Electrochemiluminescence Biosensors Using Screen-Printed Electrodes
Abstract
:1. Introduction
Screen Printed Electrodes (SPEs)
2. ECL Immunosensors
- The label-free configuration is usually the simplest. They are based on the immobilization of a capture antibody over the electrode surface followed by the specific analyte recognition by the capture specific antibody, resulting in the retention of the analyte over the electrode surface. This simplest change over the electrode surface affects the ECL signal, generating an increase of the ECL emission if the analyte acts as co-reactant of the ECL used system or in the case that the diffusion of luminophore or co-reactant will be favored as a consequence of less steric hindrance.
- Competitive configuration is based on the competition of labeled and unlabeled analytes for a limited number of antibody binding sites [59]. Only one antibody is used in a competitive configuration, which is usually attached to the electrode surface. Labeled analytes are usually modified with luminophore species, obtaining ECL signals which decrease with the higher concentration of the analyte of interest, as a consequence of fewer labeled analytes attached over the electrode surface.
- Sandwich type configuration is one of the most widely used on ECL immunosensor. It is based on the immobilization of a capture-specific antibody on the electrode surface (or over magnetic beads in some cases). After that, the specific antigen (analyte) is bonded over the capture antibody. Then, a secondary labeled antibody (detection antibody) reacts with the previous immobilized analyte. Considering the different labels attached to secondary antibodies, different configurations of sandwich-type ECL immunosensors have been developed:
- ○
- The traditional sandwich type immunosensor is based on the linkage to the secondary antibody of a luminophore molecule or a nanomaterial, which acts as luminophore itself or is used as support to attach a great number of luminophore molecules.
- ○
- The quench-type electrochemiluminescence immunosensor uses a secondary antibody linked to an element capable of generating resonance energy transfer (RET) phenomena of the emitting specie [60,61]. The element capable of quenching the ECL emission is usually a nanomaterial or a composite nanomaterial, decreasing the ECL emission when higher amounts of analytes are present in the sample.
- ○
- A kind of sandwich-type ECL immunosensor that has recently appeared is named Faraday-cage-type electrochemiluminescence immunosensor [62,63]. The more important difference from the traditional sandwich-type immunosensor is that faraday-cage-type immunosensor uses a conductive two-dimensional nanomaterial (e.g., graphene, among others) simultaneously coated with a luminophore and a recognition component such as detection antibody, which could directly overlap on the electrode surface. In that configuration, electrons could flow freely from the working electrode surface to the detection element, extending the outer Helmholtz plane (OHP) of the electrode. This strategy allows the two-dimensional nanomaterial coated by thousands of luminophore molecules, being all of them electrochemically “effective” and really close to the working electrode surface.
2.1. [Ru(bpy)3]2+ ECL Systems
2.2. Luminol ECL Systems
2.3. S2O82− ECL Systems
2.4. Other ECL System
3. ECL Enzymatic Biosensors
ECL Enzymatic Biosensors Based on SPEs
4. ECL DNA Biosensors
4.1. [Ru(bpy)3]2+, Luminol and Nanomaterials ECL Systems
4.2. ECL DNA Biosensors Based on SPEs
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miao, W. Electrogenerated Chemiluminescence and Its Biorelated Applications. Chem. Rev. 2008, 108, 2506–2553. [Google Scholar] [CrossRef]
- Sojic, N.; Arbault, S.; Bouffier, L.; Kuhn, A. Applications of electrogenerated chemiluminescence in analytical chemistry. In Luminescence in Electrochemistry: Applications in Analytical Chemistry, Physics and Biology; Miomandre, F., Audebert, P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 257–291. [Google Scholar] [CrossRef]
- Choi, J.-P.; Bard, A.J. Electrogenerated chemiluminescence (ECL) 79.: Reductive-oxidation ECL of tris(2,2′-bipyridine)ruthenium(II) using hydrogen peroxide as a coreactant in pH 7.5 phosphate buffer solution. Anal. Chim. Acta 2005, 541, 141–148. [Google Scholar] [CrossRef]
- Chang, M.-M.; Saji, T.; Bard, A.J. Electrogenerated chemiluminescence. 30. Electrochemical oxidation of oxalate ion in the presence of luminescers in acetonitrile solutions. J. Am. Chem. Soc. 1977, 99, 5399–5403. [Google Scholar] [CrossRef]
- Rubinstein, I.; Bard, A.J. Electrogenerated chemiluminescence. 37. Aqueous ecl systems based on tris(2,2′-bipyridine)ruthenium(2+) and oxalate or organic acids. J. Am. Chem. Soc. 1981, 103, 512–516. [Google Scholar] [CrossRef]
- Noffsinger, J.B.; Danielson, N.D. Generation of chemiluminescence upon reaction of aliphatic amines with tris(2,2′-bipyridine)ruthenium(III). Anal. Chem. 1987, 59, 865–868. [Google Scholar] [CrossRef]
- Leland, J.K.; Powell, M.J. Electrogenerated Chemiluminescence: An Oxidative-Reduction Type ECL Reaction Sequence Using Tripropyl Amine. J. Electrochem. Soc. 2019, 137, 3127–3131. [Google Scholar] [CrossRef]
- Zu, Y.; Bard, A.J. Electrogenerated Chemiluminescence. 66. The Role of Direct Coreactant Oxidation in the Ruthenium Tris(2,2′)bipyridyl/Tripropylamine System and the Effect of Halide Ions on the Emission Intensity. Anal. Chem. 2000, 72, 3223–3232. [Google Scholar] [CrossRef]
- Kanoufi, F.; Zu, Y.; Bard, A.J. Homogeneous Oxidation of Trialkylamines by Metal Complexes and Its Impact on Electrogenerated Chemiluminescence in the Trialkylamine/Ru(bpy)32+ System. J. Phys. Chem. B 2001, 105, 210–216. [Google Scholar] [CrossRef]
- White, H.S.; Bard, A.J. Electrogenerated chemiluminescence. 41. Electrogenerated chemiluminescence and chemiluminescence of the Ru(2,21-bpy)32+-S2O82- system in acetonitrile-water solutions. J. Am. Chem. Soc. 1982, 104, 6891–6895. [Google Scholar] [CrossRef]
- Becker, W.G.; Seung, H.S.; Bard, A.J. Electrogenerated chemiluminescence: Part XLIII. Aromatic hydrocarbon/peroxydisulfate systems in acetonitrile-benzene solutions. J. Electroanal. Chem. Interfacial Electrochem. 1984, 167, 127–140. [Google Scholar] [CrossRef]
- Fabrizio, E.F.; Prieto, I.; Bard, A.J. Hydrocarbon Cation Radical Formation by Reduction of Peroxydisulfate. J. Am. Chem. Soc. 2000, 122, 4996–4997. [Google Scholar] [CrossRef]
- Miao, W.; Choi, J.-P.; Bard, A.J. Electrogenerated Chemiluminescence 69: The Tris(2,2‘-bipyridine)ruthenium(II), (Ru(bpy)32+)/Tri-n-propylamine (TPrA) System RevisitedA New Route Involving TPrA•+ Cation Radicals. J. Am. Chem. Soc. 2002, 124, 14478–14485. [Google Scholar] [CrossRef] [PubMed]
- Hiramoto, K.; Villani, E.; Iwama, T.; Komatsu, K.; Inagi, S.; Inoue, K.Y.; Nashimoto, Y.; Ino, K.; Shiku, H. Recent Advances in Electrochemiluminescence-Based Systems for Mammalian Cell Analysis. Micromachines 2020, 11, 530. [Google Scholar] [CrossRef]
- Ye, R.; Huang, L.; Qiu, B.; Song, Z.; Lin, Z.; Chen, G. Cathodic electrochemiluminescent behavior of luminol at nafion–nano-TiO2 modified glassy carbon electrode. Luminescence 2011, 26, 531–535. [Google Scholar] [CrossRef]
- Yin, X.-B.; Dong, S.; Wang, E. Analytical applications of the electrochemiluminescence of tris (2,2′-bipyridyl) ruthenium and its derivatives. TrAC Trends Anal. Chem. 2004, 23, 432–441. [Google Scholar] [CrossRef]
- Morita, H.; Konishi, M. Electrogenerated Chemiluminescence Derivatization Reagents for Carboxylic Acids and Amines in High-Performance Liquid Chromatography Using Tris(2,2‘-bipyridine)ruthenium(II). Anal. Chem. 2002, 74, 1584–1589. [Google Scholar] [CrossRef]
- Li, F.; Cui, H.; Lin, X.-Q. Determination of adrenaline by using inhibited Ru(bpy)32+ electrochemiluminescence. Anal. Chim. Acta 2002, 471, 187–194. [Google Scholar] [CrossRef]
- Forster, R.J.; Hogan, C.F. Electrochemiluminescent Metallopolymer Coatings: Combined Light and Current Detection in Flow Injection Analysis. Anal. Chem. 2000, 72, 5576–5582. [Google Scholar] [CrossRef]
- Liu, J.; Cao, W.; Yang, X.; Wang, E. Determination of diphenhydramine by capillary electrophoresis with tris(2,2′-bipyridyl)ruthenium(II) electrochemiluminescence detection. Talanta 2003, 59, 453–459. [Google Scholar] [CrossRef]
