Abstract
Hypoxanthine (hpx) is an important molecule for both biochemistry research and biomedical applications. It is involved in several biological processes associated to energy and purine metabolism and has been proposed as a biomarker for a variety of disease states. Consequently, the discovery and development of systems suitable for the detection of hypoxanthine is pretty appealing in this research field. Thus, we have obtained a stable diruthenium (III) compound in its dehydrated and hydrated forms with formula [{Ru(µ-Cl)(µ-hpx)}2Cl4] (1a) and [{Ru(µ-Cl)(µ-hpx)}2Cl4]·2H2O (1b), respectively. This purine-based diruthenium(III) system was prepared from two very different starting materials, namely, inosine and azathioprine, the latter being an immunosuppressive drug. Remarkably, it was observed that an unusual azathioprine hydrolysis occurs in the presence of ruthenium, thus generating hypoxanthine instead of the expected 6-mercaptopurine antimetabolite, so that the hpx molecule is linked to two ruthenium(III) ions. 1a and 1b were characterized through IR, SEM, powder and single-crystal X-ray Diffraction and Cyclic Voltammetry (CV). The electrochemical studies allowed us to detect the hpx molecule when coordinated to ruthenium in the reported compound. The grade of sensitivity, repeatability and stability reached by this diruthenium system make it potentially useful and could provide a first step to develop new sensor devices suitable to detect hypoxanthine.
1. Introduction
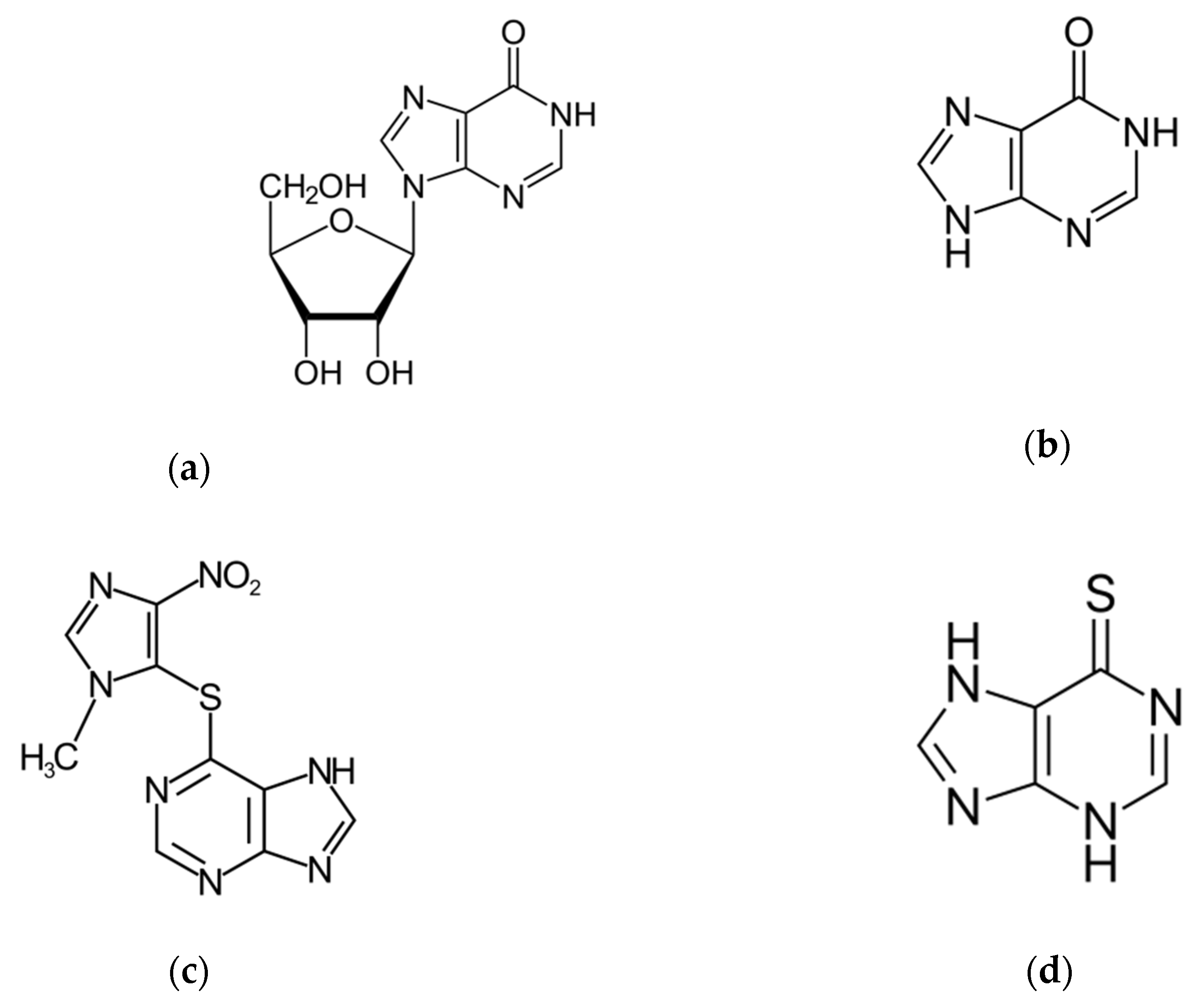
Hypoxanthine (6-hydroxypurine) is a deaminated form of adenine and a constituent of the nucleoside inosine (Scheme 1). It is formed during purine metabolism and is found in both tissues and body fluids of human beings and animals. The identification of hypoxanthine (hpx) as a biomarker for hypoxia has long been known [1,2]. More recently, hpx has also been proposed as a biomarker for colorectal cancer [3], Alzheimer’s disease [4], multiple sclerosis [5] and cardiac ischemia [6] and as checkpoint metabolite for other inflammatory processes [7] and toxicity levels [8]. In addition, hpx has been studied as a strong predictor of performance in highly trained athletes in sports and physical exertion, the hpx concentration indicating the training status and adaptation in consecutive phases of long training cycles [9,10]. Moreover, the determination of levels of hpx in meat and fish products has been established to be an important and convenient indicator of freshness and quality control in the food industry [11,12].
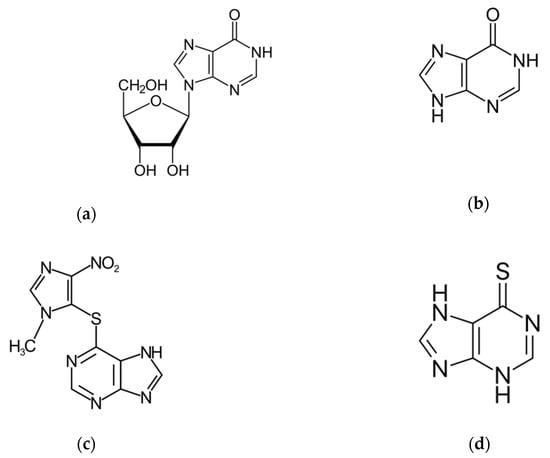
Scheme 1.
Molecular structure of: (a) Inosine, (b) Hypoxanthine, (c) Azathioprine and (d) 6-Mercaptopurine.
As a continuation of our interest in investigating biomolecule-based complexes of several metal ions [13,14,15,16,17] and their implementation in devices [18,19], we have studied the synthesis of ruthenium with some purine-based compounds that either contain hypoxanthine or could generate this analyte. Thus, we have investigated the products of the hydrolysis reactions with inosine and azathioprine (Scheme 1).
