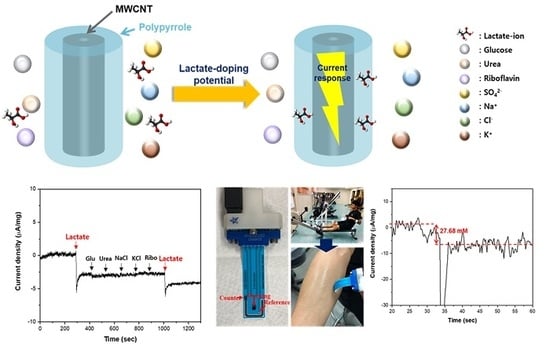
Selective Nonenzymatic Amperometric Detection of Lactic Acid in Human Sweat Utilizing a Multi-Walled Carbon Nanotube (MWCNT)-Polypyrrole Core-Shell Nanowire
Abstract
:1. Introduction
2. Materials and Methods
2.1. Surface Modificaiton of Multi-Wall Carbon Nanotube (MWCNT)
2.2. Synthesis of MWCNT-Polypyrrole Core-Shell Nanowires
2.3. Characterization and Electrochemical Measurements
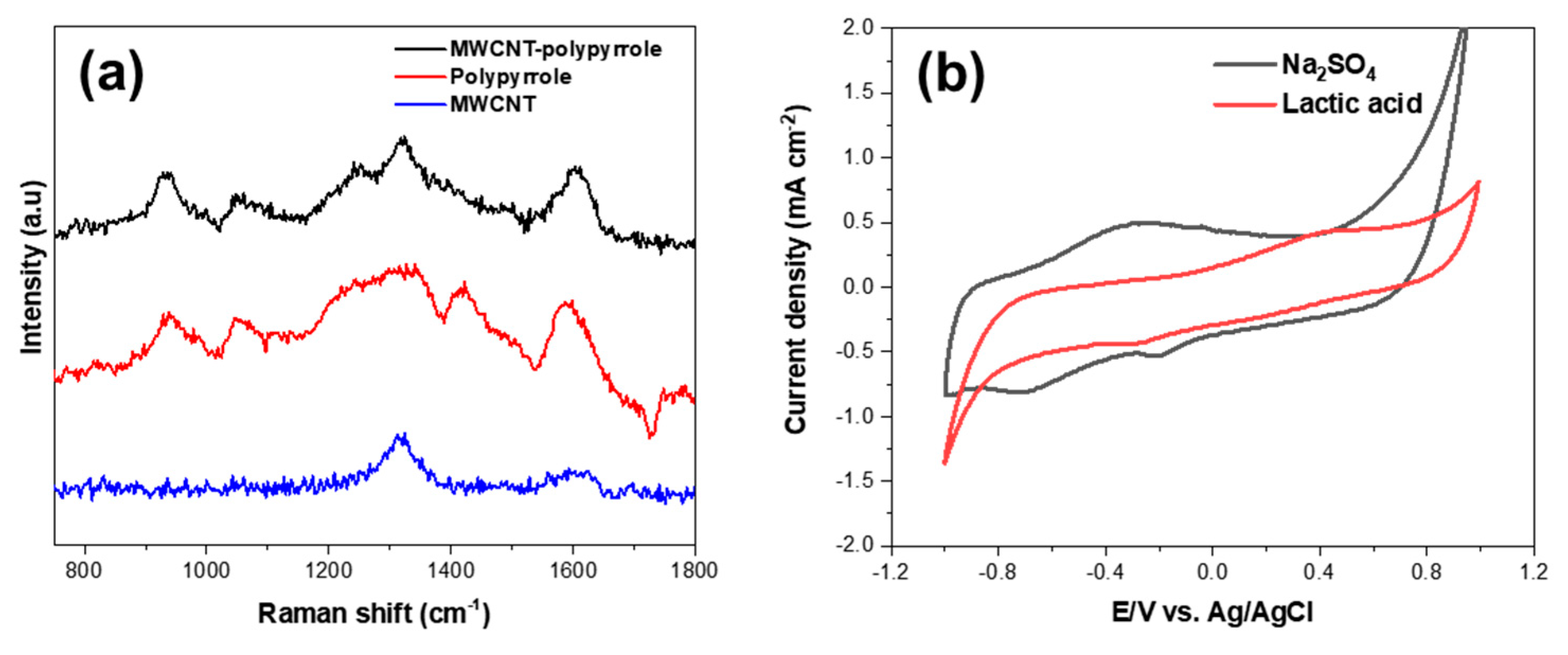
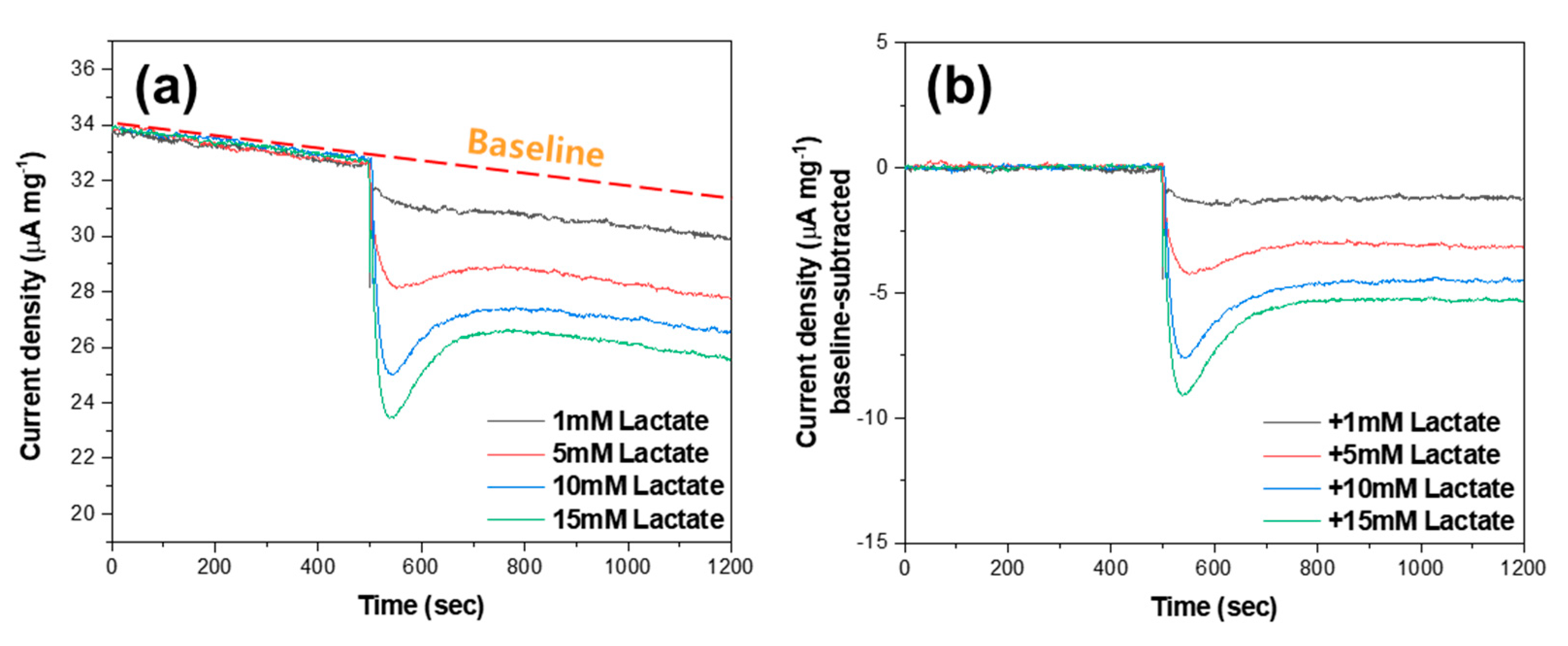
3. Results and Discussion


4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pundir, C.; Narwal, V.; Batra, B. Determination of lactic acid with special emphasis on biosensing methods: A review. Biosens. Bioelectron. 2016, 86, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Labib, M.; Sargent, E.H.; Kelley, S.O. Electrochemical methods for the analysis of clinically relevant biomolecules. Chem. Rev. 2016, 116, 9001–9090. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.R.; Ahumada, F.; Garay, F.; Baruzzi, A.M. Amperometric biosensor for direct blood lactate detection. Anal. Chem. 2010, 82, 5568–5572. [Google Scholar] [CrossRef]
- Jia, W.; Bandodkar, A.J.; Valdés-Ramírez, G.; Windmiller, J.R.; Yang, Z.; Ramirez, J.; Chan, G.; Wang, J. Electrochemical tattoo biosensors for real time noninvasive lactate monitoring. Anal. Chem. 2013, 85, 6553–6560. [Google Scholar] [CrossRef] [PubMed]
- Pribil, M.M.; Laptev, G.U.; Karyakina, E.E.; Karyakin, A.A. Noninvasive hypoxia monitor based on gene-free engineering of lactate oxidase for analysis of undiluted sweat. Anal. Chem. 2014, 86, 5215–5219. [Google Scholar] [CrossRef]
- Zaryanov, N.V.; Nikitina, V.N.; Karpova, E.V.; Karyakina, E.E.; Karyakin, A.A. Nonenzymatic sensor for lactate detection in human sweat. Anal. Chem. 2017, 89, 11198–11202. [Google Scholar] [CrossRef]
- Nesakumar, N.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Fabrication of lactate biosensor based on lactate dehydrogenase immobilized on cerium oxide nanoparticles. J. Coll. Interface Sci. 2013, 410, 158–164. [Google Scholar] [CrossRef]
