Exhaled Breath Analysis for Diabetes Diagnosis and Monitoring: Relevance, Challenges and Possibilities
Abstract
:1. Introduction
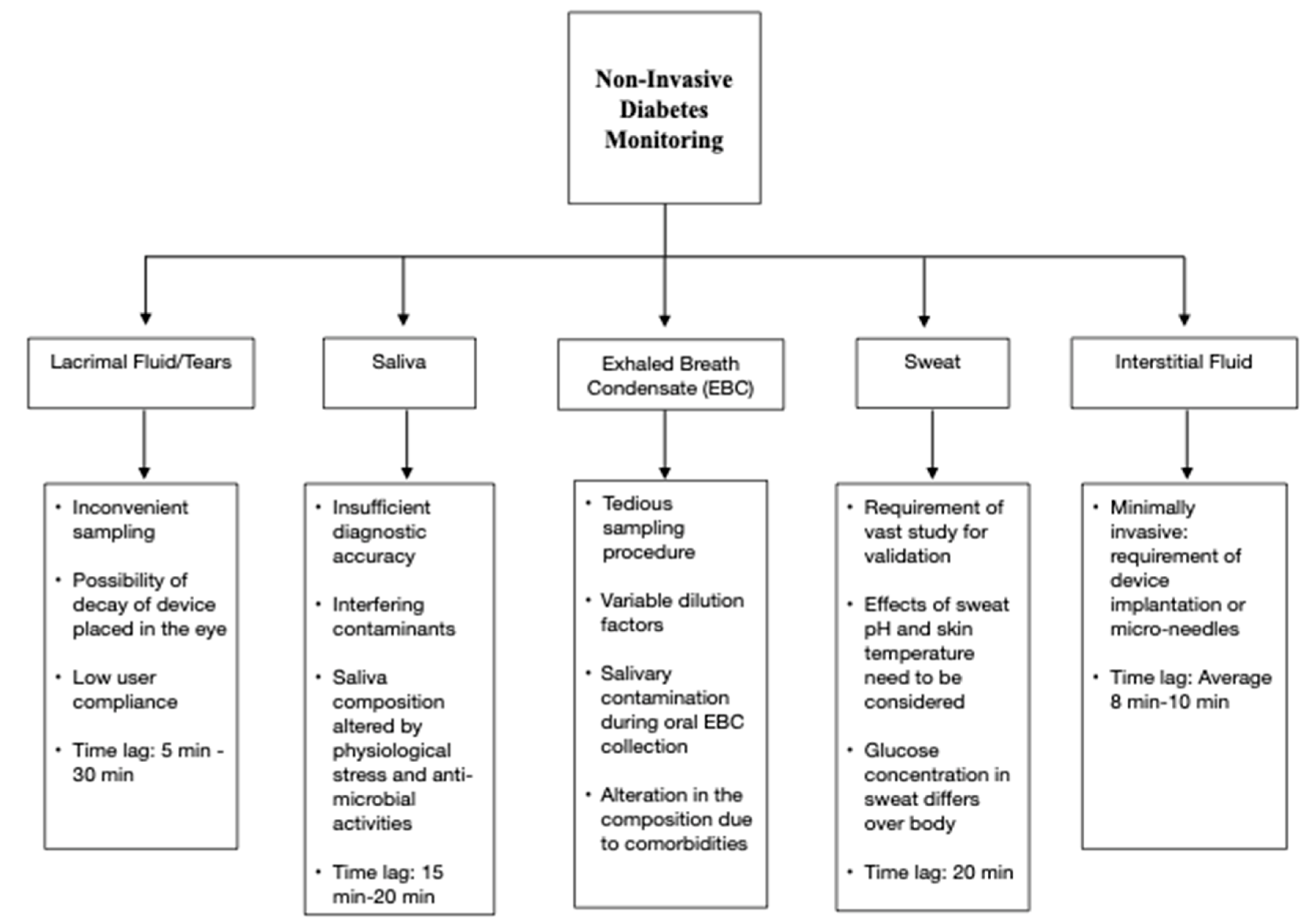
2. Non-Invasive Diabetes Monitoring Devices
| Technology | Device | Participants/Number of Paired Measurements | Performance | Measurement Area | Comments | References |
|---|---|---|---|---|---|---|
| NIR Spectroscopy | Wizmi | 32 women 224 paired glucose measurements | MARD: 7.23% | Wrist |
| [24] |
| Ultrasound + Thermal + Electromagnetic | GlucoTrack | 91 subjects | MARD: 23.4% 97.3% readings lie in clinically acceptable zones in Clarke Error Grid | Earlobe |
| [17,24,25] |
| Ultrasound + Thermal + Electromagnetic | Egm1000™ | 36 T2DM patients 11 people with prediabetes 188 paired glucose measurements | MARD: 13.8% | Earlobe |
| [18,26] |
| Fluorescence | EverSense | 23 subjects | MARD: 14.8% | Subcutaneous implant in the upper arm |
| [27,28] |
| Reverse Iontophoresis | SugarBEAT | 13,639 paired glucose measurements | MARD: 13.39% | Skin |
| [29,30,31] |
| Photo Thermal Detection | Diamontech D-Base | 59 healthy subjects 41 subjects with diabetes | 99.1% precise measurements | Finger |
| [32] |
| Tissue Photography Analysis | Tensortip Combo Glucometer | 19 subjects | MARD: 17.1% | Finger |
| [33,34] |
| Subcutaneous Wired Enzyme Glucose Sensing | Abbott FreeStyle® Libre | 144 subjects | MARD: 9.2% | Upper arm skin (Sensor uses thin filament inserted just under the skin) |
| [35,36,37] |
| Radio Wave Spectroscopy | Glucowise™ | N/A | N/A | Skin between thumb and forefinger or earlobe |
| [38] |
| Infrared Spectroscopy | Tech4Life Enterprises Non-Invasive Glucometer | N/A | N/A | Finger |
| [39] |
| Photoplethysmography | HELO Extense | N/A | N/A | Finger |
| [40] |
| MIR spectroscopy/Optical Parametric Oscillation | Light Touch Technology | N/A | 99% of measured values are within A zone and B zone defined by the ISO 15197 standard | Hand |
| [41] |
| SkinTaste Technology: Biosensors and array of micropoints | K’Watch Glucose | N/A | N/A | Wrist |
| [42] |
| Radiofrequency Sensor Technology | Alertgy | N/A | N/A | Wrist |
| [43] |
| Bio RFID Technology: Spectroscopy | UBAND-Know Labs | N/A | 4.3% mean difference compared to FreeStyle Libre | Wrist |
| [44] |
| Photoplethysmography | LifePlus: LifeLeaf | N/A | N/A | Wrist |
| [45,46] |
| Tear Sensor | Noviosense | 24 T1DM subjects | MARD = 16.7% | Lower Eyelid |
| [47,48,49] |
| Sensors based on photonics sensing technology | Indigo Diabetes | N/A | N/A | Subcutaneous implant |
| [50] |
3. Potential Breath Biomarkers of Diabetes
3.1. Standalone Breath Biomarkers of Diabetes
| Type of Diabetes | Potential Breath Biomarkers | References |
|---|---|---|
| T1DM | Acetone Ethanol Carbon Monoxide Isoprene Propane Methyl Nitrate Pentanal Isopropanol Dimethyl Sulphide | [10,63,70,72,73,75,76,80,81] |
| T2DM | Acetone Isopropanol Ethylene Ammonia Carbon Monoxide Toluene m-Xylene 2,3,4-trimethylhexane 2,6,8-trimethyldecane Tridecane Undecane | [62,72,76,77,78,79] |
3.2. Breath Biomarker Clusters of Diabetes
4. Sensing Methodologies for Breath Analysis
4.1. Chemiresistive Sensing
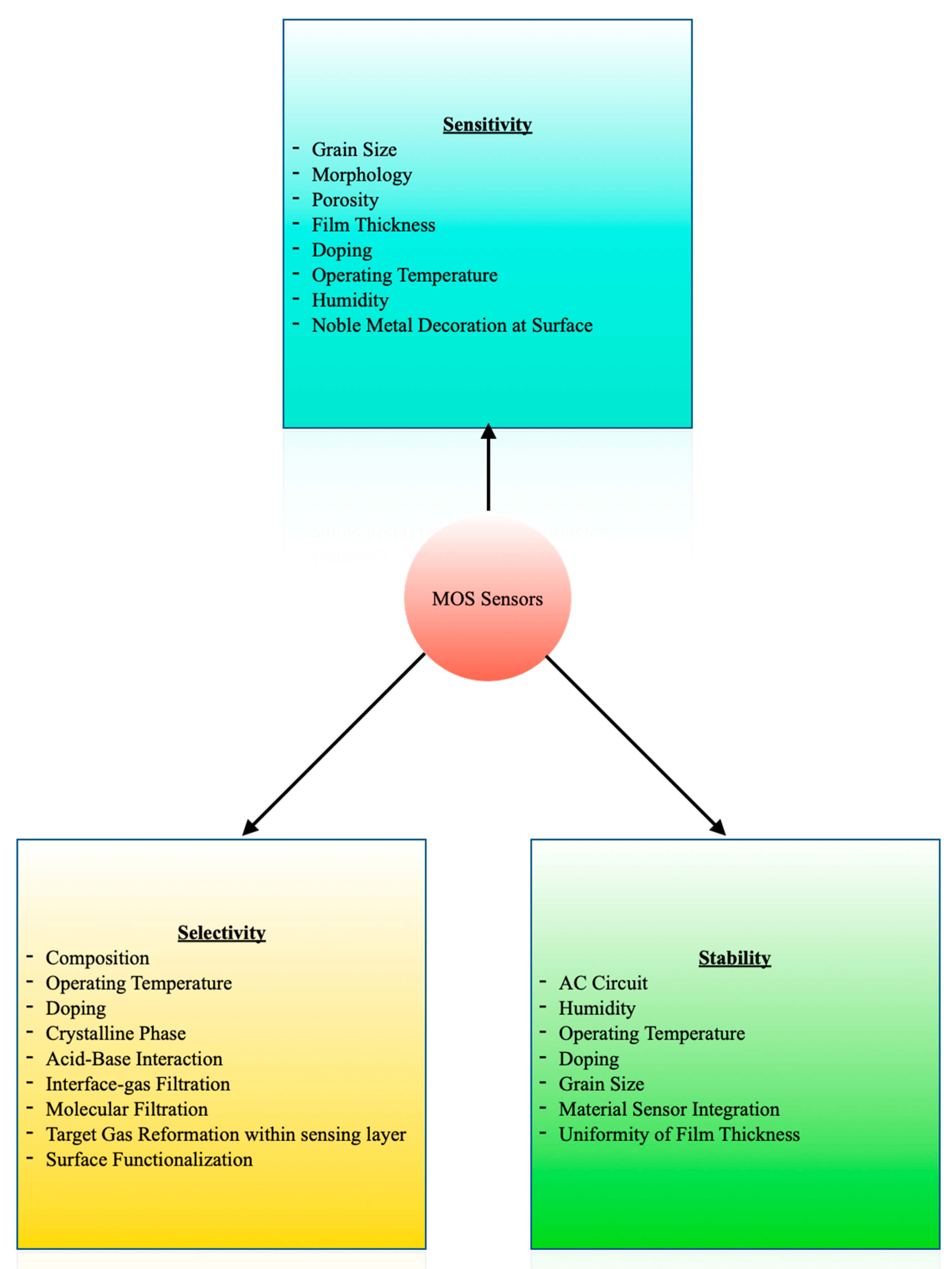
4.1.1. MOS Sensors
