Advances in Sensing Technologies for Monitoring of Bone Health
Abstract
:1. Introduction
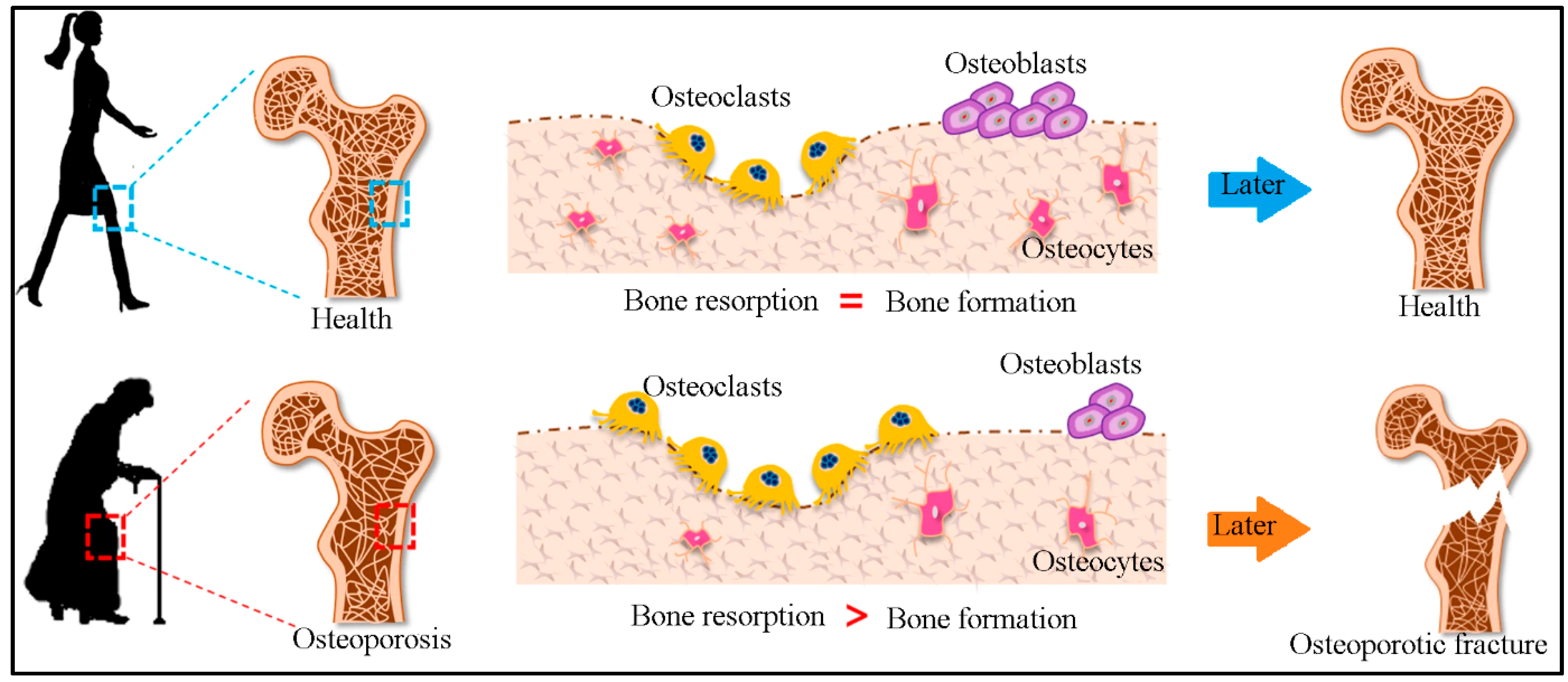
2. Bone Remodeling
3. Bone Diseases
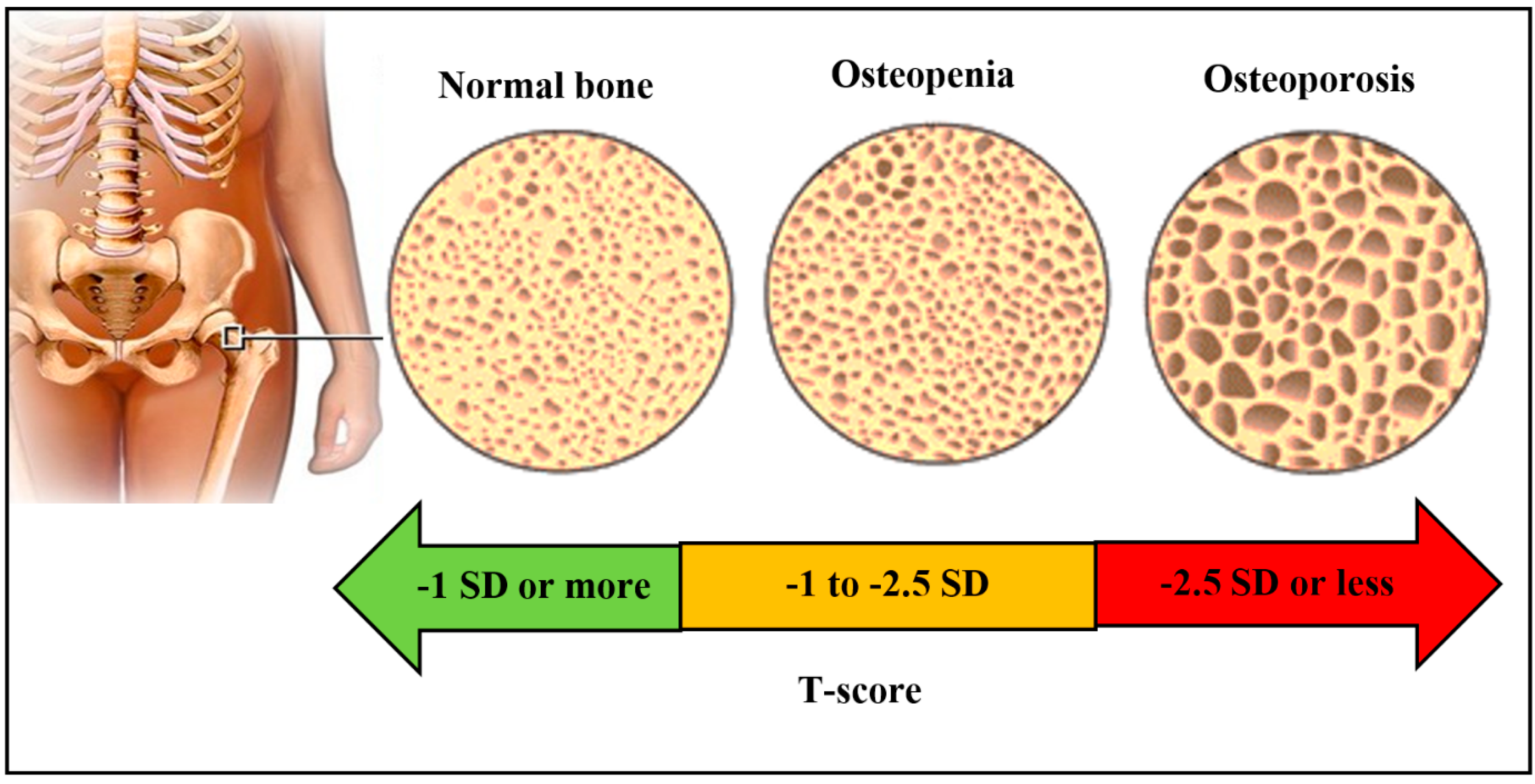
3.1. Osteoporosis
3.2. Bone Cancer and Infections
3.3. Osteogenesis Imperfecta
3.4. Paget’s Diseases of Bone
3.5. Osteomalacia
3.6. Osteopetrosis
3.7. Fibrous Dysplasia
3.8. Scoliosis
3.9. Osteomyelitis
4. Current Diagnostic Tests for Bone Health Monitoring
4.1. Bone Densitometry
4.2. Bone Scan
4.3. Bone X-ray (Radiography)
4.4. Calcium Blood Test
4.5. Bone Biopsy
5. Biochemical Markers for Bone Health Monitoring
5.1. Biochemical Markers for Bone Formation
5.1.1. Bone-Specific Alkaline Phosphatase (BALP)
5.1.2. Procollagen I Peptides
5.1.3. Osteocalcin
5.2. Biochemical Markers of Bone Resorption
5.2.1. Hydroxyproline (OHP)
5.2.2. Collagen Cross-Link Molecules
5.2.3. Hydroxylysine Glycosides
5.2.4. Telopeptides of Type I Collagen
5.2.5. Bone Sialoprotein (BSP)
5.2.6. Tartrate-Resistant Acid Phosphatase (TRACP)
5.2.7. Cathepsin K
6. Traditional Techniques for Measurement of Bone Turnover Markers
6.1. Enzyme-Linked Immunosorbent Assay (ELISA)
6.2. Radioimmunoassay (RIA)
6.3. High-Performance Liquid Chromatography (HPLC)
7. Sensors for Diagnosis of Bone Health
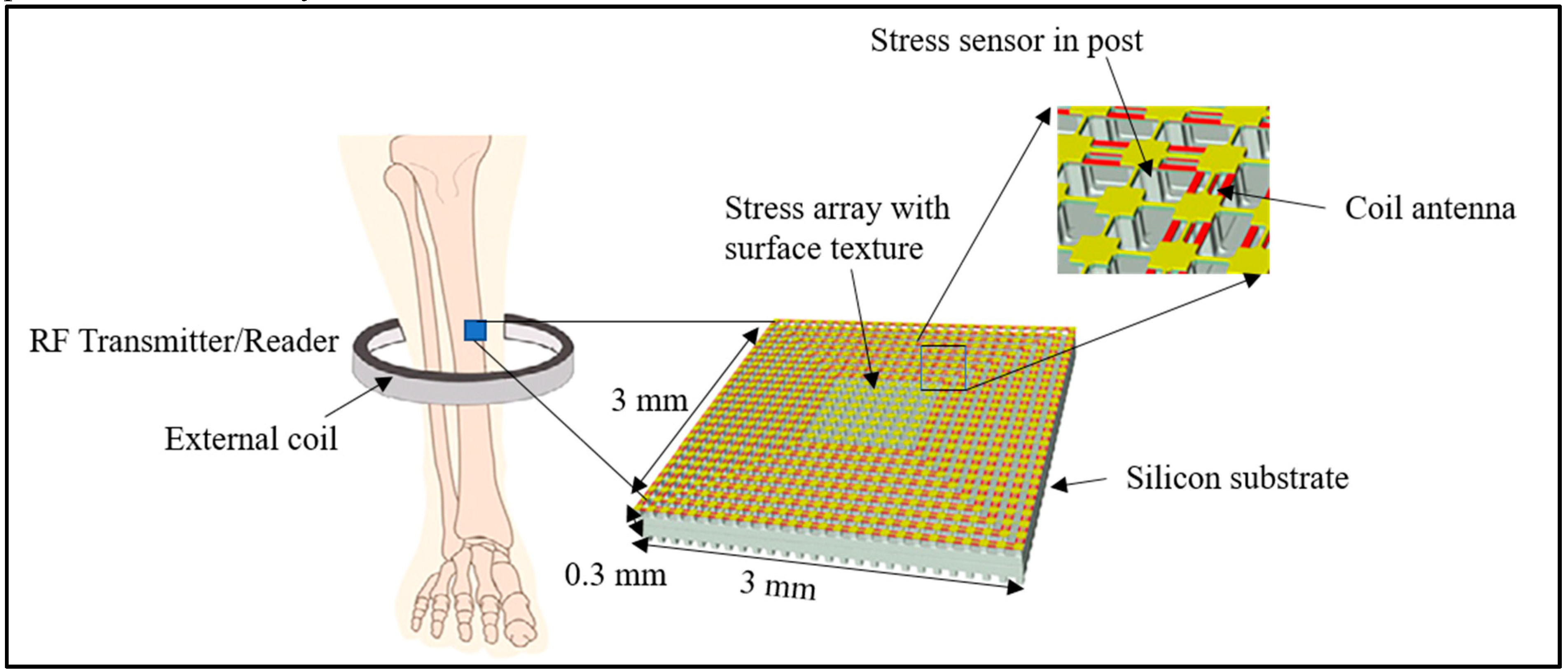
7.1. Physical Sensors
7.2. Biosensors
7.2.1. Electrochemical Biosensors
7.2.2. Acoustic Biosensors
7.2.3. Other Sensors
8. Conclusions and Future Trends
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Florencio-silva, R.; Rodrigues, G.; Sasso-cerri, E.; Simões, M.J.; Cerri, P.S.; Cells, B. Biology of Bone Tissue: Structure, Function, and Factors that Influence Bone Cells. BioMed Res. Int. 2015, 2015, 421746. [Google Scholar] [CrossRef] [Green Version]
- Datta, H.K.; Ng, W.F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biomechanical and Molecular Regulation of Bone Remodeling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.R.; Chen, C.H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 5–13. [Google Scholar] [CrossRef] [Green Version]
