Investigating the Regulation of Neural Differentiation and Injury in PC12 Cells Using Microstructure Topographic Cues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design and Preparation of Topological Microstructures
2.2. Design and Production of the Upper Layer of the Microchip
2.3. Cell Culture and Seeding
2.4. Immunofluorescence and Imaging
2.5. Total RNA Isolate and RT-qPCR
2.6. Data Analysis
3. Results and Discussion
3.1. Characterization of Topological Microstructures in PDMS Replicas
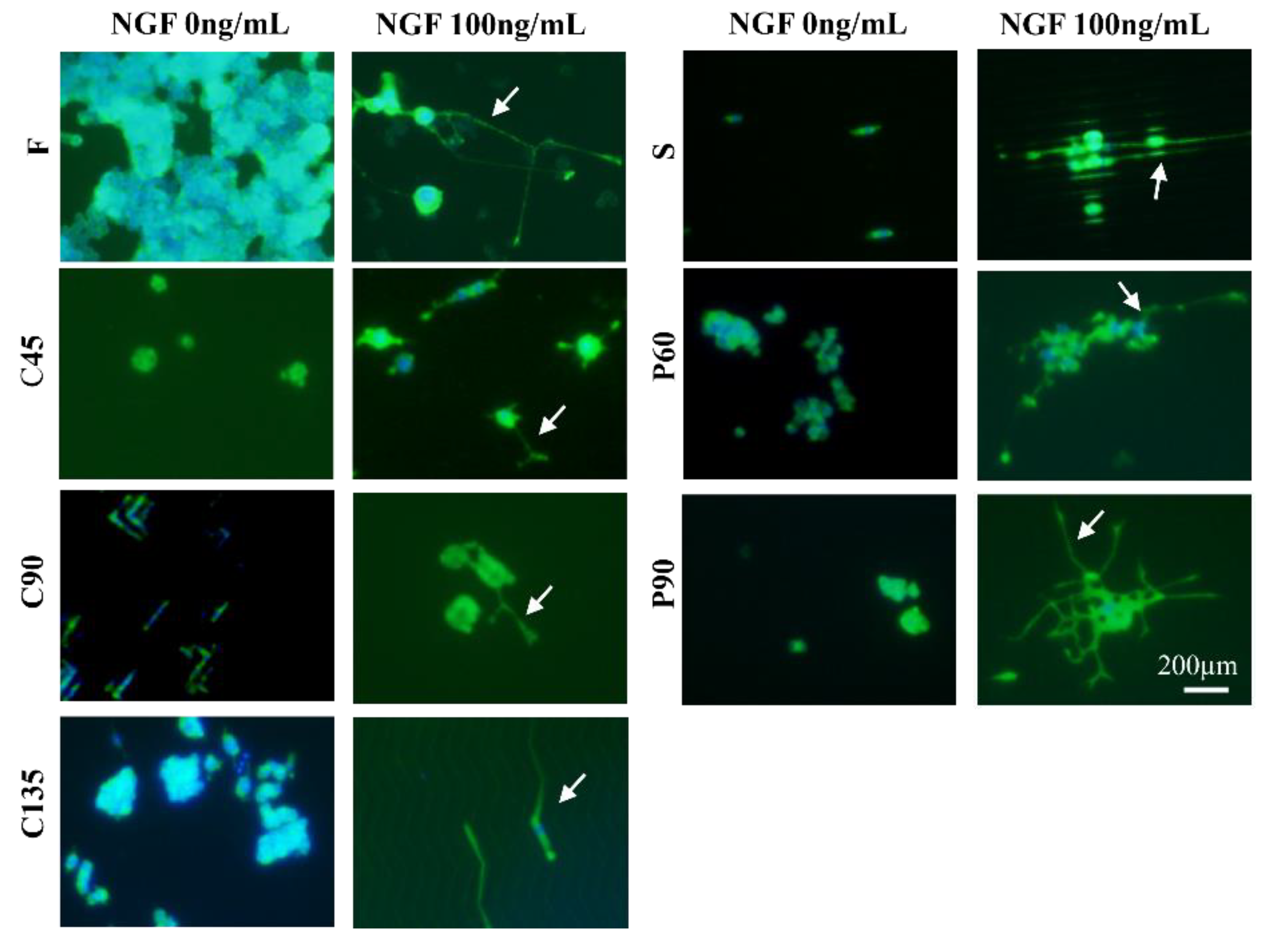
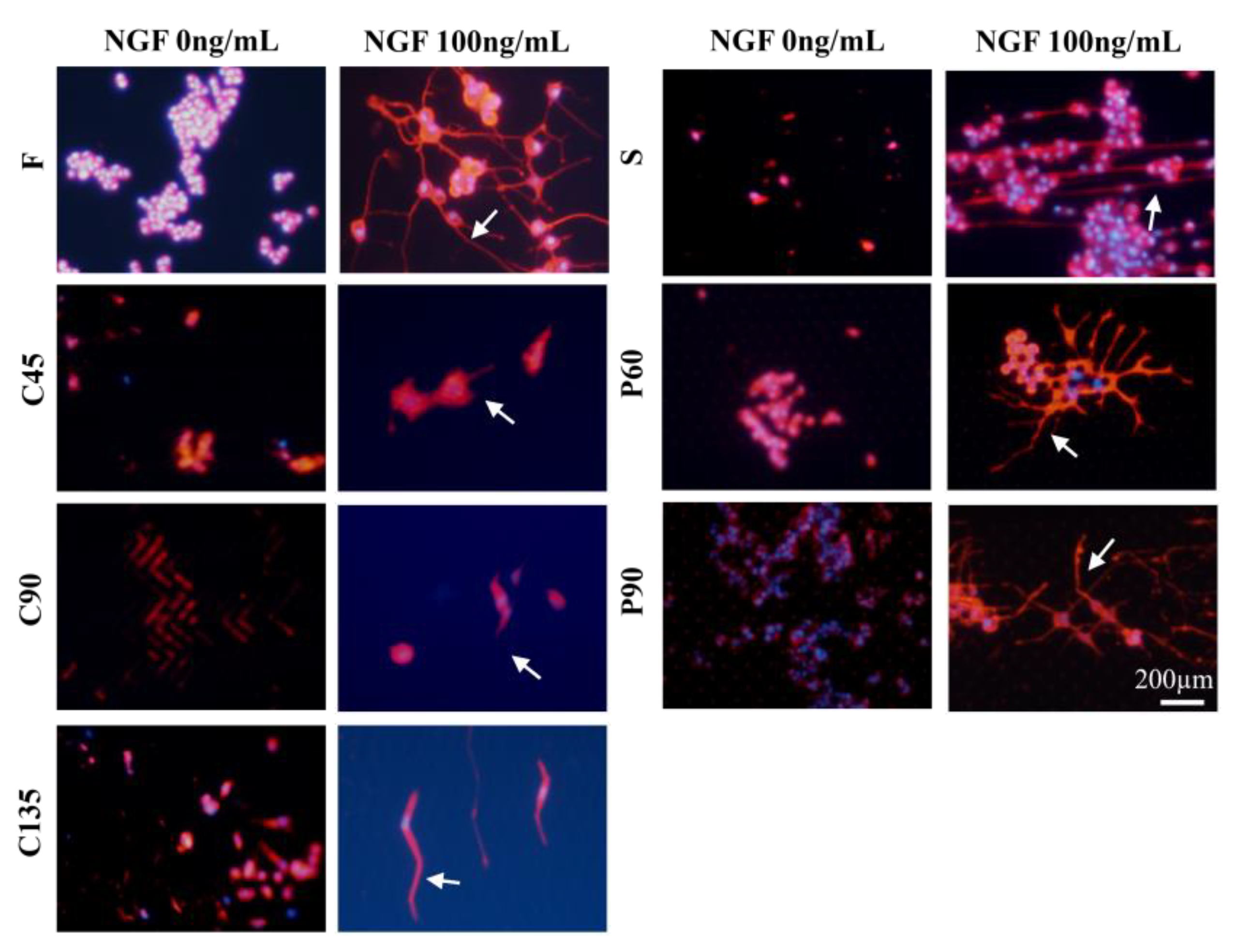
3.2. Differentiation of PC12 Cells in Different Topological Microstructures
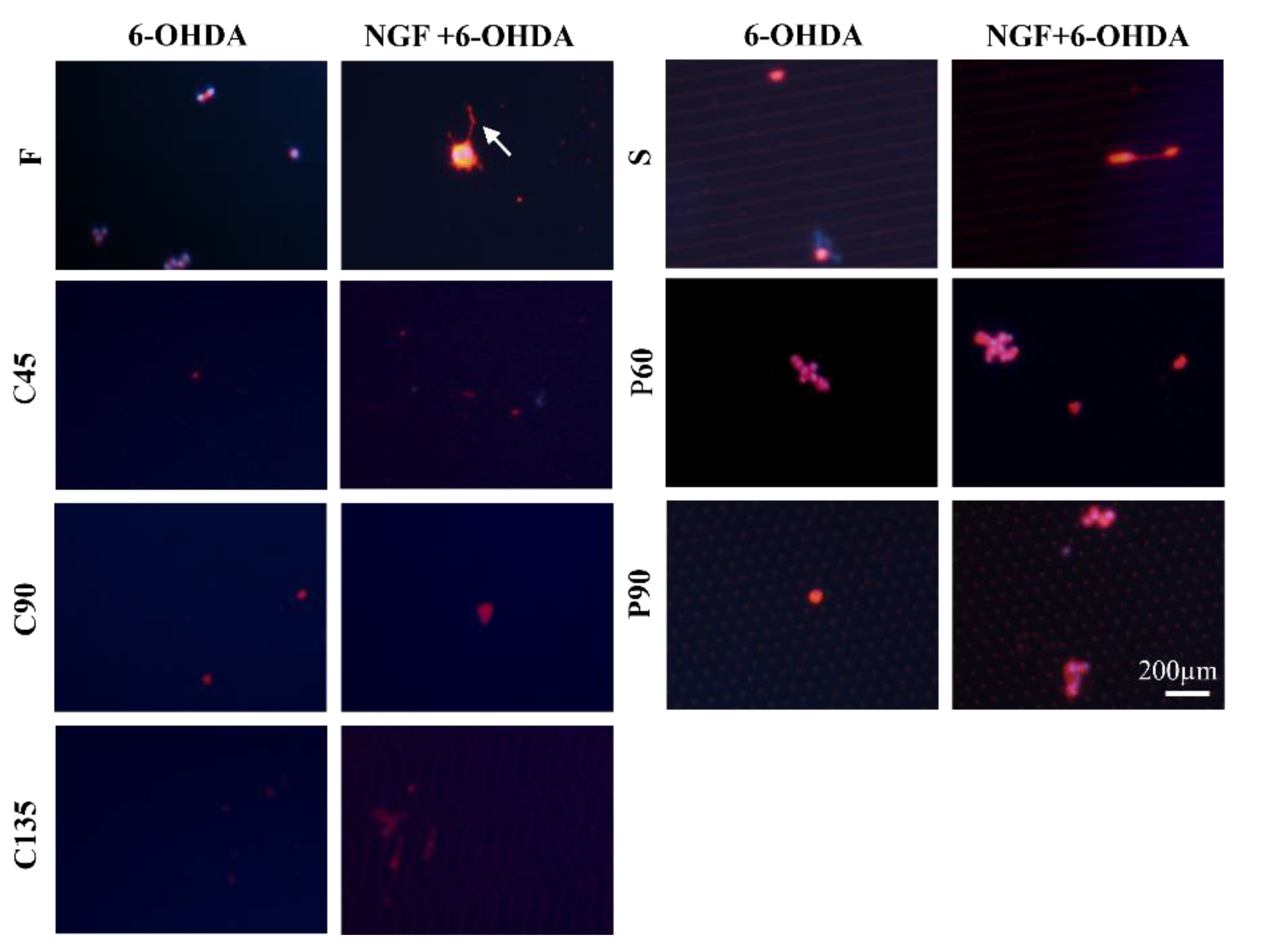
3.3. Cellular Neurotoxin Injury of PC12 Cells on Different Topological Microstructures
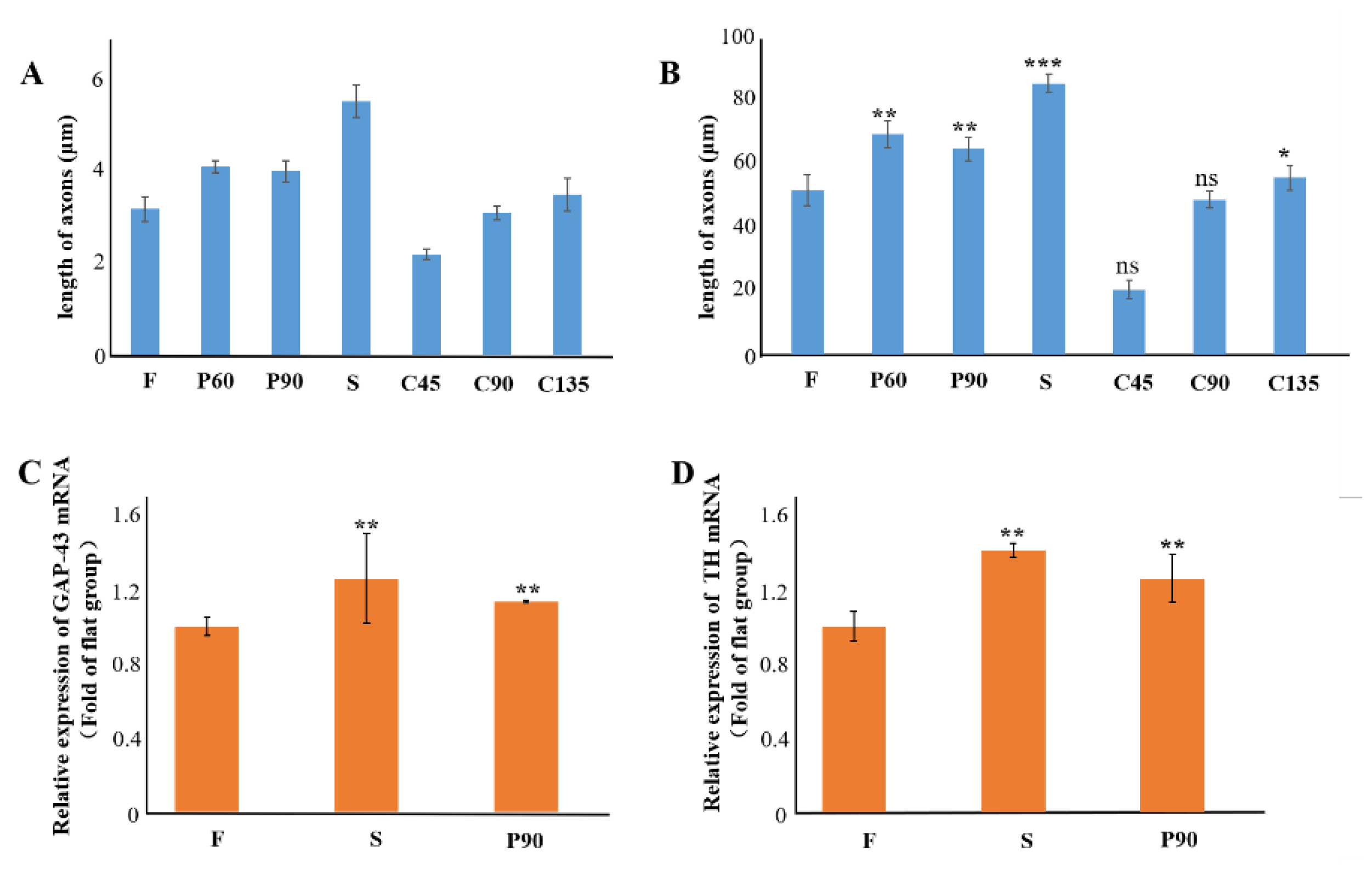
3.4. Analysis on the Trend of Neuronal Axon Differentiation on a Microchip
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Yang, C.Y.; Huang, W.Y.; Chen, L.H.; Liang, N.W.; Wang, H.C.; Lu, J.; Wang, X.; Wang, T.W. Neural tissue engineering: The influence of scaffold surface topography and extracellular matrix microenvironment. J. Mater. Chem. B 2021, 9, 567–584. [Google Scholar] [CrossRef]
- Kovacs, G.G. Concepts and classification of neurodegenerative diseases. Handb. Clin. Neurol. 2017, 145, 301–307. [Google Scholar]
