Enhancement of the Peroxidase-Like Activity of Iodine-Capped Gold Nanoparticles for the Colorimetric Detection of Biothiols
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Determination of Cysteine in an Aqueous Solution
2.3. Detection of Cysteine in Real Samples
3. Results and Discussion
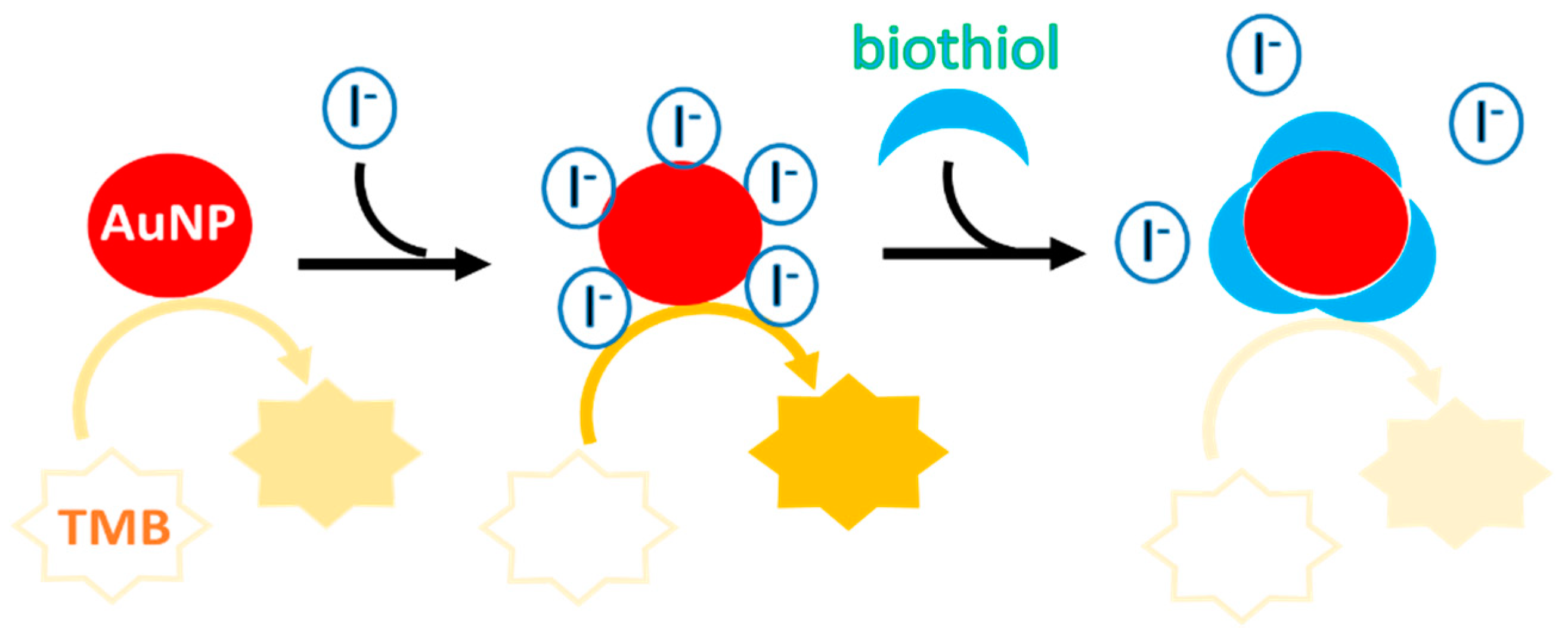
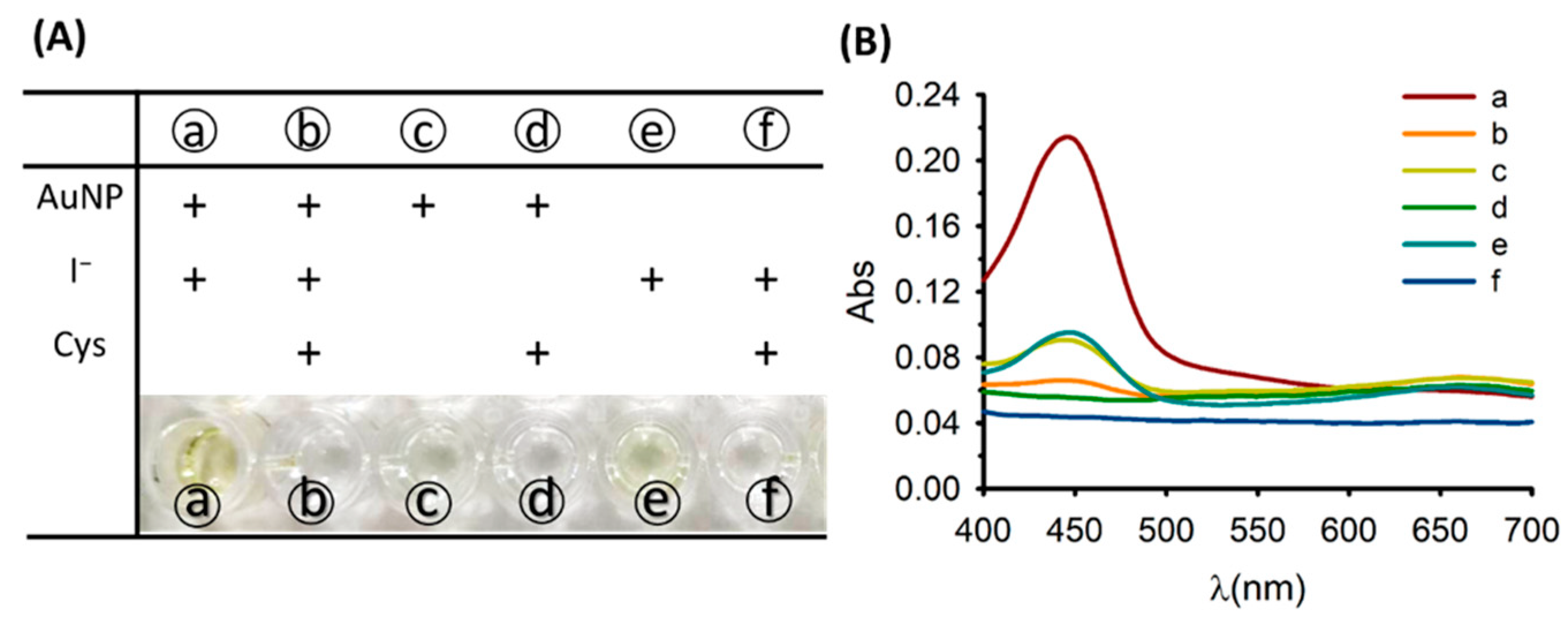
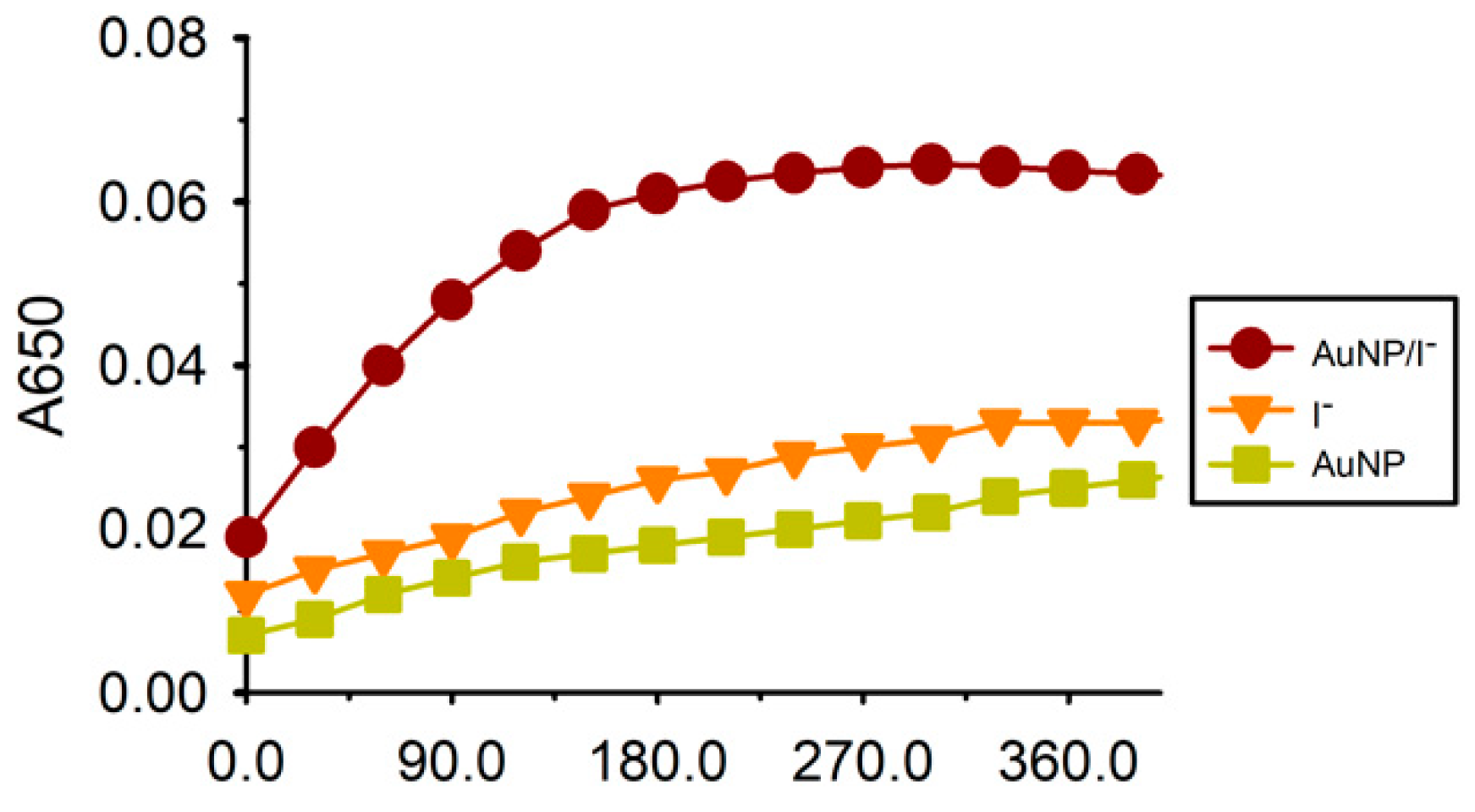
3.1. Principle of the Colorimetric Cysteine Assay
3.2. Optimization of Experimental Conditions
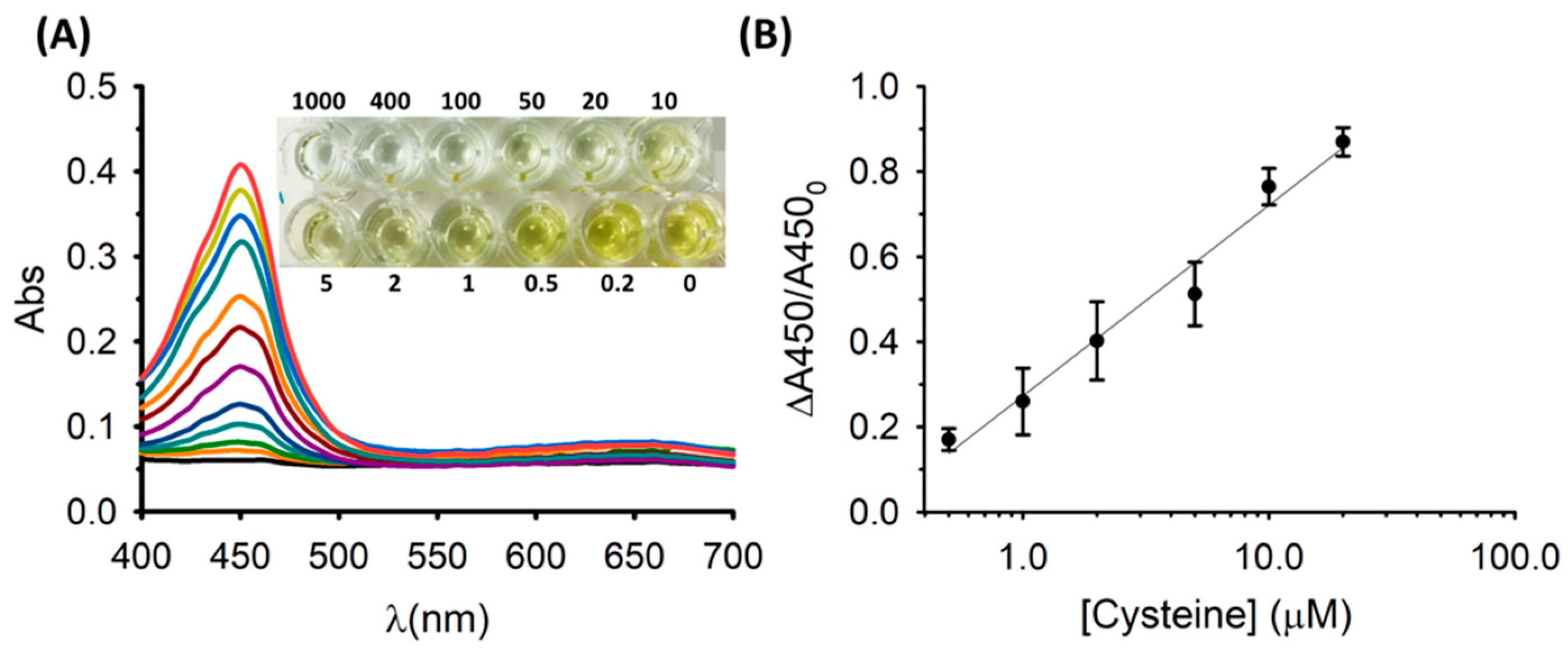
3.3. Colorimetric Sensing of Cysteine
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, N.R.; Rathod, V.K. Enzyme catalyzed synthesis of cosmetic esters and its intensification: A review. Process Biochem. 2015, 50, 1793–1806. [Google Scholar] [CrossRef]
- Mat Yusoff, M.; Gordon, M.H.; Niranjan, K. Aqueous enzyme assisted oil extraction from oilseeds and emulsion de-emulsifying methods: A review. Trends Food Sci. Technol. 2015, 41, 60–82. [Google Scholar] [CrossRef]
- Bucur, B.; Munteanu, F.D.; Marty, J.L.; Vasilescu, A. Advances in Enzyme-Based Biosensors for Pesticide Detection. Biosensors 2018, 8, 27. [Google Scholar] [CrossRef] [Green Version]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Cormode, D.P.; Gao, L.; Koo, H. Emerging Biomedical Applications of Enzyme-Like Catalytic Nanomaterials. Trends Biotechnol. 2018, 36, 15–29. [Google Scholar] [CrossRef]
- Wu, L.; Wan, G.; Hu, N.; He, Z.; Shi, S.; Suo, Y.; Wang, K.; Xu, X.; Tang, Y.; Wang, G. Synthesis of Porous CoFe2O4 and Its Application as a Peroxidase Mimetic for Colorimetric Detection of H2O2 and Organic Pollutant Degradation. Nanomaterials 2018, 8, 451. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Gao, X.J.; Zhu, Y.; Muhammad, F.; Tan, S.; Cao, W.; Lin, S.; Jin, Z.; Gao, X.; Wei, H. Nitrogen-Doped Carbon Nanomaterials as Highly Active and Specific Peroxidase Mimics. Chem. Mater. 