- Marchese, R.D.; Puchalski, D.; Miller, P.; Antonello, J.; Hammond, O.; Green, T.; Rubinstein, L.J.; Caulfield, M.J.; Sikkema, D. Optimization and Validation of a Multiplex, Electrochemiluminescence-Based Detection Assay for the Quantitation of Immunoglobulin G Serotype-Specific Antipneumococcal Antibodies in Human Serum. Clin. Vaccine Immunol. 2009, 16, 387–396. [Google Scholar] [CrossRef] [Green Version]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 136, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, D.-W.; Xiu, G.; Long, Y.-T. Applications of screen-printed electrodes in current environmental analysis. Curr. Opin. Electrochem. 2017, 3, 137–143. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Integrated Affinity Biosensing Platforms on Screen-Printed Electrodes Electrografted with Diazonium Salts. Sensors 2018, 18, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, K.; Vestergaard, M.C.; Tamiya, E. Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application. Sensors 2016, 16, 1761. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Anquela, C.; García-Mendiola, T.; Abad, J.M.; Pita, M.; Pariente, F.; Lorenzo, E. Scaffold electrodes based on thioctic acid-capped gold nanoparticles coordinated Alcohol Dehydrogenase and Azure A films for high performance biosensor. Bioelectrochemistry 2015, 106, 335–342. [Google Scholar] [CrossRef]
- Rama, E.C.; Costa-García, A. Screen-printed Electrochemical Immunosensors for the Detection of Cancer and Cardiovascular Biomarkers. Electroanalysis 2016, 28, 1700–1715. [Google Scholar] [CrossRef]
- Mistry, K.K.; Layek, K.; Mahapatra, A.; RoyChaudhuri, C.; Saha, H. A review on amperometric-type immunosensors based on screen-printed electrodes. Analyst 2014, 139, 2289–2311. [Google Scholar] [CrossRef]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Serafín, V.; Valverde, A.; Martínez-García, G.; Martínez-Periñán, E.; Comba, F.; Garranzo-Asensio, M.; Barderas, R.; Yáñez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Graphene quantum dots-functionalized multi-walled carbon nanotubes as nanocarriers in electrochemical immunosensing. Determination of IL-13 receptor α2 in colorectal cells and tumor tissues with different metastatic potential. Sens. Actuators B Chem. 2019, 284, 711–722. [Google Scholar] [CrossRef]
- Martínez-Periñán, E.; Sánchez-Tirado, E.; González-Cortés, A.; Barderas, R.; Sánchez-Puelles, J.M.; Martínez-Santamaría, L.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Amperometric determination of endoglin in human serum using disposable immunosensors constructed with poly(pyrrolepropionic) acid-modified electrodes. Electrochim. Acta 2018, 292, 887–894. [Google Scholar] [CrossRef]
- García, T.; Fernández-Barrena, M.G.; Revenga-Parra, M.; Núñez, A.; Casero, E.; Pariente, F.; Prieto, J.; Lorenzo, E. Disposable sensors for rapid screening of mutated genes. Anal. Bioanal. Chem. 2010, 398, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- García-Mendiola, T.; Cerro, M.R.; López-Moreno, J.M.; Pariente, F.; Lorenzo, E. Dyes as bifunctional markers of DNA hybridization on surfaces and mutation detection. Bioelectrochemistry 2016, 111, 115–122. [Google Scholar] [CrossRef]
- García-Mendiola, T.; Bayon-Pizarro, V.; Zaulet, A.; Fuentes, I.; Pariente, F.; Teixidor, F.; Viñas, C.; Lorenzo, E. Metallacarboranes as tunable redox potential electrochemical indicators for screening of gene mutation. Chem. Sci. 2016, 7, 5786–5797. [Google Scholar] [CrossRef] [Green Version]
- García-Mendiola, T.; Bravo, I.; López-Moreno, J.M.; Pariente, F.; Wannemacher, R.; Weber, K.; Popp, J.; Lorenzo, E. Carbon nanodots based biosensors for gene mutation detection. Sens. Actuators B Chem. 2018, 256, 226–233. [Google Scholar] [CrossRef]
- García-Mendiola, T.; Requena-Sanz, S.; Martínez-Periñán, E.; Bravo, I.; Pariente, F.; Lorenzo, E. Influence of carbon nanodots on DNA-Thionine interaction. Application to breast cancer diagnosis. Electrochim. Acta 2020, 353, 136522. [Google Scholar] [CrossRef]
- García-Mendiola, T.; Gutiérrez-Sánchez, C.; Gibaja, C.; Torres, I.; Busó-Rogero, C.; Pariente, F.; Solera, J.; Razavifar, Z.; Palacios, J.J.; Zamora, F.; et al. Functionalization of a Few-Layer Antimonene with Oligonucleotides for DNA Sensing. ACS Appl. Nano Mater. 2020, 3, 3625–3633. [Google Scholar] [CrossRef]
- Chen, A.; Chatterjee, S. Nanomaterials based electrochemical sensors for biomedical applications. Chem. Soc. Rev. 2013, 42, 5425–5438. [Google Scholar] [CrossRef]
- Song, S.; Qin, Y.; He, Y.; Huang, Q.; Fan, C.; Chen, H.-Y. Functional nanoprobes for ultrasensitive detection of biomolecules. Chem. Soc. Rev. 2010, 39, 4234–4243. [Google Scholar] [CrossRef]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, Q.; Li, J.; Wang, E. Recent Advances Based on Nanomaterials as Electrochemiluminescence Probes for the Fabrication of Sensors. ChemElectroChem 2017, 4, 1639–1650. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, T.; Huang, R. Recent Advances in Electrochemiluminescence Sensors for Pathogenic Bacteria Detection. Micromachines 2019, 10, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renedo, O.D.; Alonso-Lomillo, M.A.; Martínez, M.J.A. Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Moro, G.; Bottari, F.; Van Loon, J.; Du Bois, E.; De Wael, K.; Moretto, L.M. Disposable electrodes from waste materials and renewable sources for (bio)electroanalytical applications. Biosens. Bioelectron. 2019, 146, 111758. [Google Scholar] [CrossRef] [PubMed]
- Moya, A.; Gabriel, G.; Villa, R.; Javier del Campo, F. Inkjet-printed electrochemical sensors. Curr. Opin. Electrochem. 2017, 3, 29–39. [Google Scholar] [CrossRef]
- Silva, N.F.D.; Almeida, C.M.R.; Magalhães, J.M.C.S.; Gonçalves, M.P.; Freire, C.; Delerue-Matos, C. Development of a disposable paper-based potentiometric immunosensor for real-time detection of a foodborne pathogen. Biosens. Bioelectron. 2019, 141, 111317. [Google Scholar] [CrossRef]
- Rubio-Govea, R.; Hickey, D.P.; García-Morales, R.; Rodriguez-Delgado, M.; Domínguez-Rovira, M.A.; Minteer, S.D.; Ornelas-Soto, N.; García-García, A. MoS2 nanostructured materials for electrode modification in the development of a laccase based amperometric biosensor for non-invasive dopamine detection. Microchem. J. 2020, 155, 104792. [Google Scholar] [CrossRef]
- Rungsawang, T.; Punrat, E.; Adkins, J.; Henry, C.; Chailapakul, O. Development of Electrochemical Paper-based Glucose Sensor Using Cellulose-4-aminophenylboronic Acid-modified Screen-printed Carbon Electrode. Electroanalysis 2016, 28, 462–468. [Google Scholar] [CrossRef]
- Sánchez-Calvo, A.; Costa-García, A.; Blanco-López, M.C. Paper-based electrodes modified with cobalt phthalocyanine colloid for the determination of hydrogen peroxide and glucose. Analyst 2020, 145, 2716–2724. [Google Scholar] [CrossRef]
- Tao, C.; Yen, C.-S.; Liu, J.-T.; Chen, C.-J. Analytical performance of paper electro-biosensor detection platform for point-of-care diagnosis. Cellulose 2016, 23, 3799–3808. [Google Scholar] [CrossRef]
- Yang, H.; Kong, Q.; Wang, S.; Xu, J.; Bian, Z.; Zheng, X.; Ma, C.; Ge, S.; Yu, J. Hand-drawn&written pen-on-paper electrochemiluminescence immunodevice powered by rechargeable battery for low-cost point-of-care testing. Biosens. Bioelectron. 2014, 61, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Bernalte, E.; Foster, C.W.; Brownson, D.A.; Mosna, M.; Smith, G.C.; Banks, C.E. Pencil It in: Exploring the Feasibility of Hand-Drawn Pencil Electrochemical Sensors and Their Direct Comparison to Screen-Printed Electrodes. Biosensors 2016, 6, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foster, C.W.; Brownson, D.A.C.; Ruas de Souza, A.P.; Bernalte, E.; Iniesta, J.; Bertotti, M.; Banks, C.E. Pencil it in: Pencil drawn electrochemical sensing platforms. Analyst 2016, 141, 4055–4064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kannan, B.; Jahanshahi-Anbuhi, S.; Pelton, R.H.; Li, Y.; Filipe, C.D.M.; Brennan, J.D. Printed Paper Sensors for Serum Lactate Dehydrogenase using Pullulan-Based Inks to Immobilize Reagents. Anal. Chem. 2015, 87, 9288–9293. [Google Scholar] [CrossRef]
- Credou, J.; Berthelot, T. Cellulose: From biocompatible to bioactive material. J. Mater. Chem. B 2014, 2, 4767–4788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yáñez-Sedeño, P.; Campuzano, S.A.-O.; Pingarrón, J.A.-O. Screen-Printed Electrodes: Promising Paper and Wearable Transducers for (Bio)Sensing. Biosensors 2020, 10, 76. [Google Scholar] [CrossRef]
- Wang, T.; Wang, D.; Padelford, J.W.; Jiang, J.; Wang, G. Near-Infrared Electrogenerated Chemiluminescence from Aqueous Soluble Lipoic Acid Au Nanoclusters. J. Am. Chem. Soc. 2016, 138, 6380–6383. [Google Scholar] [CrossRef]
- Cox, K.L.; Devanarayan, V.; Kriauciunas, A.; Manetta, J.; Montrose, C.; Sittampalam, S. Immunoassay Methods. Available online: https://www.ncbi.nlm.nih.gov/books/NBK92434/#:~:text=Sandwich%20Immunoassay%20(ELISA),attached%20to%20a%20solid%20surface (accessed on 18 August 2020).
