Rolling Circle Amplification as an Efficient Analytical Tool for Rapid Detection of Contaminants in Aqueous Environments
Abstract
:1. Introduction
2. Advantages and Disadvantages of the RCA Assay
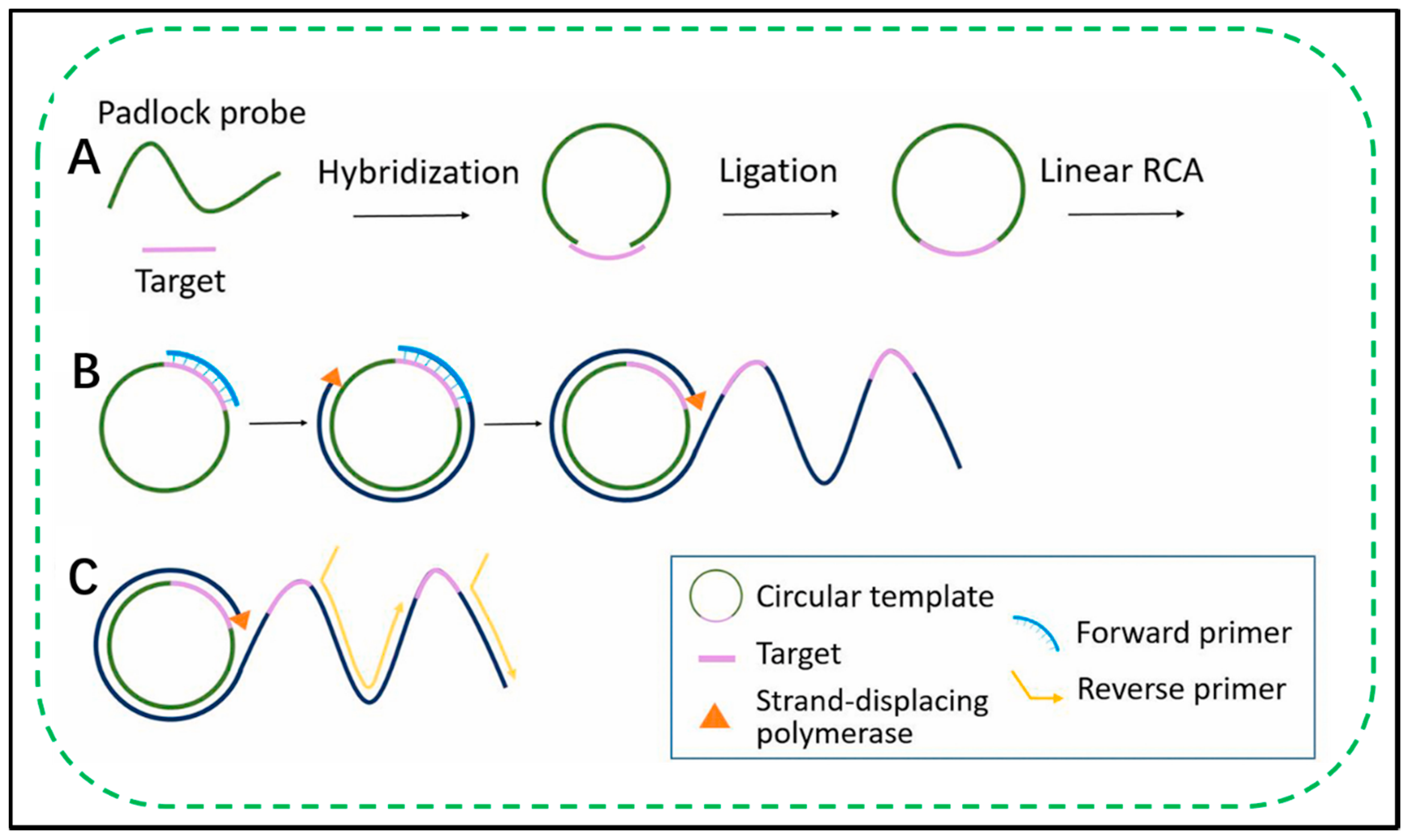
2.1. Fundamentals of RCA
2.2. Exponential RCA Amplification
2.3. Detection of the RCA Product
3. RCA Assay for the Detection of Targets in Aqueous Environments
3.1. RCA Assay for Heavy Metal Ions
3.1.1. Mercury (Hg)
3.1.2. Lead (Pb)
3.1.3. Other Ions
3.2. RCA Assay for Organic Small Molecules
3.3. RCA Assay for Nucleic Acids
3.4. RCA Assay for Peptides and Proteins
3.5. RCA Assay for Microorganisms
4. Emerging Nanotechnology for RCA Assay
4.1. DNA Technology
4.1.1. DNA Assembly Technology
4.1.2. DNA Machines
4.2. Engineering of RCA as a Portable Tool for Point-of-Use Detection
4.2.1. Microfluidic Chips
4.2.2. Paper-Based Platforms
4.2.3. Electrochemistry Platforms
4.2.4. Commercial Portable Device
5. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blair, B.D.; Crago, J.P.; Hedman, C.J.; Klaper, R.D. Pharmaceuticals and personal care products found in the Great Lakes above concentrations of environmental concern. Chemosphere 2013, 93, 2116–2123. [Google Scholar] [CrossRef] [Green Version]
- Eckert, E.M.; Di Cesare, A.; Kettner, M.T.; Arias-Andres, M.; Fontaneto, D.; Grossart, H.P.; Corno, G. Microplastics increase impact of treated wastewater on freshwater microbial community. Environ. Pollut. 2018, 234, 495–502. [Google Scholar] [CrossRef]
- Wang, Z.; Han, S.; Cai, M.; Du, P.; Zhang, Z.; Li, X. Environmental behaviour of methamphetamine and ketamine in aquatic ecosystem: Degradation, bioaccumulation, distribution, and associated shift in toxicity and bacterial community. Water Res. 2020, 174, 115585. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Huang, Q.X.; Wang, Z.F.; Zhang, K.; Tang, C.M.; Cui, J.L.; Feng, J.L.; Peng, X.Z. Occurrence and behaviour of pharmaceuticals, steroid hormones, and endocrine-disrupting personal care products in wastewater and the recipient river water of the Pearl River Delta, South China. J. Environ. Monitor. 2011, 13, 871–878. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.L.; Ngo, H.H.; Guo, W.S.; Liu, Y.W.; Zhou, J.L.; Chang, S.W.; Nguyen, D.D.; Bui, X.T.; Zhang, X.B. Bioprocessing for elimination antibiotics and hormones from swine wastewater. Sci. Total Environ. 2018, 621, 1664–1682. [Google Scholar] [CrossRef] [PubMed]
- Hamid, H.; Eskicioglu, C. Fate of oestrogenic hormones in wastewater and sludge treatment: A review of properties and analytical detection techniques in sludge matrix. Water Res. 2012, 46, 5813–5833. [Google Scholar] [CrossRef]
- Ganjali, M.R.; Faridbod, F.; Davarkhah, N.; Shahtaheri, S.J.; Norouzi, P. All Solid State Graphene Based Potentiometric Sensors for Monitoring of Mercury Ions in Waste Water Samples. Int. J. Environ. Res. 2015, 9, 333–340. [Google Scholar]
- Gavrilescu, M.; Demnerova, K.; Aamand, J.; Agathoss, S.; Fava, F. Emerging pollutants in the environment: Present and future challenges in biomonitoring, ecological risks and bioremediation. New Biotechnol. 2015, 32, 147–156. [Google Scholar] [CrossRef]
- Henderson, R.K.; Baker, A.; Murphy, K.R.; Hamblya, A.; Stuetz, R.M.; Khan, S.J. Fluorescence as a potential monitoring tool for recycled water systems: A review. Water Res. 2009, 43, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M.T. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]
- Ali, M.M.; Li, F.; Zhang, Z.; Zhang, K.; Kang, D.-K.; Ankrum, J.A.; Le, X.C.; Zhao, W. Rolling circle amplification: A versatile tool for chemical biology, materials science and medicine. Chem. Soc. Rev. 2014, 43, 3324–3341. [Google Scholar] [CrossRef]
- Lizardi, P.M.; Huang, X.H.; Zhu, Z.R.; Bray-Ward, P.; Thomas, D.C.; Ward, D.C. Mutation detection and single-molecule counting using isothermal rolling-circle amplification. Nat. Genet. 1998, 19, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Murakami, T.; Sumaoka, J.; Komiyama, M. Sensitive isothermal detection of nucleic-acid sequence by primer generation-rolling circle amplification. Nucleic Acids Res. 2009, 37, e19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, W.A.; Ali, M.M.; Brook, M.A.; Li, Y.F. Rolling circle amplification: Applications in nanotechnology and biodetection with functional nucleic acids. Angew. Chem. Int. Ed. 2008, 47, 6330–6337. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.X.; Chen, F.; Li, Q.; Wang, L.H.; Fan, C.H. Isothermal Amplification of Nucleic Acids. Chem. Rev. 2015, 115, 12491–12545. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.N.; Wang, Y.; Liu, S.; Yu, J.H.; Wang, H.Z.; Wang, Y.L.; Huang, J.D. Label-free and highly sensitive electrochemical detection of E-coli based on rolling circle amplifications coupled peroxidase-mimicking DNAzyme amplification. Biosens. Bioelectron. 2016, 75, 315–319. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Lv, S.Z.; Lu, M.H.; Tang, D.P. Photoelectrochemical biosensing of disease marker on p-type Cu-doped Zn0.3Cd0.7S based on RCA and exonuclease III amplification. Biosens. Bioelectron. 2018, 117, 590–596. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Shu, J.; He, Y.; Lin, Z.Z.; Zhang, K.Y.; Lv, S.Z.; Tang, D.P. CdTe/CdSe quantum dot-based fluorescent aptasensor with hemin/G-quadruplex DNzyme for sensitive detection of lysozyme using rolling circle amplification and strand hybridization. Biosens. Bioelectron. 2017, 87, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.P.; Lu, J.; Luo, Z.F.; Zhang, L.Y.; Liu, P.Q.; Chen, Z.G. Competitive electrochemical platform for ultrasensitive cytosensing of liver cancer cells by using nanotetrahedra structure with rolling circle amplification. Biosens. Bioelectron. 2018, 120, 8–14. [Google Scholar] [CrossRef]