- Pereira, A.C.; Aguiar, M.R.; Kisner, A.; Macedo, D.; Kubota, L.T. Amperometric biosensor for lactate based on lactate dehydrogenase and Meldola Blue coimmobilized on multi-wall carbon-nanotube. Sens. Actuators B Chem. 2007, 124, 269–276. [Google Scholar] [CrossRef]
- Mu, Y.; Jia, D.; He, Y.; Miao, Y.; Wu, H.L. Nano nickel oxide modified non-enzymatic glucose sensors with enhanced sensitivity through an electrochemical process strategy at high potential. Biosens. Bioelectron. 2011, 26, 2948–2952. [Google Scholar] [CrossRef]
- Mengarda, P.; Dias, F.A.L.; Peixoto, J.V.; Osiecki, R.; Bergamini, M.F.; Marcolino-Junior, L.H. Determination of lactate levels in biological fluids using a disposable ion-selective potentiometric sensor based on polypyrrole films. Sens. Actuators B Chem. 2019, 296, 126663. [Google Scholar] [CrossRef]
- Bobacka, J.; Gao, Z.; Ivaska, A.; Lewenstam, A. Mechanism of ionic and redox sensitivity of p-type conducting polymers. J. Electroanal. Chem. 1994, 368, 33–41. [Google Scholar] [CrossRef]
- Yang, Y.; Asiri, A.M.; Du, D.; Lin, Y. Acetylcholinesterase biosensor based on a gold nanoparticle–polypyrrole–reduced graphene oxide nanocomposite modified electrode for the amperometric detection of organophosphorus pesticides. Analyst 2014, 139, 3055–3060. [Google Scholar] [CrossRef]
- Paul, S.; Lee, Y.S.; Choi, J.A.; Kang, Y.C.; Kim, D.W. Synthesis and electrochemical characterization of polypyrrole/multi-walled carbon nanotube composite electrodes for supercapacitor applications. Bull. Korean Chem. Soc. 2010, 31, 1228–1232. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Cai, K.; Wang, J.; Shen, S. Influence of polymerization method on the thermoelectric properties of multi-walled carbon nanotubes/polypyrrole composites. Synth. Met. 2016, 211, 58–65. [Google Scholar] [CrossRef]