- Chapurlat, R.D.; Confavreux, C.B. Novel biological markers of bone: From bone metabolism to bone physiologya. Rheumatology 2016, 55, 1714–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, J.K.; Kim, H.Y.; Lee, C.Y.; Park, K.S.; Park, H.G. Label-free and washing-free alkaline phosphatase assay using a personal glucose meter. J. Biol. Eng. 2019, 13, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Claudon, A.; Vergnaud, P.; Valverde, C.; Mayr, A.; Klause, U.; Garnero, P. New automated multiplex assay for bone turnover markers in osteoporosis. Clin. Chem. 2008, 54, 1554–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clowes, J.A.; Hannon, R.A.; Yap, T.S.; Hoyle, N.R.; Blumsohn, A.; Eastell, R. Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone 2002, 30, 886–890. [Google Scholar] [CrossRef]
- Chang, Y.; Cho, B.; Kim, S.; Kim, J. Direct conversion of fibroblasts to osteoblasts as a novel strategy for bone regeneration in elderly individuals. Exp. Mol. Med. 2019, 51, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Rawadi, G.; Vayssière, B.; Dunn, F.; Baron, R.; Roman-Roman, S. BMP-2 Controls Alkaline Phosphatase Expression and Osteoblast Mineralization by a Wnt Autocrine Loop. J. Bone Miner. Res. 2003, 18, 1842–1853. [Google Scholar] [CrossRef] [PubMed]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef] [PubMed]
- Vasikaran, S.; Eastell, R.; Bruyère, O.; Foldes, A.J.; Garnero, P.; Griesmacher, A.; McClung, M.; Morris, H.A.; Silverman, S.; Trenti, T.; et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: A need for international reference standards. Osteoporos. Int. 2011, 22, 391–420. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, F.; Weiss, L.; Campbell, P.; Miller, M.; Fedder, G.K. Design of a multi-axis implantable MEMS sensor for intraosseous bone stress monitoring. J. Micromech. Microeng. 2009, 19, 085016. [Google Scholar] [CrossRef]
- Heaney, R.P.; Gallagher, J.C.; Johnston, C.C.; Neer, R.; Parfitt, A.M.; Whedon, G.D. Calcium nutrition and bone health in the elderly. Am. J. Clin. Nutr. 1982, 36, 986–1013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.M.; Heer, M.A.; Shackelford, L.C.; Sibonga, J.D.; Ploutz-Snyder, L.; Zwart, S.R. Benefits for bone from resistance exercise and nutrition in long-duration spaceflight: Evidence from biochemistry and densitometry. J. Bone Miner. Res. 2012, 27, 1896–1906. [Google Scholar] [CrossRef] [PubMed]