- Song, C.; Li, X.; Hu, L.; Shi, T.; Wu, D.; Ma, H.; Zhang, Y.; Fan, D.; Wei, Q.; Ju, H. Quench-Type Electrochemiluminescence Immunosensor Based on Resonance Energy Transfer from Carbon Nanotubes and Au-Nanoparticles-Enhanced g-C3N4 to CuO@Polydopamine for Procalcitonin Detection. ACS Appl. Mater. Interfaces 2020, 12, 8006–8015. [Google Scholar] [CrossRef]
- Xue, J.; Yang, L.; Wang, H.; Yan, T.; Fan, D.; Feng, R.; Du, B.; Wei, Q.; Ju, H. Quench-type electrochemiluminescence immunosensor for detection of amyloid β-protein based on resonance energy transfer from luminol@SnS2-Pd to Cu doped WO3 nanoparticles. Biosens. Bioelectron. 2019, 133, 192–198. [Google Scholar] [CrossRef]
- Kannan, P.; Chen, J.; Su, F.; Guo, Z.; Huang, Y. Faraday-Cage-Type Electrochemiluminescence Immunoassay: A Rise of Advanced Biosensing Strategy. Anal. Chem. 2019, 91, 14792–14802. [Google Scholar] [CrossRef]
- Guo, Z.; Sha, Y.; Hu, Y.; Yu, Z.; Tao, Y.; Wu, Y.; Zeng, M.; Wang, S.; Li, X.; Zhou, J.; et al. Faraday cage-type electrochemiluminescence immunosensor for ultrasensitive detection of Vibrio vulnificus based on multi-functionalized graphene oxide. Anal. Bioanal. Chem. 2016, 408, 7203–7211. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, M.; Mohd-Naim, N.F.; Keasberry, N.A.; Ahmed, M.U. A highly sensitive and label-free electrochemiluminescence immunosensor for beta 2-microglobulin. Anal. Methods 2017, 9, 2570–2577. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Feng, R.; Wu, D.; Zhang, Y.; Li, H.; Du, B.; Wei, Q. Ultrasensitive electrochemiluminescence immunosensor based on Ru(bpy)32+ and Ag nanoparticles doped SBA-15 for detection of cancer antigen 15-3. Sens. Actuators B Chem. 2013, 188, 462–468. [Google Scholar] [CrossRef]
- Jiang, D.; Zhang, L.; Liu, F.; Liu, C.; Liu, L.; Pu, X. An electrochemiluminescence sensor with dual signal amplification of Ru(bpy)32+ based on PtNPs and glucose dehydrogenase for diagnosis of gas gangrene. RSC Adv. 2016, 6, 19676–19685. [Google Scholar] [CrossRef]
- Li, C.; Fu, Z.; Li, Z.; Wang, Z.; Wei, W. Cross-talk-free multiplexed immunoassay using a disposable electrochemiluminescent immunosensor array coupled with a non-array detector. Biosens. Bioelectron. 2011, 27, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Habtamu, H.B.; Sentic, M.; Silvestrini, M.; De Leo, L.; Not, T.; Arbault, S.; Manojlovic, D.; Sojic, N.; Ugo, P. A Sensitive Electrochemiluminescence Immunosensor for Celiac Disease Diagnosis Based on Nanoelectrode Ensembles. Anal. Chem. 2015, 87, 12080–12087. [Google Scholar] [CrossRef] [PubMed]
- Spehar-Délèze, A.-M.; Julich, S.; Gransee, R.; Tomaso, H.; Dulay, S.B.; O’Sullivan, C.K. Electrochemiluminescence (ECL) immunosensor for detection of Francisella tularensis on screen-printed gold electrode array. Anal. Bioanal. Chem. 2016, 408, 7147–7153. [Google Scholar] [CrossRef]
- Kadimisetty, K.; Mosa, I.M.; Malla, S.; Satterwhite-Warden, J.E.; Kuhns, T.M.; Faria, R.C.; Lee, N.H.; Rusling, J.F. 3D-printed supercapacitor-powered electrochemiluminescent protein immunoarray. Biosens. Bioelectron. 2016, 77, 188–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jian, Y.; Wang, H.; Sun, X.; Zhang, L.; Cui, K.; Ge, S.; Yu, J. Electrochemiluminescence cytosensing platform based on Ru(bpy)32+@silica-Au nanocomposite as luminophore and AuPd nanoparticles as coreaction accelerator for in situ evaluation of intracellular H2O2. Talanta 2019, 199, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Gan, N.; Hou, J.; Hu, F.; Cao, Y.; Li, T.; Guo, Z.; Wang, J. A Renewable and Ultrasensitive Electrochemiluminescence Immunosenor Based on Magnetic RuL@SiO2-Au~RuL-Ab2 Sandwich-Type Nano-Immunocomplexes. Sensors 2011, 11, 7749–7762. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Gan, N.; Hu, F.; Li, T.; Zhou, H.; Li, X.; Zheng, L. A single antibody sandwich electrochemiluminescence immunosensor based on protein magnetic molecularly imprinted polymers mimicking capture probes. Sens. Actuators B Chem. 2013, 186, 300–307. [Google Scholar] [CrossRef]
- Feng, X.; Gan, N.; Zhou, J.; Li, T.; Cao, Y.; Hu, F.; Yu, H.; Jiang, Q. A novel dual-template molecularly imprinted electrochemiluminescence immunosensor array using Ru(bpy)32+-Silica@Poly-L-lysine-Au composite nanoparticles as labels for near-simultaneous detection of tumor markers. Electrochim. Acta 2014, 139, 127–136. [Google Scholar] [CrossRef]
- Hou, J.; Gan, N.; Hu, F.; Zheng, L.; Cao, Y.; Li, T. One Renewable and Magnetic Electrochemiluminescence Immunosenor Based on Tris(2,2′-bipyridine) ruthenium(II) Modified Magnetic Composite Nanoparticles Labeled Anti-AFP. Int. J. Electrochem. Sci. 2011, 6, 2845–2858. [Google Scholar]
- Zhou, H.; Yue, H.; Zhou, Y.; Wang, L.; Fu, Z. A novel disposable immunosensor based on quenching of electrochemiluminescence emission of Ru(bpy)32+ by amorphous carbon nanoparticles. Sens. Actuators B Chem. 2015, 209, 744–750. [Google Scholar] [CrossRef]
- Fang, D.; Ren, H.; Huang, Y.; Dai, H.; Huang, D.; Lin, Y. Photothermal amplified cathodic ZnO quantum dots/Ru(bpy)32+/S2O82- ternary system for ultrasensitive electrochemiluminescence detection of thyroglobulin. Sens. Actuators B Chem. 2020, 312, 127950. [Google Scholar] [CrossRef]
- Zhou, J.; Gan, N.; Li, T.; Hu, F.; Li, X.; Wang, L.; Zheng, L. A cost-effective sandwich electrochemiluminescence immunosensor for ultrasensitive detection of HIV-1 antibody using magnetic molecularly imprinted polymers as capture probes. Biosens. Bioelectron. 2014, 54, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Su, M.; Liu, H.; Ge, S.; Ren, N.; Ding, L.; Yu, J.; Song, X. An electrochemiluminescence lab-on-paper device for sensitive detection of two antigens at the MCF-7 cell surface based on porous bimetallic AuPd nanoparticles. RSC Adv. 2016, 6, 16500–16506. [Google Scholar] [CrossRef]
- Wang, S.; Ge, L.; Yan, M.; Yu, J.; Song, X.; Ge, S.; Huang, J. 3D microfluidic origami electrochemiluminescence immunodevice for sensitive point-of-care testing of carcinoma antigen 125. Sens. Actuators B Chem. 2013, 176, 1–8. [Google Scholar] [CrossRef]
