Mesoporous One-Component Gold Microshells as 3D SERS Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of CaCO3 Templates
2.3. Synthesis of Gold Nanoparticles
2.4. Immobilization of Gold Nanoparticles
2.5. Fabrication of One-Component SERS Substrates
2.6. Dynamic Light Scattering (DLS) and ζ-Potential Measurements
2.7. Brunauer, Emmett and Teller (BET) Analysis
2.8. Scanning Electron Microscopy (SEM) and Transmission Electron Microscopy (TEM)
2.9. Raman Microscopy
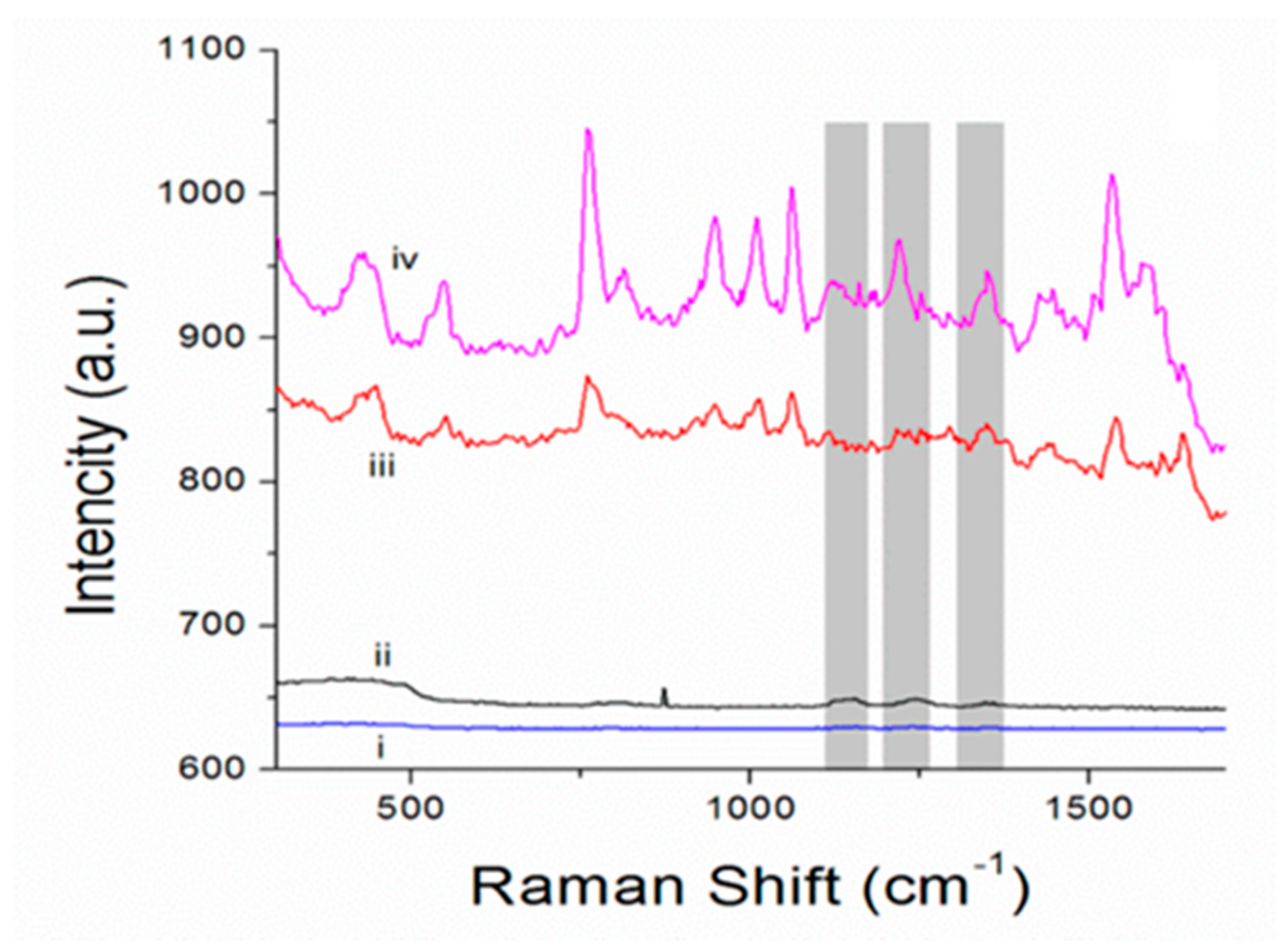
3. Results
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Langer, J.; de Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and Future of Surface-Enhanced Raman Scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Liu, W.; Tian, S.; Hong, T. Novel Surface-Enhanced Raman Spectroscopy Techniques for DNA, Protein and Drug Detection. Sensors 2019, 19, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, A.; Ghosh, A.; Barui, A. Advances in surface-enhanced Raman spectroscopy for cancer diagnosis and staging. J. Raman Spectrosc. 2020, 51, 7–36. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Q.; Ma, F.; Li, X.; Liu, F.; Zhou, M. Surface-enhanced Raman nanoparticles for tumor theranostics applications. Acta Pharm. Sin. B 2018, 8, 349–359. [Google Scholar] [CrossRef]
- Andreou, C.; Kishore, S.A.; Kircher, M.F. Surface-Enhanced Raman Spectroscopy: A New Modality for Cancer Imaging. J. Nucl. Med. 2015, 56, 1295–1299. [Google Scholar] [CrossRef] [Green Version]
- Turk, N.; Raza, A.; Wuytens, P.; Demol, H.; Van Daele, M.; Detavernier, C.; Skirtach, A.; Gevaert, K.; Baets, R. Comparison of free-space and waveguide-based SERS platforms. Nanomaterials 2019, 9, 1401. [Google Scholar] [CrossRef] [Green Version]
- Krafft, C.; Schmitt, M.; Schie, I.W.; Cialla-May, D.; Matthäus, C.; Bocklitz, T.; Popp, J. Label-Free Molecular Imaging of Biological Cells and Tissues by Linear and Nonlinear Raman Spectroscopic Approaches. Angew. Chem. Int. Ed. 2017, 56, 4392–4430. [Google Scholar] [CrossRef]
- Bontempi, N.; Carletti, L.; De Angelis, C.; Alessandri, I. Plasmon-free SERS detection of environmental CO2 on TiO2 surfaces. Nanoscale 2016, 8, 3226–3231. [Google Scholar] [CrossRef]
- Martines-Arano, H.; García-Pérez, B.E.; Vidales-Hurtado, M.A.; Trejo-Valdez, M.; Hernández-Gómez, L.H.; Torres-Torres, C. Chaotic Signatures Exhibited by Plasmonic Effects in Au Nanoparticles with Cells. Sensors 2019, 19, 4728. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Zhang, L.; Lang, X.; Yamaguchi, Y.; Iwasaki, H.; Inouye, Y.; Xue, Q.; Chen, M. Single molecule detection from a large-scale SERS-active Au79Ag21 substrate. Sci. Rep. 2011, 1, 112. [Google Scholar] [CrossRef]
- Piao, L.; Park, S.; Lee, H.B.; Kim, K.; Kim, J.; Chung, T.D. Single gold microshell tailored to sensitive surface enhanced raman scattering probe. Anal. Chem. 2010, 82, 447–451. [Google Scholar] [CrossRef]