2018, 30, 6431–6439. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, R.; Dong, X.; Zhu, S.; Zhou, R.; Yan, L.; Zhang, C.; Wang, Q.; Gu, Z.; Zhao, Y. BiO2–x Nanosheets as Radiosensitizers with Catalase-Like Activity for Hypoxia Alleviation and Enhancement of the Radiotherapy of Tumors. Inorg. Chem. 2020, 59, 3482–3493. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Zhu, J.; Zheng, Y.-Q. Co3O4 nanocrystals as an efficient catalase mimic for the colorimetric detection of glutathione. J. Mater. Chem. B 2018, 6, 6858–6864. [Google Scholar] [CrossRef]
- Zhu, S.; Lei, C.; Sun, J.; Zhao, X.E.; Wang, X.; Yan, X.; Liu, W.; Wang, H. Probing NAD+/NADH-dependent biocatalytic transformations based on oxidase mimics of MnO2. Sens. Actuators B Chem. 2019, 282, 896–903. [Google Scholar] [CrossRef]
- Chi, M.; Zhu, Y.; Jing, L.; Wang, C.; Lu, X. Fabrication of oxidase-like polyaniline-MnO2 hybrid nanowires and their sensitive colorimetric detection of sulfite and ascorbic acid. Talanta 2019, 191, 171–179. [Google Scholar] [CrossRef]
- Liang, H.; Lin, F.F.; Zhang, Z.J.; Liu, B.W.; Jiang, S.H.; Yuan, Q.P.; Liu, J. Multicopper laccase mimicking nanozymes with nucleotides as ligands. ACS Appl. Mater. Interfaces 2017, 9, 1352–1360. [Google Scholar] [CrossRef]
- Jiang, B.; Fang, L.; Wu, K.; Yan, X.; Fan, K. Ferritins as natural and artificial nanozymes for theranostics. Theranostics 2020, 10, 687–706. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, X.; Croley, T.R.; Boudreau, M.D.; He, W.; Cai, J.; Li, P.; Yin, J.J. Ferroxidase-like and antibacterial activity of PtCu alloy nanoparticles. J. Environ. Sci. Health Part C 2019, 37, 99–115. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Zhang, H.; Wu, W.; Zhang, Z.; Dong, S. Porous Co3O4 nanoplates with pH-switchable peroxidase- and catalase-like activity. Nanoscale 2018, 10, 19140–19146. [Google Scholar] [CrossRef]