- Fraser, K.B.; Rawlins, A.B.; Clark, R.G.; Alcalay, R.N.; Standaert, D.G.; Liu, N.; Parkinson’s Disease Biomarker Program Consortium; West, A.B. Ser(P)-1292 LRRK2 in Urinary Exosomes Is Elevated in Idiopathic Parkinson’s Disease. Mov. Disord. 2016, 31, 1543–1550. [Google Scholar] [CrossRef]
- Nisbet, R.M.; Gotz, J. Amyloid-β and Tau in Alzheimer’s Disease: Novel Pathomechanisms and Non-Pharmacological Treatment Strategies. J. Alzheimers Dis. 2018, 64, S517–S527. [Google Scholar] [CrossRef]
- Ricco, M.; Vezzosi, L.; Balzarini, F.; Gualerzi, G.; Ranzieri, S. Prevalence of Huntington Disease in Italy: A systematic review and meta-analysis. Acta Biomed. 2020, 91, 119–127. [Google Scholar] [PubMed]
- Sveinbjornsdottir, S.; Neurochem, J. The clinical symptoms of Parkinson’s disease. J. Neurochem. 2016, 139 (Suppl. 1), 318–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liljegren, M.; Naasan, G.; Temlett, J.; Perry, D.C.; Rankin, K.P.; Merrilees, J.; Grinberg, L.T.; Seeley, W.W.; Englund, E.; Miller, B.L. Criminal Behavior in Frontotemporal Dementia and Alzheimer Disease. JAMA Neurol. 2015, 72, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Thies, W.; Bleiler, L. Alzheimer’s Association Report 2011 Alzheimer’s disease facts and figures Alzheimer’s Association. Alzheimers Dement 2011, 7, 208–244. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, C.R.; Kamm, R.D. 3D matrix microenvironment for targeted differentiation of embryonic stem cells into neural and glial lineages. Biomaterials 2013, 34, 5995–6007. [Google Scholar] [CrossRef] [PubMed]