- Alfaro, J.F.; Weiss, L.E.; Campbell, P.G.; Miller, M.C.; Heyward, C.; Doctor, J.S.; Fedder, G.K. BioImplantable Bone Stress Sensor. In Proceedings of the 2005 IEEE Engineering in Medicine and Biology 27th Annual Conference, Shanghai, China, 17–18 January 2006; pp. 518–521. [Google Scholar]
- Mamtani, R.; Stern, P.; Dawood, I.; Cheema, S. Metals and disease: A global primary health care perspective. J. Toxicol. 2011, 2011, 319136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dongre, N.N.; Suryakar, A.N.; Patil, A.J.; Hundekari, I.A.; Devarnavadagi, B.B. Biochemical effects of lead exposure on battery manufacture workers with reference to blood pressure, calcium metabolism and bone mineral density. Indian J. Clin. Biochem. 2013, 28, 65–70. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Barceló, E.J.; Mediavilla, M.D.; Tan, D.X.; Reiter, R.J. Scientific Basis for the Potential Use of Melatonin in Bone Diseases: Osteoporosis and Adolescent Idiopathic Scoliosis. J. Osteoporos. 2010, 2010, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Webster, T.J. In Situ Sensor Advancements for Osteoporosis Prevention, Diagnosis, and Treatment. Curr. Osteoporos. Rep. 2016, 14, 386–395. [Google Scholar] [CrossRef]
- World Health Organization. IRIS (Institutional Repository for Information Sharing) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. In Report of a WHO Study Group; World Health Organizationh: Rome, Itly, (22 to 25 June 1992); 1994. [Google Scholar]
- Colicino, E.; Just, A.; Kioumourtzoglou, M.; Vokonas, P.; Cardenas, A.; Sparrow, D.; Weisskopf, M.; Nie, L.H.; Hu, H.; Schwartz, J.D.; et al. Blood DNA methylation biomarkers of cumulative lead exposure in adults. J. Expo. Sci. Environ. Epidemiol. 2019. [Google Scholar] [CrossRef]
- Rodríguez, J.; Mandalunis, P.M. A review of metal exposure and its effects on bone health. J. Toxicol. 2018, 2018, 4854152. [Google Scholar] [CrossRef]
- Khashayar, P.; Amoabediny, G.; Larijani, B.; Vanfleteren, J. Bone biosensors: Knowing the present and predicting the future. J. Micromech. Microeng. 2016, 26, 23002. [Google Scholar] [CrossRef]
- Rachner, T.D.; Khosla, S.; Hofbauer, L.C. Osteoporosis: Now and the future. Lancet 2011, 377, 1276–1287. [Google Scholar] [CrossRef] [Green Version]
- Osteopenia, Osteoporosis and Exercise. Available online: http://strongandstable.com.au/2017/10/osteopenia-osteoporosis-and-exercise/ (accessed on 17 January 2020).