- Cui, H.; Wang, W.; Duan, C.-F.; Dong, Y.-P.; Guo, J.-Z. Synthesis, Characterization, and Electrochemiluminescence of Luminol-Reduced Gold Nanoparticles and Their Application in a Hydrogen Peroxide Sensor. Chem. Eur. J. 2007, 13, 6975–6984. [Google Scholar] [CrossRef]
- Kong, W.; Zhou, H.; Ouyang, H.; Li, Z.; Fu, Z. A disposable label-free electrochemiluminescent immunosensor for transferrin detection based on a luminol-reduced gold nanoparticle-modified screen-printed carbon electrode. Anal. Methods 2014, 6, 2959–2964. [Google Scholar] [CrossRef]
- Wu, L.; Sha, Y.; Li, W.; Wang, S.; Guo, Z.; Zhou, J.; Su, X.; Jiang, X. One-step preparation of disposable multi-functionalized g-C3N4 based electrochemiluminescence immunosensor for the detection of CA125. Sens. Actuators B Chem. 2016, 226, 62–68. [Google Scholar] [CrossRef]
- Zhang, M.; Dai, W.; Yan, M.; Ge, S.; Yu, J.; Song, X.; Xu, W. Ultrasensitive electrochemiluminescence immunosensor using PtAg@carbon nanocrystals composites as labels and carbon nanotubes-chitosan/gold nanoparticles as enhancer. Analyst 2012, 137, 2112–2118. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Li, L.; Li, S.; Wang, X.; Li, M.; Wang, S.; Yu, J. 3D origami electrochemiluminescence immunodevice based on porous silver-paper electrode and nanoporous silver double-assisted signal amplification. Sens. Actuators B Chem. 2013, 188, 417–424. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Yang, H.; Ding, Y.-N.; Su, M.; Zhu, J.; Yan, M.; Yu, J.; Song, X. Gold–silver nanocomposite-functionalized graphene sensing platform for an electrochemiluminescent immunoassay of a tumor marker. RSC Adv. 2013, 3, 14701–14709. [Google Scholar] [CrossRef]
- Ma, C.; Liu, F.; Yang, H.; Ge, S.; Yu, J.; Yan, M.; Song, X. Application of SnO2 nanocrystal as novel electrochemiluminescence signal reporter for sensitive immunoassay with nanoporous PtRu alloy enhancement. Sens. Actuators B Chem. 2014, 195, 423–430. [Google Scholar] [CrossRef]
- Liu, F.; Deng, W.; Zhang, Y.; Ge, S.; Yu, J.; Song, X. Application of ZnO quantum dots dotted carbon nanotube for sensitive electrochemiluminescence immunoassay based on simply electrochemical reduced Pt/Au alloy and a disposable device. Anal. Chim. Acta 2014, 818, 46–53. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Y.; Yu, J.; Song, X.; Ge, S.; Yan, M. Application of indium tin oxide device in gold-coated magnetic iron solid support enhanced electrochemiluminescent immunosensor for determination of carcinoma embryonic antigen. Sens. Actuators B Chem. 2012, 171–172, 891–898. [Google Scholar] [CrossRef]
- Li, L.; Li, W.; Ma, C.; Yang, H.; Ge, S.; Yu, J. Paper-based electrochemiluminescence immunodevice for carcinoembryonic antigen using nanoporous gold-chitosan hybrids and graphene quantum dots functionalized Au@Pt. Sens. Actuators B Chem. 2014, 202, 314–322. [Google Scholar] [CrossRef]
- Liu, W.; Ma, C.; Yang, H.; Zhang, Y.; Yan, M.; Ge, S.; Yu, J.; Song, X. Electrochemiluminescence immunoassay using a paper electrode incorporating porous silver and modified with mesoporous silica nanoparticles functionalized with blue-luminescent carbon dots. Microchim. Acta 2014, 181, 1415–1422. [Google Scholar] [CrossRef]
- Yan, J.; Yan, M.; Ge, L.; Ge, S.; Yu, J. An origami electrochemiluminescence immunosensor based on gold/graphene for specific, sensitive point-of-care testing of carcinoembryonic antigen. Sens. Actuators B Chem. 2014, 193, 247–254. [Google Scholar] [CrossRef]
- Gutiérrez-Sánchez, C.; Pita, M.; Vaz-Domínguez, C.; Shleev, S.; De Lacey, A.L. Gold Nanoparticles as Electronic Bridges for Laccase-Based Biocathodes. J. Am. Chem. Soc. 2012, 134, 17212–17220. [Google Scholar] [CrossRef] [PubMed]
- Dhanjai; Lu, X.; Wu, L.; Chen, J.; Lu, Y. Robust Single-Molecule Enzyme Nanocapsules for Biosensing with Significantly Improved Biosensor Stability. Anal. Chem. 2020, 92, 5830–5837. [Google Scholar] [CrossRef]
- Miao, Y.; Tan, S.N. Amperometric hydrogen peroxide biosensor based on immobilization of peroxidase in chitosan matrix crosslinked with glutaraldehyde. Analyst 2000, 125, 1591–1594. [Google Scholar] [CrossRef]
- Cen, Y.-K.; Liu, Y.-X.; Xue, Y.-P.; Zheng, Y.-G. Immobilization of Enzymes in/on Membranes and their Applications. Adv. Synth. Catal. 2019, 361, 5500–5515. [Google Scholar] [CrossRef]
- Fang, C.; Li, H.; Yan, J.; Guo, H.; Yifeng, T. Progress of the Electrochemiluminescence Biosensing Strategy for Clinical Diagnosis with Luminol as the Sensing Probe. ChemElectroChem 2017, 4, 1587–1593. [Google Scholar] [CrossRef]
- Da Silva, B.F.; Pérez, S.; Gardinalli, P.; Singhal, R.K.; Mozeto, A.A.; Barceló, D. Analytical chemistry of metallic nanoparticles in natural environments. TrAC Trends Anal. Chem. 2011, 30, 528–540. [Google Scholar] [CrossRef]
- Harvey, N. Luminescence during Electrolysis. J. Phys. Chem. 1929, 33, 1456–1459. [Google Scholar] [CrossRef]
- Richter, M.M. Electrochemiluminescence (ECL). Chem. Rev. 2004, 104, 3003–3036. [Google Scholar] [CrossRef]
- Kremeskotter, J.; Wilson, R.; Schiffrin, D.J.; Luff, B.J.; Wilkinson, J.S. Detection of glucose via electrochemiluminescence in a thin-layer cell with a planar optical waveguide. Meas. Sci. Technol. 1995, 6, 1325–1328. [Google Scholar] [CrossRef]
- Zhu, L.; Li, Y.; Tian, F.; Xu, B.; Zhu, G. Electrochemiluminescent determination of glucose with a sol–gel derived ceramic–carbon composite electrode as a renewable optical fiber biosensor. Sens. Actuators B Chem. 2002, 84, 265–270. [Google Scholar] [CrossRef]
- Lin, Z.; Chen, J.; Chen, G. An ECL biosensor for glucose based on carbon-nanotube/Nafion film modified glass carbon electrode. Electrochim. Acta 2008, 53, 2396–2401. [Google Scholar] [CrossRef]
- Liu, L.; Ma, Q.; Li, Y.; Liu, Z.; Su, X. A novel signal-off electrochemiluminescence biosensor for the determination of glucose based on double nanoparticles. Biosens. Bioelectron. 2015, 63, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wu, X.; Xu, H.; Wang, Y.; Chi, Y.; Chen, G. A highly performing electrochemiluminescent biosensor for glucose based on a polyelectrolyte-chitosan modified electrode. Electrochim. Acta 2009, 54, 4582–4586. [Google Scholar] [CrossRef]