- Zhang, K.Y.; Lv, S.Z.; Lin, Z.Z.; Li, M.J.; Tang, D.P. Bio-bar-code-based photoelectrochemical immunoassay for sensitive detection of prostate-specific antigen using rolling circle amplification and enzymatic biocatalytic precipitation. Biosens. Bioelectron. 2018, 101, 159–166. [Google Scholar] [CrossRef]
- Chen, A.Y.; Ma, S.Y.; Zhuo, Y.; Chai, Y.Q.; Yuan, R. In Situ Electrochemical Generation of Electrochemiluminescent Silver Naonoclusters on Target-Cycling Synchronized Rolling Circle Amplification Platform for MicroRNA Detection. Anal. Chem. 2016, 88, 3203–3210. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Du, Y.; Yao, Y.; Wu, J.; Meng, S.; Luo, J.; Zhang, X.; Yang, D.; Wang, C.; Qian, Y.; et al. Rolling circle amplification triggered poly adenine-gold nanoparticles production for label-free electrochemical detection of thrombin. Sens. Actuators B Chem. 2018, 266, 9–18. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; Yuan, R.; Chai, Y.Q. “Off” to “On” Surface-Enhanced Raman Spectroscopy Platform with Padlock Probe-Based Exponential Rolling Circle Amplification for Ultrasensitive Detection of MicroRNA 155. Anal. Chem. 2017, 89, 2866–2872. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.L.; Shu, J.; Tang, D.P. Near-Infrared-to-Ultraviolet Light-Mediated Photoelectrochemical Aptasensing Platform for Cancer Biomarker Based on Core Shell NaYF4:Yb,Tm@TiO2 Upconversion Microrods. Anal. Chem. 2018, 90, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Lim, M.C.; Woo, M.A.; Jun, B.H. Radial Flow Assay Using Gold Nanoparticles and Rolling Circle Amplification to Detect Mercuric Ions. Nanomaterials 2018, 8, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.; Li, W.S.; Li, J.Q.; Tao, B.B.; Xu, Y.Q.; Li, H.J.; Lu, A.P.; Sun, S.G. Study on rolling circle amplification of Ebola virus and fluorescence detection based on graphene oxide. Sens. Actuators B Chem. 2016, 227, 655–659. [Google Scholar] [CrossRef]
- Zhao, J.; Lei, Y.-M.; Chai, Y.-Q.; Yuan, R.; Zhuo, Y. Novel electrochemiluminescence of perylene derivative and its application to mercury ion detection based on a dual amplification strategy. Biosens. Bioelectron. 2016, 86, 720–727. [Google Scholar] [CrossRef]
- Osborne, R.J.; Thornton, C.A. Cell-free cloning of highly expanded CTG repeats by amplification of dimerized expanded repeats. Nucleic Acids Res. 2008, 36, e24. [Google Scholar] [CrossRef]
- Wang, F.; Lu, C.H.; Liu, X.Q.; Freage, L.; Willner, I. Amplified and Multiplexed Detection of DNA Using the Dendritic Rolling Circle Amplified Synthesis of DNAzyme Reporter Units. Anal. Chem. 2014, 86, 1614–1621. [Google Scholar] [CrossRef]
- Du, Y.C.; Zhu, Y.J.; Li, X.Y.; Kong, D.M. Amplified detection of genome-containing biological targets using terminal deoxynucleotidyl transferase-assisted rolling circle amplification. Chem. Commun. 2018, 54, 682–685. [Google Scholar] [CrossRef] [Green Version]
- Inoue, J.; Shigemori, Y.; Mikawa, T. Improvements of rolling circle amplification (RCA) efficiency and accuracy using Thermus thermophilus SSB mutant protein. Nucleic Acids Res. 2006, 34, e69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.S.; Deng, T.; Chu, X.; Yang, R.H.; Jiang, J.H.; Shen, G.L.; Yu, R.Q. Rolling Circle Amplification Combined with Gold Nanoparticle Aggregates for Highly Sensitive Identification of Single-Nucleotide Polymorphisms. Anal. Chem. 2010, 82, 2811–2816. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.Q.; Bakht, S.; Devos, K.M.; Gale, M.D.; Osbourn, A. L-RCA (ligation-rolling circle amplification): A general method for genotyping of shingle nucleotide polymorphisms (SNPs). Nucleic Acids Res. 2001, 29, e116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.X.; Wang, H.; Liu, C.H.; Wang, H.H.; Duan, X.R.; Li, Z.P. Ultrasensitive genotyping with target-specifically generated circular DNA templates and RNA FRET probes. Chem. Commun. 2015, 51, 11556–11559. [Google Scholar] [CrossRef] [PubMed]
- Ko, O.; Han, S.; Lee, J.B. Selective release of DNA nanostructures from DNA hydrogel. J. Ind. Eng. Chem. 2020, 84, 46–51. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, Q.; Li, Z.P.; Gu, J.; Brennan, J.D.; Li, Y.F. Programming a topologically constrained DNA nanostructure into a sensor. Nat. Commun. 2016, 7, 12074. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Zhang, S.X.; Ouyang, C.H.; Wang, Z.M.; Wu, D.; Liu, Y.Y.; Jiang, Y.F.; Wu, Z.S. DNA nanostructures from palindromic rolling circle amplification for the fluorescent detection of cancer-related microRNAs. Talanta 2019, 192, 175–181. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Zhang, H.Z.; Wang, F.; Zhang, G.D.; Zhou, T.; Wang, X.F.; Liu, S.Z.; Liu, T.T. DNA Block Macromolecules Based on Rolling Circle Amplification Act as Scaffolds to Build Large-Scale Origami Nanostructures. Macromol. Rapid. Commun. 2018, 39, 1800263. [Google Scholar] [CrossRef]