- Puckert, C.; Tomaskovic-Crook, E.; Gambhir, S.; Wallace, G.G.; Crook, J.M.; Higgins, M.J. Molecular interactions and forces of adhesion between single human neural stem cells and gelatin methacrylate hydrogels of varying stiffness. Acta Biomater. 2020, 106, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Donahue, H.J. Cell Sensing and Response to Micro- and Nanostructured Surfaces Produced by Chemical and Topographic Patterning. Tissue Eng. 2007, 13, 1879–1891. [Google Scholar] [CrossRef]
- Zamani, F.; Amani-Tehran, M.; Latifi, M.; Shokrgozar, M.A. The influence of surface nanoroughness of electrospun PLGA nanofibrous scaffold on nerve cell adhesion and proliferation. J. Mater. Sci. Mater. Med. 2013, 24, 1551–1560. [Google Scholar] [CrossRef]
- Kulangara, K.; Adler, A.F.; Wang, H.; Chellappan, M.; Hammett, E.; Yasuda, R.; Leong, K.W. The effect of substrate topography on direct reprogramming of fibroblasts to induced neurons. Biomaterials 2014, 35, 5327–5336. [Google Scholar] [CrossRef] [Green Version]
- Xia, H.; Chen, Q.; Fang, Y.; Liu, D.; Zhong, D.; Wu, H.; Xia, Y.; Yan, Y.; Tang, W.; Sun, X. Directed neurite growth of rat dorsal root ganglion neurons and increased colocalization with Schwann cells on aligned poly (methyl methacrylate) electrospun nanofifibers. Brain Res. 2014, 1565, 18–27. [Google Scholar] [CrossRef]