- Qiu, B.; Lin, Z.; Wang, J.; Chen, Z.; Chen, J.; Chen, G. An electrochemiluminescent biosensor for glucose based on the electrochemiluminescence of luminol on the nafion/glucose oxidase/poly(nickel(II)tetrasulfophthalocyanine)/multi-walled carbon nanotubes modified electrode. Talanta 2009, 78, 76–80. [Google Scholar] [CrossRef]
- Lou, F.; Lu, Z.; Hu, F.; Li, C.M. A 3D bio-platform constructed by glucose oxidase adsorbed on Au nanoparticles assembled polyaniline nanowires to sensitively detect glucose by electrochemiluminescence. J. Electroanal. Chem. 2017, 787, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Deng, S.; Lei, J.; Ju, H. Disposable electrochemiluminescent biosensor using bidentate-chelated CdTe quantum dots as emitters for sensitive detection of glucose. Analyst 2012, 137, 140–144. [Google Scholar] [CrossRef]
- Yu, L.; Wei, X.; Fang, C.; Tu, Y. A disposable biosensor for noninvasive diabetic diagnosis rest on the Au/TiO2 nano-composite intensified electrochemiluminescence. Electrochim. Acta 2016, 211, 27–35. [Google Scholar] [CrossRef]
- Chu, H.; Wei, X.; Wu, M.; Yan, J.; Tu, Y. An electrochemiluminescent biosensor based on polypyrrole immobilized uricase for ultrasensitive uric acid detection. Sens. Actuators B Chem. 2012, 163, 247–252. [Google Scholar] [CrossRef]
- Ballesta-Claver, J.; Díaz Ortega, I.F.; Valencia-Mirón, M.C.; Capitán-Vallvey, L.F. Disposable luminol copolymer-based biosensor for uric acid in urine. Anal. Chim. Acta 2011, 702, 254–261. [Google Scholar] [CrossRef]
- Bravo, I.; Gutiérrez-Sánchez, C.; García-Mendiola, T.; Revenga-Parra, M.; Pariente, F.; Lorenzo, E. Enhanced Performance of Reagent-Less Carbon Nanodots Based Enzyme Electrochemical Biosensors. Sensors 2019, 19, 5576. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Yan, J.; Chu, H.; Wu, M.; Tu, Y. An exercise degree monitoring biosensor based on electrochemiluminescent detection of lactate in sweat. Sens. Actuators B Chem. 2010, 143, 655–659. [Google Scholar] [CrossRef]
- Claver, J.B.; Mirón, M.C.V.; Capitán-Vallvey, L.F. Disposable electrochemiluminescent biosensor for lactate determination in saliva. Analyst 2009, 134, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Marquette, C.A.; Ravaud, S.; Blum, L.J. Luminol Electrochemiluminescence-Based Biosensor for Total Cholesterol Determination in Natural Samples. Anal. Lett. 2000, 33, 1779–1796. [Google Scholar] [CrossRef]
- Zhu, S.; Lin, X.; Ran, P.; Mo, F.; Xia, Q.; Fu, Y. A glassy carbon electrode modified with C-dots and silver nanoparticles for enzymatic electrochemiluminescent detection of glutamate enantiomers. Microchim. Acta 2017, 184, 4679–4684. [Google Scholar] [CrossRef]
- Zhao, Q.; Tang, S.; Fang, C.; Tu, Y.-F. Titania nanotubes decorated with gold nanoparticles for electrochemiluminescent biosensing of glycosylated hemoglobin. Anal. Chim. Acta 2016, 936, 83–90. [Google Scholar] [CrossRef]
- Dai, H.; Chi, Y.; Wu, X.; Wang, Y.; Wei, M.; Chen, G. Biocompatible electrochemiluminescent biosensor for choline based on enzyme/titanate nanotubes/chitosan composite modified electrode. Biosens. Bioelectron. 2010, 25, 1414–1419. [Google Scholar] [CrossRef]
- Gao, W.; Chen, Y.; Xi, J.; Lin, S.; Chen, Y.; Lin, Y.; Chen, Z. A novel electrochemiluminescence ethanol biosensor based on tris(2,2′-bipyridine) ruthenium (II) and alcohol dehydrogenase immobilized in graphene/bovine serum albumin composite film. Biosens. Bioelectron. 2013, 41, 776–782. [Google Scholar] [CrossRef]
- Leca, B.; Blum, L.J. Luminol electrochemiluminescence with screen-printed electrodes for low-cost disposable oxidase-based optical sensors. Analyst 2000, 125, 789–791. [Google Scholar] [CrossRef]
- Corgier, B.P.; Marquette, C.A.; Blum, L.J. Screen-printed electrode microarray for electrochemiluminescent measurements. Anal. Chim. Acta 2005, 538, 1–7. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Xu, T.; Liao, H.; Yao, C.; Liu, Y.; Li, Z.; Chen, Z.; Pan, D.; Sun, L.; et al. Gram-scale synthesis of single-crystalline graphene quantum dots with superior optical properties. Nat. Commun. 2014, 5, 5357. [Google Scholar] [CrossRef] [Green Version]
- Mo, R.; Li, F.; Tan, X.; Xu, P.; Tao, R.; Shen, G.; Lu, X.; Liu, F.; Shen, L.; Xu, B.; et al. High-quality mesoporous graphene particles as high-energy and fast-charging anodes for lithium-ion batteries. Nat. Commun. 2019, 10, 1474. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Dong, F.; Minteer, S.D. The progress and outlook of bioelectrocatalysis for the production of chemicals, fuels and materials. Nat. Catal. 2020, 3, 225–244. [Google Scholar] [CrossRef]
- Deshmukh, M.A.; Park, S.-J.; Kang, B.-C.; Ha, T.-J. Carbon Nanohybrids for Advanced Electronic Applications. Phys. Status Solidi (a) 2020. [Google Scholar] [CrossRef]
- Valenti, G.; Rampazzo, E.; Kesarkar, S.; Genovese, D.; Fiorani, A.; Zanut, A.; Palomba, F.; Marcaccio, M.; Paolucci, F.; Prodi, L. Electrogenerated chemiluminescence from metal complexes-based nanoparticles for highly sensitive sensors applications. Coord. Chem. Rev. 2018, 367, 65–81. [Google Scholar] [CrossRef]
- Molinero-Abad, B.; Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Amperometric determination of sulfite using screen-printed electrodes modified with metallic nanoparticles. Microchim. Acta 2013, 180, 1351–1355. [Google Scholar] [CrossRef]
- Pita, M.; Gutierrez-Sanchez, C.; Toscano, M.D.; Shleev, S.; De Lacey, A.L. Oxygen biosensor based on bilirubin oxidase immobilized on a nanostructured gold electrode. Bioelectrochemistry 2013, 94, 69–74. [Google Scholar] [CrossRef]
- Fritea, L.; Tertis, M.; Sandulescu, R.; Cristea, C. Chapter Eleven—Enzyme–Graphene platforms for electrochemical biosensor design with biomedical applications. In Methods in Enzymology; Kumar, C.V., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 609, pp. 293–333. [Google Scholar]
- Martinez-Perinan, E.; Revenga-Parra, M.; Gennari, M.; Pariente, F.; Mas-Balleste, R.; Zamora, F.; Lorenzo, E. Insulin sensor based on nanoparticle-decorated multiwalled carbon nanotubes modified electrodes. Sens. Actuators B-Chem. 2016, 222, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Cancelliere, R.; Carbone, K.; Pagano, M.; Cacciotti, I.; Micheli, L. Biochar from Brewers’ Spent Grain: A Green and Low-Cost Smart Material to Modify Screen-Printed Electrodes. Biosensors 2019, 9, 139. [Google Scholar] [CrossRef]