- Mohsen, M.G.; Kool, E.T. The Discovery of Rolling Circle Amplification and Rolling Circle Transcription. Acc. Chem. Res. 2016, 49, 2540–2550. [Google Scholar] [CrossRef] [Green Version]
- Fire, A.; Xu, S.Q. Rolling Replication of Short Dna Circles. Proc. Natl. Acad. Sci. USA 1995, 92, 4641–4645. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.Y.; Daubendiek, S.L.; Zillman, M.A.; Ryan, K.; Kool, E.T. Rolling circle DNA synthesis: Small circular oligonucleotides as efficient templates for DNA polymerases. J. Am. Chem. Soc. 1996, 118, 1587–1594. [Google Scholar] [CrossRef] [Green Version]
- Blanco, L.; Bernad, A.; Lazaro, J.M.; Martin, G.; Garmendia, C.; Salas, M. Highly efficient DNA synthesis by the phage phi 29 DNA polymerase. Symmetrical mode of DNA replication. J. Biol. Chem. 1989, 264, 8935–8940. [Google Scholar] [CrossRef]
- Krzywkowski, T.; Kuhnemund, M.; Wu, D.; Nilsson, M. Limited reverse transcriptase activity of phi29 DNA polymerase. Nucleic Acids Res. 2018, 46, 3625–3632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, F.; Hernandez-Neuta, I.; Grabbe, M.; Madaboosi, N.; Albert, J.; Nilsson, M. Padlock Probe Assay for Detection and Subtyping of Seasonal Influenza. Clin. Chem. 2018, 64, 1704–1712. [Google Scholar] [CrossRef]
- Nilsson, M.; Malmgren, H.; Samiotaki, M.; Kwiatkowski, M.; Chowdhary, B.P.; Landegren, U. Padlock probes: Circularizing oligonucleotides for localized DNA detection. Science 1994, 265, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.M.; Wei, H.; Hu, T.Y.; Jiang, J.Q.; Chang, J.L.; Guan, Y.F.; Zhao, G.J. Suppression of rolling circle amplification by nucleotide analogues in circular template for three DNA polymerases. Biosci. Biotech. Bioch. 2016, 80, 1555–1561. [Google Scholar] [CrossRef] [Green Version]
- Li, D.X.; Zhang, T.T.; Yang, F.; Yuan, R.; Xiang, Y. Efficient and Exponential Rolling Circle Amplification Molecular Network Leads to Ultrasensitive and Label-Free Detection of MicroRNA. Anal. Chem. 2020, 92, 2074–2079. [Google Scholar] [CrossRef]
- Li, X.Y.; Cui, Y.X.; Du, Y.C.; Tang, A.N.; Kong, D.M. Label-Free Telomerase Detection in Single Cell Using a Five-Base Telomerase Product-Triggered Exponential Rolling Circle Amplification Strategy. ACS Sens. 2019, 4, 1090–1096. [Google Scholar] [CrossRef]
- Xu, H.; Xue, C.; Zhang, R.B.; Chen, Y.R.; Li, F.; Shen, Z.F.; Jia, L.; Wu, Z.S. Exponential rolling circle amplification and its sensing application for highly sensitive DNA detection of tumour suppressor gene. Sens. Actuators B Chem. 2017, 243, 1240–1247. [Google Scholar] [CrossRef]
- Pumford, E.A.; Lu, J.; Spaczai, I.; Prasetyo, M.E.; Zheng, E.M.; Zhang, H.; Kamei, D.T. Developments in integrating nucleic acid isothermal amplification and detection systems for point-of-care diagnostics. Biosens. Bioelectron. 2020, 170, 112674. [Google Scholar] [CrossRef]
- Ulanovsky, L.; Bodner, M.; Trifonov, E.N.; Choder, M. Curved DNA: Design, synthesis, and circularization. Proc. Natl. Acad. Sci. USA 1986, 83, 862–866. [Google Scholar] [CrossRef] [Green Version]
- Jin, G.; Wang, C.; Yang, L.; Li, X.; Guo, L.; Qiu, B.; Lin, Z.; Chen, G. Hyperbranched rolling circle amplification based electrochemiluminescence aptasensor for ultrasensitive detection of thrombin. Biosens. Bioelectron. 2015, 63, 166–171. [Google Scholar] [CrossRef]
- Wang, X.M.; Teng, D.; Guan, Q.F.; Tian, F.; Wang, J.H. Detection of genetically modified crops using multiplex asymmetric polymerase chain reaction and asymmetric hyperbranched rolling circle amplification coupled with reverse dot blot. Food Chem. 2015, 173, 1022–1029. [Google Scholar] [CrossRef]
- Yang, L.; Tao, Y.; Yue, G.; Li, R.; Qin, B.; Guo, L.; Lin, Z.; Yang, H.-H. Highly Selective and Sensitive Electrochemiluminescence Biosensor for p53 DNA Sequence Based on Nicking Endonuclease Assisted Target Recycling and Hyperbranched Rolling Circle Amplification. Anal. Chem. 2016, 88, 5097–5103. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-R.; Zhu, G.; Zhang, C.-Y. Homogeneous and Label-Free Detection of MicroRNAs Using Bifunctional Strand Displacement Amplification-Mediated Hyperbranched Rolling Circle Amplification. Anal. Chem. 2014, 86, 6703–6709. [Google Scholar] [CrossRef] [PubMed]
- Dahl, F.; Baner, J.; Gullberg, M.; Mendel-Hartvig, M.; Landegren, U.; Nilsson, M. Circle-to-circle amplification for precise and sensitive DNA analysis. Proc. Natl. Acad. Sci. USA 2004, 101, 4548–4553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Yin, Q.X.; McConnell, E.M.; Chang, Y.Y.; Brennan, J.D.; Li, Y.F. DNAzyme Feedback Amplification: Relaying Molecular Recognition to Exponential DNA Amplification. Chem. Eur. J. 2018, 24, 4473–4479. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Wang, Y.; Liu, S.; Wang, C.L.; Liang, J.X.; Li, S.S.; Qu, X.N.; Zhang, R.F.; Yu, J.H.; Huang, J.D. Triple-helix molecular-switch-actuated exponential rolling circular amplification for ultrasensitive fluorescence detection of miRNAs. Analyst 2019, 144, 5245–5253. [Google Scholar] [CrossRef]
- Jiang, Y.; Zou, S.; Cao, X. A simple dendrimer-aptamer based microfluidic platform for E. coli O157:H7 detection and signal intensification by rolling circle amplification. Sens. Actuators B Chem. 2017, 251, 976–984. [Google Scholar] [CrossRef]