- Lim, H.; Jung, J.H.; Park, Y.M.; Lee, H.N.; Kim, H.J. High-performance aqueous rechargeable sulfate- and sodium-ion battery based on polypyrrole-MWCNT core-shell nanowires and Na0.44MnO2 nanorods. Appl. Surf. Sci. 2018, 446, 131–138. [Google Scholar] [CrossRef]
- Amade, R.; Joyer, E.; Caglar, B.; Mutlu, T.; Bertran, E. Optimization of MnO2/vertically aligned carbon nanotube composite for supercapacitor application. J. Power Sour. 2011, 196, 5779–5783. [Google Scholar] [CrossRef]
- Gao, C.; Jin, Y.; Kong, H.; Whitby, R.; Acquah, S.F.A.; Chen, G.Y.; Qian, H.; Hartschuh, A.; Silva, S.R.P.; Henley, S.; et al. Polyurea-functionalized multiwalled carbon nanotubes: Synthesis, morphology, and raman spectroscopy. J. Phys. Chem. B 2005, 109, 11925–11932. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Zhang, L.; Xing, R.; Huang, H.; Qu, Y.; Jiao, T.; Zhou, J.; Peng, Q. Facile preparation and electrochemical characterization of self-assembled core-shell diamond-polypyrrole nanocomposites. Coll. Surf. A Physicochem. Eng. Asp. 2018, 555, 787–794. [Google Scholar] [CrossRef]
- Benhaddad, L.; Bernard, M.; Deslouis, C.; Makhloufi, L.; Messaoudi, B.; Pailleret, A.; Takenouti, H. Chemical synthesis of hollow sea urchin like nanostructured polypyrrole particles through a core–Shell redox mechanism using a MnO2 powder as oxidizing agent and sacrificial nanostructured template. Synth. Met. 2013, 175, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Šetka, M.; Calavia, R.; Vojkůvka, L.; Llobet, E.; Drbohlavová, J.; Vallejos, S. Raman and XPS studies of ammonia sensitive polypyrrole nanorods and nanoparticles. Sci. Rep. 2019, 9, 8465. [Google Scholar] [CrossRef]
- Biswas, S.; Drzal, L.T. Multilayered nanoarchitecture of graphene nanosheets and polypyrrole nanowires for high performance supercapacitor electrodes. Chem. Mater. 2010, 22, 5667–5671. [Google Scholar] [CrossRef]
- Ingram, M.D.; Staesche, H.; Ryder, K.S. Activated polypyrrole electrodes for high-power supercapacitor applications. Solid State Ion. 2004, 169, 51–57. [Google Scholar] [CrossRef]