- Patel, P. Biosensors and Biomarkers: Promising Tools for Cancer Diagnosis. Int. J. Biosens. Bioelectron. 2017, 3, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Evola, F.R.; Costarella, L.; Pavone, V.; Caff, G.; Cannavò, L.; Sessa, A.; Avondo, S.; Sessa, G. Biomarkers of osteosarcoma, chondrosarcoma, and ewing sarcoma. Front. Pharmacol. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nugent, M. MicroRNA and bone cancer. Adv. Exp. Med. Biol. 2015, 889, 201–230. [Google Scholar] [PubMed]
- Devogelaer, J.-P.; Coppin, C. Osteogenesis Imperfecta. Treat. Endocrinol. 2006, 5, 229–242. [Google Scholar] [CrossRef]
- Rauch, F.; Glorieux, F.H. Osteogenesis imperfecta. Lancet 2004, 363, 1377–1385. [Google Scholar] [CrossRef]
- Forlino, A.; Cabral, W.A.; Barnes, A.M.; Marini, J.C. New perspectives on osteogenesis imperfecta. Nat. Rev. Endocrinol. 2011, 7, 540–557. [Google Scholar] [CrossRef] [Green Version]
- Gkouva, L.; Andrikoula, M. Active Paget’s disease of bone with normal biomarkers of bone metabolism: A case report and review of the literature. Clin. Rheumatol. 2011, 30, 139–144. [Google Scholar] [CrossRef]
- Siris, E.; Roodman, G.D. Paget’s Disease of Bone. In Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism, 7th ed.; Rosen, C.J., Compston, J.E., Bilezikian, J.P., Bouillon, R., Clemens, T., Bauer, D.C., Ebeling, P.R., Engelke, K., Goltzman, D., Guise, T., et al., Eds.; American Society for Bone and Mineral Research: Washington, DC, USA, 2009. [Google Scholar]
- Christenson, R.H. Biochemical markers of bone metabolism: An overview. Clin. Biochem. 1997, 30, 573–593. [Google Scholar] [CrossRef]
- Basha, B.; Rao, D.S.; Han, Z.H.; Parfitt, A.M. Osteomalacia due to vitamin D depletion: A neglected consequence of intestinal malabsorption. Am. J. Med. 2000, 108, 296–300. [Google Scholar] [CrossRef]
- Bhan, A.; Qiu, S.; Rao, S.D. Bone histomorphometry in the evaluation of osteomalacia. Bone Rep. 2018, 8, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Sisco, M. Luiza Kerstenetzky 53. In Abd-Elsayed A. Pain; Springer: Cham, Switzerland, 2019; pp. 261–265. [Google Scholar]
- Adams, J.E. Radiology of rickets and osteomalacia. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: London, UK, 2018; Volume 1. [Google Scholar]
- Elbossaty, W.F. Mineralization of Bones in Osteoporosis and Osteomalacia. Ann. Clin. Lab. Res. 2017, 5, 3–6. [Google Scholar] [CrossRef]
- Noh, C.-K.; Lee, M.-J.; Kim, B.K.; Chung, Y.-S. A Case of Nutritional Osteomalacia in Young Adult Male. J. Bone Metab. 2013, 20, 51. [Google Scholar] [CrossRef] [Green Version]
- Whyte, M.P. Connective Tissue and Its Heritable Disorders: Molecular, Genetic, and Medical Aspects, 2nd ed.; Royce, P.M., Steinmann, B., Eds.; Wiley online library: Hoboken, NJ, USA, 2002; pp. 789–807. [Google Scholar]
- DiCaprio, M.R.; Enneking, W.F. Fibrous dysplasia: Pathophysiology, evaluation, and treatment. J. Bone Jt. Surg. Ser. A 2005, 87, 1848–1864. [Google Scholar] [CrossRef]
- Lane, J.M.; Khan, S.N.; O’Connor, W.J.; Nydick, M.; Hommen, J.P.; Schneider, R.; Tomin, E.; Brand, J.; Curtin, J. Bisphosphonate therapy in fibrous dysplasia. Clin. Orthop. Relat. Res. 2001, 382, 6–12. [Google Scholar] [CrossRef]
- Lustig, L.R.; Holliday, M.J.; McCarthy, E.F.; Nager, G.T. Fibrous dysplasia involving the skull base and temporal bone. Arch. Otolaryngol. Head Neck Surg. 2001, 127, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Weinstein, S.L.; Dolan, L.A.; Cheng, J.C.Y.; Danielsson, A.; Morcuende, J.A. Adolescent idiopathic scoliosis. Lancet 2008, 371, 1527–1537. [Google Scholar] [CrossRef] [Green Version]
- Burwell, R.G.; Webb, J.K. Scoliosis in children and adolescents. Curr. Paediatr. 1991, 1, 137–141. [Google Scholar] [CrossRef]
- Lombardi, G.; Akoume, M.Y.; Colombini, A.; Moreau, A.; Banfi, G. Biochemistry of adolescent idiopathic scoliosis. In Advances in Clinical Chemistry; Academic Press Elsevier: London, UK, 2011; Volume 54, pp. 165–182. [Google Scholar]
- Lew, P.D.P.; Waldvogel, P.F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Álvarez, H.C.; López, J.P.; de Rojas, L.G.; Quiroz, J.P.Y. Lumbar vertebral osteomyelitis. Rev. Cuba. Ortop. Traumatol. 2013, 27, 84–90. [Google Scholar]
- Lazzarini, L.; Mader, J.T.; Calhoun, J.H. Osteomielitis en huesos largos. Conceptos actuales. J. Bone Joint Surg. Am. 2004, 86, 2305–2318. [Google Scholar] [CrossRef] [PubMed]
- Sia, I.G.; Berbari, E.F. Osteomyelitis. Best Pract. Res. Clin. Rheumatol. 2006, 20, 1065–1081. [Google Scholar] [CrossRef] [PubMed]
- Gafni, R.I.; Baron, J. Overdiagnosis of osteoporosis in children due to misinterpretation of Dual-energy x-ray absorptiometry (DEXA). J. Pediatr. 2004, 144, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.D.; Zapalowski, C.; Kulak, C.A.M.; Bilezikian, J.P. Bone densitometry: The best way to detect osteoporosis and to monitor therapy. J. Clin. Endocrinol. Metab. 1999, 84, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