- Peng, X.; Liang, W.-B.; Wen, Z.-B.; Xiong, C.-Y.; Zheng, Y.-N.; Chai, Y.-Q.; Yuan, R. Ultrasensitive Fluorescent Assay Based on a Rolling-Circle-Amplification-Assisted Multisite-Strand-Displacement-Reaction Signal-Amplification Strategy. Anal. Chem. 2018, 90, 7474–7479. [Google Scholar] [CrossRef]
- Wang, J.; Dong, H.-Y.; Zhou, Y.; Han, L.-Y.; Zhang, T.; Lin, M.; Wang, C.; Xu, H.; Wu, Z.-S.; Jia, L. Immunomagnetic antibody plus aptamer pseudo-DNA nanocatenane followed by rolling circle amplication for highly sensitive CTC detection. Biosens. Bioelectron. 2018, 122, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, S.; Li, H.-F.; Lin, J.-M. Multi-DNAzymes-functionalized gold nanoparticles for ultrasensitive chemiluminescence detection of thrombin on microchip. Anal. Chim. Acta 2018, 1027, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.R.; Tong, P.; Li, H.; Tang, J.; Zhang, L. Ultrasensitive electrochemical detection of Pb2+ based on rolling circle amplification and quantum dots tagging. Biosens. Bioelectron. 2013, 42, 608–611. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xie, S.B.; Zhang, J.; Tang, D.Y.; Tang, Y. Immobilized-free miniaturized electrochemical sensing system for Pb2+ detection based on dual Pb2+-DNAzyme assistant feedback amplification strategy. Biosens. Bioelectron. 2018, 117, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Zhang, W.; Yan, Y.R.; Shen, B.; Zhu, D.; Lei, P.H.; Ding, S.J. A novel electrochemical biosensor for ultrasensitive and specific detection of DNA based on molecular beacon mediated circular strand displacement and rolling circle amplification. Biosens. Bioelectron. 2014, 62, 274–279. [Google Scholar] [CrossRef]
- Mittal, S.; Thakur, S.; Mantha, A.K.; Kaur, H. Bio-analytical applications of nicking endonucleases assisted signal-amplification strategies for detection of cancer biomarkers -DNA methyl transferase and microRNA. Biosens. Bioelectron. 2019, 124, 233–243. [Google Scholar] [CrossRef]
- Bialy, R.M.; Ali, M.M.; Li, Y.F.; Brennan, J.D. Protein-Mediated Suppression of Rolling Circle Amplification for Biosensing with an Aptamer-Containing DNA Primer. Chem. Eur. J. 2020, 26, 5085–5092. [Google Scholar] [CrossRef]
- Fan, T.T.; Mao, Y.; Liu, F.; Zhang, W.; Lin, J.S.; Yin, J.X.; Tan, Y.; Huang, X.T.; Jiang, Y.Y. Label-free fluorescence detection of circulating microRNAs based on duplex-specific nuclease-assisted target recycling coupled with rolling circle amplification. Talanta 2019, 200, 480–486. [Google Scholar] [CrossRef]
- Duy, J.; Smith, R.L.; Collins, S.D.; Connell, L.B. A field-deployable colorimetric bioassay for the rapid and specific detection of ribosomal RNA. Biosens. Bioelectron. 2014, 52, 433–437. [Google Scholar] [CrossRef]
- Wen, Y.Q.; Xu, Y.; Mao, X.H.; Wei, Y.L.; Song, H.Y.; Chen, N.; Huang, Q.; Fan, C.H.; Li, D. DNAzyme-Based Rolling-Circle Amplification DNA Machine for Ultrasensitive Analysis of MicroRNA in Drosophila Larva. Anal. Chem. 2012, 84, 7664–7669. [Google Scholar] [CrossRef]
- Tang, L.H.; Liu, Y.; Ali, M.M.; Kang, D.K.; Zhao, W.A.; Li, J.H. Colorimetric and Ultrasensitive Bioassay Based on a Dual-Amplification System Using Aptamer and DNAzyme. Anal. Chem. 2012, 84, 4711–4717. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Xu, Q.F.; Lu, X.Q.; Zhang, C.Y. A Label-Free Bioluminescent Sensor for Real-Time Monitoring Polynucleotide Kinase Activity. Anal. Chem. 2014, 86, 8481–8488. [Google Scholar] [CrossRef] [PubMed]
- Mashimo, Y.; Mie, M.; Suzuki, S.; Kobatake, E. Detection of small RNA molecules by a combination of branched rolling circle amplification and bioluminescent pyrophosphate assay. Anal. Bioanal. Chem. 2011, 401, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tong, P.; Lin, Y.; Lu, W.; He, Y.; Lu, M.; Zhang, L.; Chen, G. Highly sensitive fluorescent sensor for mercury based on hyperbranched rolling circle amplification. Analyst 2015, 140, 907–911. [Google Scholar] [CrossRef]
- Xie, S.; Tang, Y.; Tang, D. Highly sensitive electrochemical detection of mercuric ions based on sequential nucleic acid amplification and guanine nanowire formation. Anal. Methods 2017, 9, 5478–5483. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, H.; Chen, F.; Bai, M.; Zhao, J.; Zhao, Y. Trifunctional molecular beacon-mediated quadratic amplification for highly sensitive and rapid detection of mercury(II) ion with tunable dynamic range. Biosens. Bioelectron. 2016, 86, 892–898. [Google Scholar] [CrossRef]
- Lv, J.; Xie, S.; Cai, W.; Zhang, J.; Tang, D.; Tang, Y. Highly effective target converting strategy for ultrasensitive electrochemical assay of Hg2+. Analyst 2017, 142, 4708–4714. [Google Scholar] [CrossRef]
- Wu, S.; Yu, Q.; He, C.; Duan, N. Colorimetric aptasensor for the detection of mercury based on signal intensification by rolling circle amplification. Spectrochim. Acta Part A 2020, 224, 117387. [Google Scholar] [CrossRef]
- Lim, J.W.; Kim, T.-Y.; Choi, S.-W.; Woo, M.-A. 3D-printed rolling circle amplification chip for on-site colorimetric detection of inorganic mercury in drinking water. Food Chem. 2019, 300, 125177. [Google Scholar] [CrossRef]
- Lu, W.; Lin, C.; Yang, J.; Wang, X.; Yao, B.; Wang, M. A DNAzyme assay coupled with effective magnetic separation and rolling circle amplification for detection of lead cations with a smartphone camera. Anal. Bioanal. Chem. 2019, 411, 5383–5391. [Google Scholar] [CrossRef]