- Yuan, F.; Zhao, H.M.; Zang, H.M.; Ye, F.; Quan, X. Three-dimensional graphene supported bimetallic nanocomposites with DNA regulated-flexibly switchable peroxidase-like activity. ACS Appl. Mater. Interfaces 2016, 8, 9855–9864. [Google Scholar] [CrossRef]
- McVey, C.; Logan, N.; Thanh, N.T.; Elliott, C.; Cao, C. Unusual switchable peroxidase-mimicking nanozyme for the determination of proteolytic biomarker. Nano Res. 2019, 12, 509–516. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhang, X.; Liu, B.; Liu, J. Molecular imprinting on inorganic nanozymes for hundred-fold enzyme specificity. J. Am. Chem. Soc. 2017, 139, 5412–5419. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Mathur, A.; Wang, Y.; Maheshwari, V.; Su, H.; Liu, J. Fluoride-capped nanoceria as a highly efficient oxidase-mimicking nanozyme: Inhibiting product adsorption and increasing oxygen vacancies. Nanoscale 2019, 3, 17841–17850. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, M.; Liu, J. Highly selective fluorescent sensing of phosphite through recovery of poisoned nickel oxide nanozyme. Anal. Chem. 2020, 92, 3118–3124. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Wu, T.H.; Yang, C.H.; Lin, C.W.; Chen, C.Y. Gold Nanoparticle-Based Colorimetric Strategies for Chemical and Biological Sensing Applications. Nanomaterials 2019, 9, 861. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Wang, G.; Takarada, T.; Maeda, M. Target-Recycling-Amplified Colorimetric Detection of Pollen Allergen Using Non-Cross-Linking Aggregation of DNA-Modified Gold Nanoparticles. ACS Sens. 2019, 4, 363–369. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Chen, C.Y.; Lin, C.W. DNA base-stacking assay utilizing catalytic hairpin assembly-induced gold nanoparticle aggregation for colorimetric protein sensing. Chem. Commun. 2016, 52, 4167–4170. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Chen, C.Y.; Chuang, T.L.; Wu, T.H.; Wei, S.C.; Liao, H.; Lin, C.W. Aptamer-based colorimetric detection of proteins using a branched DNA cascade amplification strategy and unmodified gold nanoparticles. Biosens. Bioelectron. 2016, 78, 200–205. [Google Scholar] [CrossRef]
- Mulder, D.W.; Phiri, M.M.; Vorster, B.C. Gold Nanostar Colorimetric Detection of Fructosyl Valine as a Potential Future Point of Care Biosensor Candidate for Glycated Haemoglobin Detection. Biosensors 2019, 9, 100. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.C.; Lee, C.H.; Wu, T.H.; Chen, C.P.; Chen, C.Y.; Lin, C.W. Reversion of gold nanoparticle aggregates for the detection of Cu2+ and its application in immunoassays. Analyst 2017, 142, 4684–4690. [Google Scholar] [CrossRef]
- Shayesteh, O.H.; Khosroshahi, A.G. A polyA aptamer-based label-free colorimetric biosensor for the detection of kanamycin in human serum. Anal. Methods 2020, 12, 1858–1867. [Google Scholar] [CrossRef]
- Cai, J.; Ding, L.; Gong, P.; Huang, J. A colorimetric detection of microRNA-148a in gastric cancer by gold nanoparticle–RNA conjugates. Nanotechnology 2019, 31, 095501. [Google Scholar] [CrossRef]
- Liu, Q.; Li, L.; Zhao, Y.; Chen, Z. Colorimetric detection of DNA at the nanomolar level based on enzyme-induced gold nanoparticle de-aggregation. Microchim. Acta 2018, 185, 301. [Google Scholar] [CrossRef]
- Wu, T.H.; Chang, C.C.; Valliant, J.; Bruyant, A.; Lin, C.W. DNA biosensor combining single-wavelength colorimetry and digital lock-in amplifier within a smartphone. Lab Chip 2016, 16, 4527–4533. [Google Scholar] [CrossRef] [PubMed]
- Coleman, B.; Coarsey, C.; Asghar, W. Cell phone based colorimetric analysis for point-of-care settings. Analyst 2019, 144, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.H.; Luo, B.Y.; He, S.B.; Chen, R.T.; Lin, Z.; Peng, H.P.; Xia, X.H.; Chen, W. Redox Recycling-Triggered Peroxidase-Like Activity Enhancement of Bare Gold Nanoparticles for Ultrasensitive Colorimetric Detection of Rare-Earth Ce3+ Ion. Anal. Chem. 2019, 91, 4039–4046. [Google Scholar] [CrossRef]