- Demirel, G.; Usta, H.; Yilmaz, M.; Çelik, M.; Alidagi, H.A.; Buyukserin, F. Surface-enhanced Raman spectroscopy (SERS): An adventure from plasmonic metals to organic semiconductors as SERS platforms. J. Mater. Chem. C 2018, 6, 5314–5335. [Google Scholar] [CrossRef]
- Son, H.Y.; Kim, K.R.; Lee, J.B.; Le Kim, T.H.; Jang, J.; Kim, S.J.; Yoon, M.S.; Kim, J.W.; Nam, Y.S. Bioinspired Synthesis of Mesoporous Gold-silica Hybrid Microspheres as Recyclable Colloidal SERS Substrates. Sci. Rep. 2017, 7, 14728. [Google Scholar] [CrossRef] [Green Version]
- Han, D.; Lim, S.Y.; Kim, B.J.; Piao, L.; Chung, T.D. Mercury(ii) detection by SERS based on a single gold microshell. Chem. Commun. 2010, 46, 5587–5589. [Google Scholar] [CrossRef]
- Lai, Y.; Sun, S.; He, T.; Schlücker, S.; Wang, Y. Raman-encoded microbeads for spectral multiplexing with SERS detection. RSC Adv. 2015, 5, 13762–13767. [Google Scholar] [CrossRef] [Green Version]
- Hwang, H.; Kim, S.-H.; Yang, S.-M. Microfluidic fabrication of SERS-active microspheres for molecular detection. Lab Chip 2011, 11, 87–92. [Google Scholar] [CrossRef]
- Rice, D.; Mouras, R.; Gleeson, M.; Liu, N.; Tofail, S.A.M.; Soulimane, T.; Silien, C. APTES duality and nanopore seed regulation in homogeneous and nanoscale-controlled reduction of Ag shell on SiO2 microparticle for quantifiable single particle SERS. ACS Omega 2018, 3, 13028–13035. [Google Scholar] [CrossRef] [Green Version]
- Kamyshinsky, R.; Marchenko, I.; Parakhonskiy, B.; Yashchenok, A.; Chesnokov, Y.; Mikhutkin, A.; Gorin, D.; Vasiliev, A.L.; Bukreeva, T. Composite materials based on Ag nanoparticles in situ synthesized on the vaterite porous matrices. Nanotechnology 2018, 30, 035603. [Google Scholar] [CrossRef]
- Yashchenok, A.; Borisova, D.; Parakhonskiy, B.; Masic, A.; Pinchasik, B.; Möhwald, H.; Skirtach, A. Nanoplasmonic smooth silica versus porous calcium carbonate bead biosensors for detection of biomarkers. Ann. Phys. 2012, 524, 723–732. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Abalymov, A.; Ivanova, A.; Khalenkow, D.; Skirtach, A.G. Magnetic and silver nanoparticle functionalized calcium carbonate particles—Dual functionality of versatile, movable delivery carriers which can surface-enhance Raman signals. J. Appl. Phys. 2019, 126, 203102. [Google Scholar] [CrossRef]
- Stetciura, I.Y.; Markin, A.V.; Ponomarev, A.N.; Yakimansky, A.; Demina, T.; Grandfils, C.; Volodkin, D.; Gorin, D. New surface-enhanced Raman scattering platforms: Composite calcium carbonate microspheres coated with astralen and silver nanoparticles. Langmuir 2013, 29, 4140–4147. [Google Scholar] [CrossRef]
- Stetciura, I.Y.; Yashchenok, A.; Masic, A.; Lyubin, E.; Inozemtseva, O.A.; Drozdova, M.; Markvichova, E.A.; Khlebtsov, B.; Fedyanin, A.A.; Sukhorukov, G.B.; et al. Composite SERS-based satellites navigated by optical tweezers for single cell analysis. Analyst 2015, 140, 4981–4986. [Google Scholar] [CrossRef] [Green Version]