- Bartl, R.; Bartl, C. The Osteoporosis Manual: Prevention, Diagnosis and Management; Springer: Cham, Switzerland, 2019; pp. 67–75. [Google Scholar]
- Cummings, S.R.; Bates, D.; Black, D.M. Clinical use of bone densitometry: Scientific review. J. Am. Med. Assoc. 2002, 288, 1889–1897. [Google Scholar] [CrossRef] [Green Version]
- van den Wyngaert, T.; Strobel, K.; Kampen, W.U.; Kuwert, T.; Van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; Beheshti, M. The EANM practice guidelines for bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef] [Green Version]
- Brenner, A.I.; Koshy, J.; Morey, J.; Lin, C.; Dipoce, J. The bone scan. Semin. Nucl. Med. 2012, 42, 11–26. [Google Scholar] [CrossRef] [Green Version]
- Handmaker, H.; Leonards, R. The bone scan in inflammatory osseous disease. Semin. Nucl. Med. 1976, 6, 95–105. [Google Scholar] [CrossRef]
- Kiuru, M.J.; Pihlajamaki, H.K.; Hietanen, H.J.; Ahovuo, J.A. MR imaging, bone scintigraphy, and radiography in bone stress injuries of the pelvis and the lower extremity. Acta Radiol. 2002, 43, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Handa, A.; Uchiyama, Y.; Shinpuku, E.; Watanabe, M. Comparison of three plain radiography methods for evaluating proximal humerus bone strength in women. J. Orthop. Sci. 2019, 24, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Todorović-Tirnanić, M.; Obradović, V.; Han, R.; Goldner, B.; Stanković, D.; Sekulić, D.; Lazić, T.; Djordjević, B. Diagnostic approach to reflex sympathetic dystrophy after fracture: Radiography or bone scintigraphy? Eur. J. Nucl. Med. 1995, 22, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J.; Khaltaev, N. A reference standard for the description of osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Vijayanathan, S.; Butt, S.; Gnanasegaran, G.; Groves, A.M. Advantages and Limitations of Imaging the Musculoskeletal System by Conventional Radiological, Radionuclide, and Hybrid Modalities. Semin. Nucl. Med. 2009, 39, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, A.; Müller, M.; Heuser, A.; Kolevica, A.; Glüer, C.C.; Both, M.; Laue, C.; Hehn, U.; Kloth, S.; Shroff, R.; et al. Calcium isotope ratios in blood and urine: A new biomarker for the diagnosis of osteoporosis. Bone Rep. 2019, 10, 100200. [Google Scholar] [CrossRef]
- Channon, M.B.; Gordon, G.W.; Morgan, J.L.L.; Skulan, J.L.; Smith, S.M.; Anbar, A.D. Using natural, stable calcium isotopes of human blood to detect and monitor changes in bone mineral balance. Bone 2015, 77, 69–74. [Google Scholar] [CrossRef]
- Morgan, J.L.L.; Gordon, G.W.; Arrua, R.C.; Skulan, J.L.; Anbar, A.D.; Bullen, T.D. High-precision measurement of variations in calcium isotope ratios in urine by multiple collector inductively coupled plasma mass spectrometry. Anal. Chem. 2011, 83, 6956–6962. [Google Scholar] [CrossRef]
- Wan-Ibrahim, W.I.; Singh, V.A.; Hashim, O.H.; Abdul-Rahman, P.S. Biomarkers for bone tumors: Discovery from genomics and proteomics studies and their challenges. Mol. Med. 2015, 21, 861–872. [Google Scholar] [CrossRef]
- Bover, J.; Ureña-Torres, P.; Alonso, A.M.L.; Torregrosa, J.V.; Rodríguez-García, M.; Castro-Alonso, C.; Górriz, J.L.; Benito, S.; López-Báez, V.; Cora, M.J.L.; et al. Osteoporosis, bone mineral density and CKD-MBD (II): Therapeutic implications. Nefrologia 2019, 39, 227–242. [Google Scholar] [CrossRef]
- Hannon, R.A.; Eastell, R. Bone markers and current laboratory assays. Cancer Treat. Rev. 2006, 32, 7–14. [Google Scholar] [CrossRef]
- Nielson, C.M.; Jacobs, J.M.; Orwoll, E.S. Proteomic studies of bone and skeletal health outcomes. Bone 2019, 126, 18–26. [Google Scholar] [CrossRef]
- Naylor, K.; Eastell, R. Bone turnover markers: Use in osteoporosis. Nat. Rev. Rheumatol. 2012, 8, 379–389. [Google Scholar] [CrossRef]
- Sharp, C.A.; Linder, C.; Magnusson, P. Analysis of human bone alkaline phosphatase isoforms: Comparison of isoelectric focusing and ion-exchange high-performance liquid chromatography. Clin. Chim. Acta 2007, 379, 105–112. [Google Scholar] [CrossRef]
- Burr, D.B. Bone Morphology and Organization. In Basic and Applied Bone Biology, 2nd ed.; Burr, D.B., Allen, M.R., Eds.; Academic Press: Indianapolis, IN, USA, 2013; pp. 3–26. [Google Scholar]
- Eastell, R.; Garnero, P.; Audebert, C.; Cahall, D.L. Reference intervals of bone turnover markers in healthy premenopausal women: Results from a cross-sectional European study. Bone 2012, 50, 1141–1147. [Google Scholar] [CrossRef]
- Oranger, A.; Colaianni, G.; Grano, M. Bone cells. Imaging Prosthet. Jt. A Comb. Radiol. Clin. Perspect. 2014, 14, 3–13. [Google Scholar]
- Stella, D.; Brown, J.; Coleman, R. The role of biomarkers in the management of bone-homing malignancies. J. Bone Oncol. 2017, 9, 1–9. [Google Scholar]