- Tang, D.; Xia, B.; Tang, Y.; Zhang, J.; Zhou, Q. Metal-ion-induced DNAzyme on magnetic beads for detection of lead(II) by using rolling circle amplification, glucose oxidase, and readout of pH changes. Microchim. Acta 2019, 186, 318. [Google Scholar] [CrossRef]
- Cheng, X.; Yu, X.; Chen, L.; Zhang, H.; Wu, Y.; Fu, F. Visual detection of ultra-trace levels of uranyl ions using magnetic bead-based DNAzyme recognition in combination with rolling circle amplification. Microchim. Acta 2017, 184, 4259–4267. [Google Scholar] [CrossRef]
- Li, X.; Song, J.; Xue, Q.-W.; You, F.-H.; Lu, X.; Kong, Y.-C.; Ma, S.-Y.; Jiang, W.; Li, C.-Z. A Label-Free and Sensitive Fluorescent Qualitative Assay for Bisphenol A Based on Rolling Circle Amplification/Exonuclease III-Combined Cascade Amplification. Nanomaterials 2016, 6, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Q.; Li, P.; Gao, Z.; Yau Li, S.F. Rapid, sensitive and highly specific label-free fluorescence biosensor for microRNA by branched rolling circle amplification. Sens. Actuators B Chem. 2019, 281, 424–431. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Luan, C.; Fu, F.; Chen, B.; Liu, H.; Zhao, Y. Porous Hydrogel Encapsulated Photonic Barcodes for Multiplex MicroRNA Quantification. Adv. Funct. Mater. 2018, 28, 1704458. [Google Scholar] [CrossRef]
- Xu, L.-P.; Chen, Y.; Yang, G.; Shi, W.; Dai, B.; Li, G.; Cao, Y.; Wen, Y.; Zhang, X.; Wang, S. Ultratrace DNA Detection Based on the Condensing-Enrichment Effect of Superwettable Microchips. Adv. Mater. 2015, 27, 6878–6884. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, R.; Xie, L.; Li, Q.; Zhou, W.; Wang, R.; Ye, J.; Wang, D.; Xue, N.; Lin, X.; et al. A smart device for label-free and real-time detection of gene point mutations based on the high dark phase contrast of vapour condensation. Lab Chip 2015, 15, 3891–3896. [Google Scholar] [CrossRef]
- He, Z.; Wei, J.; Gan, C.; Liu, W.; Liu, Y. A rolling circle amplification signal-enhanced immunosensor for ultrasensitive microcystin-LR detection based on a magnetic graphene-functionalized electrode. RSC Adv. 2017, 7, 39906–39913. [Google Scholar] [CrossRef] [Green Version]
- Hui, C.Y.; Liu, M.; Li, Y.; Brennan, J.D. A Paper Sensor Printed with Multifunctional Bio/Nano Materials. Angew. Chem. Int. Ed. 2018, 57, 4549–4553. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.; Wang, Y.; Zhou, J.; Li, C. Establishment and application of hyperbranched rolling circle amplification coupled with lateral flow dipstick for the sensitive detection of Karenia mikimotoi. Harmful Algae 2019, 84, 151–160. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, R.; Wang, Y.; Chen, G.; Guo, C.; Zhou, J. Comparative detection of Karenia mikimotoi by exponential rolling circle amplification (E-RCA) and double-ligation E-RCA. J. Appl. Psychol. 2019, 31, 505–518. [Google Scholar] [CrossRef]
- Zhang, C.; Chen, G.; Wang, Y.; Sun, R.; Nie, X.; Zhou, J. MHBMDAA: Membrane-based DNA array with high resolution and sensitivity for toxic microalgae monitoring. Harmful Algae 2018, 80, 107–116. [Google Scholar] [CrossRef]
- Najafzadeh, M.J.; Vicente, V.A.; Feng, P.; Naseri, A.; Sun, J.; Rezaei-Matehkolaei, A.; de Hoog, G.S. Rapid Identification of Seven Waterborne Exophiala Species by RCA DNA Padlock Probes. Mycopathologia 2018, 183, 669–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Yang, K.; Zhao, X.; Lin, Z.; Liu, Z.; Luo, S.; Zhang, Y.; Wang, Y.; Fu, W. Terahertz spectroscopy for the isothermal detection of bacterial DNA by magnetic bead-based rolling circle amplification. Analyst 2017, 142, 4661–4669. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Zhang, C.; Wang, Y.; Guo, C.; Zhou, J.; Chen, G. Application of hyperbranched rolling circle amplification (HRCA) and HRCA-based strip test for the detection of Chattonella marina. Environ. Sci. Pollut. Res. 2017, 24, 15678–15688. [Google Scholar] [CrossRef]
- Pearson, V.M.; Caudle, S.B.; Rokyta, D.R. Viral recombination blurs taxonomic lines: Examination of single-stranded DNA viruses in a wastewater treatment plant. PeerJ 2016, 4, 18. [Google Scholar] [CrossRef]
- Chen, G.; Cai, P.; Zhang, C.; Wang, Y.; Zhang, S.; Guo, C.; Lu, D.D. Hyperbranched rolling circle amplification as a novel method for rapid and sensitive detection of Amphidinium carterae. Harmful Algae 2015, 47, 66–74. [Google Scholar] [CrossRef]
- Bejhed, R.S.; Zardán Gómez de la Torre, T.; Svedlindh, P.; Strömberg, M. Optomagnetic read-out enables easy, rapid, and cost-efficient qualitative biplex detection of bacterial DNA sequences. Biotechnol. J. 2015, 10, 469–472. [Google Scholar] [CrossRef] [Green Version]
- Ge, C.; Yuan, R.; Yi, L.; Yang, J.; Zhang, H.; Li, L.; Nian, W.; Yi, G. Target-induced aptamer displacement on gold nanoparticles and rolling circle amplification for ultrasensitive live Salmonella typhimurium electrochemical biosensing. J. Electroanal.Chem. 2018, 826, 174–180. [Google Scholar] [CrossRef]
- Gao, Z.F.; Liu, R.; Wang, J.; Dai, J.; Huang, W.-H.; Liu, M.; Wang, S.; Xia, F.; Jiang, L. Controlling Droplet Motion on an Organogel Surface by Tuning the Chain Length of DNA and Its Biosensing Application. Chem 2018, 4, 2929–2943. [Google Scholar] [CrossRef] [Green Version]
- Mao, K.; Zhang, H.; Wang, Z.L.; Cao, H.R.; Zhang, K.K.; Li, X.Q.; Yang, Z.G. Nanomaterial-based aptamer sensors for arsenic detection. Biosens. Bioelectron. 2020, 148, 111785. [Google Scholar] [CrossRef] [PubMed]