The nucleoside inosine is formed by a hpx molecule that is connected to a ribose ring through N(9)-glycosidic bond. It belongs to a group of purine antimetabolites recommended for treatment of measles and herpes infections, among others [20]. Studies on the inosine hydrolysis have been previously reported [21,22]. Azathioprine is a slow-release prodrug of the antimetabolite 6-mercaptopurine (Scheme 1), which is used as an anticancer and immunosuppressive drug [23,24]. It is used as an established clinical agent for the treatment of several pathologies, such as rheumatoid arthritis, ulcerative colitis, systemic lupus and Crohn’s disease, and also as an anti-rejection medication in human organ transplantation [23,24]. The N-methylnitroimidazolyl group of the azathioprine molecule protects the active form 6-mercaptopurine. Azathioprine undergoes hydrolysis in vivo to alter the metabolism and distribution of the drug toward its target [23,24].
Previously reported methods of determination of hpx include high-performance liquid chromatography [25,26,27,28], capillary electrophoresis [29,30], spectrophotometry [31,32] and electrochemiluminescence [33,34], which are usually costly and laborious and require a long analysis time. On the other hand, electrochemical methods offer several advantages, such as relatively simple and cheap instrumentation, high selectivity and sensitivity, high stability and rapid response time [35,36,37]. Nevertheless, given the wide range of potentially interfering purine-based compounds that can affect the detection of hpx generated in biological processes, more selective methods of analysis are needed.
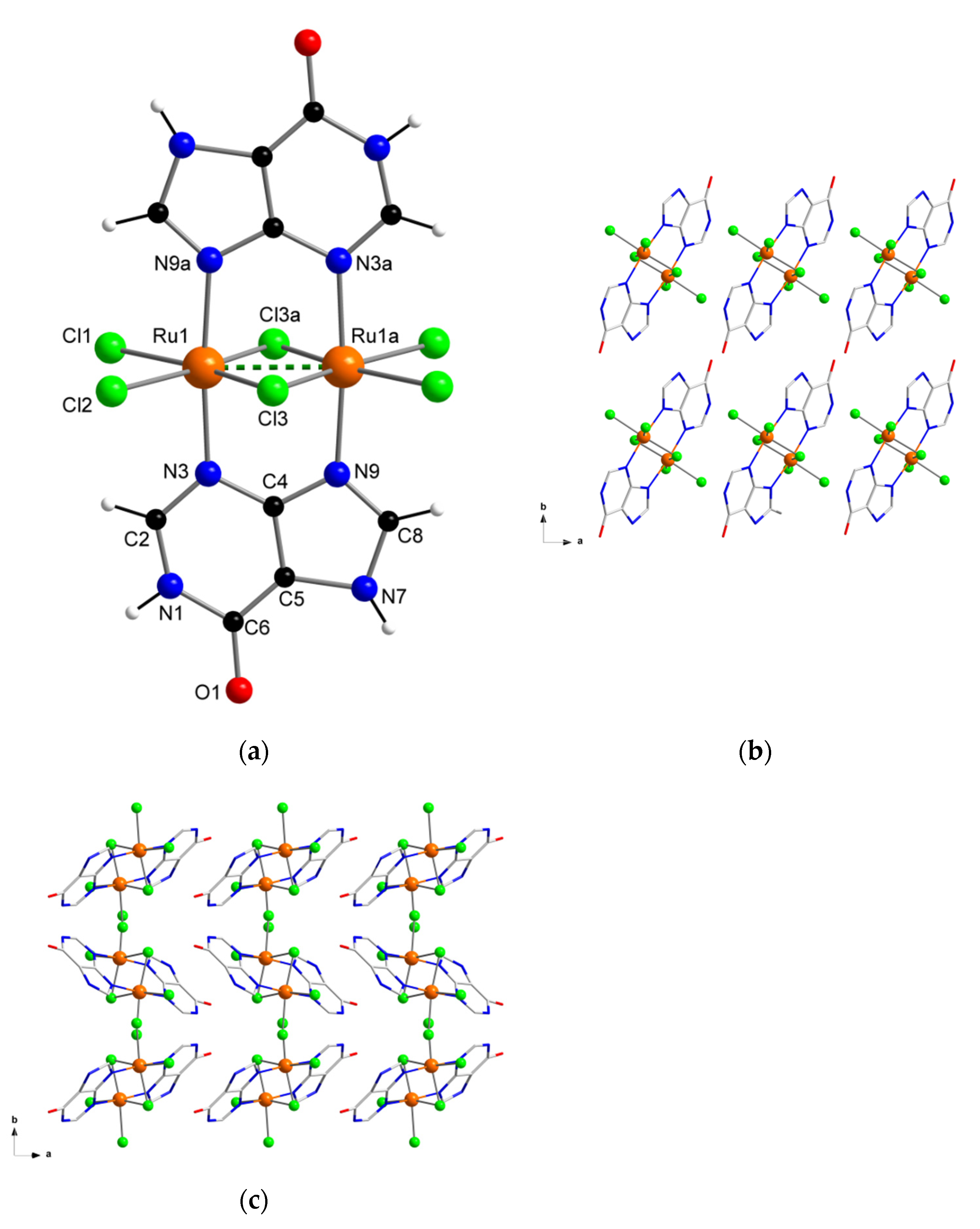
Herein, we report the preparation, characterization and electrochemical properties of a new diruthenium system (Figure 1), which shows great stability and also selectivity for the hypoxanthine molecule, and therefore, could establish a first step to develop new sensor devices suitable for hypoxanthine detection.
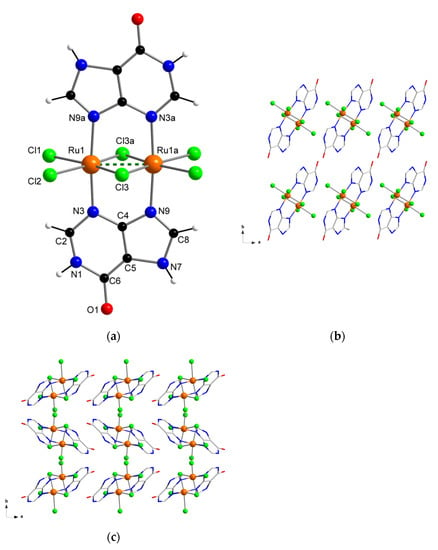
Figure 1.
(a) Molecular structure of the [{Ru(µ-Cl)(µ-hpx)}2Cl4] complex in 1a and 1b; (b) view along the crystallographic c axis of a fragment of the crystal packing of 1a; (c) view along the crystallographic c axis of a fragment of the crystal packing of 1b.