- Ivaska, K.K.; Käkönen, S.M.; Gerdhem, P.; Obrant, K.J.; Pettersson, K.; Väänänen, H.K. Urinary osteocalcin as a marker of bone metabolism. Clin. Chem. 2005, 51, 618–628. [Google Scholar] [CrossRef]
- Ingram, R.T.; Park, Y.K.; Clarke, B.L.; Fitzpatrick, L.A. Age- and gender-related changes in the distribution of osteocalcin in the extracellular matrix of normal male and female bone. Possible involvement of osteocalcin in bone remodeling. J. Clin. Investig. 1994, 93, 989–997. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; PadmaVijayasri, A.; Rekha, P.S. Early Diagnosis of Osteoporosis in Postmenopausal Women Using Bone Markers. IOSR J. Dent. Med. Sci. 2015, 14, 102–106. [Google Scholar]
- Swaminathan, R. Biochemical markers of bone turnover. Clin. Chim. Acta 2001, 313, 95–105. [Google Scholar] [CrossRef]
- Lu, J.; Wang, M.; Wang, Z.; Fu, Z.; Lu, A.; Zhang, G. Advances in the discovery of cathepsi. inhibitors on bone resorption. J. Enzyme Inhib. Med. Chem. 2018, 33, 890–904. [Google Scholar] [CrossRef]
- Robins, S.P.; Black, D.; Paterson, C.R.; Reid, D.M.; Duncan, A.; Seibel, M.J. Evaluation of urinary hydroxypyridinium crosslink measurements as resorption markers in metabolic bone diseases. Eur. J. Clin. Investig. 1991, 21, 310–315. [Google Scholar] [CrossRef]
- Seibel, M.J. Biochemical markers of bone turnover part II: Clinical applications in the management of osteoporosis. Clin. Biochem. Rev. 2006, 27, 123–138. [Google Scholar]
- Indumati, V.; Patil, V. Biochemical markers of bone remodeling in osteoporosis-current concepts. J. Clin. Diagn. Res. 2010, 4, 2089–2097. [Google Scholar]
- Wu, C.H.; Chang, Y.F.; Chen, C.H.; Lewiecki, E.M.; Wüster, C.; Reid, I.; Tsai, K.S.; Matsumoto, T.; Mercado-Asis, L.B.; Chan, D.C.; et al. Consensus Statement on the Use of Bone Turnover Markers for Short-Term Monitoring of Osteoporosis Treatment in the Asia- Pacific Region. J. Clin. Densitom. 2019. [CrossRef]
- Herrmann, M.; Seibel, M. The amino- and carboxyterminal cross-linked telopeptides of collagen type I, NTX-I and CTX-I: A comparative review. Clin. Chim. Acta 2008, 393, 57–75. [Google Scholar] [CrossRef]
- Maeno, Y.; Inaba, M.; Okuno, S.; Yamakawa, T.; Ishimura, E.; Nishizawa, Y. Serum concentrations of cross-linked N-telopeptides of type I collagen: New marker for bone resorption in hemodialysis patients. Clin. Chem. 2005, 51, 2312–2317. [Google Scholar] [CrossRef] [Green Version]
- Ganss, B.; Kim, R.H.; Sodek, J. Bone sialoprotein. Crit. Rev. Oral Biol. Med. 1999, 10, 79–98. [Google Scholar] [CrossRef]
- Halleen, J.M.; Alatalo, S.L.; Suominen, H.; Cheng, S.; Janckila, A.J.; Väänänen, H.K. Tartrate-resistant acid phosphatase 5b: A novel serum marker of bone resorption. J. Bone Miner. Res. 2000, 15, 1337–1345. [Google Scholar] [CrossRef]
- Nenonen, A.; Cheng, S.; Ivaska, K.K.; Alatalo, S.L.; Lehtimäki, T.; Schmidt-Gayk, H.; Uusi-Rasi, K.; Heinonen, A.; Kannus, P.; Sievänen, H. Serum TRACP 5b is a useful marker for monitoring alendronate treatment: Comparison with other markers of bone turnover. J. Bone Miner. Res. 2005, 20, 1804–1812. [Google Scholar] [CrossRef] [PubMed]
- Holzer, G.; Noske, H.; Lang, T.; Holzer, L.; Willinger, U. Soluble cathepsin K: A novel marker for the prediction of nontraumatic fractures? J. Lab. Clin. Med. 2005, 146, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, Y.; Nakamura, T.; Ohta, H.; Kushida, K.; Gorai, I.; Shiraki, M.; Fukunaga, M.; Hosoi, T.; Miki, T.; Chaki, O.; et al. Guidelines for the use of biochemical markers of bone turnover in osteoporosis (2004). J. Bone Miner. Metab. 2005, 23, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Grange, R.D.; Thompson, J.P.; Lambert, D.G.; Mahajan, R.P. Radioimmunoassay, enzyme and non-enzyme-based immunoassays. Br. J. Anaesth. 2014, 112, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Engvall, E. The ELISA, enzyme-linked immunosorbent assay. Clin. Chem. 2010, 56, 319–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afsarimanesh, N.; Mukhopadhyay, S.C.; Kruger, M. Sensing technologies for monitoring of bone-health: A review. Sens. Actuators A Phys. 2018, 274, 165–178. [Google Scholar] [CrossRef]
- Melkko, J.; Niemi, S.; Risteli, L.; Risteli, J. Radioimmunoassay of the carboxyterminal propeptide of human type I procollagen. Clin. Chem. 1990, 36, 1328–1332. [Google Scholar] [CrossRef]
- Risteli, J.; Elomaa, I.; Niemi, S.; Novamo, A.; Risteli, L. Radioimmunoassay for the pyridinoline cross-linked carboxy-terminal telopeptide of type I collagen: A new serum marker of bone collagen degradation. Clin. Chem. 1993, 39, 635–640. [Google Scholar] [CrossRef]
- Raiti, S.; Davis, W.T. The principles and application of radioimmunoassay with special reference to the gonadotropins. Obstet. Gynecol. Surv. 1969, 24, 289–310. [Google Scholar] [CrossRef]
- Goldsmith, S.J. Radioimmunoassay: Review of basic principles. Semin. Nucl. Med. 1975, 5, 125–152. [Google Scholar] [CrossRef]
