
3.7. Reproducibility and Stability
3.8. Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dinesh, S.; Shikha, G.; Bhavana, G.; Nidhi, S.; Dileep, S. Biological activities of purine analogues: A review. J. Pharm. Sci. Innov. 2012, 1, 29–34. [Google Scholar]
- Saenger, W. Structure and function of nucleosides and nucleotides. Angew. Chemie Int. Ed. Engl. 1973, 12, 591–601. [Google Scholar] [CrossRef]
- Yang, Y.; Geldmacher, D.S.; Herrup, K. DNA replication precedes neuronal cell death in Alzheimer’s disease. J. Neurosci. 2001, 21, 2661–2668. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.Y.; Ugozzoli, L.; Pal, B.K.; Wallace, R.B. Allele-specific enzymatic amplification of β-globin genomic DNA for diagnosis of sickle cell anemia. Proc. Natl. Acad. Sci. USA 1989, 86, 2757–2760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rommens, J.M.; Kerem, B.; Alon, N.; Grzelczak, Z.; Zielenski, J.; Lok, S.; Plavsic, N.; Chou, J.; Drumm, M.L.; Iannuzzi, M.C.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar]
- Brotons, A.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Iniesta, J. Carbon materials for the electrooxidation of nucleobases, nucleosides and nucleotides toward cytosine methylation detection: A review. Anal. Methods 2016, 8, 702–715. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.; Luo, L.; Ding, Y.; Liu, B.; Xu, D. Simultaneous determination of purine and pyrimidine bases in DNA using poly(3,4-ethylenedioxythiophene)/graphene composite film. J. Electroanal. Chem. 2014, 735, 51–56. [Google Scholar] [CrossRef]
- Thangaraj, R.; Senthil Kumar, A. Simultaneous detection of guanine and adenine in DNA and meat samples using graphitized mesoporous carbon modified electrode. J. Solid State Electrochem. 2013, 17, 583–590. [Google Scholar] [CrossRef]
- Anu Prathap, M.U.; Srivastava, R.; Satpati, B. Simultaneous detection of guanine, adenine, thymine, and cytosine at polyaniline/MnO2 modified electrode. Electrochim. Acta 2013, 114, 285–295. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D. PGR Tips on writing effective CVs. Phys. Rev. Lett. 2004, 306, 666–669. [Google Scholar]
- Zhang, M.; Smith, A.; Gorski, W. Carbon nanotube-chitosan system for electrochemical sensing based on dehydrogenase enzymes. Anal. Chem. 2004, 76, 5045–5050. [Google Scholar] [CrossRef]
- Chen, L.; Tang, Y.; Wang, K.; Liu, C.; Luo, S. Direct electrodeposition of reduced graphene oxide on glassy carbon electrode and its electrochemical application. Electrochem. Commun. 2011, 13, 133–137. [Google Scholar] [CrossRef]
- Mani, V.; Govindasamy, M.; Chen, S.M.; Chen, T.W.; Kumar, A.S.; Huang, S.T. Core-shell heterostructured multiwalled carbon nanotubes@reduced graphene oxide nanoribbons/chitosan, a robust nanobiocomposite for enzymatic biosensing of hydrogen peroxide and nitrite. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kosynkin, D.V.; Higginbotham, A.L.; Sinitskii, A.; Lomeda, J.R.; Dimiev, A.; Price, B.K.; Tour, J.M. Longitudinal unzipping of carbon nanotubes to form graphene nanoribbons. Nature 2009, 458, 872–876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosynkin, D.V.; Lu, W.; Sinitskii, A.; Pera, G.; Sun, Z.; Tour, J.M. Highly conductive graphene nanoribbons by longitudinal splitting of carbon nanotubes using potassium vapor. ACS Nano 2011, 5, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Xiong, K.; Fan, Q.; Wu, T.; Shi, H.; Chen, L.; Yan, M. Enhanced bovine serum albumin absorption on the N-hydroxysuccinimide activated graphene oxide and its corresponding cell affinity. Mater. Sci. Eng. C 2017, 81, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Sun, S.; Li, H.; Wang, M.; Jia, R. Electrochemical bisphenol A sensor based on core-shell multiwalled carbon nanotubes/graphene oxide nanoribbons. Sens. Actuators B Chem. 2015, 209, 275–280. [Google Scholar] [CrossRef]
- Sharma, V.K.; Jelen, F.; Trnkova, L. Functionalized solid electrodes for electrochemical biosensing of purine nucleobases and their analogues: A review. Sensors 2015, 15, 1564–1600. [Google Scholar] [CrossRef] [Green Version]
- Sun, W.; Xi, M.; Zhang, L.; Zhan, T.; Gao, H.; Jiao, K. Electrochemical behaviors of thymine on a new ionic liquid modified carbon electrode and its detection. Electrochim. Acta 2010, 56, 222–226. [Google Scholar] [CrossRef]
- Jankowska-Śliwińska, J.; Dawgul, M.; Kruk, J.; Pijanowska, D.G. Comparison of electrochemical determination of purines and pyrimidines by means of carbon, graphite and gold paste electrodes. Int. J. Electrochem. Sci. 2017, 12, 2329–2343. [Google Scholar] [CrossRef]
- Oliveira-Brett, A.M.; Piedade, J.A.P.; Silva, L.A.; Diculescu, V.C. Voltammetric determination of all DNA nucleotides. Anal. Biochem. 2004, 332, 321–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, W.; Li, Y.; Duan, Y.; Jiao, K. Direct electrocatalytic oxidation of adenine and guanine on carbon ionic liquid electrode and the simultaneous determination. Biosens. Bioelectron. 2008, 24, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Ju, H.X.; Chen, H.Y. Simultaneous determination of guanine and adenine in DNA using an electrochemically pretreated glassy carbon electrode. Anal. Chim. Acta 2002, 461, 243–250. [Google Scholar] [CrossRef]
- Fan, Y.; Huang, K.J.; Niu, D.J.; Yang, C.P.; Jing, Q.S. TiO2-graphene nanocomposite for electrochemical sensing of adenine and guanine. Electrochim. Acta 2011, 56, 4685–4690. [Google Scholar] [CrossRef]
- Liu, H.; Wang, G.; Chen, D.; Zhang, W.; Li, C.; Fang, B. Fabrication of polythionine/NPAu/MWNTs modified electrode for simultaneous determination of adenine and guanine in DNA. Sens. Actuators B Chem. 2008, 128, 414–421. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Luo, G. The electrocatalytic oxidation of thymine at α-cyclodextrin incorporated carbon nanotube-coated electrode. Electroanalysis 2003, 15, 1129–1133. [Google Scholar] [CrossRef]
- Ruan, C.X.; Lou, J.; Duan, Y.Y.; Sun, W. Electrochemical oxidation of cytosine on carbon paste electrode and its determination. J. Chin. Chem. Soc. 2010, 57, 1056–1060. [Google Scholar] [CrossRef]
- Zen, J.M.; Chang, M.R.; Ilangovan, G. Simultaneous determination of guanine and adenine contents in DNA, RNA and synthetic oligonucleotides using a chemically modified electrode. Analyst 1999, 124, 679–684. [Google Scholar] [CrossRef]