- Yang, X.; Li, K.; Zhang, X.; Liu, C.; Guo, B.; Wen, W.; Gao, X. Nanofiber Membrane supported lung-on-a-chip Microdevice for Anti-cancer Drug Testing. Lab Chip 2018, 18, 486–495. [Google Scholar] [CrossRef]
- Yao, K.; Li, W.; Li, K.; Wu, Q.; Gu, Y.; Zhao, L.; Zhang, Y.; Gao, X. Simple Fabrication of Multicomponent Heterogeneous Fibers for Cell Co-Culture via Microfluidic Spinning. Macromol. Biosci. 2020, 20, e1900395. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Bai, Y.; You, M.; Hu, J.; Yao, C.; Cao, L.; Xu, F. Fully integrated microfluidic devices for qualitative, quantitative and digital nucleic acids testing at point of care. Biosens. Bioelectron. 2021, 177, 112952. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.B.; Chien, C.C.; You, H.L.; Kuo, F.C.; Lee, M.S.; Lee, G.B. Rapid antimicrobial susceptibility tests on an integrated microfluidic device for precision medicine of antibiotics. Biosens. Bioelectron. 2021, 176, 112890. [Google Scholar] [CrossRef]
- Wu, R.; Kim, T. Review of microfluidic approaches for fabricating intelligent fiber devices: Importance of shape characteristics. Lab Chip 2021, 21, 1217–1240. [Google Scholar] [CrossRef] [PubMed]
- Peyrin, J.; Deleglise, B.; Saias, L.; Vignes, M.; Gougis, P.; Magnifico, S.; Betuing, S.; Pietri, M.; Caboche, J.; Vanhoutte, P.; et al. Axon diodes for the reconstruction of oriented neuronal networks in microfluidic chambers. Lab. Chip 2011, 11, 3663–3673. [Google Scholar] [CrossRef]