2. Materials and Methods
2.1. Reagents and Instruments
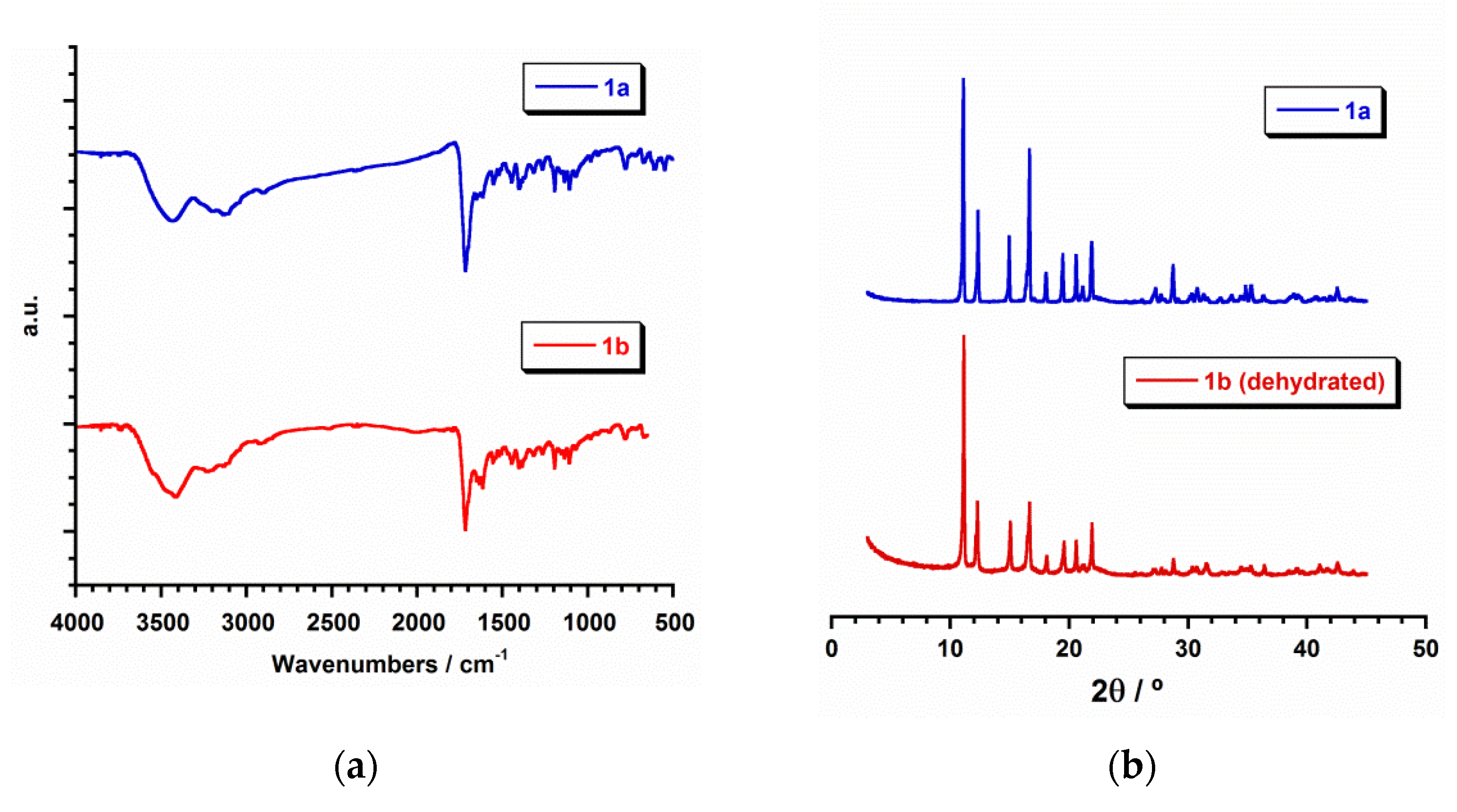
All of the manipulations were performed under aerobic conditions. Azathioprine and inosine were purchased from Alfa Aesar and Sigma Aldrich, respectively. Ruthenium precursors, RuCl3·H2O and K2[RuCl5(H2O)], and the rest of materials were used as-received and were of reagent grade. Elemental analyses (C, H, N) and X-ray microanalysis were performed by the Central Service for the Support to Experimental Research (SCSIE) at the University of Valencia. Scanning electron microscopy (SEM) images and results were obtained from a Hitachi S-4800 field emission scanning electron microscope. Infrared spectra (IR) of 1a and 1b (in the Supplementary Materials) were recorded with a PerkinElmer Spectrum 65 FT-IR spectrometer in the range of 400 to 4000 cm−1 (Figure 2).
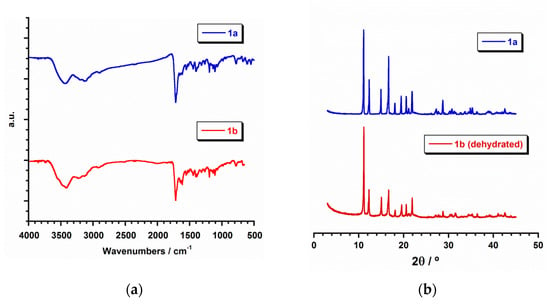
Figure 2.
(a) IR spectra (Transmission mode) for 1a (blue) and 1b (red); (b) plot of the experimental powder X-ray diffraction (PXRD) patterns profile (2θ/°) in the range 0–50° for 1a (blue) and dehydrated 1b (red).
Electrochemical studies were performed by using an Autolab/PGSTAT 204 scanning potentiostat operating at a scan rate range of 10–250 mV s−1. Cyclic voltammograms were carried out by using 0.1 M NBu4PF6 as supporting electrolyte and 0.001 M solutions of 1a, inosine and azathioprine in dry dimethylformamide (dmf). The working electrode was a glassy carbon disk (0.32 cm2) that was polished with 1.0 μm of diamond powder, sonicated, washed with absolute ethanol and acetone and air dried. The reference electrode was AgCl/Ag, separated from the test solution by a salt bridge containing the solvent/supporting electrolyte, with platinum as an auxiliary electrode. All experiments were performed in standard electrochemical cells at 25 °C under argon. The investigated potential range was in the range of −2.0 to +2.0 V vs. AgCl/Ag. Ferrocene (Fc) was added as internal standard at the end of all the measurements. The formal potentials were measured at a scan rate of 200 mV s−1 and were referred to the ferrocenium/ferrocene (Fc+/Fc) redox couple.
2.2. Preparation of the Compounds
2.2.1. Synthesis of [{Ru(µ-Cl)(µ-hpx)}2Cl4] (1a)
A solvothermal reaction of K2[RuCl5(H2O)] (3.52 mg, 0.01 mmol) and inosine (2.66 mg, 0.01 mmol) was performed in HCl (4 mL, 3 M) at 90 °C for 3 days, followed by a 12-h cooling process to room temperature. Dark brown crystals of 1a were obtained and were suitable for X-ray data collection. Yield: ca. 55%. Anal. Calcd. for C10H8Cl6N8O2Ru2 (1a): C, 17.5; H, 1.2; N, 16.3. Found: C, 17.7; H, 1.3; N, 16.5. IR peaks (KBr pellets, ν/cm−1): 3202(m), 3132(m), 3110(m), 3046(m), 2905(m), 1718(vs), 1653(m), 1555(m), 1522(w), 1447(m), 1407(m), 1318(m), 1267(m), 1196(s), 1160(w), 1137(m), 1118(s), 984(w), 779(m), 723(w), 674(m), 610(m), 550(m), 478(w) and 412(w).