- Wu, K.; Fei, J.; Bai, W.; Hu, S. Direct electrochemistry of DNA, guanine and adenine at a nanostructured film-modified electrode. Anal. Bioanal. Chem. 2003, 376, 205–209. [Google Scholar] [CrossRef]







| Analyte | I | II | LOD (µM) | Dynamic Range (µM) |
|---|---|---|---|---|
| G | ΔIp1 = 0.3651x + 0.066 R² = 0.9919 | ΔIp2 = 0.0621x + 1.493 R² = 0.9901 | 0.002 | 0.05–256.0 |
| A | ΔIp1 = 0.1963x + 0.144 R² = 0.9911 | ΔIp2 = 0.0606x + 0.881 R² = 0.9916 | 0.023 | 0.05–172.0 |
| T | ΔIp1 = 0.008x + 0.4086 R² = 0.9944 | ΔIp2 = 0.0008x + 2.947 R² = 0.9923 | 1.330 | 5.0–855.0 |
| C | ΔIp = 0.0186x + 0.4199 R² = 0.9909 | 0.641 | 2.5–342.0 |
| Analyte | Modified Working Electrode | LOD (µM) | Dynamic Range (µM) | Ref. |
|---|---|---|---|---|
| G | GCE | 0.06 | 0.2–10 | [21] |
| CILE a | 0.00787 | 0.3–50 | [22] | |
| Pretreated GCE b | 0.03 | 0.08–2.7 | [23] | |
| TiO2-graphene/GCE | 0.15 | 0.5–200 | [24] | |
| GCE/GONRs-CH | 0.0018 | 0.013–256 | This study | |
| A | GCE | 0.07 | 0.2–10 | [21] |
| CILE | 0.25 | 1.5–70 | [22] | |
| Pretreated GCE | 0.03 | 0.08–2.7 | [23] | |
| TiO2-graphene/GCE | 0.10 | 0.5–200 | [24] | |
| PTH/NPAu/MWNTs c | 0.008 | 0.05–5 | [25] | |
| GCE/GONRs-CH | 0.023 | 0.11–172 | This study | |
| T | GCE | 0.89 | 1–20 | [21] |
| PANI/MnO2 d | 1.3 | 10–100 | [9] | |
| α-CD/CNT/CE e | 5 | 25–1800 | [26] | |
| PEDOT/GR/GCE f | 0.33 | 2.5–650 | [7] | |
| GCE/GONRs-CH | 1.330 | 6.0–855 | This study | |
| C | GCE | 1.76 | 1–20 | [21] |
| PANI/MnO2 | 1.3 | 10–100 | [9] | |
| CPE g | 1.9 | 10–1000 | [27] | |
| PEDOT/GR/GCE | 0.33 | 1–1000 | [7] | |
| GCE/GONRs-CH | 0.640 | 3.5–342 | This study |
| Analyte | Detected (µM) | Spiked (µM) | Determined (µM) | Recovery (%) | |
| Single Nucleotides | A | 25.9 ± 3.89 | 25 | 23.85 ± 0.30 | 95.41 ± 1.2 |
| T | 24.4 ± 4.92 | 50 | 48.91 ± 1.03 | 97.90 ± 2.06 | |
| G | 24.9 ± 2.87 | 25 | 24.08 ± 0.22 | 96.34 ± 0.88 | |
| C | 22.5 ± 1.10 | 15 | 14.44 ± 0.44 | 96.28 ± 2.93 | |
| dsDNA | A | 13.04 ± 1.47 | 25 | 23.80 ± 0.13 | 95.26 ± 0.52 |
| T | 11.38 ± 2.28 | 40 | 39.8 ± 1.37 | 99.50 ± 3.4 | |
| G | 19.23 ± 1.10 | 25 | 22.8 ± 0.39 | 91.14 ± 1.7 | |
| C | 23.75 ± 2.79 | 20 | 20.9 ± 2.75 | 104.7 ± 13.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Li, S.; Noroozifar, M.; Kerman, K. Graphene Oxide Nanoribbons in Chitosan for Simultaneous Electrochemical Detection of Guanine, Adenine, Thymine and Cytosine. Biosensors 2020, 10, 30. https://doi.org/10.3390/bios10040030
Zhou J, Li S, Noroozifar M, Kerman K. Graphene Oxide Nanoribbons in Chitosan for Simultaneous Electrochemical Detection of Guanine, Adenine, Thymine and Cytosine. Biosensors. 2020; 10(4):30. https://doi.org/10.3390/bios10040030
Chicago/Turabian StyleZhou, Jiayun, Shaopei Li, Meissam Noroozifar, and Kagan Kerman. 2020. "Graphene Oxide Nanoribbons in Chitosan for Simultaneous Electrochemical Detection of Guanine, Adenine, Thymine and Cytosine" Biosensors 10, no. 4: 30. https://doi.org/10.3390/bios10040030
APA StyleZhou, J., Li, S., Noroozifar, M., & Kerman, K. (2020). Graphene Oxide Nanoribbons in Chitosan for Simultaneous Electrochemical Detection of Guanine, Adenine, Thymine and Cytosine. Biosensors, 10(4), 30. https://doi.org/10.3390/bios10040030