- Afsarimanesh, N.; Mukhopadhyay, S.C.; Kruger, M. State-of-the-art of sensing technologies for monitoring of bone-health. Smart Sens. Meas. Instrum. 2019, 30, 7–31. [Google Scholar]
- Vare, S.R.; Shelke, M.M.; Bidkar, J.S.; Dama, G.Y. HPLC: A Simple And Advance Methods Of Separation And Validation. World J. Pharm. Res. 2019, 8, 478–496. [Google Scholar]
- Seibel, M.J.; Woitge, H.W.; Farahmand, I.; Oberwittler, H.; Ziegler, R. Automated and manual assays for urinary crosslinks of collagen: Which assay to use? Exp. Clin. Endocrinol. Diabetes 1998, 106, 143–148. [Google Scholar] [CrossRef]
- Wingren, C.; Borrebaeck, C.A.K. Antibody Microarrays: Current Status and Key Technological Advances. Omi. A J. Integr. Biol. 2006, 10, 411–427. [Google Scholar] [CrossRef]
- Klosterhoff, B.S.; Ghee Ong, K.; Krishnan, L.; Hetzendorfer, K.M.; Chang, Y.H.; Allen, M.G.; Guldberg, R.E.; Willett, N.J. Wireless implantable sensor for noninvasive, longitudinal quantification of axial strain across rodent long bone defects. J. Biomech. Eng. 2017, 139, 1–8. [Google Scholar] [CrossRef]
- Luka, G.; Ahmadi, A.; Najjaran, H.; Alocilja, E.; DeRosa, M.; Wolthers, K.; Malki, A.; Aziz, H.; Althani, A.; Hoorfar, M. Microfluidics integrated biosensors: A leading technology towards lab-on-A-chip and sensing applications. Sensors 2015, 15, 30011–30031. [Google Scholar] [CrossRef] [Green Version]
- Arlett, J.L.; Myers, E.B.; Roukes, M.L. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Wen, Y.H.; Yang, G.Y.; Bailey, V.J.; Lin, G.; Tang, W.C.; Keyak, J.H. Mechanically robust micro-fabricated strain gauges for use on bones. In Proceedings of the IEEE/EMBS Special Topic Conference on Microtechnology in Medicine and Biology, Oahu, HI, USA, 12–15 May 2005; pp. 302–304. [Google Scholar]
- Umbrecht, F.; Wägli, P.; Dechand, S.; Gattiker, F.; Neuenschwander, J.; Sennhauser, U.; Hierold, C. Wireless implantable passive strain sensor: Design, fabrication and characterization. J. Micromech. Microeng. 2010, 20, 085005. [Google Scholar] [CrossRef]
- Lin, G.; Chang, S.; Kuo, C.H.; Magda, J.; Solzbacher, F. Free swelling and confined smart hydrogels for applications in chemomechanical sensors for physiological monitoring. Sens. Actuators B Chem. 2009, 136, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Oess, N.P.; Weisse, B.; Nelson, B.J. Magnetoelastic Strain Sensor for Optimized Assessment of Bone Fracture Fixation. IEEE Sens. J. 2009, 9, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Bichurin, M.; Petrov, R.; Leontiev, V.; Semenov, G.; Sokolov, O. Magnetoelectric current sensors. Sensors 2017, 17, 1271. [Google Scholar] [CrossRef] [Green Version]
- Hughes, S.; Dobson, J.; el Haj, A.J. Magnetic targeting of mechanosensors in bone cells for tissue engineering applications. J. Biomech. 2007, 40 (Suppl. 1), 96–104. [Google Scholar] [CrossRef] [PubMed]
- Naughton, G.K.; Mansbridge, J.N.; Horwitz, D.L.; Zeltinger, J.; Cerny, D.J. Monitorable Three-Dimensional Scaffolds And Tissue Culture Systems. International Application No. PCT/US2000/017542, 4 January 2001. [Google Scholar]
- Singh, P.; Shrivastava, A. Optical Biosensor Based on Microbendings Technique: An Optimized Mean to Measure the Bone Strength. Adv. Opt. Technol. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Singh, P. SPR biosensors: Historical perspectives and current challenges. Sens. Actuators B Chem. 2016, 229, 110–130. [Google Scholar] [CrossRef]
- Tombelli, S. Piezoelectric biosensors for medical applications. In Biosensors for Medical Applications; Higson, S., Ed.; Woodhead Publishing Series in Biomaterials: Cambridge, UK, 2012; pp. 41–64. [Google Scholar]
- Pohanka, M. The piezoelectric biosensors: Principles and applications, a review. Int. J. Electrochem. Sci. 2017, 12, 496–506. [Google Scholar] [CrossRef]
- Dey, D.; Goswami, T. Optical biosensors: A revolution towards quantum nanoscale electronics device fabrication. J. Biomed. Biotech. 2011, 2011, 1–7. [Google Scholar] [CrossRef]
- Bunde, R.L.; Jarvi, E.J.; Rosentreter, J.J. Piezoelectric quartz crystal biosensors. Talanta 1998, 46, 1223–1236. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Luo, J.K.; Nguyen, N.T.; Walton, A.J.; Flewitt, A.J.; Zu, X.T.; Li, Y.; McHale, G.; Matthews, A.; Iborra, E. Advances in piezoelectric thin films for acoustic biosensors, acoustofluidics and lab-on-chip applications. Prog. Mater. Sci. 2017, 89, 31–91. [Google Scholar] [CrossRef] [Green Version]
- Sroga, G.E.; Vashishth, D. Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr. Osteoporos. Rep. 2012, 10, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Nurunnabi, M.; Morshed, M.; Paul, A.; Polini, A.; Kuila, T.; Al Hariri, M.; Lee, Y.K.; Jaffa, A.A. Recent advances in application of biosensors in tissue engineering. Biomed Res. Int. 2014, 2014, 307519. [Google Scholar] [CrossRef] [Green Version]
- Sabr, A.K. Biosensors. Am. J. Biomed. Eng. 2016, 6, 170–179. [Google Scholar]