- Aragay, G.; Pons, J.; Merkoci, A. Recent Trends in Macro-, Micro-, and Nanomaterial-Based Tools and Strategies for Heavy-Metal Detection. Chem. Rev. 2011, 111, 3433–3458. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. A review on detection of heavy metal ions in water—An electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Kim, H.N.; Ren, W.X.; Kim, J.S.; Yoon, J. Fluorescent and colorimetric sensors for detection of lead, cadmium, and mercury ions. Chem. Soc. Rev. 2012, 41, 3210–3244. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Wu, Y.; Wang, L.; Zhan, X.; Zhou, P. A mini-review on functional nucleic acids-based heavy metal ion detection. Biosens. Bioelectron. 2016, 86, 353–368. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Chen, C.; Yin, R.; Shen, Y.; Mao, K.; Yang, Z.; Feng, X.; Zhang, H. Bioaccumulation of Hg in Rice Leaf Facilitates Selenium Bioaccumulation in Rice (Oryza sativa L.) Leaf in the Wanshan Mercury Mine. Environ. Sci. Technol. 2020, 54, 3228–3236. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Wang, J.; Du, R.; Liu, Y.; Chi, J.; He, X.; Huang, K.; Luo, Y.; Xu, W. A test strip platform based on a whole-cell microbial biosensor for simultaneous on-site detection of total inorganic mercury pollutants in cosmetics without the need for predigestion. Biosens. Bioelectron. 2020, 150, 111899. [Google Scholar] [CrossRef]
- Ma, X.Y.; Miao, P. Silver nanoparticle@DNA tetrahedron-based colorimetric detection of HIV-related DNA with cascade strand displacement amplification. J. Mater. Chem. B 2019, 7, 2608–2612. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Fan, D.Q.; Liu, Y.Q.; Dong, S.J. Cascaded multiple amplification strategy for ultrasensitive detection of HIV/HCV virus DNA. Biosens. Bioelectron. 2017, 87, 116–121. [Google Scholar] [CrossRef]
- Hu, X.L.; Li, C.; Feng, C.; Mao, X.X.; Xiang, Y.; Li, G.X. One-step colorimetric detection of an antibody based on protein-induced unfolding of a G-quadruplex switch. Chem. Commun. 2017, 53, 4692–4694. [Google Scholar] [CrossRef]
- Chong, H.Q.; Ching, C.B. Development of Colorimetric-Based Whole-Cell Biosensor for Organophosphorus Compounds by Engineering Transcription Regulator DmpR. ACS Synth. Biol. 2016, 5, 1290–1298. [Google Scholar] [CrossRef]
- Tao, Y.; Li, M.Q.; Kim, B.; Auguste, D.T. Incorporating gold nanoclusters and target-directed liposomes as a synergistic amplified colorimetric sensor for HER2-positive breast cancer cell detection. Theranostics 2017, 7, 899–911. [Google Scholar] [CrossRef]
- Ye, X.S.; Shi, H.; He, X.X.; Wang, K.M.; He, D.G.; Yan, L.A.; Xu, F.Z.; Lei, Y.L.; Tang, J.L.; Yu, Y.R. Iodide-Responsive Cu-Au Nanoparticle-Based Colorimetric Platform for Ultrasensitive Detection of Target Cancer Cells. Anal. Chem. 2015, 87, 7141–7147. [Google Scholar] [CrossRef]
- Liu, L.; Lin, H.W. Paper-Based Colorimetric Array Test Strip for Selective and Semiquantitative Multi-Ion Analysis: Simultaneous Detection of Hg2+, Ag+, and Cu2+. Anal. Chem. 2014, 86, 8829–8834. [Google Scholar] [CrossRef]
- Ji, R.; Niu, W.; Chen, S.; Xu, W.; Ji, X.; Yuan, L.; Zhao, H.; Geng, M.; Qiu, J.; Li, C. Target-inspired Pb2+-dependent DNAzyme for ultrasensitive electrochemical sensor based on MoS2-AuPt nanocomposites and hemin/G-quadruplex DNAzyme as signal amplifier. Biosens. Bioelectron. 2019, 144, 111560. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.F.; Wang, Y.T.; Hua, X.X.; Huang, Y.Q.; Feng, X.M.; Fan, Q.L.; Huang, W. Rapid Detection of Lead Ion (II) Based on Cationic Conjugated Polymer and Aptamer. Chin. J. Anal. Chem. 2016, 44, 1092–1098. [Google Scholar]
- Tsekenis, G.; Filippidou, M.K.; Chatzipetrou, M.; Tsouti, V.; Zergioti, I.; Chatzandroulis, S. Heavy metal ion detection using a capacitive micromechanical biosensor array for environmental monitoring. Sens. Actuators B Chem. 2015, 208, 628–635. [Google Scholar] [CrossRef]
- Chen, X.; Ba, Y.; Ma, L.J.; Cai, X.; Yin, Y.; Wang, K.H.; Guo, J.G.; Zhang, Y.J.; Chen, J.N.; Guo, X.; et al. Characterization of microRNAs in serum: A novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008, 18, 997–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, H.F.; Lei, J.P.; Ding, L.; Wen, Y.Q.; Ju, H.X.; Zhang, X.J. MicroRNA: Function, Detection, and Bioanalysis. Chem. Rev. 2013, 113, 6207–6233. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Huang, K.J.; Niu, K.X. Recent advances in signal amplification strategy based on oligonucleotide and nanomaterials for microRNA detection-a review. Biosens. Bioelectron. 2018, 99, 612–624. [Google Scholar] [CrossRef]
- Ma, D.D.; Huang, C.X.; Zheng, J.; Tang, J.R.; Li, J.S.; Yang, J.F.; Yang, R.H. Quantitative detection of exosomal microRNA extracted from human blood based on surface-enhanced Raman scattering. Biosens. Bioelectron. 2018, 101, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.C.; Zhang, P.; Chai, Y.Q.; Yuan, R. Bi-directional DNA Walking Machine and Its Application in an Enzyme-Free Electrochemiluminescence Biosensor for Sensitive Detection of MicroRNAs. Anal. Chem. 2017, 89, 5036–5042. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.Y.; Han, B.; Liu, W.C.; Zhou, J.F.; Liu, K.W.; Yang, D.P.; Tang, D.P. Liposome-amplified photoelectrochemical immunoassay for highly sensitive monitoring of disease biomarkers based on a split-type strategy. Biosens. Bioelectron. 2018, 99, 230–236. [Google Scholar] [CrossRef]