2.2.2. Synthesis of [{Ru(µ-Cl)(µ-hpx)}2Cl4]·2H2O (1b)
RuCl3·H2O (6.60 mg, 0.03 mmol) and azathioprine (6.80 mg, 0.03 mmol) reacted through a solvothermal reaction in HCl (4 mL, 3 M) at 90 °C for 3 days, followed by a 12-h cooling process to room temperature. Dark brown crystals of 1b were thus obtained, which were suitable for X-ray data collection. Yield: ca. 72%. Anal. Calcd. for C10H12Cl6N8O4Ru2 (1b): C, 16.6; H, 1.7; N, 15.5. Found: C, 16.7; H, 1.9; N, 15.2. IR peaks (KBr pellets, ν/cm−1): 3433(br), 3202(m), 3133(m), 3110(m), 3047(m), 2905(m), 1718(vs), 1653(m), 1615(m), 1554(m), 1522(w), 1447(m), 1407(m), 1318(m), 1267(m), 1196(s), 1160(w), 1137(m), 1118(s), 984(w), 779(m), 723(w), 674(m), 610(m), 550(m), 478(w) and 412(w).
2.3. X-ray Data Collection and Structure Refinement
X-ray diffraction data from single crystals of dimensions 0.18 × 0.06 × 0.04 (1a) and 0.27 × 0.14 × 0.11 mm3 (1b) were collected on a Bruker D8 Venture diffractometer with graphite-monochromated Mo-Kα radiation (λ = 0.71073 Å). Crystal parameters and refinement results for 1a and 1b are summarized in Table 1. The structures were solved by standard direct methods and subsequently completed by Fourier recycling by using the SHELXTL software packages. The obtained models were refined with version 2017/1 of SHELXL against F2 on all data by full-matrix least squares [38]. In the two samples, all non-hydrogen atoms were anisotropically refined, whereas the hydrogen atoms of the hpx molecules were set in calculated positions and refined isotropically by using the riding model. The graphical manipulations were performed with the DIAMOND program [39]. The CCDC codes for 1a and 1b are 2046049 and 2046050, respectively. In addition, X-ray powder diffraction (PXRD) measurements were performed through a capillary sample holder in a PANalytical Empyrean diffractometer containing a hybrid monochromator (Cu-Kα1 radiation) and a PIXcel detector.

Table 1.
Summary of the crystal data and structure refinement parameters for 1a and 1b.
3. Results and Discussion
3.1. Synthetic Procedure
By reacting K2[RuCl5(H2O)] with inosine (for 1a) and RuCl3·H2O with azathioprine (for 1b) in hydrochloric acid (3 M) solutions, we obtained the same diruthenium(III) complex, but in its dehydrated and hydrated forms [{Ru(µ-Cl)(µ-hpx)}2Cl4] (1a) and [{Ru(µ-Cl)(µ-hpx)}2Cl4]·2H2O (1b), respectively. In both cases, the synthesis process was performed by heating the reaction mixture at 90 °C through a solvothermal method, followed by a 12-h cooling process to room temperature as the crystallization technique. Thus, dark brown crystals of 1a and 1b were obtained in satisfactory yields. Inosine was chosen in this work as starting material because it is a suitable source of hypoxanthine (Scheme 1), and given the synthetic conditions, this purine nucleoside can easily undergo hydrolysis through a N(9)-glycosidic bond with a rapid release of hypoxanthine along with the ribose sugar [21]. The released hypoxanthine molecule acts as a ligand towards the ruthenium metal ions, generating the stable complex 1a. In the case of the synthesis with azathioprine, 1b was obtained as an unexpected product in a reaction which was intended to replace some of the Cl groups linked to the ruthenium(III) ions by the potentially chelating 6-mercaptopurine antimetabolite (Scheme 1). Instead, the species 1b was formed. The hydrolytic reaction that azathioprine undergoes during its mechanism of drug action, that is, the cleavage of the N-methylnitroimidazolyl group from the sulfur atom, and therefore, the release of 6-mercaptopurine [23,24], in the presence of ruthenium(III) ions and in an acid medium, would be modified. After releasing the 6-mercaptopurine molecule, water attacks it, generating hypoxanthine, which in turn would lead to the formation of the species 1b, so that the reported syntheses constitute new preparative methods to obtain purine-based Ru(III) compounds.
3.2. Description of the Crystal Structure
The crystal structure and exact chemical composition of 1a and 1b were established by single-crystal X-ray diffraction. 1a and 1b crystallize in the monoclinic system with space group P21/c (Table 1). Their structures are made up of the neutral dinuclear [{Ru(µ-Cl)(µ-hpx)}2Cl4] units. Only in 1b there are solvent molecules of crystallization, which are H2O molecules. In their asymmetric units, half a [{Ru(µ-Cl)(µ-hpx)}2Cl4] complex is in both 1a and 1b, and one H2O molecule of crystallization is also present in 1b (Figure 1).
In the dinuclear complex of 1a and 1b, each six-coordinate RuIII ion is bonded to four chloride ions and two nitrogen atoms (N3 and N9) from two hpx molecules in a distorted octahedral environment. No significant differences are found in the Ru–Cl [average value, 2.329(1) Å in 1a and 2.310(1) Å in 1b] and Ru–N [2.066(1) Å in 1a and 2.061(1) Å in 1b] bond lengths, which are similar in both compounds and are in agreement with those values found in previously reported RuIII systems [40,41]. The two RuIII ions are linked each other through two hpx molecules and a double Ru–Cl–Ru bridge and the short intramolecular Ru···Ru distance that is generated (ca. 2.6 Å) indicates the formation of a metal-metal bond (dashed line in Figure 1a). The hpx molecule in 1a and 1b is planar and its bond lengths and angles agree with those found in the literature for similar dinuclear complexes [42,43].
In the crystal lattice of 1a and 1b the dinuclear [{Ru(µ-Cl)(µ-hpx)}2Cl4] units pack in different ways (Figure 1b,c), because of their pseudopolymorphism. Thus, π···π stacking interactions between neighboring rings of coordinated hpx molecules occur in 1b [with the shortest intercentroid distance being ca. 3.85 Å], whereas no π⋯π type interactions are observed in 1a. Cl···π interactions between adjacent [{Ru(µ-Cl)(µ-hpx)}2Cl4] units take place in both RuIII compounds [Cl···π distances varying in the ranges 3.16–3.37 and 3.46–3.73 Å for 1a and 1b, respectively]. In addition, H-bonding interactions contribute to stabilizing the crystal structure in both systems. Finally, the powder X-ray diffraction (PXRD) patterns of 1a and 1b, which are quite different to that of the hpx molecule [44], confirmed the homogeneity of their bulk samples (Figure 2).