4.1.2. Other Chemiresistive Materials
4.2. Electrochemical Sensing
4.3. Piezoelectric Sensors
4.4. Optical Sensing
4.5. FET Sensing
4.6. Wearable Sensing
5. Discussion
- Mixed expiratory breath includes all the phases of breath and is prone to environmental, nose, and mouth contaminants.
- Removal of the estimated dead space from the breath results in the late expiratory breath. It has a better concentration of endogenous VOCs.
- End-tidal breath has the highest level of exhaled CO2 and is the richest in endogenous VOCs.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Diabetes Federation. IDF Diabetes Atlas, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019. [Google Scholar]
- American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2017, 40, S11–S24. [Google Scholar] [CrossRef] [Green Version]
- Buchanan, T.A.; Xiang, A.; Kjos, S.L.; Watanabe, R. What Is Gestational Diabetes? Diabetes Care 2007, 30 (Suppl. 2), S105–S111. [Google Scholar] [CrossRef] [Green Version]
- Beran, D.; Ewen, M.; Laing, R. Constraints and challenges in access to insulin: A global perspective. Lancet Diabetes Endocrinol. 2016, 4, 275–285. [Google Scholar] [CrossRef]
- The IQ Group Global. Addressing the Challenges of Invasive Glucose Monitoring. 2019. Available online: https://theiqgroupglobal.com/wp-content/uploads/2019/11/IQGroupGlobal_Addressing-the-challenges-of-invasive-glucose-monitoring_Diabetes_Whitepaper.pdf (accessed on 21 August 2021).
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef]
- Beduk, T.; Durmus, C.; Hanoglu, S.B.; Beduk, D.; Salama, K.N.; Goksel, T.; Turhan, K.; Timur, S. Breath as the mirror of our body is the answer really blowing in the wind? Recent technologies in exhaled breath analysis systems as non-invasive sensing platforms. TrAC Trends Anal. Chem. 2021, 143, 116329. [Google Scholar] [CrossRef]
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Davis, M.D.; Fowler, S.J.; Montpetit, A.J. Exhaled breath testing—A tool for the clinician and researcher. Paediatr. Respir. Rev. 2019, 29, 37–41. [Google Scholar] [CrossRef]
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The clinical potential of exhaled breath analysis for diabetes mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usman, F.; Dennis, J.O.; Ahmed, A.Y.; Meriaudeau, F.; Ayodele, O.B.; Rabih, A.A.S. A Review of Biosensors for Non-Invasive Diabetes Monitoring and Screening in Human Exhaled Breath. IEEE Access 2019, 7, 5963–5974. [Google Scholar] [CrossRef]
- Obeidat, Y. The Most Common Methods for Breath Acetone Concentration Detection: A Review. IEEE Sens. J. 2021, 21, 14540–14558. [Google Scholar] [CrossRef]
- Koureas, M.; Kalompatsios, D.; Amoutzias, G.D.; Hadjichristodoulou, C.; Gourgoulianis, K.; Tsakalof, A. Comparison of Targeted and Untargeted Approaches in Breath Analysis for the Discrimination of Lung Cancer from Benign Pulmonary Diseases and Healthy Persons. Molecules 2021, 26, 2609. [Google Scholar] [CrossRef] [PubMed]
- Non-Invasive Glucose Monitoring Devices Market by Product and Geography-Forecast and Analysis 2021–2025. Available online: https://www.technavio.com/report/non-invasive-glucose-monitoring-devices-market-size-industry-analysis (accessed on 21 August 2021).