- Bertoncello, P.; Ugo, P. Recent Advances in Electrochemiluminescence with Quantum Dots and Arrays of Nanoelectrodes. ChemElectroChem 2017, 4, 1663–1676. [Google Scholar] [CrossRef]
- Soldatkina, O.V.; Soldatkin, O.O.; Kasap, B.O.; Kucherenko, D.Y.; Kucherenko, I.S.; Kurc, B.A.; Dzyadevych, S.V. A Novel Amperometric Glutamate Biosensor Based on Glutamate Oxidase Adsorbed on Silicalite. Nanoscale Res. Lett. 2017, 12, 260. [Google Scholar] [CrossRef] [Green Version]
- Hezard, T.; Fajerwerg, K.; Evrard, D.; Collière, V.; Behra, P.; Gros, P. Gold nanoparticles electrodeposited on glassy carbon using cyclic voltammetry: Application to Hg(II) trace analysis. J. Electroanal. Chem. 2012, 664, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Sánchez, C.; Mediavilla, M.; Guerrero-Esteban, T.; Revenga-Parra, M.; Pariente, F.; Lorenzo, E. Direct covalent immobilization of new nitrogen-doped carbon nanodots by electrografting for sensing applications. Carbon 2020, 159, 303–310. [Google Scholar] [CrossRef]
- Bravo, I.; García-Mendiola, T.; Revenga-Parra, M.; Pariente, F.; Lorenzo, E. Diazonium salt click chemistry based multiwall carbon nanotube electrocatalytic platforms. Sens. Actuators B Chem. 2015, 211, 559–568. [Google Scholar] [CrossRef]
- Castrovilli, M.C.; Bolognesi, P.; Chiarinelli, J.; Avaldi, L.; Cartoni, A.; Calandra, P.; Tempesta, E.; Giardi, M.T.; Antonacci, A.; Arduini, F.; et al. Electrospray deposition as a smart technique for laccase immobilisation on carbon black-nanomodified screen-printed electrodes. Biosens. Bioelectron. 2020, 163, 112299. [Google Scholar] [CrossRef] [PubMed]
- Antuña-Jiménez, D.; González-García, M.B.; Hernández-Santos, D.; Fanjul-Bolado, P. Screen-Printed Electrodes Modified with Metal Nanoparticles for Small Molecule Sensing. Biosensors 2020, 10, 9. [Google Scholar] [CrossRef] [Green Version]
- Tian, C.; Wang, L.; Luan, F.; Zhuang, X. An electrochemiluminescence sensor for the detection of prostate protein antigen based on the graphene quantum dots infilled TiO2 nanotube arrays. Talanta 2019, 191, 103–108. [Google Scholar] [CrossRef]
- Habekost, A. Rapid and sensitive spectroelectrochemical and electrochemical detection of glyphosate and AMPA with screen-printed electrodes. Talanta 2017, 162, 583–588. [Google Scholar] [CrossRef]
- Guerrero-Esteban, T.; Gutiérrez-Sánchez, C.; Revenga-Parra, M.; Pau, J.L.; Pariente, F.; Lorenzo, E. Enhanced electrochemiluminescence by ZnO nanowires for taurine determination. Talanta 2019, 204, 63–69. [Google Scholar] [CrossRef]
- Long, Y.-M.; Bao, L.; Peng, Y.; Zhang, Z.-L.; Pang, D.-W. Self-co-reactant and ion-annihilation electrogenerated chemiluminescence of carbon nanodots. Carbon 2018, 129, 168–174. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Ma, C.; Zhu, J.-J. Carbon-based dots for electrochemiluminescence sensing. Mater. Chem. Front. 2020, 4, 369–385. [Google Scholar] [CrossRef]
- Liu, X.; Fang, C.; Yan, J.; Li, H.; Tu, Y. A sensitive electrochemiluminescent biosensor based on AuNP-functionalized ITO for a label-free immunoassay of C-peptide. Bioelectrochemistry 2018, 123, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yuan, R.; Chai, Y.; Chen, S.; Zhong, H.; Wang, C.; Cheng, Y. A biosensor for cholesterol based on gold nanoparticles-catalyzed luminol electrogenerated chemiluminescence. Biosens. Bioelectron. 2012, 32, 288–292. [Google Scholar] [CrossRef]
- Li, J.-J.; Shang, L.; Jia, L.-P.; Ma, R.-N.; Zhang, W.; Jia, W.-L.; Wang, H.-S.; Xu, K.-H. An ultrasensitive electrochemiluminescence sensor for the detection of HULC based on Au@Ag/GQDs as a signal indicator. J. Electroanal. Chem. 2018, 824, 114–120. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, Q.; Zhang, C.-Y. Nanomaterial-based biosensors for DNA methyltransferase assay. J. Mater. Chem. B 2020, 8, 3488–3501. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Fan, Y.; Zhang, H.; Chen, S.; Yuan, R. An ultrasensitive signal-on electrochemiluminescence biosensor based on Au nanoclusters for detecting acetylthiocholine. Anal. Bioanal. Chem. 2019, 411, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Salehnia, F.; Hosseini, M.; Ganjali, M.R. Enhanced electrochemiluminescence of luminol by an in situ silver nanoparticle-decorated graphene dot for glucose analysis. Anal. Methods 2018, 10, 508–514. [Google Scholar] [CrossRef]
- Du, X.; Jiang, D.; Chen, S.; Dai, L.; Zhou, L.; Hao, N.; You, T.; Mao, H.; Wang, K. CeO2 nanocrystallines ensemble-on-nitrogen-doped graphene nanocomposites: One-pot, rapid synthesis and excellent electrocatalytic activity for enzymatic biosensing. Biosens. Bioelectron. 2017, 89, 681–688. [Google Scholar] [CrossRef]
- García, T.; Revenga-Parra, M.; Sobrino, B.; Carracedo, A.; Alonso, C.; Lorenzo, E.; Pariente, F. Electrochemical DNA base pairs quantification and endonuclease cleavage detection. Biosens. Bioelectron. 2011, 27, 40–45. [Google Scholar] [CrossRef]
- García-Mendiola, T.; Barreiro Martínez, T.; Pariente, F.; Molano, J.; Lorenzo, E. Screening of Specific Gene Mutations Associated with Cystic Fibrosis. Electroanalysis 2014, 26, 1362–1372. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N. Electrochemical nanobiosensing in whole blood: Recent advances. TrAC Trends Anal. Chem. 2016, 80, 167–176. [Google Scholar] [CrossRef]
- Carter, M.T.; Bard, A.J. Electrochemical investigations of the interaction of metal chelates with DNA. 3. Electrogenerated chemiluminescent investigation of the interaction of tris(1,10-phenanthroline)ruthenium(II) with DNA. Bioconjugate Chem. 1990, 1, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.; Bard, A.J. Electrochemical studies of the interaction of metal chelates with DNA. 4. Voltammetric and electrogenerated chemiluminescent studies of the interaction of tris(2,2’-bipyridine)osmium(II) with DNA. Anal. Chem. 1990, 62, 2658–2662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, G.; Lian, G.; Luo, F.; Xie, Q.; Lin, Z.; Chen, G. Electrochemiluminescence biosensor for miRNA-21 based on toehold-mediated strand displacement amplification with Ru(phen)32+ loaded DNA nanoclews as signal tags. Biosens. Bioelectron. 2020, 147, 111789. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, Y.; Zhang, H.; Wang, Z. Electrochemiluminescence Biosensor for Nucleolin Imaging in a Single Tumor Cell Combined with Synergetic Therapy of Tumor. ACS Sens. 2020, 5, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Khoshfetrat, S.M.; Ranjbari, M.; Shayan, M.; Mehrgardi, M.A.; Kiani, A. Wireless Electrochemiluminescence Bipolar Electrode Array for Visualized Genotyping of Single Nucleotide Polymorphism. Anal. Chem. 2015, 87, 8123–8131. [Google Scholar] [CrossRef]