3.3. Scanning Electron Microscopy (SEM)
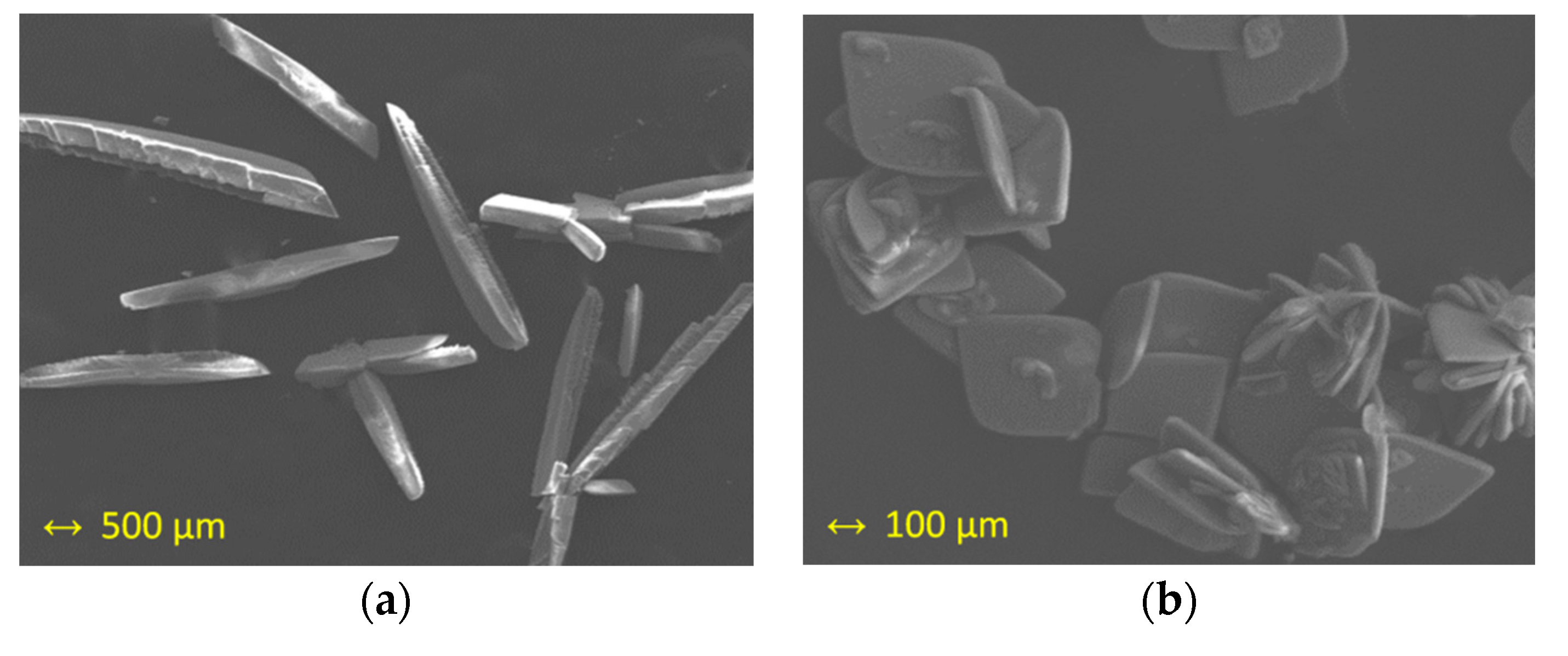
The study of X-ray microanalysis through of scanning electron microscopy (SEM) gave an Ru/Cl molar ratio of 1:3 for both studied samples (1a and 1b) and was performed as previously done for other ruthenium systems [45,46]. Sulfur was not detected in 1a nor 1b. The pseudopolymorphism of 1a and 1b was evident in the recorded images that are given in Figure 3, where crystals of 1a are shown as needles, whereas crystals of 1b are displayed as crystallized plates.
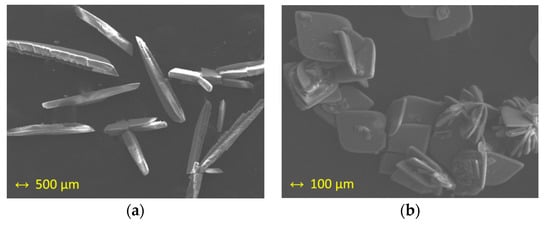
Figure 3.
Scanning electron microscopy (SEM) images of: (a) crystals of 1a; (b) crystals of 1b.
3.4. Cyclic Voltammetry (CV)
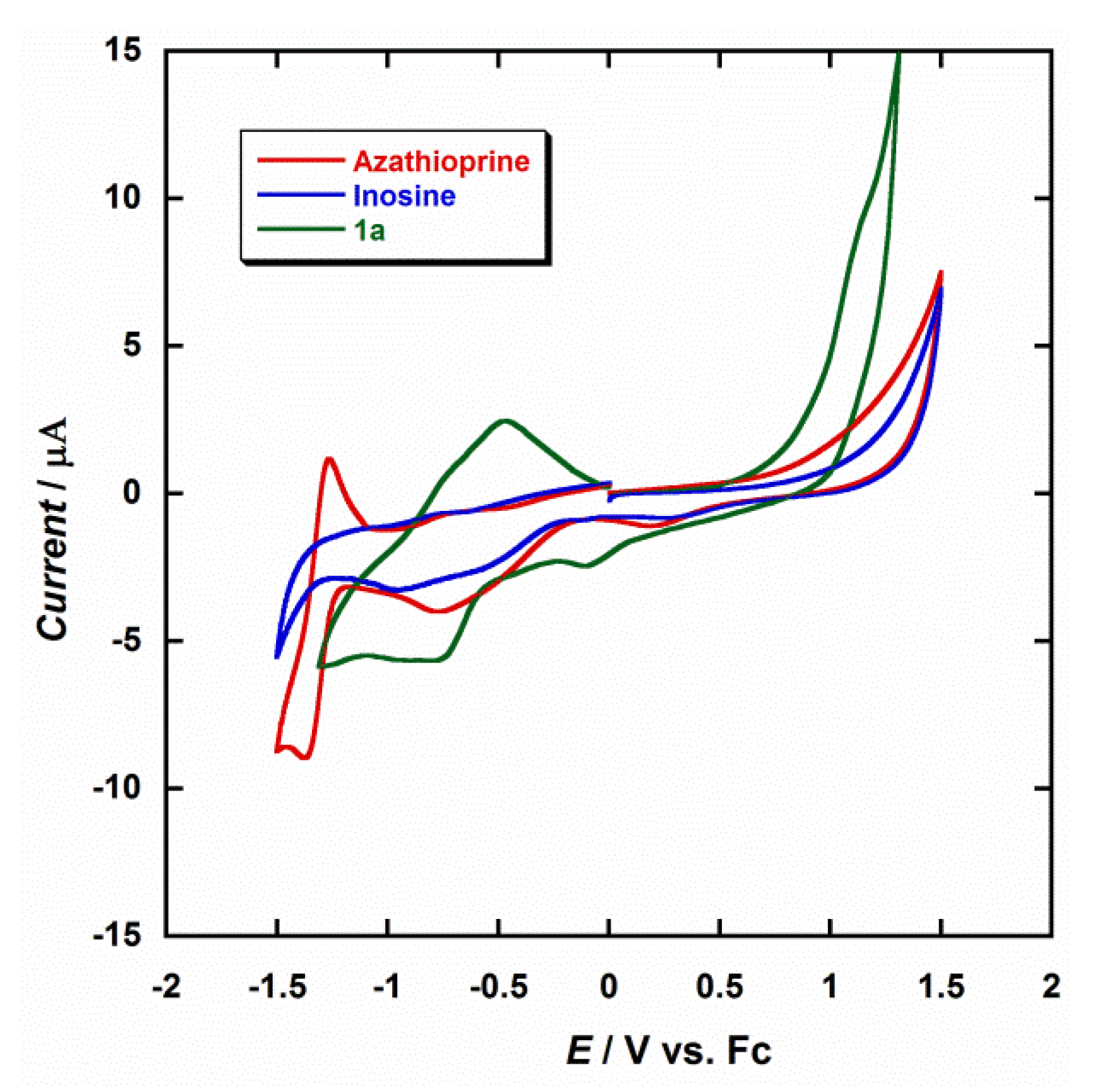
The electrochemical properties of the diruthenium(III) complex were investigated through cyclic voltammetry (CV) in dry dmf and at room temperature. Figure 4 shows a comparison of CV curves obtained in the same conditions for 1a, inosine and azathioprine. The electrochemical behavior of azathioprine has been studied in detail in previous works [47,48,49,50]; nevertheless, we have included it for the purpose of our investigation. The most characteristic feature observed during the electrochemical reduction of azathioprine is the peak detected at a potential of about −1.30 V, which can be assigned to the reduction of the nitro group (-NO2) to the corresponding hydroxylamine (-NHOH) that the N-methylnitroimidazolyl moiety of the molecule contains [47,48,49,50]. The CV curve obtained for inosine is very similar to that of hypoxantine, which makes the detection of hypoxantine in the presence of inosine quite difficult [51,52].