- Gonzales, W.V.; Mobashsher, A.T.; Abbosh, A. The Progress of Glucose Monitoring—A Review of Invasive to Minimally and Non-Invasive Techniques, Devices and Sensors. Sensors 2019, 19, 800. [Google Scholar] [CrossRef] [Green Version]
- Reiterer, F.; Polterauer, P.; Schoemaker, M.; Schmelzeisen-Redecker, G.; Freckmann, G.; Heinemann, L.; del Re, L. Significance and Reliability of MARD for the Accuracy of CGM Systems. J. Diabetes Sci. Technol. 2017, 11, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Harman-Boehm, I.; Gal, A.; Raykhman, A.M.; Naidis, E.; Mayzel, Y. Noninvasive Glucose Monitoring: Increasing Accuracy by Combination of Multi-Technology and Multi-Sensors. J. Diabetes Sci. Technol. 2010, 4, 583–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosli, H.; Madani, B. Performance evaluation of egm1000TM non-invasive glucose monitoring device in patients with type 2 diabetes and subjects with prediabetes. Int. J. Med. Dev. Ctries. 2021, 5, 1020–1028. [Google Scholar] [CrossRef]
- Gupta, V.; Kaur, A. Salivary glucose levels in diabetes mellitus patients: A case-control study. J. Oral Maxillofac. Pathol. 2020, 24, 187. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Jiang, N.; Kazarian, S.G.; Tasoglu, S.; Yetisen, A.K. Progress in Biomedical Engineering Optical sensors for continuous glucose monitoring. Prog. Biomed. Eng. 2021, 3, 022004. [Google Scholar] [CrossRef]
- Ephraim, R.K.D.; Anto, E.O.; Acheampong, E.; Fondjo, L.A.; Barnie, R.B.; Sakyi, S.A.; Asare, A. Fasting salivary glucose levels is not a better measure for identifying diabetes mellitus than serum or capillary blood glucose levels: Comparison in a Ghanaian population. Heliyon 2019, 5, 1286. [Google Scholar] [CrossRef] [Green Version]
- Chung, M.; Fortunato, G.; Radacsi, N. Wearable flexible sweat sensors for healthcare monitoring: A review. J. R. Soc. Interface 2019, 16, 20190217. [Google Scholar] [CrossRef]
- van Enter, B.J.; von Hauff, E. Challenges and perspectives in continuous glucose monitoring. Chem. Commun. 2018, 54, 5032–5045. [Google Scholar] [CrossRef]
- Hadar, E.; Chen, R.; Toledano, Y.; Tenenbaum-Gavish, K.; Atzmon, Y.; Hod, M. Noninvasive, continuous, real-time glucose measurements compared to reference laboratory venous plasma glucose values. J. Matern.-Fetal Neonatal Med. 2018, 32, 3393–3400. [Google Scholar] [CrossRef]
- GlucoTrack|Your Track to Health!...TM. Available online: http://www.glucotrack.com/ (accessed on 21 August 2021).
- EGM-1000-Star Medik Sdn Bhd. Available online: https://starmedik.com/products/home-care/egm-1000/ (accessed on 21 August 2021).
- Jafri, R.Z.; Balliro, C.A.; El-Khatib, F.; Maheno, M.M.; Hillard, M.A.; O’Donovan, A.; Selagamsetty, R.; Zheng, H.; Damiano, E.R.; Russell, S.J. A Three-Way Accuracy Comparison of the Dexcom G5, Abbott Freestyle Libre Pro, and Senseonics Eversense Continuous Glucose Monitoring Devices in a Home-Use Study of Subjects with Type 1 Diabetes. Diabetes Technol. Ther. 2020, 22, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Health Care Providers|Eversense CGM. Available online: https://www.ascensiadiabetes.com/eversense/healthcare-providers/ (accessed on 23 August 2021).
- sugarBEAT. Nemaura Medical Clinical Presentation of sugarBEAT®. A Prospective Single Centre Evaluation of the Accuracy and Safety of the sugarBEAT® Non-Invasive Continuous Glucose Monitor (CGM) System; sugarBEAT: Loughborough, UK, 2018. [Google Scholar]
- sugarBEAT. Meet sugarBEAT. Available online: https://sugarbeat.com/ (accessed on 21 August 2021).
- Nemaura Announces CE Mark Approval of SugarBEAT®-Nemaura Medical Nemaura Medical. Available online: https://nemauramedical.com/nemaura-announces-ce-mark-approval-of-sugarbeat/ (accessed on 23 August 2021).
- DiaMonTech:: Non-Invasive Blood Glucose Monitoring. Available online: https://www.diamontech.de/ (accessed on 21 August 2021).
- Segman, Y. Device and Method for Noninvasive Glucose Assessment. J. Diabetes Sci. Technol. 2018, 12, 1159–1168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CoG-Hybrid Glucometer|Cnoga Digital Care. Available online: https://www.cnogacare.co/cog-hybrid-glucometer (accessed on 21 August 2021).
- Karinka, S.A.; Bailey, T.S.; Brazg, R.L.; Budiman, E.S.; Castorino, K.; Christiansen, M.P.; Liljenquist, D.R.; Liu, H. 910-P: Improved Accuracy of 14-Day Factory-Calibrated FreeStyle Libre System with New Glucose Algorithm. Diabetes 2019, 68 (Suppl. 1), 910-P. [Google Scholar] [CrossRef]
- FreeStyle Libre 2 System|CGM with Real-Time Glucose Alarms. Available online: https://www.freestyle.abbott/us-en/products/freestyle-libre-2.html (accessed on 21 August 2021).
- FreeStyle Libre 14 Day System|CGM Diabetes Monitor. Available online: https://www.freestyle.abbott/us-en/products/freestyle-14-day.html (accessed on 23 August 2021).
- glucoWISE®: Meet the New Non-Invasive Glucose Monitor that Helps You Take Control of Your Life. Available online: https://gluco-wise.com/ (accessed on 21 August 2021).
- Glucometer|Non Invasive Glucose Monitor|Tech4Life. Available online: https://tech4lifeenterprises.com/non-invasive-glucometer/ (accessed on 21 August 2021).
- Heloextense–WGN. Available online: https://website.worldgn.com/heloextense/ (accessed on 21 August 2021).
- Development Product Information|Light-Touch-Tech—Light Touch Technology Co., Ltd. Available online: http://www.light-tt.co.jp/product?lang=en (accessed on 21 August 2021).
- K’Watch Glucose-PKVitality. Available online: https://www.pkvitality.com/ktrack-glucose/ (accessed on 21 August 2021).
- Technology-AlertgyTM. Available online: https://www.alertgy.com/technology/ (accessed on 21 August 2021).
- UBAND—Know Labs. Available online: https://www.knowlabs.co/bio-rfid (accessed on 21 August 2021).
- Home-LifePlus. Available online: https://www.lifeplus.ai/ (accessed on 21 August 2021).
- About Us-LifePlus. Available online: https://www.lifeplus.ai/about-us/ (accessed on 23 August 2021).
- Kownacka, A.E.; Vegelyte, D.; Joosse, M.; Anton, N.; Toebes, B.J.; Lauko, J.; Buzzacchera, I.; Lipinska, K.; Wilson, D.A.; Geelhoed-Duijvestijn, N.; et al. Clinical Evidence for Use of a Noninvasive Biosensor for Tear Glucose as an Alternative to Painful Finger-Prick for Diabetes Management Utilizing a Biopolymer Coating. Biomacromolecule 2018, 19, 4504–4511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geelhoed-Duijvestijn, P.; Vegelyte, D.; Kownacka, A.; Anton, N.; Joosse, M.; Wilson, C. Performance of the Prototype NovioSense Noninvasive Biosensor for Tear Glucose in Type 1 Diabetes. J. Diabetes Sci. Technol. 2020, 15, 1932296820964844. [Google Scholar] [CrossRef]
- Noviosense|Tear Glucose Sensor. Available online: http://noviosense.com/ (accessed on 21 August 2021).
- Hassle-Free Glucose Monitoring with Our Next-Gen Sensor-Indigo. Available online: https://indigomed.com/ (accessed on 21 August 2021).