- Francisco, H.; Yellen, B.B.; Halverson, D.S.; Friedman, G.; Gallo, G. Regulation of axon guidance and extension by three-dimensional constraints. Biomaterials 2007, 28, 3398–3407. [Google Scholar] [CrossRef] [Green Version]
- Moe, A.A.; Suryana, M.; Marcy, G.; Lim, S.K.; Ankam, S.; Goh, J.Z.; Jin, J.; Teo, B.K.; Law, J.B.; Low, H.Y.; et al. Microarray with Micro- and Nano-topographies Enables Identififi cation of the Optimal Topography for Directing the Differentiation of Primary Murine Neural Progenitor Cells. Small 2012, 8, 3050–3061. [Google Scholar] [CrossRef]
- Li, H.; Tang, Z.; Chu, P.; Song, Y.; Yang, Y.; Sun, B.; Niu, M.; Qaed, E.; Shopit, A.; Han, G.; et al. Neuroprotective effect of Phosphocreatine on oxidative stress and mitochondrial dysfunction induced apoptosis in vitro and in vivo: Involvement of dual PI3K/Akt and Nrf2/HO-1 pathways. Free Radic. Biol. Med. 2018, 120, 228–238. [Google Scholar] [CrossRef]
- Nguyen, T.; Ngo, T.A.; Dang, D.B.; Wolff, A. Optimising the supercritical angle fluorescence structures in polymer microfluidic biochips for highly sensitive pathogen detection: A case study on Escherichia coli. Lab. Chip 2019, 19, 3825–3833. [Google Scholar] [CrossRef] [Green Version]
- Arora, A.; Simone, G.; Salieb-Beugelaar, G.B.; Kim, J.T.; Manz, A. Latest developments in micro total analysis systems. Lab Chip 2010, 82, 4830–4847. [Google Scholar] [CrossRef] [PubMed]