- Zhao, H.; Qu, Y.; Yuan, F.; Quan, X. A visible and label-free colorimetric sensor for miRNA-21 detection based on peroxidase-like activity of graphene/gold-nanoparticle hybrids. Anal. Methods 2016, 8, 2005–2012. [Google Scholar] [CrossRef]
- Jiang, C.; Li, Z.; Wu, Y.; Guo, W.; Wang, J.; Jiang, Q. Colorimetric Detection of Hg2+ Based on Enhancement of Peroxidase-like Activity of Chitosan-Gold Nanoparticles. Bull. Korean Chem. Soc. 2018, 39, 625–630. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, Y.; Ding, D.; Guo, R. Structural effects of amphiphilic protein/gold nanoparticle hybrid based nanozyme on peroxidase-like activity and silver-mediated inhibition. RSC Adv. 2016, 6, 112435–112444. [Google Scholar] [CrossRef]
- Hu, J.; Ni, P.; Dai, H.; Sun, Y.; Wang, Y.; Jiang, S.; Li, Z. Aptamer-based colorimetric biosensing of abrin using catalytic gold nanoparticles. Analyst 2015, 140, 3581–3586. [Google Scholar] [CrossRef]
- Chang, C.C.; Chen, C.P.; Lee, C.H.; Chen, C.Y.; Lin, C.W. Colorimetric detection of human chorionic gonadotropin using catalytic gold nanoparticles and a peptide aptamer. Chem. Commun. 2014, 50, 14443–14446. [Google Scholar] [CrossRef] [Green Version]
- Long, Y.J.; Li, Y.F.; Liu, Y.; Zheng, J.J.; Tang, J.; Huang, C.Z. Visual observation of the mercury-stimulated peroxidase mimetic activity of gold nanoparticles. Chem. Commun. 2011, 47, 11939–11941. [Google Scholar] [CrossRef]
- Zhan, L.; Li, C.M.; Wu, W.B.; Huang, C.Z. A colorimetric immunoassay for respiratory syncytial virus detection based on gold nanoparticles–graphene oxide hybrids with mercury-enhanced peroxidase-like activity. Chem. Commun. 2014, 50, 11526–11528. [Google Scholar] [CrossRef]