- Harvey, C.J.; LeBouf, R.F.; Stefaniak, A.B. Formulation and stability of a novel artificial human sweat under conditions of storage and use. Toxicol. Vitr. 2010, 24, 1790–1796. [Google Scholar] [CrossRef]
- Narwal, V.; Sharma, M.; Rani, S.; Pundir, C. An ultrasensitive amperometric determination of lactate by lactate dehydrogenase nanoparticles immobilized onto Au electrode. Int. J. Biol. Macromol. 2018, 115, 767–775. [Google Scholar] [CrossRef]
- Manna, B.; Raj, C.R. Covalent functionalization and electrochemical tuning of reduced graphene oxide for the bioelectrocatalytic sensing of serum lactate. J. Mater. Chem. B 2016, 4, 4585–4593. [Google Scholar] [CrossRef]
- Lamas-Ardisana, P.J.; Loaiza, Ó.A.; Añorga, L.; Jubete, E.; Borghei, M.; Ruiz, V.; Ochoteco, E.; Cabanero, G.; Grande, H.J. Disposable amperometric biosensor based on lactate oxidase immobilised on platinum nanoparticle-decorated carbon nanofiber and poly(diallyldimethylammonium chloride) films. Biosens. Bioelectron. 2014, 56, 345–351. [Google Scholar] [CrossRef]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- Shkotova, L.V.; Piechniakova, N.Y.; Kukla, O.L.; Dzyadevych, S. Thin-film amperometric multibiosensor for simultaneous determination of lactate and glucose in wine. Food Chem. 2016, 197, 972–978. [Google Scholar] [CrossRef]
- Bravo, I.; Revenga-Parra, M.; Weber, K.; Popp, J.; Pariente, F.; Lorenzo, E. One-step reduced/quinone functionalized graphene oxide as reagentless lactate biosensing platform. Sens. Actuators B Chem. 2018, 267, 533–541. [Google Scholar] [CrossRef]
- Parra-Alfambra, A.M.; Casero, E.; Vázquez, L.; Quintana, C.; Del Pozo, M.; Petit-Domínguez, M.D. MoS2 nanosheets for improving analytical performance of lactate biosensors. Sens. Actuators B Chem. 2018, 274, 310–317. [Google Scholar] [CrossRef]
- Kim, S.; Kim, K.; Kim, H.J.; Lee, H.N.; Park, T.J.; Park, Y.M. Non-enzymatic electrochemical lactate sensing by NiO and Ni(OH)2 electrodes: A mechanistic investigation. Electrochim. Acta 2018, 276, 240–246. [Google Scholar] [CrossRef]