- Hulme, J.P.; An, S.; Goddard, N.; Miyahara, Y.; Oki, A. Fabrication of a flexible multi-referenced surface plasmon sensor using room temperature nanoimprint lithography. Curr. Appl. Phys. 2019, 2, e185–e188. [Google Scholar] [CrossRef]
- Owens, C.E.; Hart, A.J. High-precision modular microfluidics by micromilling of interlocking injection-molded blocks. Lab Chip 2018, 18, 890–901. [Google Scholar] [CrossRef] [PubMed]
- Peter, L.; Marcus, S.G.; Anurag, M.; Mae, R.W.; Healy, K.E.; Raghavan, R. μOrgano: A lego-like plug & play system for modular multi-organ-chips. PLoS ONE 2015, 10, e0139587. [Google Scholar]
- Ji, Q.; Zhang, J.M.; Liu, Y.; Li, X.; Lv, P.; Jin, D.; Duan, H. A modular microfluidic device via multimaterial 3D printing for emulsion generation. Sci. Rep. 2018, 8, 4791. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, X.; Zhang, Y.; Wen, W.; Gao, X. 3D Microstructure Inhibits Mesenchymal Stem Cells Homing to the Site of Liver Cancer Cells on a Microchip. Genes 2017, 8, 218. [Google Scholar] [CrossRef] [Green Version]
- Spillane, M.; Ketschek, A.; Merianda, T.T.; Twiss, J.L.; Gallo, G. Mitochondria Coordinate Sites of Axon Branching through Localized Intra-axonal Protein Synthesis. Cell Rep. 2013, 5, 1564–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, L.Y.; Fan, X.R.; Shi, Z.J.; Fan, Z.C.; Luo, J.; Lin, N.; Liu, C.Y.; Wu, L.; Zeng, X.R.; Cao, J.M.; et al. Hyperpolarization-Activated Cyclic Nucleotide-Gated Ion (HCN) Channels Regulate PC12 Cell Differentiation toward Sympathetic Neuron. Front. Cell. Neurosci. 