- Shah, J.; Purohit, R.; Singh, R.; Karakoti, A.S.; Singh, S. ATP-enhanced peroxidase-like activity of gold nanoparticles. J. Colloid Interface Sci. 2015, 456, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Wang, G.; Takarada, T.; Maeda, M. Iodine-Mediated Etching of Triangular Gold Nanoplates for Colorimetric Sensing of Copper Ion and Aptasensing of Chloramphenicol. ACS Appl. Mater. Interfaces 2017, 9, 34518–34525. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, W.; Wang, Q.; Ma, J.; Su, X.; Jiang, Q.; Sun, X. Iodine-Labeled Au Nanorods with High Radiochemical Stability for Imaging-Guided Radiotherapy and Photothermal Therapy. ACS Appl. Nano Mater. 2019, 2, 1374–1381. [Google Scholar] [CrossRef]
- Komoto, Y.; Fujii, S.; Hara, K.; Kiguchi, M. Single Molecular Bridging of Au Nanogap Using Aryl Halide Molecules. J. Phys. Chem. C 2013, 117, 24277–24282. [Google Scholar] [CrossRef]
- Gündüz, T.; Kılıç, E.; Köseoğlu, F.; Öztaş, S. Titrations in non-aqueous media. Part XIII. Potentiometric and conductimetric titrations of α-amino acids with perchloric acid in acetic acid and acetonitrile-acetic acid solvents. Analyst 1988, 113, 1313–1316. [Google Scholar] [CrossRef]
- Liu, M.; Yan, W.; Lin, J.M.; Hashi, Y.; Liu, L.B.; Wei, Y. On-line liquid chromatography–mass spectrometry with dilution line to achieve large volume urine injection for the improvement of sensitivity. J. Chromatogr. A 2008, 1198, 87–94. [Google Scholar] [CrossRef]
- Chen, Y.M.; Cheng, T.L.; Tseng, W.L. Fluorescence turn-on detection of iodide, iodate and total iodine using fluorescein-5-isothiocyanate-modified gold nanoparticles. Analyst 2009, 134, 2106–2112. [Google Scholar] [CrossRef]
- Wei, S.C.; Hsu, P.H.; Lee, Y.F.; Lin, Y.W.; Huang, C.C. Selective Detection of Iodide and Cyanide Anions Using Gold-Nanoparticle-Based Fluorescent Probes. ACS Appl. Mater. Interfaces 2012, 4, 2652–2658. [Google Scholar] [CrossRef]