- Adiguzel, Y.; Kulah, H. Breath sensors for lung cancer diagnosis. Biosens. Bioelectron. 2015, 65, 121–138. [Google Scholar] [CrossRef]
- Tai, H.; Wang, S.; Duan, Z.; Jiang, Y. Evolution of breath analysis based on humidity and gas sensors: Potential and challenges. Sens. Actuators B Chem. 2020, 318, 128104. [Google Scholar] [CrossRef]
- Bikov, A.; Lazar, Z.; Schandl, K.; Antus, B.; Losonczy, G.; Horvath, I. Exercise changes volatiles in exhaled breath assessed by an electronic nose. Acta Physiol. Hung. 2011, 98, 321–328. [Google Scholar] [CrossRef]
- Heaney, L.M.; Lindley, M.R. Translation of exhaled breath volatile analyses to sport and exercise applications. Metabolomics 2017, 13, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ajibola, O.A.; Smith, D.; Španěl, P.; Ferns, G.A.A. Effects of dietary nutrients on volatile breath metabolites. J. Nutr. Sci. 2013, 2, 1–15. [Google Scholar] [CrossRef] [Green Version]
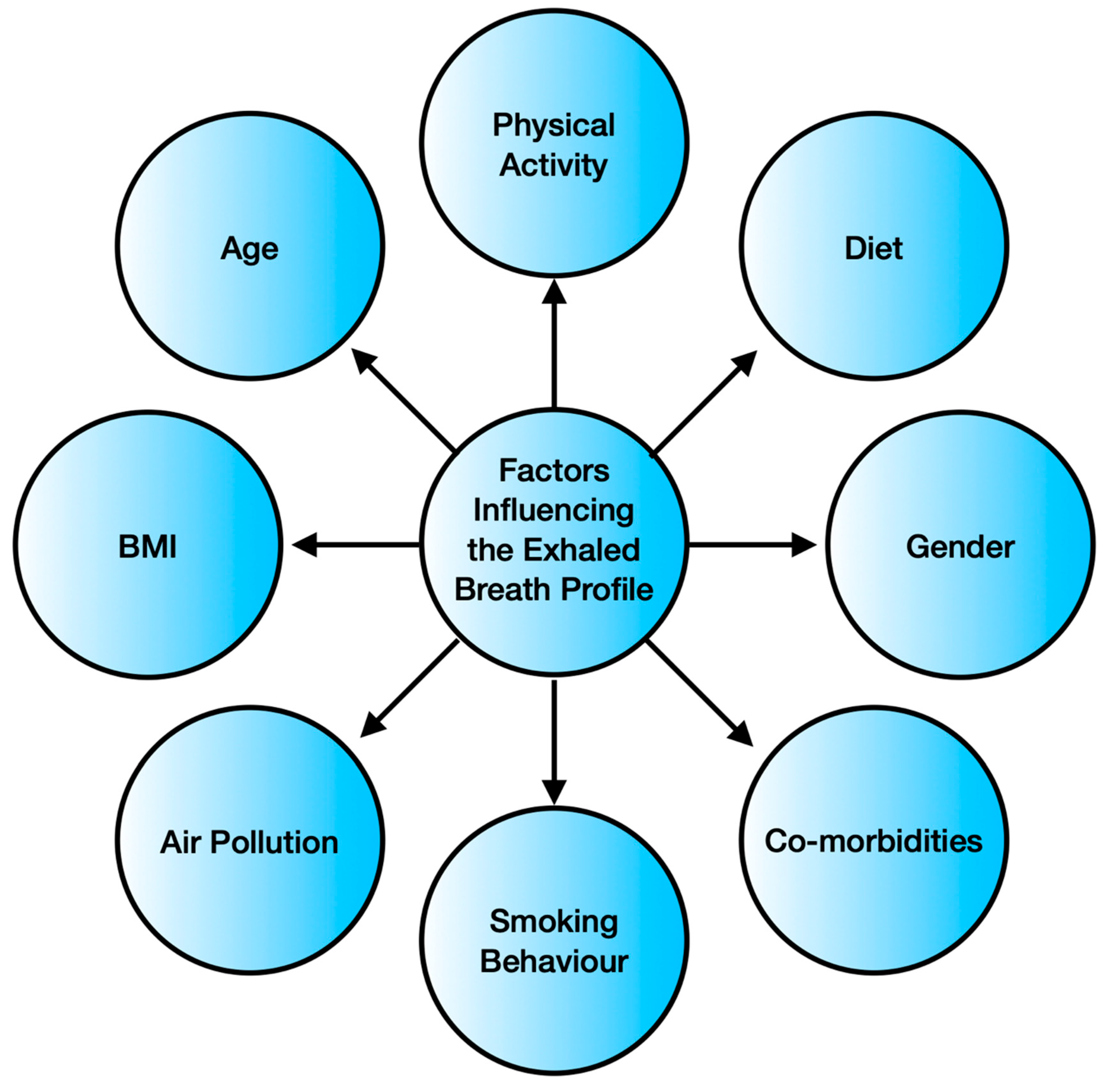
- Blanchet, L.; Smolinska, A.; Baranska, A.; Tigchelaar, E.; Swertz, M.; Zhernakova, A.; Dallinga, J.W.; Wijmenga, C.; Schooten, F.J. van. Factors that influence the volatile organic compound content in human breath. J. Breath Res. 2017, 11, 016013. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, Q.; Chen, B.; Han, Y.; Cheng, L.; Shen, Y.; Hao, P.; Zhang, Z. Factors influencing breath analysis results in patients with diabetes mellitus. J. Breath Res. 2019, 13, 046012. [Google Scholar] [CrossRef]
- Yokokawa, T.; Sato, T.; Suzuki, S.; Oikawa, M.; Yoshihisa, A.; Kobayashi, A.; Yamaki, T.; Kunii, H.; Nakazato, K.; Suzuki, H.; et al. Elevated exhaled acetone concentration in stage C heart failure patients with diabetes mellitus. BMC Cardiovasc. Disord. 2017, 17, 1–6. [Google Scholar] [CrossRef]
- Montuschi, P.; Paris, D.; Melck, D.; Lucidi, V.; Ciabattoni, G.; Raia, V.; Calabrese, C.; Bush, A.; Barnes, P.J.; Motta, A. NMR spectroscopy metabolomic profiling of exhaled breath condensate in patients with stable and unstable cystic fibrosis. Thorax 2012, 67, 222–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcondes-Braga, F.G.; Batista, G.L.; Bacal, F.; Gutz, I. Exhaled Breath Analysis in Heart Failure. Curr. Heart Fail. Rep. 2016, 13, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Dent, A.G.; Sutedja, T.G.; Zimmerman, P.V. Exhaled breath analysis for lung cancer. J. Thorac. Dis. 2013, 5, S540. [Google Scholar] [CrossRef]
- Deng, C.; Zhang, J.; Yu, X.; Zhang, W.; Zhang, X. Determination of acetone in human breath by gas chromatography–mass spectrometry and solid-phase microextraction with on-fiber derivatization. J. Chromatogr. B 2004, 810, 269–275. [Google Scholar] [CrossRef]
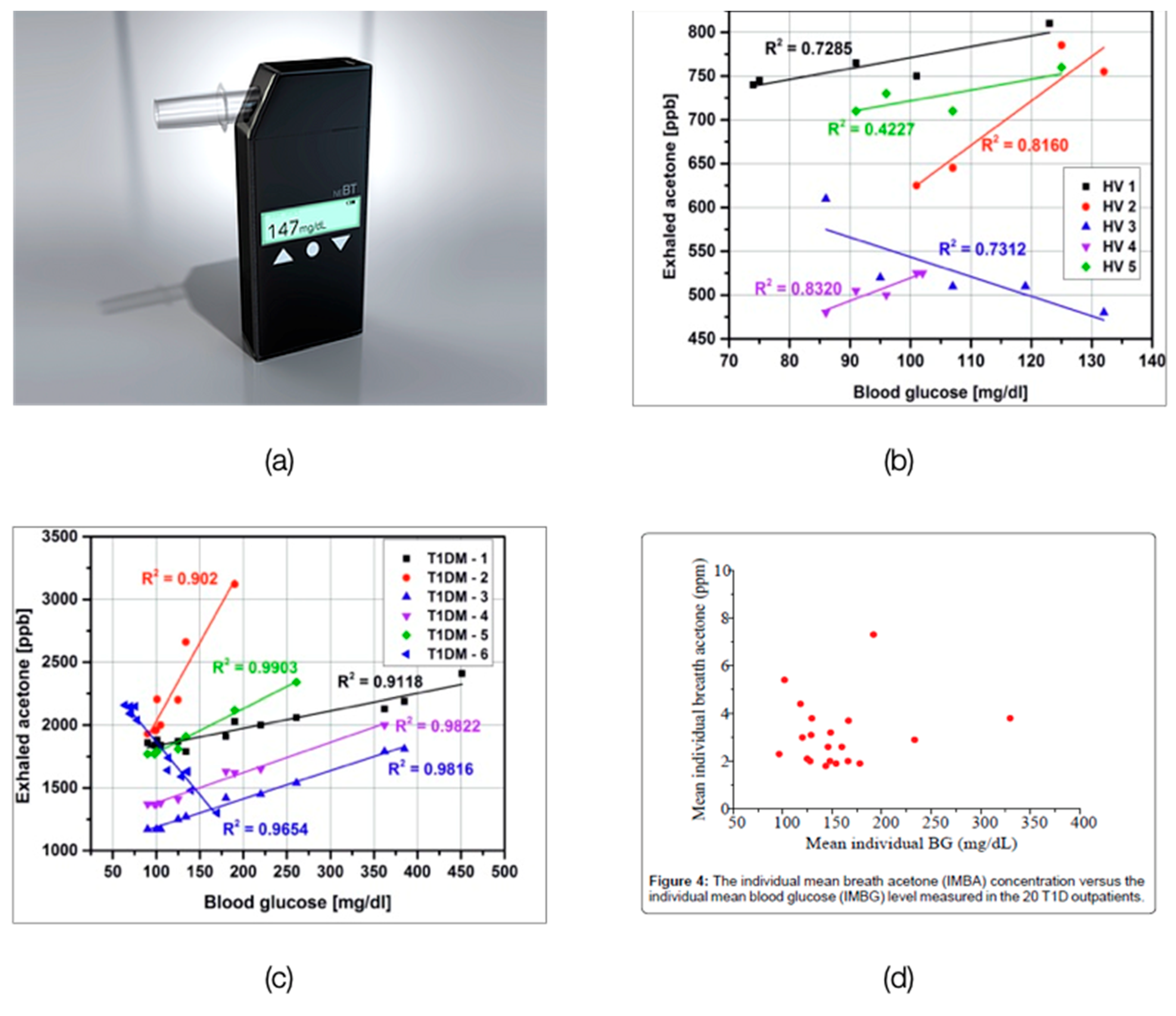
- Wang, C.; Mbi, A.; Shepherd, M. A study on breath acetone in diabetic patients using a cavity ringdown breath analyzer: Exploring correlations of breath acetone with blood glucose and glycohemoglobin A1C. IEEE Sens. J. 2010, 10, 54–63. [Google Scholar] [CrossRef]
- Turner, C.; Walton, C.; Hoashi, S.; Evans, M. Breath acetone concentration decreases with blood glucose concentration in type I diabetes mellitus patients during hypoglycaemic clamps. J. Breath Res. 2009, 3, 046004. [Google Scholar] [CrossRef]
- Priefer, R.; Rust, M. Utilizing single-use technology for diabetes monitoring via breath acetone. In Single-Use Technologies II: Bridging Polymer Science to Biotechnology Applications; ECI Digital Archives: New York, NY, USA, 2017. [Google Scholar]
- Shokrekhodaei, M.; Quinones, S. Review of Non-Invasive Glucose Sensing Techniques: Optical, Electrical and Breath Acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef] [Green Version]
- New England Breath Technologies, Inc. Diabetes Screener & Monitor. Available online: https://www.breathhealth.net/ (accessed on 21 August 2021).
- Rydosz, A. Micropreconcentrator in LTCC Technology with Mass Spectrometry for the Detection of Acetone in Healthy and Type-1 Diabetes Mellitus Patient Breath. Metabolites 2014, 4, 921–931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.; Wang, Z.; Yuan, Y.; Chen, Z.; Zhao, X.; Li, Y.; Wang, C. Continuous Monitoring of Breath Acetone, Blood Glucose and Blood Ketone in 20 Type 1 Diabetic Outpatients Over 30 Days. J. Anal. Bioanal. Tech. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Galassetti, P.R.; Novak, B.; Nemet, D.; Rose-Gottron, C.; Cooper, D.M.; Meinardi, S.; Newcomb, R.; Zaldivar, F.; Blake, D.R. Breath Ethanol and Acetone as Indicators of Serum Glucose Levels: An Initial Report. Diabetes Technol. Ther. 2005, 7, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Simic, M.; Ajdukovic, N.; Veselinovic, I.; Mitrovic, M.; Djurendic-Brenesel, M. Endogenous ethanol production in patients with Diabetes Mellitus as a medicolegal problem. Forensic Sci. Int. 2012, 216, 97–100. [Google Scholar] [CrossRef]