- Sirivisoot, S.; Yao, C.; Xiao, X.; Sheldon, B.W.; Webster, T.J. Developing Biosensors for Monitoring Orthopedic Tissue Growth. MRS Online Proc. Libr. Arch. 2006, 950. [Google Scholar] [CrossRef] [Green Version]
- Qi, M.; Zhang, Y.; Cao, C.M.; Zhang, M.X.; Liu, S.H.; Liu, G.Z. Decoration of RGO nanosheets with aryldiazonium salt and gold nanoparticles towards a label-free amperometric immunosensor for detecting cytokine TNF-α in live cells. Anal. Chem. 2016, 88, 9614–9621. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Katyal, V.; Malik, V.; Asatkar, A.; Inwati, G.; Mukherjee, T.K. Nanobiosensors: Concepts and Variations. ISRN Nanomater. 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Essays Biochem. 2016, 60, 69–80. [Google Scholar] [PubMed] [Green Version]
- Batool, R.; Rhouati, A.; Nawaz, M.H.; Hayat, A.; Marty, J.L. A review of the construction of nano-hybrids for electrochemical biosensing of glucose. Biosensors 2019, 9, 46. [Google Scholar] [CrossRef] [Green Version]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Ramanathan, M.; Patil, M.; Epur, R.; Yun, Y.; Shanov, V.; Schulz, M.; Heineman, W.R.; Datta, M.K.; Kumta, P.N. Gold-coated carbon nanotube electrode arrays: Immunosensors for impedimetric detection of bone biomarkers. Biosens. Bioelectron. 2016, 77, 580–588. [Google Scholar] [CrossRef]
- Yun, Y.H.; Bhattacharya, A.; Watts, N.B.; Schulz, M.J. A label-free electronic biosensor for detection of bone turnover markers. Sensors 2009, 9, 7957–7969. [Google Scholar] [CrossRef] [Green Version]
- Afsarimanesh, N.; Zia, A.I.; Mukhopadhyay, S.C.; Kruger, M.; Yu, P.L.; Kosel, J.; Kovacs, Z. Smart sensing system for the prognostic monitoring of bone health. Sensors 2016, 16, 976. [Google Scholar] [CrossRef] [Green Version]
- Afsarimanesh, N.; Mukhopadhyay, S.C.; Kruger, M. Molecularly imprinted polymer-based electrochemical biosensor for bone loss detection. IEEE Trans. Biomed. Eng. 2018, 65, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Kabala, S.I.; Yagar, H.; Ozcan, H.M. A new biosensor for osteoporosis detection. Prep. Biochem. Biotechnol. 2019, 49, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Sappia, L.; Felice, B.; Sanchez, M.A.; Martí, M.; Madrid, R.; Pividori, I. Electrochemical sensor for alkaline phosphatase as biomarker for clinical and in vitro applications. Sens. Actuators B Chem. 2019, 281, 221–228. [Google Scholar] [CrossRef]
- Chandra, P.E.; Sokolove, J.; Hipp, B.G.; Lindstrom, T.M.; Elder, J.T.; Reveille, J.D.; Eberl, H.; Klause, U.; Robinson, W.H. Novel multiplex technology for diagnostic characterization of rheumatoid arthritis. Arthritis Res. Ther. 2011, 13, R102. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Kim, K.B.; Kim, Y.I. Love wave SAW biosensors for detection of antigen-antibody binding and comparison with SPR biosensor. Food Sci. Biotechnol. 2011, 20, 1413–1418. [Google Scholar] [CrossRef]
- Shrivastava, S.; Prakash, R. Assessment of bone condition by acoustic emission technique: A review. J. Biomed. Sci. Eng. 2009, 2, 144–154. [Google Scholar] [CrossRef]
- Lentle, B.C.; Aldrich, J.E.; Akhtar, A. Diagnosis of osteoporosis using acoustic emissions. U.S. Patent 6,024,711; issued, 15 February 2000. [Google Scholar]
- Drafts, B. Acoustic wave technology sensors. IEEE Trans. Microw. Theory Tech. 2001, 49, 795–802. [Google Scholar] [CrossRef]
- Nirschl, M.; Rantala, A.; Tukkiniemi, K.; Auer, S.; Hellgren, A.C.; Pitzer, D.; Schreiter, M.; Vikholm-Lundin, I. CMOS-integrated film bulk acoustic resonators for label-free biosensing. Sensors 2010, 10, 4180–4193. [Google Scholar] [CrossRef]
- García-González, D.L.; Aparicio, R. Sensors: From biosensors to the electronic nose. Grasas y Aceites 2002, 53, 96–114. [Google Scholar] [CrossRef]
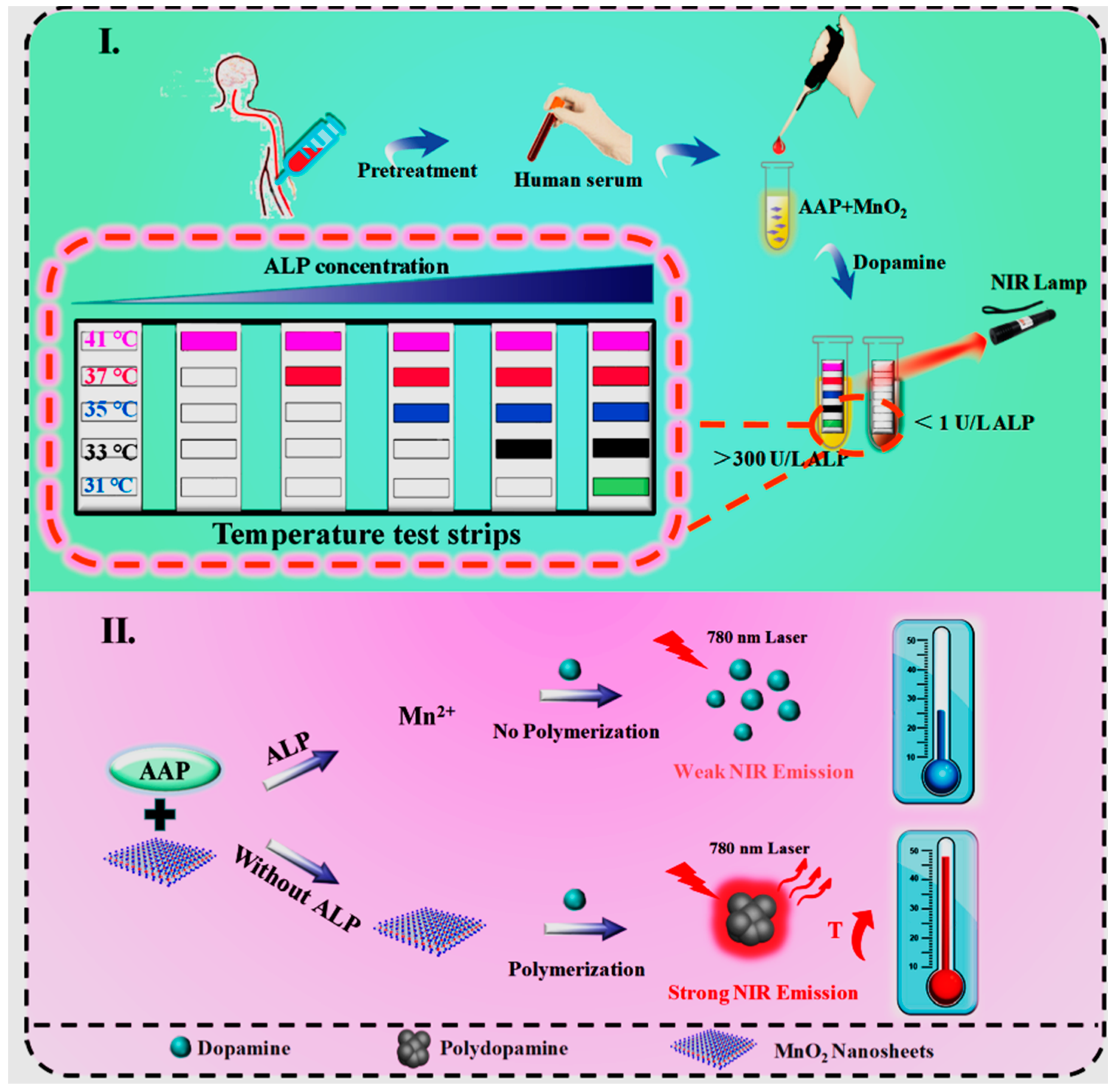
- Liu, X.; Zou, L.; Yang, X.; Wang, Q.; Zheng, Y.; Geng, X.; Liao, G.; Nie, W.; Wang, K. Point-of-Care Assay of Alkaline Phosphatase Enzymatic Activity Using a Thermometer or Temperature Discoloration Sticker as Readout. Anal. Chem. 2019, 91, 7943–7949. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, S.; Bandyopadhyay-Ghosh, S.; Ghosh, S.B.; Liu, G. Advances in Sensing Technologies for Monitoring of Bone Health. Biosensors 2020, 10, 42. https://doi.org/10.3390/bios10040042
Rani S, Bandyopadhyay-Ghosh S, Ghosh SB, Liu G. Advances in Sensing Technologies for Monitoring of Bone Health. Biosensors. 2020; 10(4):42. https://doi.org/10.3390/bios10040042
Chicago/Turabian StyleRani, Seema, Sanchita Bandyopadhyay-Ghosh, Subrata Bandhu Ghosh, and Guozhen Liu. 2020. "Advances in Sensing Technologies for Monitoring of Bone Health" Biosensors 10, no. 4: 42. https://doi.org/10.3390/bios10040042
APA StyleRani, S., Bandyopadhyay-Ghosh, S., Ghosh, S. B., & Liu, G. (2020). Advances in Sensing Technologies for Monitoring of Bone Health. Biosensors, 10(4), 42. https://doi.org/10.3390/bios10040042