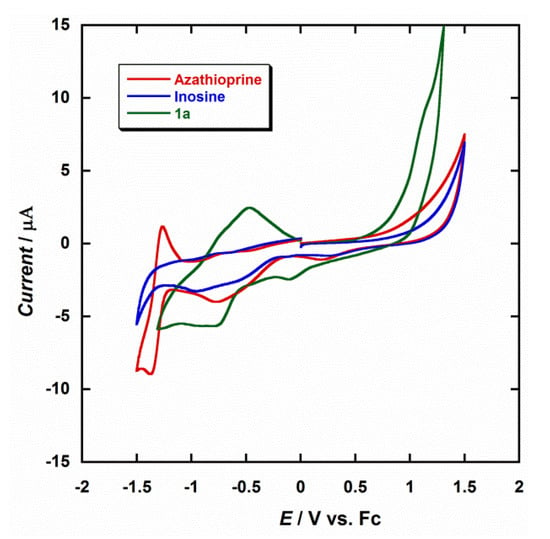
Figure 4.
Cyclic voltammograms of 1a (green), inosine (blue) and azathioprine (red) in dry dmf (0.1 M NBu4PF6) at 25 °C and scan rate 200 mV/s.
Diruthenium complexes exhibiting metal–metal bonding have been extensively investigated for decades [53]. In general, this type of Ru2 compounds show electronic structures and redox properties that are unique [54,55,56]. Only one or up to three redox processes can be observed in the CV curves of these systems, depending on the solvent and the supporting electrolyte [54,55,56]. In dimethylformamide (dmf), only a single reduction would be expected, which would occur between 0.0 V and −0.30 V, so that the first reduction peak observed at −0.11 V for 1a would be associated to the RuIII/RuII reduction process, which is very close to that reported for similar Ru2III systems [54]. A second peak at −0.82 V would be generated by the influence of the analyte, in this case the hpx molecule (Figure 4). For testing repeatability of the CV curve of the studied Ru2III system (1a), it was measured five times. Thus, a relative standard deviation of approximately 1.5% of the current response was obtained. It is evident from Figure 4 that there is a marked difference between the reported reductions waves for these three compounds, their current peaks being found to be well-resolved at the employed conditions, and the coordination of the hpx molecule being responsible of the CV curve observed for 1a. Therefore, given the possibility of coexistence of hypoxanthine and inosine, or azathioprine, in singular biological processes or biomedical studies, our results can be used for the designing and development of new ruthenium-based devices that act as sensors for the detection of hypoxanthine.
4. Conclusions
In summary, a new purine-based diruthenium(III) system of formula [{Ru(µ-Cl)(µ-hpx)}2Cl4] (hpx = hypoxanthine) has been prepared from two different starting materials, namely, inosine and azathioprine, the latter being an immunosuppressant drug. Remarkably, it was observed that during the synthetic process azathioprine undergoes hydrolytic reaction that in the presence of ruthenium generates hpx, instead of the expected 6-mercaptopurine antimetabolite, which in turn was detected through the formation of the diruthenium(III) system. The diruthenium(III) system was obtained in its dehydrated and hydrated forms and characterized by IR, SEM, powder and single-crystal X-ray diffraction and cyclic voltammetry (CV). The study on the CV curves allowed us to detect hpx when coordinated to ruthenium in the reported compound. The grade of sensitivity, repeatability and stability reached by this diruthenium system make it potentially useful and could provide a first step to develop sensor devices suitable to detect the hpx molecule. Further investigations on the synthesis and characterization of this type of ruthenium systems is now in progress for similar target molecules in our group.
Supplementary Materials
X-ray crystallographic data in CIF format for compounds 1a and 1b are available online at https://www.mdpi.com/2079-6374/11/1/19/s1.
Author Contributions
I.C. and J.M.-L. conceived the idea and obtained funding for the project. M.O.-A. performed the synthesis, the X-ray data collection and the rest of the physical measurements. J.M.-L. analyzed the data associated with all the experiments and wrote the manuscript, which all authors discussed and commented on. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the VLC-BIOCLINIC Program (2017) of the University of Valencia [Subprogram A “Acciones Exploratorias”, Project 02-2017-A] and the Spanish Ministry of Science, Innovation and Universities [Project PID2019-109735GB-I00].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors M.O.-A. and J.M.-L. thank the Spanish “FPI fellowships” and “Ramón y Cajal” programs, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saugstad, O.D. Hypoxanthine as a Measurement of Hypoxia. Pediatr. Res. 1975, 9, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Nagao, H.; Nishizawa, H.; Tanaka, Y.; Fukata, T.; Mizushima, T.; Furuno, M.; Bamba, T.; Tsushima, Y.; Fujishima, Y.; Kita, S.; et al. Hypoxanthine Secretion from Human Adipose Tissue and its Increase in Hypoxia. Obesity 2018, 26, 1168–1178. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Sánchez-Espiridion, B.; Lin, M.; White, L.; Mishra, L.; Raju, G.S.; Kopetz, S.; Eng, C.; Hildebrandt, M.A.T.; Chang, D.W.; et al. Global and targeted serum metabolic profiling of colorectal cancer progression. Cancer 2017, 123, 4066–4074. [Google Scholar] [CrossRef] [PubMed]
- Chouraki, V.; Preis, S.R.; Yang, Q.; Beiser, A.; Li, S.; Larson, M.G.; Weinstein, G.; Wang, T.J.; Gerszten, R.E.; Vasan, R.S.; et al. Association of amine biomarkers with incident dementia and Alzheimer’s disease in the Framingham Study. Alzheimer’s Dement. 2017, 13, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Lazzarino, G.; Amorini, A.M.; Petzold, A.; Gasperini, C.; Ruggieri, S.; Quartuccio, M.E.; Lazzarino, G.; Di Stasio, E.; Tavazzi, B. Serum Compounds of Energy Metabolism Impairment Are Related to Disability, Disease Course and Neuroimaging in Multiple Sclerosis. Mol. Neurobiol. 2017, 54, 7520–7533. [Google Scholar] [CrossRef] [PubMed]
- Farthing, D.E.; Farthing, C.A.; Xi, L. Inosine and hypoxanthine as novel biomarkers for cardiac ischemia: From bench to point-of-care. Exp. Biol. Med. 2015, 240, 821–831. [Google Scholar] [CrossRef]
- Lee, J.S.; Wang, R.X.; Alexeev, E.E.; Lanis, J.M.; Battista, K.D.; Glover, L.E.; Colgan, S.P. Hypoxanthine is a checkpoint stress metabolite in colonic epithelial energy modulation and barrier function. J. Biol. Chem. 2018, 293, 6039–6051. [Google Scholar] [CrossRef] [PubMed]
- Casali, E.; Berni, P.; Spisni, A.; Baricchi, R.; Pertinhez, T.A. Hypoxanthine: A new paradigm to interpret the origin of transfusion toxicity. Blood Transfus. 2016, 14, 555–556. [Google Scholar]
- Zieliński, J.; Krasińska, B.; Kusy, K. Hypoxanthine as a Predictor of Performance in Highly Trained Athletes. Int. J. Sports Med. 2013, 34, 1079–1086. [Google Scholar] [CrossRef]
- Zieliński, J.; Kusy, K. Hypoxanthine: A Universal Metabolic Indicator of Training Status in Competitive Sports. Exerc. Sport Sci. Rev. 2015, 43, 214–221. [Google Scholar] [CrossRef]
- Lawal, A.T.; Adeloju, S.B. Polypyrrole-Based Xanthine Oxidase Potentiometric Biosensor for Hypoxanthine. J. Appl. Sci. 2008, 8, 2599–2605. [Google Scholar] [CrossRef]