- Paredi, P.; Biernacki, W.; Invernizzi, G.; Kharitonov, S.A.; Barnes, P.J. Exhaled Carbon Monoxide Levels Elevated in Diabetes and Correlated With Glucose Concentration in Blood: A New Test for Monitoring the Disease? CHEST 1999, 116, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Peverall, R.; Richmond, G.; Blaikie, T.P.J.; Taylor, D.; Hancock, G.; Evans, M.L. Exhaled Breath Isoprene Rises during Hypoglycemia in Type 1 Diabetes. Diabetes Care 2016, 39, e97–e98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trefz, P.; Schmidt, S.C.; Sukul, P.; Schubert, J.K.; Miekisch, W.; Fischer, D.-C. Non-Invasive Assessment of Metabolic Adaptation in Paediatric Patients Suffering from Type 1 Diabetes Mellitus. J. Clin. Med. 2019, 8, 1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, B.J.; Blake, D.R.; Meinardi, S.; Rowland, F.S.; Pontello, A.; Cooper, D.M.; Galassetti, P.R. Exhaled methyl nitrate as a noninvasive marker of hyperglycemia in type 1 diabetes. Proc. Natl. Acad. Sci. USA 2007, 104, 15613–15618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, G.T.; Yang, C.L.; Lin, C.H.; Chen, C.C.; Shih, C.H. Applications of Hadamard transform-gas chromatography/mass spectrometry to the detection of acetone in healthy human and diabetes mellitus patient breath. Talanta 2014, 120, 386–390. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Liu, Y.; Cheng, S.; Duan, Y. Exhaled isopropanol: New potential biomarker in diabetic breathomics and its metabolic correlations with acetone. RSC Adv. 2017, 7, 17480–17488. [Google Scholar] [CrossRef] [Green Version]
- Petrus, M.; Popa, C.; Bratu, A.-M. Organic Volatile Compounds Used in Type 2 Diabetes. In Type 2 Diabetes—From Pathophysiol. To Cyber Syst.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Q.; Li, W.; Zhao, Z.; Yuan, X.; Huang, Y.; Duan, Y. Discovery of potential biomarkers in exhaled breath for diagnosis of type 2 diabetes mellitus based on GC-MS with metabolomics. RSC Adv. 2014, 4, 25430–25439. [Google Scholar] [CrossRef]
- Leopold, J.H.; van Hooijdonk, R.T.; Sterk, P.J.; Abu-Hanna, A.; Schultz, M.J.; Bos, L.D. Glucose prediction by analysis of exhaled metabolites—A systematic review. BMC Anesthesiol. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Pal, S.; Mitra, M. Significance of Exhaled Breath Test in Clinical Diagnosis: A Special Focus on the Detection of Diabetes Mellitus. J. Med. Biol. Eng. 2016, 36, 605–624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minh, T.D.C.; Oliver, S.R.; Ngo, J.; Flores, R.; Midyett, J.; Meinardi, S.; Carlson, M.K.; Rowland, F.S.; Blake, D.R.; Galassetti, P.R. Noninvasive measurement of plasma glucose from exhaled breath in healthy and type 1 diabetic subjects. Am. J. Physiol.-Endocrinol. Metab. 2011, 300, E1166. [Google Scholar] [CrossRef] [Green Version]
- Mansouri, S.; Boulares, S.; Alhadidi, T. Non-invasive Measurement of Blood Glucose by Breath Analysis. IEEJ Trans. Electr. Electron. Eng. 2020, 15, 1457–1464. [Google Scholar] [CrossRef]
- Zhou, X.; Xue, Z.; Chen, X.; Huang, C.; Bai, W.; Lu, Z.; Wang, T. Nanomaterial-based gas sensors used for breath diagnosis. J. Mater. Chem. B 2020, 8, 3231–3248. [Google Scholar] [CrossRef] [PubMed]
- Saasa, V.; Malwela, T.; Beukes, M.; Mokgotho, M.; Liu, C.-P.; Mwakikunga, B. Sensing Technologies for Detection of Acetone in Human Breath for Diabetes Diagnosis and Monitoring. Diagnostics 2018, 8, 12. [Google Scholar] [CrossRef] [Green Version]
- Yoon, J.-W.; Lee, J.-H. Toward breath analysis on a chip for disease diagnosis using semiconductor-based chemiresistors: Recent progress and future perspectives. Lab Chip 2017, 17, 3537–3557. [Google Scholar] [CrossRef]
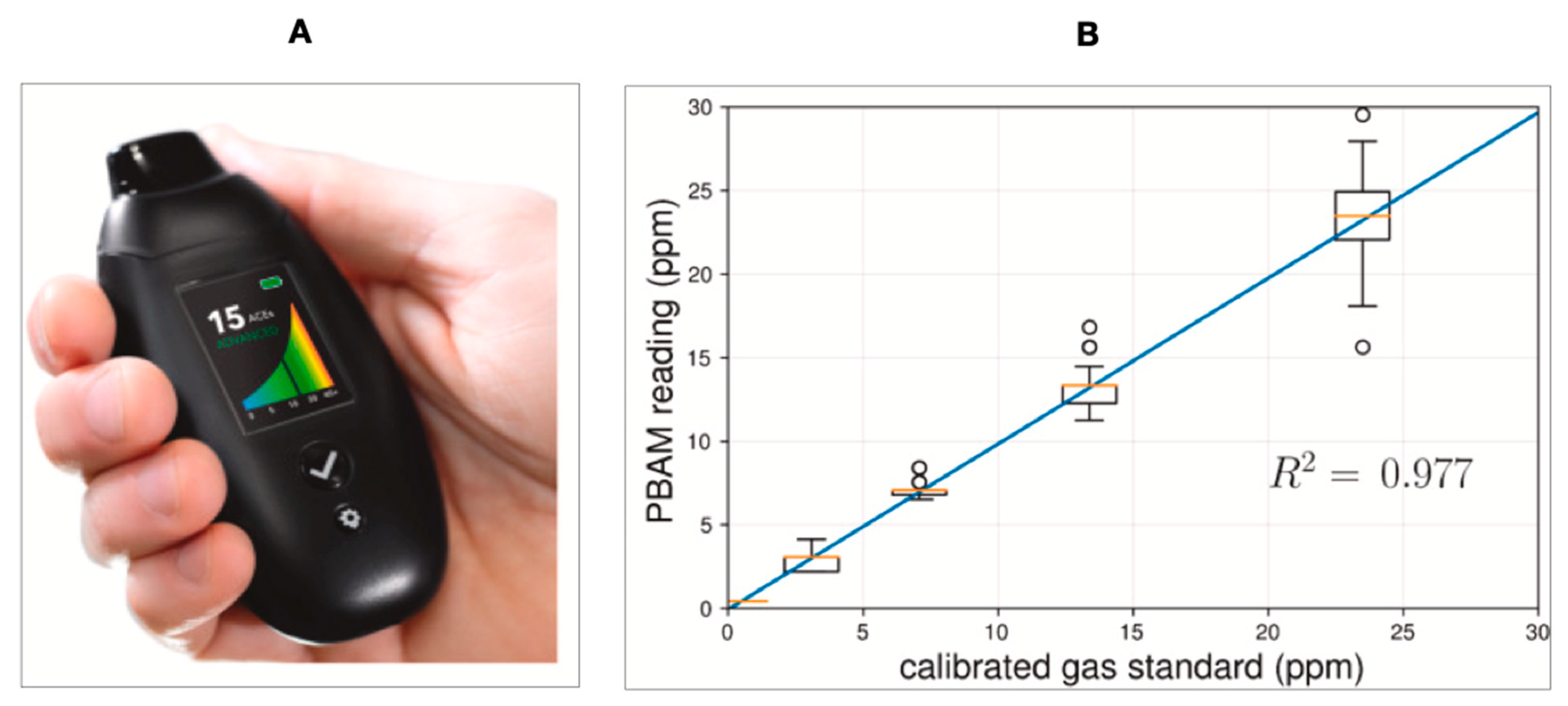
- Suntrup, D.J., III; Ratto, T.V.; Ratto, M.; McCarter, J.P. Characterization of a high-resolution breath acetone meter for ketosis monitoring. PeerJ 2020, 8, e9969. [Google Scholar] [CrossRef]
- The Science Behind Keyto, Part One—How the Keyto Breath Sensor Works|Keyto. Available online: https://getkeyto.com/science-behind-keyto-how-the-breath-sensor-works/ (accessed on 21 August 2021).
- Yang, D.; Gopal, R.A.; Lkhagvaa, T.; Choi, D. Metal-oxide gas sensors for exhaled-breath analysis: A review. Meas. Sci. Technol. 2021, 32, 102004. [Google Scholar] [CrossRef]