- Nie, Y.; Zhang, X.; Zhang, Q.; Liang, Z.; Ma, Q.; Su, X. A novel high efficient electrochemiluminescence sensor based on reductive Cu(I) particles catalyzed Zn-doped MoS2 QDs for HPV 16 DNA determination. Biosens. Bioelectron. 2020, 160, 112217. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, X.; Zhao, J.; Chen, S.; Yuan, R. An ultrasensitive sensing platform for microRNA-155 based on H2O2 quenched hydroxide-dependent ECL emission of PFO Pdots. Biosens. Bioelectron. 2020, 150, 111872. [Google Scholar] [CrossRef]
- Li, J.; Tan, S.; Kooger, R.; Zhang, C.; Zhang, Y. MicroRNAs as novel biological targets for detection and regulation. Chem. Soc. Rev. 2014, 43, 506–517. [Google Scholar] [CrossRef]
- Xia, N.; Zhang, Y.; Wei, X.; Huang, Y.; Liu, L. An electrochemical microRNAs biosensor with the signal amplification of alkaline phosphatase and electrochemical–chemical–chemical redox cycling. Anal. Chim. Acta 2015, 878, 95–101. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Y.; Liu, H.; Xia, N. An ultrasensitive electrochemical miRNAs sensor based on miRNAs-initiated cleavage of DNA by duplex-specific nuclease and signal amplification of enzyme plus redox cycling reaction. Sens. Actuators B Chem. 2015, 208, 137–142. [Google Scholar] [CrossRef]
- Xia, N.; Liu, K.; Zhou, Y.; Li, Y.; Yi, X. Sensitive detection of microRNAs based on the conversion of colorimetric assay into electrochemical analysis with duplex-specific nuclease-assisted signal amplification. Int. J. Nanomed. 2017, 12, 5013–5022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Xia, N.; Liu, H.; Kang, X.; Liu, X.; Xue, C.; He, X. Highly sensitive and label-free electrochemical detection of microRNAs based on triple signal amplification of multifunctional gold nanoparticles, enzymes and redox-cycling reaction. Biosens. Bioelectron. 2014, 53, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Fu, C.; Huang, C.; Li, N.; Wang, Y.; Ge, S.; Yu, J. Paper-based closed Au-Bipolar electrode electrochemiluminescence sensing platform for the detection of miRNA-155. Biosens. Bioelectron. 2020, 150, 111917. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Y.; Shan, Y.; Jiang, M.; Jin, X.; Gong, M.; Xu, J. A novel and sensitive electrogenerated chemiluminescence biosensor for detection of p16INK4a gene based on the functional paste-like nanofibers composites-modified screen-printed carbon electrode. J. Electroanal. Chem. 2018, 823, 368–377. [Google Scholar] [CrossRef]
- Neethirajan, S.; Kobayashi, I.; Nakajima, M.; Wu, D.; Nandagopal, S.; Lin, F. Microfluidics for food, agriculture and biosystems industries. Lab Chip 2011, 11, 1574–1586. [Google Scholar] [CrossRef]
- Azam, N.F.N.; Roy, S.; Lim, S.A.; Uddin Ahmed, M. Meat species identification using DNA-luminol interaction and their slow diffusion onto the biochip surface. Food Chem. 2018, 248, 29–36. [Google Scholar] [CrossRef]
- Anderson, P.D.; Bokor, G. Bioterrorism: Pathogens as Weapons. J. Pharm. Pract. 2012, 25, 521–529. [Google Scholar] [CrossRef]
- Klietmann, W.F.; Ruoff, K.L. Bioterrorism: Implications for the clinical microbiologist. Clin. Microbiol. Rev. 2001, 14, 364–381. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.; Wilkins, E. Electrochemical Biosensors for Detection of Biological Warfare Agents. Electroanalysis 2003, 15, 157–167. [Google Scholar] [CrossRef]
- Spehar-Délèze, A.-M.; Gransee, R.; Martinez-Montequin, S.; Bejarano-Nosas, D.; Dulay, S.; Julich, S.; Tomaso, H.; O’Sullivan, C.K. Electrochemiluminescence DNA sensor array for multiplex detection of biowarfare agents. Anal. Bioanal. Chem. 2015, 407, 6657–6667. [Google Scholar] [CrossRef]

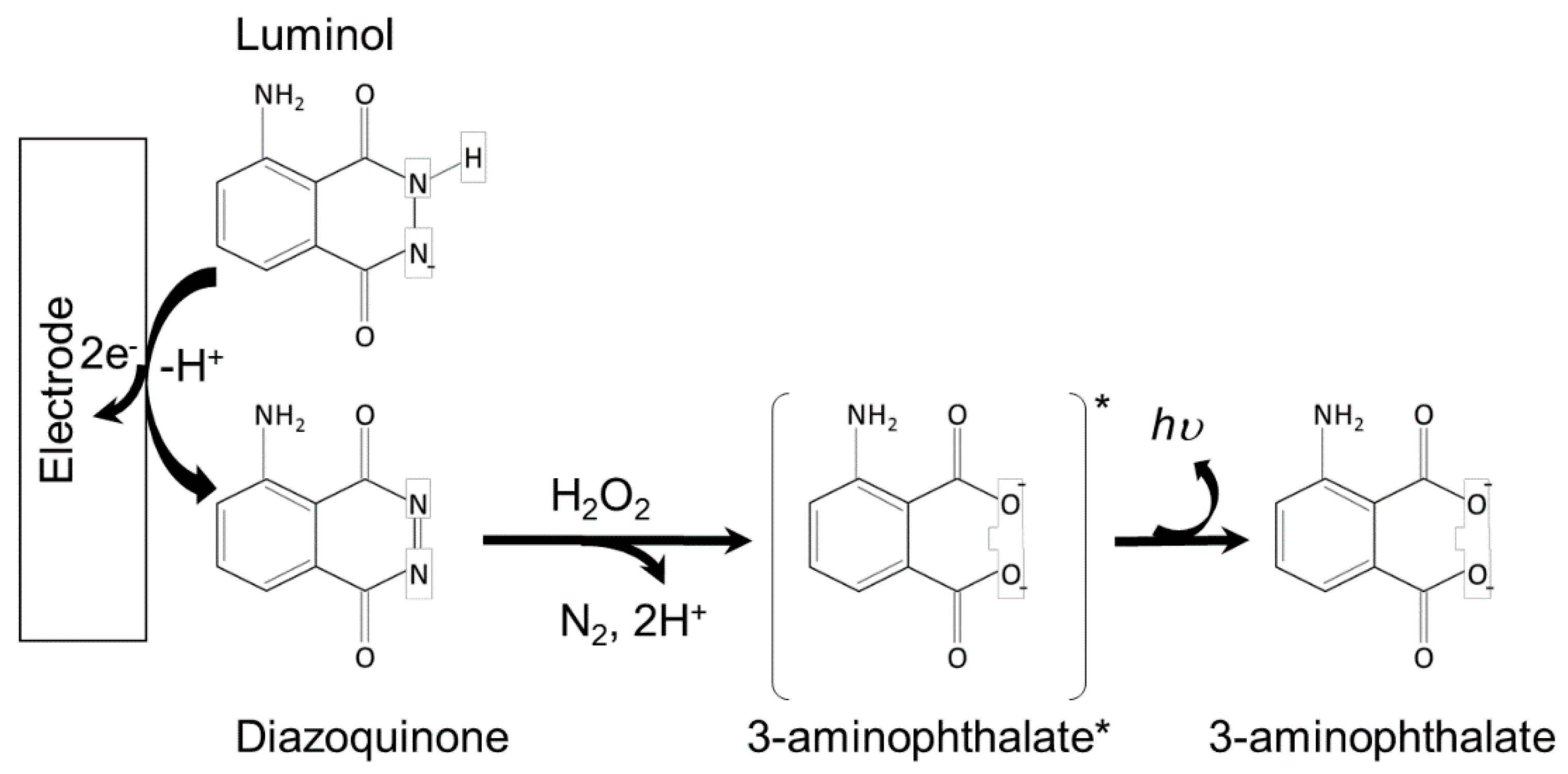
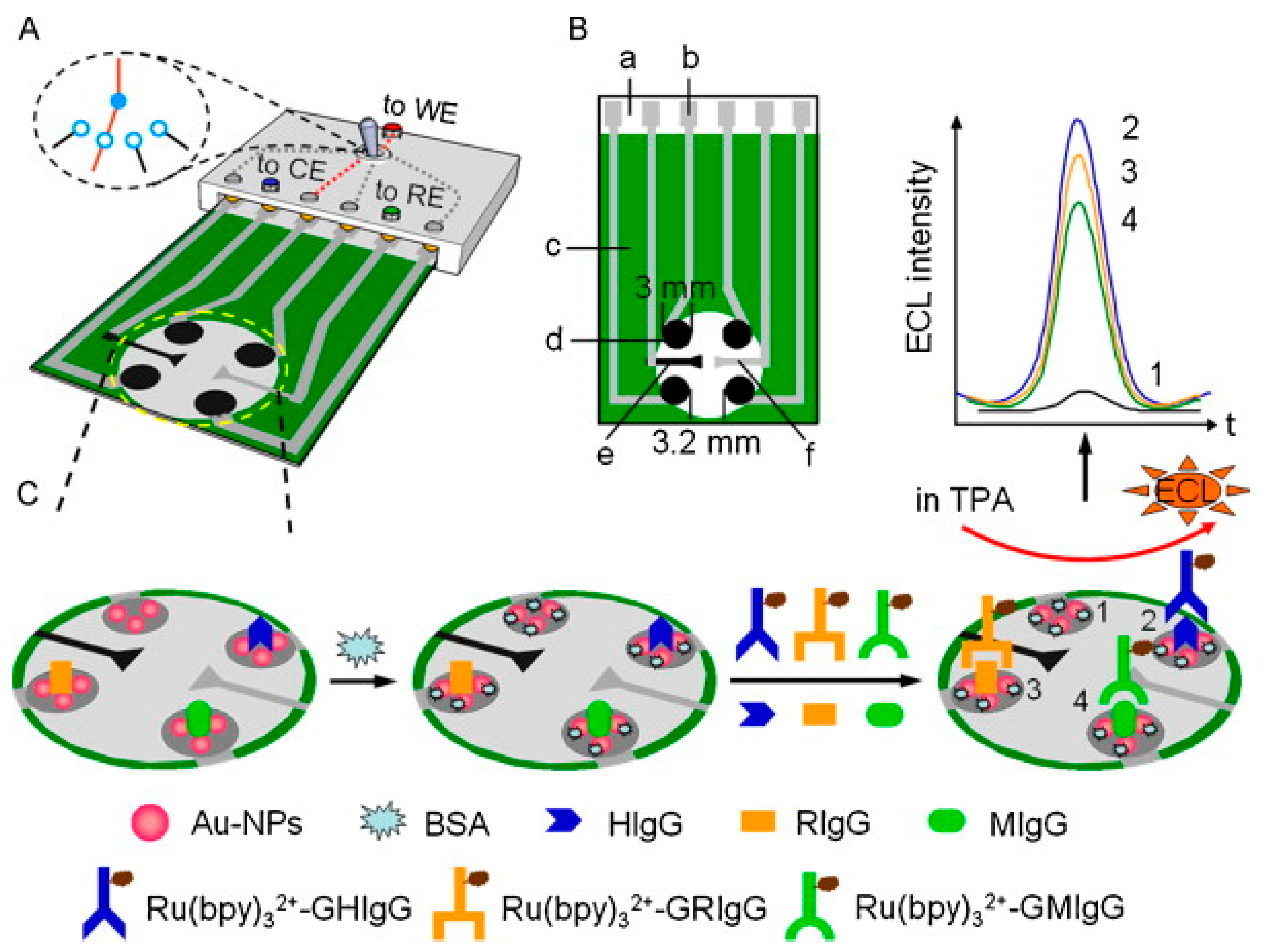






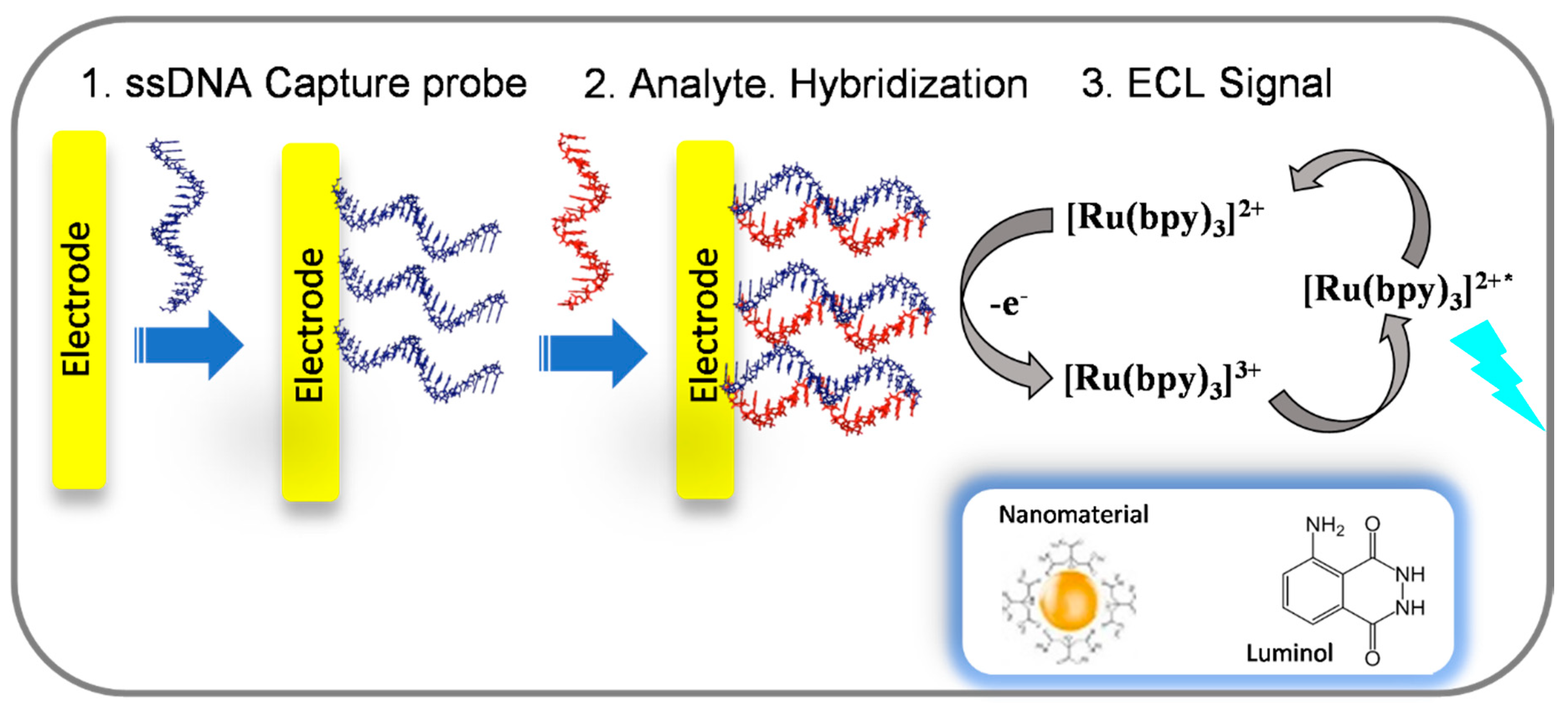


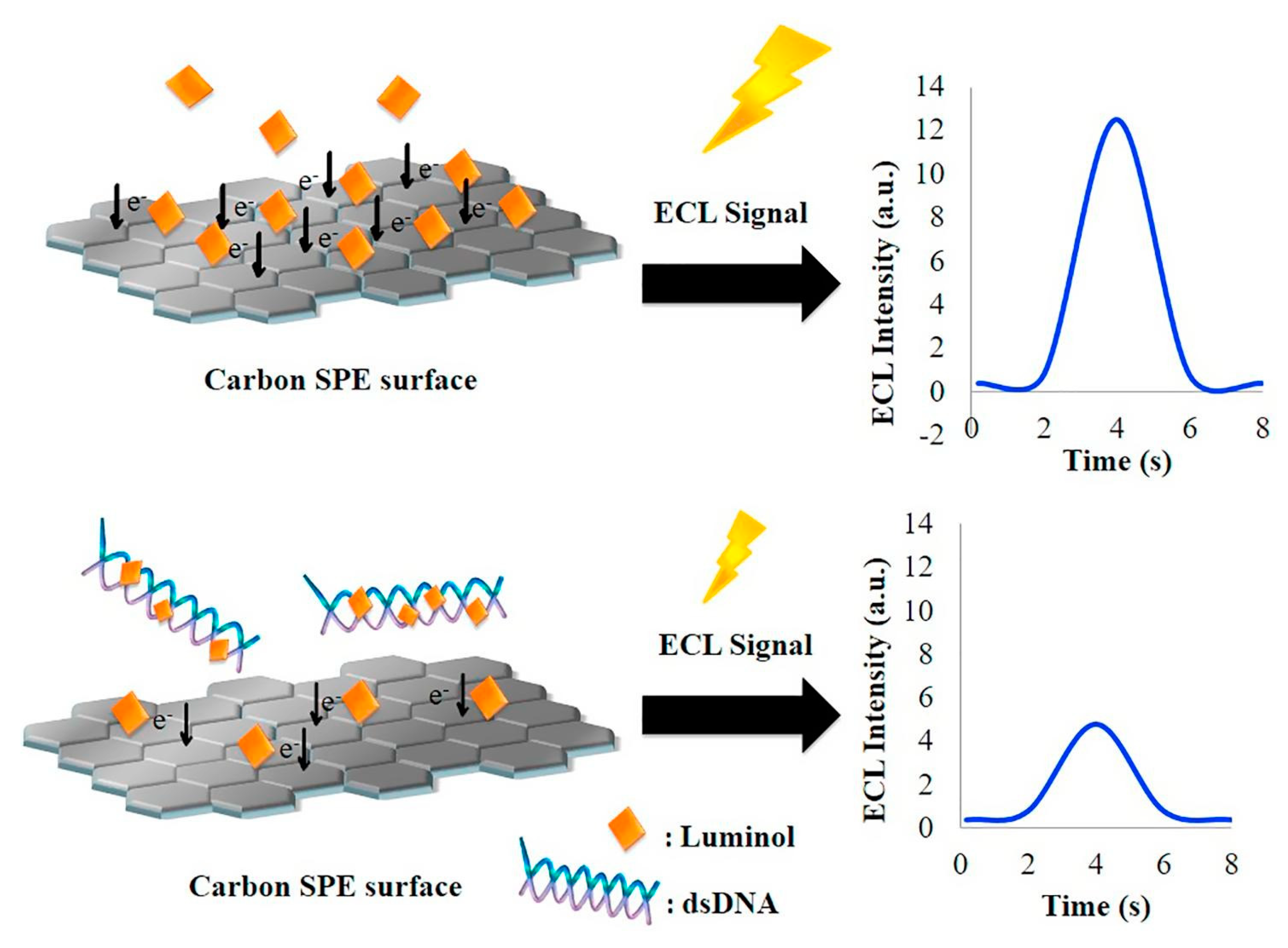
| Luminophore | Analyte | Sensing Type | Detection Limit | Reference |
|---|---|---|---|---|
| [Ru(bpy)3]2+ | beta 2-microglobulin | Direct immunosensor-Label-free immunosensor CdSe QDs-Co-reactant AuNPs decorated carbon nano-onions- amplified the ECL signals TPrA-Co-reactant | 1 fg/mL | [64] |
| [Ru(bpy)3]2+ | Clostridium Perfringens | Sandwich type immunosensor Pt Nps as luminophore support Ferrite particles support detection antibody and Glucose Dehydrogenase | 102 CFU/mL | [66] |
| [Ru(bpy)3]2+ | human, rabbit and Goat immunoglobulins | Competitive immunoassay Detection antibody covalently bonded to [Ru(bpy)3]2+ | 2.9, 6.1 and 6.5 ng/mL | [67] |
| [Ru(bpy)3]2+ | antitransglutaminase type-2 antibodies | Indirect immunosensor Biotinylated Detection antibody linked to streptavidin-[Ru(bpy)3]2+ | 0.47 ng/mL | [68] |
| [Ru(bpy)3]2+ | carbohydrate antigen 199 | Sandwich type immunosensor Detection antibody bonded to AuNPs covered by [Ru(bpy)3]2+ | 0.0055 U/mL | [52] |
| [Ru(bpy)3]2+ | Francisella tularensis | Sandwich type immunosensor silica-encapsulated [Ru(bpy)3]2+ bonded to detection antibody | 70 CFU/mL | [69] |
| [Ru(bpy)3]2+ | prostate specific antigen (PSA), prostate specific membrane antigen (PSMA) and platelet factor-4 (PF-4) | Sandwich type immunosensor Silica nanoparticles coated with [Ru(bpy)3]2+ bounded to the detection antibody | 300–500 fg/mL | [70] |
| [Ru(bpy)3]2+ | MCF-7 cell | Direct immunosensor Silica and gold nanoparticles bounded to [Ru(bpy)3]2+ | 30 Cell/mL | [71] |
| [Ru(bpy)3]2+ | alpha-fetoprotein | Sandwich type immunosensor Silica and gold nanoparticles bounded to [Ru(bpy)3]2+Biotinylated capture antibody linked to streptavidin-coated magnetic particles | 0.02 ng/mL | [72] |