| Electrode | Sensitivity (μA/mM) | Limit of Detection (LOD, μM) | Applied Potential (V vs. Ag/AgCl) | Reference |
|---|---|---|---|---|
| LDH NPs-Au | 3.45 | 0.01 | 0.10 | [24] |
| LDH-PhNHOH/rGO | 10.57 | 2.50 | 0.04 | [25] |
| LOx-Pt NPs/CNF/PDDA | 36 | 11.1 | 0.50 | [26] |
| LOx-CS/MWCNT | 3.417 | 22.6 | 0.20 | [27] |
| LOx-BSA/GA/Au | 37.1 | 5.0 | 0.75 | [28] |
| LOx-rGO/DHS | 0.0735 | 2.9 | 0.10 | [29] |
| LOx-MoS2 | 6.22 | 17.0 | 0.30 | [30] |
| 3-aminophenylboronic acid (3-APBA) | - | 1500 | - | [6] |
| NiO | 9.08/cm2 | 53 | 0.45 | [31] |
| Polypyrrole/MWCNT | 2.9 | 51 | 0.68 | This study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.M.; Lim, H.; Lee, H.-N.; Park, Y.M.; Park, J.-S.; Kim, H.-J. Selective Nonenzymatic Amperometric Detection of Lactic Acid in Human Sweat Utilizing a Multi-Walled Carbon Nanotube (MWCNT)-Polypyrrole Core-Shell Nanowire. Biosensors 2020, 10, 111. https://doi.org/10.3390/bios10090111
Choi YM, Lim H, Lee H-N, Park YM, Park J-S, Kim H-J. Selective Nonenzymatic Amperometric Detection of Lactic Acid in Human Sweat Utilizing a Multi-Walled Carbon Nanotube (MWCNT)-Polypyrrole Core-Shell Nanowire. Biosensors. 2020; 10(9):111. https://doi.org/10.3390/bios10090111
Chicago/Turabian StyleChoi, Young Min, Hana Lim, Ho-Nyun Lee, Young Min Park, Jin-Seong Park, and Hyun-Jong Kim. 2020. "Selective Nonenzymatic Amperometric Detection of Lactic Acid in Human Sweat Utilizing a Multi-Walled Carbon Nanotube (MWCNT)-Polypyrrole Core-Shell Nanowire" Biosensors 10, no. 9: 111. https://doi.org/10.3390/bios10090111
APA StyleChoi, Y. M., Lim, H., Lee, H.-N., Park, Y. M., Park, J.-S., & Kim, H.-J. (2020). Selective Nonenzymatic Amperometric Detection of Lactic Acid in Human Sweat Utilizing a Multi-Walled Carbon Nanotube (MWCNT)-Polypyrrole Core-Shell Nanowire. Biosensors, 10(9), 111. https://doi.org/10.3390/bios10090111