- Chen, Y.; Li, M.; Yan, W.; Zhuang, X.; Ng, K.W.; Cheng, X. Sensitive and Low-Power Metal Oxide Gas Sensors with a Low-Cost Microelectromechanical Heater. ACS Omega 2021, 6, 1216–1222. [Google Scholar] [CrossRef] [PubMed]
- Siebert, L.; Wolff, N.; Ababii, N.; Terasa, M.I.; Lupan, O.; Vahl, A.; Duppel, V.; Qiu, H.; Tienken, M.; Mirabelli, M.; et al. Facile fabrication of semiconducting oxide nanostructures by direct ink writing of readily available metal microparticles and their application as low power acetone gas sensors. Nano Energy 2020, 70, 104420. [Google Scholar] [CrossRef]
- Das, S.; Mahapatra, P.L.; Mondal, P.P.; Das, T.; Pal, M.; Saha, D. A highly sensitive cobalt chromite thick film based trace acetone sensor with fast response and recovery times for the detection of diabetes from exhaled breath. Mater. Chem. Phys. 2021, 262, 124291. [Google Scholar] [CrossRef]
- NHanh, H.; van Duy, L.; Hung, C.M.; Xuan, C.T.; van Duy, N.; Hoa, N.D. High-performance acetone gas sensor based on Pt–Zn2SnO4 hollow octahedra for diabetic diagnosis. J. Alloys Compd. 2021, 886, 161284. [Google Scholar] [CrossRef]
- Brahma, S.; Yeh, Y.W.; Huang, J.L.; Liu, C.P. Cu-doped p-type ZnO nanostructures as unique acetone sensor at room temperature (~25 °C). Appl. Surf. Sci. 2021, 564, 150351. [Google Scholar] [CrossRef]
- Kim, K.; Choi, P.G.; Itoh, T.; Masuda, Y. Catalyst-free Highly Sensitive SnO2 Nanosheet Gas Sensors for Parts per Billion-Level Detection of Acetone. ACS Appl. Mater. Interfaces 2020, 12, 51637–51644. [Google Scholar] [CrossRef]
- Xu, H.; Gao, J.; Li, M.; Zhao, Y.; Zhang, M.; Zhao, T.; Wang, L.; Jiang, W.; Zhu, G.; Qian, X.; et al. Mesoporous WO3 Nanofibers with Crystalline Framework for High-Performance Acetone Sensing. Front. Chem. 2019, 7, 266. [Google Scholar] [CrossRef]
- Johnson, M.; Koirala, S.; Zhang, R.A.; Wang, Q. Nanomaterial-Based Sensing Technology for the Application in Breath Analyzer as for Early Disease Detection and Prevention. Recent Trends Biotechnol. MedDocs Publ. 2021, 2, 1–8. [Google Scholar]
- Freddi, S.; Emelianov, A.V.; Bobrinetskiy, I.I.; Drera, G.; Pagliara, S.; Kopylova, D.S.; Chiesa, M.; Santini, G.; Mores, N.; Moscato, U.; et al. Development of a Sensing Array for Human Breath Analysis Based on SWCNT Layers Functionalized with Semiconductor Organic Molecules. Adv. Healthc. Mater. 2020, 9, 2000377. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, X.; Xu, L.; Zhu, S.; Yang, S.; Chen, X.; Dong, B.; Bai, X.; Lu, G.; Song, H. Graphene quantum dot-functionalized three-dimensional ordered mesoporous ZnO for acetone detection toward diagnosis of diabetes. Nanoscale 2019, 11, 11496–11504. [Google Scholar] [CrossRef]
- Mishra, R.K.; Choi, G.-J.; Choi, H.-J.; Gwag, J.-S. ZnS Quantum Dot Based Acetone Sensor for Monitoring Health-Hazardous Gases in Indoor/Outdoor Environment. Micromachines 2021, 12, 598. [Google Scholar] [CrossRef]
- Chuang, M.Y.; Lin, Y.T.; Tung, T.W.; Chang, L.Y.; Zan, H.W.; Meng, H.F.; Lu, C.J.; Tao, Y.T. Room-temperature-operated organic-based acetone gas sensor for breath analysis. Sens. Actuators B Chem. 2018, 260, 593–600. [Google Scholar] [CrossRef]
- Lavanya, N.; Leonardi, S.G.; Marini, S.; Espro, C.; Kanagaraj, M.; Reddy, S.L.; Sekar, C.; Neri, G. MgNi2O3 nanoparticles as novel and versatile sensing material for non-enzymatic electrochemical sensing of glucose and conductometric determination of acetone. J. Alloys Compd. 2020, 817, 152787. [Google Scholar] [CrossRef]
- Jiang, L.; Lv, S.; Tang, W.; Zhao, L.; Wang, C.; Wang, J.; Wang, T.; Guo, X.; Liu, F.; Wang, C.; et al. YSZ-based acetone sensor using a Cd2SnO4 sensing electrode for exhaled breath detection in medical diagnosis. Sens. Actuators B Chem. 2021, 345, 130321. [Google Scholar] [CrossRef]
- Liu, T.; Guan, H.; Wang, T.; Liang, X.; Liu, F.; Liu, F.; Zhang, C.; Lu, G. Mixed potential type acetone sensor based on GDC used for breath analysis. Sens. Actuators B Chem. 2021, 326, 128846. [Google Scholar] [CrossRef]
- Fu, Y.; He, H.; Zhao, T.; Dai, Y.; Han, W.; Ma, J.; Xing, L.; Zhang, Y.; Xue, X. A Self-Powered Breath Analyzer Based on PANI/PVDF Piezo-Gas-Sensing Arrays for Potential Diagnostics Application. Nano-Micro Lett. 2018, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, M.; Chien, P.J.; Toma, K.; Arakawa, T.; Mitsubayashi, K. An acetone bio-sniffer (gas phase biosensor) enabling assessment of lipid metabolism from exhaled breath. Biosens. Bioelectron. 2015, 73, 208–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chien, P.-J.; Suzuki, T.; Ye, M.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Ultra-Sensitive Isopropanol Biochemical Gas Sensor (Bio-Sniffer) for Monitoring of Human Volatiles. Sensors 2020, 20, 6827. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, F.; Prabhakar, A.; Qin, X.; Forzani, E.S.; Tao, N. Colorimetric Sensor for Online Accurate Detection of Breath Acetone. ACS Sens. 2020, 6, 450–453. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, J.; Bu, H.; Zhu, P.; Jiang, J.; Wu, Y.; Li, R. Dual-Channel Microarray Sensor System for Lung Cancer-Related Volatile Organic Compounds Identification in Exhaled Breath. Available online: https://www.preprints.org/manuscript/201907.0150/v1 (accessed on 21 August 2021).