- Hernández-Cázares, A.S.; Aristoy, M.C.; Toldrá, F. Hypoxanthine-based enzymatic sensor for determination of pork meat freshness. Food Chem. 2010, 123, 949–954. [Google Scholar] [CrossRef]
- Escrivà, E.; García-Lozano, J.; Martínez-Lillo, J.; Nuñez, H.; Server-Carrió, J.; Soto, L.; Carrasco, R.; Cano, J. Synthesis, Crystal Structure, Magnetic Properties, and Theoretical Studies of [{Cu(mepirizole)Br}2(μ-OH)(μ-pz)] (Mepirizole = 4-Methoxy-2-(5-methoxy-3-methyl-1H-pyrazol-1-yl)-6-methylpyrimidine; pz = Pyrazolate), a Novel μ-Pyrazolato−μ-Hydroxo-Dibridged Copper(II) Complex. Inorg. Chem. 2003, 42, 8328–8336. [Google Scholar] [PubMed]
- Armentano, D.; Marino, N.; Mastropietro, T.F.; Martínez-Lillo, J.; Cano, J.; Julve, M.; Lloret, F.; De Munno, G. Self-Assembly of a Chiral Carbonate- and Cytidine-Containing Dodecanuclear Copper(II) Complex: A Multiarm-Supplied Globular Capsule. Inorg. Chem. 2008, 47, 10229–10231. [Google Scholar] [CrossRef] [PubMed]
- Marino, N.; Armentano, D.; Mastropietro, T.F.; Julve, M.; De Munno, G.; Martínez-Lillo, J. Cubane-Type CuII4 and MnII2MnIII2 Complexes Based on Pyridoxine: A Versatile Ligand for Metal Assembling. Inorg. Chem. 2013, 52, 11934–11943. [Google Scholar] [CrossRef] [PubMed]
- Armentano, D.; Barquero, M.A.; Rojas-Dotti, C.; Moliner, N.; De Munno, G.; Brechin, E.K.; Martínez-Lillo, J. Enhancement of Intermolecular Magnetic Exchange through Halogen···Halogen Interactions in Bisadeninium Rhenium(IV) Salts. Cryst. Growth Des. 2017, 17, 5342–5348. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Castro, I.; Lloret, F.; Martínez-Lillo, J. Field-induced slow relaxation of magnetisation in two one-dimensional homometallic dysprosium(III) complexes based on alpha- and beta-amino acids. Dalton Trans. 2020, 49, 9155–9163. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Dotti, C.; Martínez-Lillo, J. Thioester-functionalised and oxime-based hexametallic manganese(III) single-molecule magnets. RSC Adv. 2017, 7, 48841–48847. [Google Scholar] [CrossRef]
- Tyagi, P.; Riso, C.; Amir, U.; Rojas-Dotti, C.; Martínez-Lillo, J. Exploring room-temperature transport of single-molecule magnet-based molecular spintronics devices using the magnetic tunnel junction as a device platform. RSC Adv. 2020, 10, 13006–13015. [Google Scholar] [CrossRef]
- Chen, P.; Goldberg, D.E.; Kolb, B.; Lanser, M.; Benowitz, L.I. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc. Natl. Acad. Sci. USA 2002, 99, 9031–9036. [Google Scholar] [CrossRef]
- Kline, P.C.; Schramm, V.L. Purine Nucleoside Phosphorylase. Inosine Hydrolysis, Tight Binding of the Hypoxanthine Intermediate, and Third-the-Sites Reactivity. Biochemistry 1992, 31, 5964–5973. [Google Scholar] [CrossRef] [PubMed]
- Jelińska, A. Kinetics of hydrolysis of inosine in aqueous solutions. React. Kinet. Catal. Lett. 2001, 72, 93–100. [Google Scholar] [CrossRef]
- Chifotides, H.T.; Dunbar, K.R.; Matonic, J.H.; Katsaros, N. Unusual structural features of tetrakis(µ-carboxylato)dirhodium(II), an antitumor agent, bound to azathioprine, a biologically active mercaptopurine derivative. Inorg. Chem. 1992, 31, 4628–4634. [Google Scholar] [CrossRef]
- Karran, P.; Attard, N. Thiopurines in current medical practice: Molecular mechanisms and contributions to therapy-related cancer. Nat. Rev. Cancer 2008, 8, 24–36. [Google Scholar] [CrossRef]
- Jones, N.R.; Murray, J.; Livingston, E.I.; Murray, C.K. Rapid estimations of hypoxanthine concentrations as indices of the freshness of chill-stored fish. J. Sci. Food Agric. 1964, 15, 763–774. [Google Scholar] [CrossRef]
- Cooper, N.; Khosravan, R.; Erdmann, C.; Fiene, J.; Lee, J.W. Quantification of uric acid, xanthine and hypoxanthine in human serum by HPLC for pharmacodynamic studies. J. Chromatogr. B 2006, 837, 1–10. [Google Scholar] [CrossRef]
- Farthing, D.; Sica, D.; Gehr, T.; Wilson, B.; Fakhry, I.; Larus, T.; Farthing, C.; Karnes, H.T. An HPLC method for determination of inosine and hypoxanthine in human plasma from healthy volunteers and patients presenting with potential acute cardiac ischemia. J. Chromatogr. B 2007, 854, 158–164. [Google Scholar] [CrossRef]
- Xiang, L.W.; Li, J.; Lin, J.M.; Li, H.F. Determination of gouty arthritis’ biomarkers in human urine using reversed-phase high-performance liquid chromatography. J. Pharm. Anal. 2014, 4, 153–158. [Google Scholar] [CrossRef]
- Bory, C.; Chantin, C.; Boulieu, R. Comparison of capillary electrophoretic and liquid chromatographic determination of hypoxanthine and xanthine for the diagnosis of xanthinuria. J. Chromatogr. A 1996, 730, 329–331. [Google Scholar] [CrossRef]
- Bory, C.; Chantin, C.; Boulieu, R. Capillary Electrophoretic Analysis of Hypoxanthine and Xanthine for the Diagnosis of Xanthinuria. In Purine and Pyrimidine Metabolism in Man IX; Griesmacher, A., Müller, M.M., Chiba, P., Eds.; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 1998; Volume 431. [Google Scholar]
- Klinenberg, J.R.; Goldfinger, S.; Bradley, K.H.; Seegmiller, J.E. An enzymatic spectrophotometric method for the determination of xanthine and hypoxanthine. Clin. Chem. 1967, 10, 834–846. [Google Scholar] [CrossRef]
- Khajehsharifi, H.; Pourbasheer, E. Simultaneous spectrophotometric determination of xanthine, hypoxanthine and uric acid in real matrix by orthogonal signal correction-partial least squares. J. Iran. Chem. Soc. 2011, 8, 1113–1119. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, S.; Lei, J.; Xu, Q.; Ju, H. Carbon nanospheres enhanced electrochemiluminescence of CdS quantum dots for biosensing of hypoxanthine. Talanta 2011, 85, 2154–2158. [Google Scholar] [CrossRef] [PubMed]
- Zuo, F.; Zhang, H.; Xie, J.; Chen, S.; Yuan, R. A sensitive ratiometric electrochemiluminescence biosensor for hypoxanthine detection by in situ generation and consumption of coreactants. Electrochim. Acta 2018, 271, 173–179. [Google Scholar] [CrossRef]
- Dou, Z.-Y.; Cui, L.-L.; He, X.Q. Electrochimical determination of uric acid, xanthine and hypoxanthine by poly(xylitol) modified glassy carbon electrode. J. Cent. South Univ. 2014, 21, 870–876. [Google Scholar] [CrossRef]
- Mardini-Farias, P.A.; Castro, A.A. Determination of Xanthine in the Presence of Hypoxanthine by Adsorptive Stripping Voltammetry at the Mercury Film Electrode. Anal. Chem. Insights 2014, 9, 49–55. [Google Scholar] [CrossRef]
- Juban, K.B.B.; Billones, J.B. Simultaneous Electrochemical Determination of Hypoxanthine and Xanthine by Poly(Threonine) Film-Modified Electrode. Anal. Bioanal. Electrochem. 2015, 7, 149–160. [Google Scholar]
- SHELXTL-2017/1, Bruker Analytical X-ray Instruments; Bruker: Madison, WI, USA, 2017.