- Markina, N.E.; Markin, A.V.; Zakharevich, A.M.; Goryacheva, I. Calcium carbonate microparticles with embedded silver and magnetite nanoparticles as new SERS-active sorbent for solid phase extraction. Microchim. Acta 2017, 184, 3937–3944. [Google Scholar] [CrossRef]
- Vikulina, A.; Voronin, D.V.; Fakhrullin, R.F.; Vinokurov, V.A.; Volodkin, D. Naturally derived nano- and micro-drug delivery vehicles: Halloysite, vaterite and nanocellulose. New J. Chem. 2020, 44, 5638–5655. [Google Scholar] [CrossRef] [Green Version]
- Trushina, D.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO3 vaterite microparticles for biomedical and personal care applications. Mater. Sci. Eng. C 2014, 45, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Trushina, D.; Bukreeva, T.V.; Antipina, M.N. Size-controlled synthesis of vaterite calcium carbonate by the mixing method: Aiming for nanosized particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Parakhonskiy, B.V.; Yashchenok, A.M.; Donatan, S.; Volodkin, D.V.; Tessarolo, F.; Antolini, R.; Möhwald, H.; Skirtach, A.G. Macromolecule loading into spherical, elliptical, star-like and cubic calcium carbonate carriers. Chem. Phys. Chem. 2014, 15, 2817–2822. [Google Scholar] [CrossRef] [PubMed]
- Feoktistova, N.; Rose, J.; Prokopović, V.Z.; Vikulina, A.; Skirtach, A.G.; Volodkin, D. Controlling the vaterite CaCO3 crystal pores. Design of tailor-made polymer based microcapsules by hard templating. Langmuir 2016, 32, 4229–4238. [Google Scholar] [CrossRef] [Green Version]
- Chong, K.Y.; Chia, C.H.; Zakaria, S.; Sajab, M.S. Vaterite calcium carbonate for the adsorption of Congo red from aqueous solutions. J. Environ. Chem. Eng. 2014, 2, 2156–2161. [Google Scholar] [CrossRef]
- Balabushevich, N.G.; Kovalenko, E.A.; Mikhalchik, E.V.; Filatova, L.Y.; Volodkin, D.; Vikulina, A.S. Mucin adsorption on vaterite CaCO3 microcrystals for the prediction of mucoadhesive properties. J. Colloid Interface Sci. 2019, 545, 330–339. [Google Scholar] [CrossRef]
- Feoktistova, N.A.; Vikulina, A.S.; Balabushevich, N.G.; Skirtach, A.G.; Volodkin, D. Bioactivity of catalase loaded into vaterite CaCO3 crystals via adsorption and co-synthesis. Mater. Des. 2020, 185, 108223. [Google Scholar] [CrossRef]
- Campbell, J.; Abnett, J.; Kastania, G.; Volodkin, D.; Vikulina, A.S. Which biopolymers are better for the fabrication of multilayer capsules? A comparative study using vaterite CaCO3 as templates. ACS Appl. Mater. Interfaces 2021, 13, 3259–3269. [Google Scholar] [CrossRef]
- Demina, P.A.; Voronin, D.V.; Lengert, E.V.; Abramova, A.M.; Atkin, V.S.; Nabatov, B.V.; Semenov, A.P.; Shchukin, D.G.; Bukreeva, T.V. Freezing-induced loading of TiO2 into porous vaterite microparticles: Preparation of CaCO3/TiO2 composites as templates to assemble UV-responsive microcapsules for wastewater treatment. ACS Omega 2020, 5, 4115–4124. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Y.; Cui, Y.; Duan, M.; Liu, M. Study on the non-linear refraction of silver nanoparticles with aggregation effect. Opt. Commun. 2005, 249, 311–317. [Google Scholar] [CrossRef]