- Ji, L.; Wang, J.; Zhu, L.; Zu, Y.; Kong, J.; Chen, Z. Differentiation of biothiols from other sulfur-containing biomolecules using iodide-capped gold nanoparticles. RSC Adv. 2016, 6, 25101–25109. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, Y.; Zhen, Y.; Guo, R. Halide ion-induced switching of gold nanozyme activity based on Au–X interactions. Langmuir 2017, 33, 6372–6381. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Hao, Y.; Yang, S.; Tian, H.; Sun, B.; Liu, Y. A novel reaction-based fluorescent probe for the detection of cysteine in milk and water samples. Food Chem. 2018, 262, 67–71. [Google Scholar] [CrossRef]
- Das, S.; Ghosh, A.; Kundu, S.; Saha, S.; Sarkar, H.S.; Sahoo, P. Development of a new fluorescent probe for cysteine detection in processed food samples. Anal. Bioanal. Chem. 2019, 411, 6203–6212. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.; Thomson, J.E.; Karina, A.; Salter, C.; Johnston, C.; Davies, S.G.; Compton, R.G. Selective electrochemical determination of cysteine with a cyclotricatechylene modified carbon electrode. Analyst 2015, 140, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Li, G.; Wang, Y.; Wang, G.; Qu, L. Amperometric L-cysteine sensor based on a carbon paste electrode modified with Y2O3 nanoparticles supported on nitrogen-doped reduced graphene oxide. Microchim. Acta 2016, 183, 1351–1357. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, S.; Wei, S.C.; Chu-Su, Y.; Lin, C.W. Surface plasmon resonance detection of silver ions and cysteine using DNA intercalator-based amplification. Anal. Bioanal. Chem. 2012, 402, 2827–2835. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Shen, W.; Lu, C.; Liang, H.; Yuan, Q. Surface plasmon resonance additivity of gold nanoparticles for colorimetric identification of cysteine and homocysteine in biological fluids. Talanta 2013, 115, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, B.; Lu, C.; Lin, J.M. Determination of cysteine, homocysteine, cystine, and homocystine in biological fluids by HPLC using fluorosurfactant-capped gold nanoparticles as postcolumn colorimetric reagents. J. Sep. Sci. 2014, 37, 30–36. [Google Scholar] [CrossRef]
- Wei, L.; Lu, X.; Kang, X.; Song, Y. Determination of Glutathione and Cysteine in Human Breast Milk by High-Performance Liquid Chromatography with Chemiluminescence Detection for Evaluating the Oxidative Stress and Exposure to Heavy Metals of Lactating Women. Anal. Lett. 2020, 16, 1–12. [Google Scholar] [CrossRef]
- Brigden, M.L.; Edgell, D.; McPherson, M.; Leadbeater, A.; Hoag, G. High incidence of significant urinary ascorbic acid concentrations in a west coast population—Implications for routine urinalysis. Clin. Chem. 1992, 38, 426–431. [Google Scholar] [CrossRef]
- Vitamin C and Urine Test Strips. Available online: https://www.roche-diagnostics.cz/content/dam/diagnostics_czechrepublic/cs_CZ/documents/produkty/PD/mocova/Vitamin_C_a_mocove_prouzky.pdf (accessed on 21 August 2020).

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-C.; Hsu, T.-L.; Chen, C.-P.; Chen, C.-Y. Enhancement of the Peroxidase-Like Activity of Iodine-Capped Gold Nanoparticles for the Colorimetric Detection of Biothiols. Biosensors 2020, 10, 113. https://doi.org/10.3390/bios10090113
Chang C-C, Hsu T-L, Chen C-P, Chen C-Y. Enhancement of the Peroxidase-Like Activity of Iodine-Capped Gold Nanoparticles for the Colorimetric Detection of Biothiols. Biosensors. 2020; 10(9):113. https://doi.org/10.3390/bios10090113
Chicago/Turabian StyleChang, Chia-Chen, Tsz-Lian Hsu, Chie-Pein Chen, and Chen-Yu Chen. 2020. "Enhancement of the Peroxidase-Like Activity of Iodine-Capped Gold Nanoparticles for the Colorimetric Detection of Biothiols" Biosensors 10, no. 9: 113. https://doi.org/10.3390/bios10090113