- Dave, V.P.; Ngo, T.A.; Pernestig, A.-K.; Tilevik, D.; Kant, K.; Nguyen, T.; Wolff, A.; Bang, D.D. MicroRNA amplification and detection technologies: Opportunities and challenges for point of care diagnostics. Lab. Investig. 2019, 99, 452–469. [Google Scholar] [CrossRef]
- Zhou, D.; Zhang, H.; Lin, H.; Jiang, J.; Lu, Z. New High Throughput Method to Analyze the Methylation Pattern of Individual DNA Molecules. Nanosci. Nanotech. Let. 2018, 10, 1554–1561. [Google Scholar] [CrossRef]
- Schwarzenbach, R.P.; Egli, T.; Hofstetter, T.B.; von Gunten, U.; Wehrli, B. Global Water Pollution and Human Health. Annu. Rev. Environ. Resour. 2010, 35, 109–136. [Google Scholar] [CrossRef]
- Hammond, J.L.; Formisano, N.; Estrela, P.; Carrara, S.; Tkac, J. Electrochemical biosensors and nanobiosensors. Biosens. Technol. Detect. Biomol. 2016, 60, 69–80. [Google Scholar]
- Jyoti, A.; Tomar, R.S. Detection of pathogenic bacteria using nanobiosensors. Environ. Chem. Lett. 2017, 15, 1–6. [Google Scholar] [CrossRef]
- Ranjbar, S.; Shahrokhian, S. Design and fabrication of an electrochemical aptasensor using Au nanoparticles/carbon nanoparticles/cellulose nanofibres nanocomposite for rapid and sensitive detection of Staphylococcus aureus. Bioelectrochemistry 2018, 123, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, B.; Feng, P. Rapid Detection of Food-borne Pathogenic Bacteria. Annu. Rev. Microbiol. 1994, 48, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Umesha, S.; Manukumar, H.M. Advanced molecular diagnostic techniques for detection of food-borne pathogens: Current applications and future challenges. Crit. Rev. Food Sci. Nutr. 2018, 58, 84–104. [Google Scholar] [CrossRef] [PubMed]
- Rohde, A.; Hammerl, J.A.; Appel, B.; Dieckmann, R.; Al Dahouk, S. FISHing for bacteria in food—A promising tool for the reliable detection of pathogenic bacteria? Food Microbiol. 2015, 46, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Kasprzyk-Hordern, B.; Frost, C.G.; Estrela, P.; Thomas, K.V. Community Sewage Sensors for Monitoring Public Health. Environ. Sci. Technol. 2015, 49, 5845–5846. [Google Scholar] [CrossRef] [Green Version]
- Bickman, S.R.; Campbell, K.; Elliott, C.; Murphy, C.; O’Kennedy, R.; Papst, P.; Lochhead, M.J. An Innovative Portable Biosensor System for the Rapid Detection of Freshwater Cyanobacterial Algal Bloom Toxins. Environ. Sci. Technol. 2018, 52, 11691–11698. [Google Scholar] [CrossRef] [Green Version]
- Preece, E.P.; Hardy, F.J.; Moore, B.C.; Bryan, M. A review of microcystin detections in Estuarine and Marine waters: Environmental implications and human health risk. Harmful Algae 2017, 61, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Shi, L.; Kuang, H.; Xu, C. DNA-Driven Nanoparticle Assemblies for Biosensing and Bioimaging. Top Curr. Chem. 2020, 378, 18. [Google Scholar] [CrossRef]
- Tian, B.; Gao, F.; Fock, J.; Dufva, M.; Hansen, M.F. Homogeneous circle-to-circle amplification for real-time optomagnetic detection of SARS-CoV-2 RdRp coding sequence. Biosens. Bioelectron. 2020, 165, 112356. [Google Scholar] [CrossRef] [PubMed]
- Mao, K.; Zhang, H.; Yang, Z. Can a Paper-Based Device Trace COVID-19 Sources with Wastewater-Based Epidemiology? Environ. Sci. Technol. 2020, 54, 3733–3735. [Google Scholar] [CrossRef]
- Na, W.; Nam, D.; Lee, H.; Shin, S. Rapid molecular diagnosis of infectious viruses in microfluidics using DNA hydrogel formation. Biosens. Bioelectron. 2018, 108, 9–13. [Google Scholar] [CrossRef]
- Lu, C.-H.; Willner, B.; Willner, I. DNA Nanotechnology: From Sensing and DNA Machines to Drug-Delivery Systems. ACS Nano 2013, 7, 8320–8332. [Google Scholar] [CrossRef]
- De Avila, B.E.F.; Angell, C.; Soto, F.; Lopez-Ramirez, M.A.; Baez, D.F.; Xie, S.B.; Wang, J.; Chen, Y. Acoustically Propelled Nanomotors for Intracellular siRNA Delivery. ACS Nano 2016, 10, 4997–5005. [Google Scholar] [CrossRef] [PubMed]
- Shimron, S.; Cecconello, A.; Lu, C.-H.; Willner, I. Metal Nanoparticle-Functionalized DNA Tweezers: From Mechanically Programmed Nanostructures to Switchable Fluorescence Properties. Nano Lett. 2013, 13, 3791–3795. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, H.; Huang, H.; Deng, J.; Fang, L.; Luo, J.; Zhang, S.; Huang, J.; Liang, W.; Zheng, J. A sensitive electrochemical strategy via multiple amplification reactions for the detection of E. coli O157: H7. Biosens. Bioelectron. 2020, 147, 111752. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Fan, F.; Hou, Y.; Yang, H.; Meng, X.; Zhang, Y.; Ren, F. Microfluidic chip and its application in autophagy detection. Trends Anal. Chem. 2019, 117, 300–315. [Google Scholar] [CrossRef]
- Heo, H.Y.; Chung, S.; Kim, Y.T.; Kim, D.H.; Seo, T.S. A valveless rotary microfluidic device for multiplex point mutation identification based on ligation-rolling circle amplification. Biosens. Bioelectron. 2016, 78, 140–146. [Google Scholar] [CrossRef]
- Liu, M.; Hui, C.Y.; Zhang, Q.; Gu, J.; Kannan, B.; Jahanshahi-Anbuhi, S.; Filipe, C.D.; Brennan, J.D.; Li, Y. Target-Induced and Equipment-Free DNA Amplification with a Simple Paper Device. Angew. Chem. Int. Ed. Engl. 2016, 55, 2709–2713. [Google Scholar] [CrossRef]