| [Ru(bpy)3]2+ | hemoglobin | Molecularly imprinted polymer- antibody Sandwich type immunosensor [Ru(bpy)3]2+@silica@AuNPs conjugated with the detection antibody magnetic molecularly imprinted polymers | 0.023 pg/mL | [73] |
| [Ru(bpy)3]2+ | carbohydrate antigen-199 and carcinoembryonic antigen | Molecularly imprinted polymer- antibody Sandwich type immunosensor [Ru(bpy)3]2+@silica@AuNPs conjugated with the detection antibody | 0.01 U/L and 0.02 pg/mL | [74] |
| [Ru(bpy)3]2+ | alpha-fetoprotein | Sandwich type immunosensor [Ru(bpy)3]2+-MWCNT-AuNPs-Detection antibody Fe3O4@Au nanoparticles linked to capture antibody | 3 pg/mL | [75] |
| [Ru(bpy)3]2+ | mouse IgG | Competitive immunosensor amorphous carbon nanoparticles with quenching effect covalently linked to mouse IgG | 0.35 ng/mL | [76] |
| [Ru(bpy)3]2+ | thyroglobulin | Sandwich type immunosensor TiO2 nanodots modified electrode, where the capture antibody is immobilized. Bioconjugate formed by MXenes layers covered by [Ru(bpy)3]2+ and ZnO quantum dots, linked to the detection antibody | 1 fg/mL | [77] |
| Luminol | human immunodeficiency virus type 1 antibody (HIV-1) | Competitive immunosensor Molecularly imprinted polymer as capture probe Horse radish peroxidase-HIV-1 | 1:60,000 dilution ratio of standard positive serum | [78] |
| Luminol | alpha-fetoprotein | Sandwich type immunosensor Luminol groups adsorber over AuPd NPs linked to detection antibody | 0.005 ng/mL | [79] |
| Luminol | carcinoma antigen 125 | Sandwich type immunosensor Luminol labeled detection antibody | 0.0074 U/mL | [80] |
| Luminol | transferrin (TRF) | Label-free immunosensor Enhacement of luninol ECL when TRF is attached to the capture antibody | 0.033 ng/mL | [82] |
| Semiconductor graphite-like carbon nitride/S2O82− co-reactant | carbohydrate antigen 125 | Label-free immunosensor Carboxilate g-C3N4/aminated Fe3O4/capture antibody | 0.4 mU/mL | [83] |
| Semiconductor carbon nanocrystals (CNCs)/S2O82− co-reactant | prostate specific antigen (PSA) | Sandwich type immunosensor PtAg@CNCs linked to detection antibody | 0.6 pg/mL | [84] |
| CdTe quantum dots/S2O82− co-reactant | carcinoembryonic antigen | Sandwich type immunosensor Detection antibody linked to CdTe quantum dots/AgNPs | 2.5 mU/mL | [86] |
| SnO2 nanocrystal/S2O82− co-reactant | carcinoembryonic antigen | Sandwich type immunosensor SnO2@PtRu linked to detection antibody | 0.72 pg/mL | [87] |
| ZnO quantum dots/S2O82− co-reactant | prostate specific antigen (PSA) | Sandwich type immunosensor ZnO@CNT linked to detection antibody | 0.61 pg/mL | [88] |
| CdTe quantum dots (QDs) | carcinoma embryonic antigen | Sandwich type immunosensor CdTe QDs linked to detection antibody Capture antibody linked to AuNPs@Fe3O4 particles. | 0.38 pg/mL | [89] |
| graphene quantum dots (GQDs)/H2O2 as co-reactant | carcinoma embryonic antigen | Sandwich type immunosensor Au@Pt core–shell nanoparticles modified with graphene quantum dots (GQDs) | 0.6 pg/mL | [90] |
| Carbon dots/TEA as co-reactant | cancer antigen 125 | Sandwich type immunosensor Detection antibody linked to carbon nanodots modified nanoporous silica nanoparticles | 4.3 mU/mL | [91] |
| phenyleneethynylene derivatives (P-acid)/TEA as co-reactant | carcinoembryonic antigen | Sandwich type immunosensor P-acid/Pt–AgANPs linked to detection antibody | 0.3 pg/mL | [92] |
| Luminophore | Enzyme | Analyte | Sensing Type | Detection Limit | Reference |
|---|---|---|---|---|---|
| Luminol | Glucose oxidase | Glucose | Surface-unpassivated CdTe QDs | 0.3 mM | [108] |
| Luminol | Glucose oxidase | Glucose | Au and TiO2 nano-composite | 0.22 mM | [109] |
| Luminol | Uricase | Uric acid | 3,3,5,5-tetramethylbenzidine and chitosan | 0.44 µM | [111] |
| Luminol | Lactate oxidase | Lactate | Methocel membrane | 5 µM | [114] |
| Luminol | Choline oxidase | Choline | Poly(vinyl alcohol) bearing styrylpyridinium groups (PVA–SbQ) photocrosslinked polymer | 0.2 µM | [120] |
| Luminol | Glucose oxidase and Lactate oxidase | Glucose Lactate | PVA–SbQ (poly(vinyl alcohol) | 3 µM 10 µM | [121] |
| Luminophore | Analyte | Sensing Type | Detection Limit | Reference |
|---|---|---|---|---|
| [Ru(phen)3]2+ | mRNA-21 | Toehold-mediated strand displacement (TMSD) | 0.65 fM | [156] |
| Luminol | nucleolin in a single HeLa cell | Mesoporous silica nanoparticles (MSN) loaded with doxorubicin (DOX) and phorbol 12-myristate 13-acetate (PMA) | [157] | |
| Luminol | SNP detection | Luminol-platinum nanoparticles | 2−600 pM | [158] |
| Nanomaterials: MoS2 QDs | HPV16 DNA | Cu(I) reductive particles catalyzed Zn-doped MoS2 QDs | 0.03 nmol/L | [159] |
| Nanomaterials: PFO Pdots | microRNA-155 | Quenching effect of H2O2 | 12.2 aM | [160] |
| [Ru(phen)3]2+/TPrA | mRNA-21 | Wax-printing technology, screen printing method and in-situ AuNPs growth | 0.67 pM | [166] |
| [Ru(bpy)3]2+/silver nanoparticles (AgNPs) | p16INK4a | Paste-like nanofibers composites-modified carbon SPE | 0.05 pM | [167] |
| Luminol | Susscrofa (Porcine) DNA | Loop-mediated isothermal amplification | 0.1 pg/μL | [169] |
| [Ru(bpy)3]2+ | pathogens | Sandwich-type assay | 0.6–1.2 nmol/L | [173] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Periñán, E.; Gutiérrez-Sánchez, C.; García-Mendiola, T.; Lorenzo, E. Electrochemiluminescence Biosensors Using Screen-Printed Electrodes. Biosensors 2020, 10, 118. https://doi.org/10.3390/bios10090118
Martínez-Periñán E, Gutiérrez-Sánchez C, García-Mendiola T, Lorenzo E. Electrochemiluminescence Biosensors Using Screen-Printed Electrodes. Biosensors. 2020; 10(9):118. https://doi.org/10.3390/bios10090118
Chicago/Turabian StyleMartínez-Periñán, Emiliano, Cristina Gutiérrez-Sánchez, Tania García-Mendiola, and Encarnación Lorenzo. 2020. "Electrochemiluminescence Biosensors Using Screen-Printed Electrodes" Biosensors 10, no. 9: 118. https://doi.org/10.3390/bios10090118
APA StyleMartínez-Periñán, E., Gutiérrez-Sánchez, C., García-Mendiola, T., & Lorenzo, E. (2020). Electrochemiluminescence Biosensors Using Screen-Printed Electrodes. Biosensors, 10(9), 118. https://doi.org/10.3390/bios10090118