- Hong, S.; Wu, M.; Hong, Y.; Jeong, Y.; Jung, G.; Shin, W.; Park, J.; Kim, D.; Jang, D.; Lee, J.H. FET-type gas sensors: A review. Sens. Actuators B Chem. 2021, 330, 129240. [Google Scholar] [CrossRef]
- Sharma, B.; Kim, J.-S. MEMS based highly sensitive dual FET gas sensor using graphene decorated Pd-Ag alloy nanoparticles for H2 detection. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Miyahara, Y. Field-Effect Transistors for Gas Sensing. In Different Types of Field-Effect Transistors-Theory and Applications; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Boussaid, F.; Bermak, A.; Tsui, C.Y. Room-Temperature Dual-mode CMOS Gas-FET Sensor for Diabetes Detection. In Proceedings of the 2018 IEEE International Symposium on Circuits and Systems (ISCAS), Florence, Italy, 27–30 May 2018. [Google Scholar] [CrossRef]
- Wu, E.; Xie, Y.; Yuan, B.; Hao, D.; An, C.; Zhang, H.; Wu, S.; Hu, X.; Liu, J.; Zhang, D. Specific and Highly Sensitive Detection of Ketone Compounds Based on p-Type MoTe2 under Ultraviolet Illumination. ACS Appl. Mater. Interfaces 2018, 10, 35664–35669. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. [Google Scholar] [CrossRef]
- Bag, A.; Lee, N.E. Recent Advancements in Development of Wearable Gas Sensors. Adv. Mater. Technol. 2021, 6, 2000883. [Google Scholar] [CrossRef]
- Xu, H.; Xiang, J.X.; Lu, Y.F.; Zhang, M.K.; Li, J.J.; Gao, B.B.; Zhao, Y.J.; Gu, Z.Z. Multifunctional Wearable Sensing Devices Based on Functionalized Graphene Films for Simultaneous Monitoring of Physiological Signals and Volatile Organic Compound Biomarkers. ACS Appl. Mater. Interfaces 2018, 10, 11785–11793. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y. Cotton-based wearable PEDOT:PSS electronic sensor for detecting acetone vapor. Flex. Print. Electron. 2017, 2, 042001. [Google Scholar] [CrossRef]
- Wang, L.; Jackman, J.A.; Park, J.H.; Tan, E.-L.; Cho, N.-J. A flexible, ultra-sensitive chemical sensor with 3D biomimetic templating for diabetes-related acetone detection. J. Mater. Chem. B 2017, 5, 4019–4024. [Google Scholar] [CrossRef]
- Andrysiewicz, W.; Krzeminski, J.; Skarżynski, K.; Marszalek, K.; Sloma, M.; Rydosz, A. Flexible Gas Sensor Printed on a Polymer Substrate for Sub-ppm Acetone Detection. Electron. Mater. Lett. 2020, 16, 146–155. [Google Scholar] [CrossRef] [Green Version]
- Salim, A.; Lim, S. Recent advances in noninvasive flexible and wearable wireless biosensors. Biosens. Bioelectron. 2019, 141, 111422. [Google Scholar] [CrossRef]
- Zou, Y.; Bo, L.; Li, Z. Recent progress in human body energy harvesting for smart bioelectronic system. Fundam. Res. 2021, 1, 364–382. [Google Scholar] [CrossRef]
- Xue, H.; Yang, Q.; Wang, D.; Luo, W.; Wang, W.; Lin, M.; Liang, D.; Luo, Q. A wearable pyroelectric nanogenerator and self-powered breathing sensor. Nano Energy 2017, 38, 147–154. [Google Scholar] [CrossRef]
- Sarno, R.; Sabilla, S.I.; Wijaya, D.R. Electronic Nose for Detecting Multilevel Diabetes using Optimized Deep Neural Network. Eng. Lett. 2020, 28, 31–42. [Google Scholar]
- Bahos, F.A.; Sainz-Vidal, A.; Sánchez-Pérez, C.; Saniger, J.M.; Gràcia, I.; Saniger-Alba, M.M.; Matatagui, D. ZIF Nanocrystal-Based Surface Acoustic Wave (SAW) Electronic Nose to Detect Diabetes in Human Breath. Biosensors 2018, 9, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wulandari, S.A.; Pramitasari, R.; Madnasri, S.; Susilo. Electronic noses for diabetes mellitus detection: A review. In Proceedings of the 2020 International Seminar on Application for Technology of Information and Communication (iSemantic), Semarang, Indonesia, 19–20 September 2020; pp. 364–369. [Google Scholar] [CrossRef]
- Shrestha, S.; Harold, C.; Boubin, M.; Lawrence, L. Smart wristband with integrated chemical sensors for detecting glucose levels using breath volatile organic compounds. Smart Biomed. Physiol. Sens. Technol. XVI 2019, 11020, 110200R. [Google Scholar] [CrossRef]
- About the SniffPhone Project|SniffPhone. Available online: https://www.sniffphone.eu/content/about-sniffphone-project (accessed on 21 August 2021).