2019, 13, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayaka, S.; Toko, M.; Ko, F. Ko Fujimori. Protection of 6-OHDA neurotoxicity by PGF2α through FP-ERK-Nrf2 signaling in SH-SY5Y cells. Toxicology 2021, 450, 152686. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Li, W.; Gong, X.; Hu, G.; Ge, J.; Wu, J.; Gao, X. Investigating the Regulation of Neural Differentiation and Injury in PC12 Cells Using Microstructure Topographic Cues. Biosensors 2021, 11, 399. https://doi.org/10.3390/bios11100399
Sun X, Li W, Gong X, Hu G, Ge J, Wu J, Gao X. Investigating the Regulation of Neural Differentiation and Injury in PC12 Cells Using Microstructure Topographic Cues. Biosensors. 2021; 11(10):399. https://doi.org/10.3390/bios11100399
Chicago/Turabian StyleSun, Xindi, Wei Li, Xiuqing Gong, Guohui Hu, Junyi Ge, Jinbo Wu, and Xinghua Gao. 2021. "Investigating the Regulation of Neural Differentiation and Injury in PC12 Cells Using Microstructure Topographic Cues" Biosensors 11, no. 10: 399. https://doi.org/10.3390/bios11100399
APA StyleSun, X., Li, W., Gong, X., Hu, G., Ge, J., Wu, J., & Gao, X. (2021). Investigating the Regulation of Neural Differentiation and Injury in PC12 Cells Using Microstructure Topographic Cues. Biosensors, 11(10), 399. https://doi.org/10.3390/bios11100399