- DIAMOND 4.5.0, Crystal Impact GbR; Crystal Impact: Bonn, Germany, 2018.
- Armentano, D.; Martínez-Lillo, J. Hexachlororhenate(IV) salts of ruthenium(III) cations: X-ray structure and magnetic properties. Inorg. Chim. Acta 2012, 380, 118–124. [Google Scholar] [CrossRef]
- Orts-Arroyo, M.; Castro, I.; Lloret, F.; Martínez-Lillo, J. Molecular Self-Assembly in a Family of Oxo-Bridged Dinuclear Ruthenium(IV) Systems. Cryst. Growth Des. 2020, 20, 2044–2056. [Google Scholar] [CrossRef]
- Dubler, E.; Hänggi, G.; Schmalle, H. Synthesis and structure of dimeric metal complexes with N(3)/N(9)-chelating hypoxanthine ligands and with bridging water molecules: [M2(µ-hyxan)2(SO4)2(µ-H2O)2(H2O)2] (M = copper, cadmium, zinc; hyxan = hypoxanthine). Inorg. Chem. 1990, 29, 2518–2523. [Google Scholar] [CrossRef]
- Hänggi, G.; Schmalle, H.; Dubler, E. Structure of [Co2(µ-hypoxanthine)2(SO4)2(µ-H2O)2(H2O)2]. Acta Cryst. 1992, C48, 1008–1012. [Google Scholar]
- Reid, J.; Bond, T.; Wang, S.; Zhou, J.; Hu, A. Synchrotron powder diffraction, X-ray absorption and 1H nuclear magnetic resonance data for hypoxanthine. Powder Diffr. 2015, 30, 278–285. [Google Scholar] [CrossRef]
- Mohite, S.S.; Patil-Deshmukh, A.B.; Chavan, S.S. Synthesis and characterization of Ru(III) complexes with 2-((E)-((4-((4-bromophenyl)ethynyl)phenyl)imino)methyl-4-((E)-phenyldiazenyl)phenol and their use as a precursor for RuO2 nanoparticles. J. Mol. Struct. 2019, 1176, 386–393. [Google Scholar] [CrossRef]
- Sur, V.P.; Mazumdar, A.; Kopel, P.; Mukherjee, S.; Vítek, P.; Michalkova, H.; Vaculovičová, M.; Moulick, A. A Novel Ruthenium Based Coordination Compound Against Pathogenic Bacteria. Int. J. Mol. Sci. 2020, 21, 2656. [Google Scholar] [CrossRef] [PubMed]
- Shahrokhian, S.; Ghalkhani, M. Electrochemical study of Azathioprine at thin carbon nanoparticle composite film electrode. Electrochem. Commun. 2009, 11, 1425–1428. [Google Scholar] [CrossRef]
- Rao, C.N.; Balaji, K.; Venkateswarlu, P. Determination of azathioprine and rocuronium in biological fluid samples by voltammetry. Biomed. Pharmacol. J. 2009, 2, 117–121. [Google Scholar]
- Jalali, F.; Rasaee, G. Electrochemical, spectroscopic, and theoretical studies on theinteraction between azathioprine and DNA. Int. J. Biol. Macromol. 2015, 81, 427–434. [Google Scholar] [CrossRef]
- Asadian, E.; Iraji Zad, A.; Shahrokhian, S. Voltammetric studies of Azathioprine on the surface of graphite electrodemodified with graphene nanosheets decorated with Ag nanoparticles. Mater. Sci. Eng. C 2016, 58, 1098–1104. [Google Scholar] [CrossRef]
- Oliveira-Brett, A.M.; Silva, L.A.; Farace, G.; Vadgama, P.; Brett, C.M.A. Voltammetric and impedance studies of inosine-5’-monophosphate and hypoxanthine. Bioelectrochemistry 2003, 59, 49–56. [Google Scholar] [CrossRef]
- Revin, S.B.; John, S.A. Selective determination of inosine in the presence of uric acid and hypoxanthine using modified electrode. Anal. Biochem. 2012, 421, 278–284. [Google Scholar] [CrossRef]
- Cotton, F.A.; Pedersen, E. Magnetic and electrochemical properties of transition metal complexes with multiple metal-to-metal bonds. II. Tetrabutyratodiruthenium(n+) with n = 0 and 1. Inorg. Chem. 1975, 14, 388–391. [Google Scholar] [CrossRef]
- Malinski, T.; Chang, D.; Feldmann, F.N.; Bear, J.L.; Kadish, K.M. Electrochemical studies of a novel ruthenium(II, III) dimer, trifluoroacetamidatoruthenium chloride (Ru2(HNOCCF3)4Cl). Inorg. Chem. 1983, 22, 3225–3233. [Google Scholar] [CrossRef]
- Hiraoka, Y.; Ikeue, T.; Sakiyama, H.; Guégan, F.; Luneau, D.; Gillon, B.; Hiromitsu, I.; Yoshioka, D.; Mikuriya, M.; Kataoka, Y.; et al. An unprecedented up-field shift in the 13C NMR spectrum of the carboxyl carbons of the lantern-type dinuclear complex TBA[Ru2(O2CCH3)4Cl2] (TBA+ = tetra(n-butyl)ammonium cation). Dalton Trans. 2015, 44, 13439–13443. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, Y.; Mikami, S.; Sakiyama, H.; Mitsumi, M.; Kawamoto, T.; Handa, M. A neutral paddlewheel-type diruthenium(III) complex with benzamidinato ligands: Synthesis, crystal structure, magnetism, and electrochemical and absorption properties. Polyhedron 2017, 136, 87–92. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).