- Tankasala, D.; Linnes, J.C. Noninvasive glucose detection in exhaled breath condensate. Transl. Res. 2019, 213, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Lawal, O.; Ahmed, W.M.; Nijsen, T.M.E.; Goodacre, R.; Fowler, S.J. Exhaled breath analysis: A review of ‘breath-taking’ methods for off-line analysis. Metabolomics 2017, 13, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Singh, V.; Grandy, J.; Pawliszyn, J. Recent advances in breath analysis to track human health by new enrichment technologies. J. Sep. Sci. 2020, 43, 226–240. [Google Scholar] [CrossRef]
- Lord, H.L.; Zhan, W.; Pawliszyn, J. Fundamentals and applications of needle trap devices: A critical review. Anal. Chim. Acta 2010, 677, 3–18. [Google Scholar] [CrossRef]
- Trefz, P.; Rösner, L.; Hein, D.; Schubert, J.K.; Miekisch, W. Evaluation of needle trap micro-extraction and automatic alveolar sampling for point-of-care breath analysis. Anal. Bioanal. Chem. 2013, 405, 3105–3115. [Google Scholar] [CrossRef]
- Kononov, A.; Korotetsky, B.; Jahatspanian, I.; Gubal, A.; Vasiliev, A.; Arsenjev, A.; Nefedov, A.; Barchuk, A.; Gorbunov, I.; Kozyrev, K.; et al. Online breath analysis using metal oxide semiconductor sensors (electronic nose) for diagnosis of lung cancer. J. Breath Res. 2019, 14, 016004. [Google Scholar] [CrossRef] [PubMed]
- Dharmawardana, N.; Woods, C.; Watson, D.I.; Yazbeck, R.; Ooi, E.H. A review of breath analysis techniques in head and neck cancer. Oral Oncol. 2020, 104, 104654. [Google Scholar] [CrossRef] [PubMed]


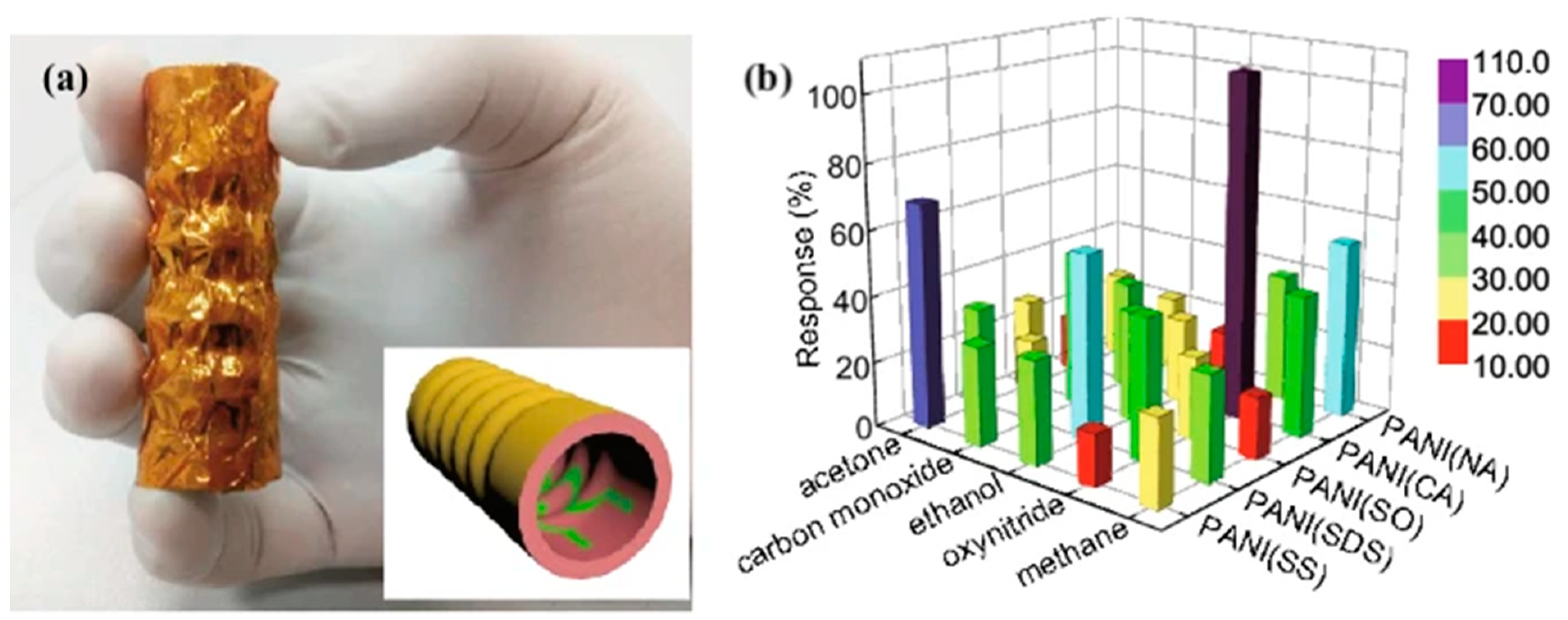



| Serial Number | Disease | Biomarkers Overlapping with Diabetes Breath Biomarkers | References |
|---|---|---|---|
| 1. | Cystic Fibrosis | Ethanol, isopropanol, acetone, methanol | [59] |
| 2. | Heart Failure | Acetone, ethanol | [60] |
| 3. | Lung Cancer | Methanol, ethanol, acetone, isoprene, isopropanol, propane, undecane | [61] |
| Biomarker Clusters | Healthy/T1DM/T2DM Subjects | Method Used | Research Outcome | References |
|---|---|---|---|---|
| Acetone, methyl nitrate, ethanol, and ethylbenzene | 17 healthy, 8 T1DM subjects | Gas Chromatography | Mean Correlation Coefficients All = 0.883 Healthy Subjects = 0.836 T1DM Subjects = 0.950 | [82] |
| 2-pentyl nitrate, propane, methanol, and acetone | 17 healthy, 8 T1DM subjects | Gas Chromatography | Mean Correlation Coefficients All = 0.869 Healthy Subjects = 0.829 T1DM Subjects = 0.990 | [82] |
| Acetone, ethanol, and propane | 130 healthy, 70 subjects with diabetes | Analog Semiconductor Sensors | Mean Correlation Coefficients All = 0.25 Healthy subjects = 0.97 Subjects with diabetes = 0.35 | [83] |
| Isopropanol, 2.3.4-trimethylhexane, 2,6,8-trimethyldecane, tridecane, and undecane | 39 healthy, 48 T2DM subjects | Gas Chromatography—Mass Spectrometry | Sensitivity = 97.9% Specificity = 100% | [79] |
| Material | Operating Temperature | Detection Limit | Response Time/Recovery Time | References |
|---|---|---|---|---|
| Stable cobalt chromite (CoCr2O4) | 300 °C | 1 ppm | 1.65 s/62 s (1 ppm) | [92] |
| Pt−Zn2SnO4 hollow octahedra | 350 °C | Theoretical detection limit: 1.276 ppb for Pt10–ZTO sensor (Pt loading amount of 1 wt%) | 14 s/607 s (100 ppm) | [93] |
| Cu-doped p-type ZnO nanostructures | Room Temperature | 1 ppm | 450 s/100 s | [94] |
| SnO2 nanosheet structure, with mainly exposed (101) crystal facets | 280 °C | 110 ppb | 40 s/610 s (1 ppm) | [95] |
| WO3 | 300 °C | <1 ppm | 24 s/27 s | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dixit, K.; Fardindoost, S.; Ravishankara, A.; Tasnim, N.; Hoorfar, M. Exhaled Breath Analysis for Diabetes Diagnosis and Monitoring: Relevance, Challenges and Possibilities. Biosensors 2021, 11, 476. https://doi.org/10.3390/bios11120476
Dixit K, Fardindoost S, Ravishankara A, Tasnim N, Hoorfar M. Exhaled Breath Analysis for Diabetes Diagnosis and Monitoring: Relevance, Challenges and Possibilities. Biosensors. 2021; 11(12):476. https://doi.org/10.3390/bios11120476
Chicago/Turabian StyleDixit, Kaushiki, Somayeh Fardindoost, Adithya Ravishankara, Nishat Tasnim, and Mina Hoorfar. 2021. "Exhaled Breath Analysis for Diabetes Diagnosis and Monitoring: Relevance, Challenges and Possibilities" Biosensors 11, no. 12: 476. https://doi.org/10.3390/bios11120476
APA StyleDixit, K., Fardindoost, S., Ravishankara, A., Tasnim, N., & Hoorfar, M. (2021). Exhaled Breath Analysis for Diabetes Diagnosis and Monitoring: Relevance, Challenges and Possibilities. Biosensors, 11(12), 476. https://doi.org/10.3390/bios11120476