- Huang, S.; Feng, M.; Li, J.; Liu, Y.; Xiao, Q. Voltammetric determination of attomolar levels of a sequence derived from the genom of hepatitis B virus by using molecular beacon mediated circular strand displacement and rolling circle amplification. Microchim. Acta 2018, 185, 206. [Google Scholar] [CrossRef]
- Shen, C.; Liu, S.; Li, X.; Zhao, D.; Yang, M. Immunoelectrochemical detection of thehuman epidermal growth factor receptor 2 (HER2) via gold nanoparticle-based rolling circle amplification. Microchim. Acta 2018, 185, 547. [Google Scholar] [CrossRef]
- Yi, X.; Li, L.; Peng, Y.; Guo, L. A universal electrochemical sensing system for small biomolecules using target-mediated sticky ends-based ligation-rolling circle amplification. Biosens. Bioelectron. 2014, 57, 103–109. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, F.; Na, N.; Ouyang, J. Detection of p53 DNA using commercially available personal glucose meters based on rolling circle amplification coupled with nicking enzyme signal amplification. Anal. Chim. Acta 2019, 1060, 64–70. [Google Scholar] [CrossRef]






| Features | Conventional PCR Assay | Real Time-PCR Assay | RCA Assay |
|---|---|---|---|
| Sensitivity | Sensitive | Highly sensitive | Highly sensitive |
| Specificity | Specific | Specific | Specific |
| Temperature conditions | Thermal cycle | Thermal cycle | Isothermal |
| Inhibition by biological samples | Yes | Yes | No |
| Instruments required | Thermocycler | Thermocycler | Not required |
| Post-assay analysis | Required | Required | Generally not required |
| Amplicon detection methods | Gel electrophoresis | Real-time detection/amplification graph | Gel electrophoresis, Turbidity measurement by visual inspection or using a real-time turbidimeter; dye-based visual detection |
| Qualitative detection | Yes | Yes | Yes |
| Quantitative detection | No | Yes | Semi-quantitative |
| Portability | Partially | Yes | Yes |
| Overall assay time | 3–5 h | 2.5–4 h | 1–1.5 h |
| Cost effectiveness | Less expensive | Expensive | Less expensive |
| Targets | Detection Signal | Detection Range | LOD | Reference | |
|---|---|---|---|---|---|
| Heavy metal ions | Hg2+ | Fluorescence | 0.42 pM–42.5 nM | 0.14 pM | [74] |
| Heavy metal ions | Hg2+ | Electrochemical | 0.2 pM–100 nM | 0.097 pM | [75] |
| Heavy metal ions | Hg2+ | Fluorescence | 0–20 nM | 200 pM | [76] |
| Heavy metal ions | Hg2+ | ECL | 0.1 pM–0.1 μM | 33 fM | [27] |
| Heavy metal ions | Hg2+ | Electrochemical | 1 pM–1 μM | 0.684 pM | [77] |
| Heavy metal ions | Hg2+ | Colorimetry | 2.5–100 nM | 1.6 nM | [78] |
| Heavy metal ions | Hg2+ | Colorimetry | 0–14 μg L−1 | 3.3 μg L−1 | [79] |
| Heavy metal ions | Pb2+ | Fluorescence | 1.0–100 nM | 1 nM | [80] |
| Heavy metal ions | Pb2+ | pH values | 1.0–100 nM | 0.91 nM | [81] |
| Heavy metal ions | Pb2+ | Fluorescence | 0.1–50 nM | 0.03 nM | [60] |
| Heavy metal ions | UO2 2+ | Colorimetry | 0.02–15 ng mL−1 | 1.0 pg mL−1 | [82] |
| Organic small molecules | Bisphenol A (BPA) | Fluorescence | 1 nM–0.1 fM | 5.4 × 10−17 M | [83] |
| Nucleic acids | miRNA | Fluorescence | 50–500 fM | 25 fM | [84] |
| Nucleic acids | miRNA | Fluorescence | 10–106fM | 20 fM | [85] |
| Nucleic acids | R6G | Fluorescence | 10−16–10−11M | 8.7 × 10−18 M | [86] |
| Nucleic acids | gene point mutation | Fluorescence | 1 μM | [87] | |
| Peptides and proteins | microcystin-LR | Electrochemical | 0.01–50 μg L−1 | 0.007 μg L−1 | [88] |
| Peptides and proteins | glutamate dehydrogenase (GDH) | Fluorescence | 10–100 nm | 3 nM | [89] |
| Microorganisms | Karenia mikimotoi | Lateral flow assay | 1–1000 cells mL−1 | 0.1 cell mL−1 | [90] |
| Microorganisms | Karenia mikimotoi | Colorimetry | 1–1000 cells mL−1 | 1 cell mL−1 | [91] |
| Microorganisms | Harmful algal blooms (HABs) | Colorimetry | 0.1–1000 cells mL−1 | 0.1 cell mL−1 | [92] |
| Microorganisms | Exophiala | Electrophoresis | - | single-nucleotide level | [93] |
| Microorganisms | 16S rDNA | THz absorption | 10−10–10−7 M | 0.6 × 10−10 M | [94] |
| Microorganisms | Chattonella marina | Fluorescence | 10–105 cells mL−1 | 10 cell mL−1 | [95] |
| Microorganisms | circular ssDNA viruses | Whole-genome sequencing | - | [96] | |
| Microorganisms | Amphidinium carterae | Electrophoresis | 100 ng mL−1–1 fg mL−1 | 281 copies | [97] |
| Microorganisms | bacterial DNA sequences | Optical (laser) | - | one bacterial DNA sequence | [98] |
| Living bacteria | Salmonella typhimurium | Current | 20–2 × 108 CFU mL−1 | 16 CFU mL−1 | [99] |
| Other targets | ATP | Droplet motion | 50 pM–5 mM | 5 nM | [100] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Zhang, H.; Cao, H.; Jiang, Y.; Mao, K.; Yang, Z. Rolling Circle Amplification as an Efficient Analytical Tool for Rapid Detection of Contaminants in Aqueous Environments. Biosensors 2021, 11, 352. https://doi.org/10.3390/bios11100352
Zhang K, Zhang H, Cao H, Jiang Y, Mao K, Yang Z. Rolling Circle Amplification as an Efficient Analytical Tool for Rapid Detection of Contaminants in Aqueous Environments. Biosensors. 2021; 11(10):352. https://doi.org/10.3390/bios11100352
Chicago/Turabian StyleZhang, Kuankuan, Hua Zhang, Haorui Cao, Yu Jiang, Kang Mao, and Zhugen Yang. 2021. "Rolling Circle Amplification as an Efficient Analytical Tool for Rapid Detection of Contaminants in Aqueous Environments" Biosensors 11, no. 10: 352. https://doi.org/10.3390/bios11100352