- Xie, C.; Li, Y.-Q.; Tang, W.; Newton, R.J. Study of dynamical process of heat denaturation in optically trapped single microorganisms by near-infrared Raman spectroscopy. J. Appl. Phys. 2003, 94, 6138. [Google Scholar] [CrossRef] [Green Version]
- Sundaraganesan, N.; Kalaichelvan, S.; Meganathan, C.; Joshua, B.D.; Cornard, J.-P. FT-IR, FT-Raman spectra and ab initio HF and DFT calculations of 4-N,N′-dimethylamino pyridine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 71, 898–906. [Google Scholar] [CrossRef]
- Saveleva, M.; Prikhozhdenko, E.; Gorin, D.; Skirtach, A.G.; Yashchenok, A.; Parakhonskiy, B. Polycaprolactone-based, porous CaCO3 and Ag nanoparticle modified scaffolds as a SERS platform with molecule-specific adsorption. Front. Chem. 2020, 7, 888. [Google Scholar] [CrossRef] [Green Version]
- Negro, L.D.; Feng, N.-N.; Gopinath, A. Electromagnetic coupling and plasmon localization in deterministic aperiodic arrays. J. Opt. A Pure Appl. Opt. 2008, 10, 10. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, S.; Cole, R.M.; Speed, J.D.; Pelfrey, S.H.; Russell, A.E.; Bartlett, P.N.; Barnett, S.M.; Baumberg, J.J. Understanding the Surface-Enhanced Raman Spectroscopy “Background”. J. Phys. Chem. C 2010, 114, 7242–7250. [Google Scholar] [CrossRef] [Green Version]
- Feoktistova, N.A.; Balabushevich, N.; Skirtach, A.G.; Volodkin, D.; Vikulina, A.S. Inter-protein interactions govern protein loading into porous vaterite CaCO3 crystals. Phys. Chem. Chem. Phys. 2020, 22, 9713–9722. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Liang, D.; Jin, Q.; Feng, J.; Tang, X. Bioorthogonal SERS nanotags as a precision theranostic platform for in vivo SERS imaging and cancer photothermal therapy. Bioconjugate Chem. 2020, 31, 182–193. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikulina, A.S.; Stetsyura, I.Y.; Onses, M.S.; Yilmaz, E.; Skirtach, A.G.; Volodkin, D. Mesoporous One-Component Gold Microshells as 3D SERS Substrates. Biosensors 2021, 11, 380. https://doi.org/10.3390/bios11100380
Vikulina AS, Stetsyura IY, Onses MS, Yilmaz E, Skirtach AG, Volodkin D. Mesoporous One-Component Gold Microshells as 3D SERS Substrates. Biosensors. 2021; 11(10):380. https://doi.org/10.3390/bios11100380
Chicago/Turabian StyleVikulina, Anna S., Inna Y. Stetsyura, M. Serdar Onses, Erkan Yilmaz, Andre G. Skirtach, and Dmitry Volodkin. 2021. "Mesoporous One-Component Gold Microshells as 3D SERS Substrates" Biosensors 11, no. 10: 380. https://doi.org/10.3390/bios11100380
APA StyleVikulina, A. S., Stetsyura, I. Y., Onses, M. S., Yilmaz, E., Skirtach, A. G., & Volodkin, D. (2021). Mesoporous One-Component Gold Microshells as 3D SERS Substrates. Biosensors, 11(10), 380. https://doi.org/10.3390/bios11100380







