High-Sensitivity High-Throughput Detection of Nucleic Acid Targets on Metasurface Fluorescence Biosensors
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. The Present Metasurface-Sensor Applicable Situation
3.2. Comparison with Other DNA-Sensing Results
3.3. Concluding Remarks
4. Materials and Methods
4.1. OligoDNAs
4.2. Binding Protein Molecules
4.3. MF-Flow Protocol
4.4. FL Measurement
5. Patents
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Ab | antibody |
| aM | attomolar (molar M = mol/L) |
| BOX | buried oxide |
| CCD | charge-coupled device |
| CDC | Centers for Disease Control and Prevention |
| DNA | deoxyribonucleic acid |
| FL | fluorescence |
| fM | femotomolar |
| GC | guanine-cytosine |
| LED | light-emitting diode |
| LOD | limit of detection |
| MF | microfluidic |
| miRNA | microRNA |
| NGS | next-generation sequencer |
| NIHT | National Institute of Health, Thailand |
| nM | nanomolar |
| OD | optical density |
| PBS | phosphate-buffered saline |
| PDMS | polydimethylsiloxane |
| pM | picomolar |
| POCT | point-of-cure testing |
| RNA | ribonucleic acid |
| SEM | scanning electron microscopy |
| SOI | silicon on insulator |
| SPR | surface plasmon resonance |
| ssDNA | single-strand DNA |
| T | thymine |
| TE8.0 | TE buffer, pH 8.0 |
References
- Udugama, B.; Kadhiresan, P.; Kozlowski, H.N.; Malekjahani, A.; Osborne, M.; Li, V.Y.C.; Chen, H.; Mubareka, S.; Gubbay, J.B.; Chan, W.C.W. Diagnosing COVID-19: The Disease and Tools for Detection. ACS Nano 2020, 14, 3822–3835. [Google Scholar] [CrossRef] [Green Version]
- Ji, T.; Liu, Z.; Wang, G.; Guo, X.; khan, S.A.; Lai, C.; Chen, H.; Huang, S.; Xia, S.; Chen, B.; et al. Detection of COVID-19: A review of the current literature and future perspectives. Biosens. Bioelectron. 2020, 166, 112455. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Uspal, W.E.; Wei, T. Airborne Transmission of COVID-19: Aerosol Dispersion, Lung Deposition, and Virus-Receptor Interactions. ACS Nano 2020, 14, 16502–16524. [Google Scholar] [CrossRef] [PubMed]
- LOD of SARS-CoV-2 by RT-PCR. Available online: https://www.niid.go.jp/niid/ja/covid-19/9482-covid14-15.html (accessed on 12 December 2020).
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.H.; Choi, M.; Ku, K.B.; Lee, C.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid Detection of COVID-19 Causative Virus (SARS-CoV-2) in Human Nasopharyngeal Swab Specimens Using Field-Effect Transistor-Based Biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alafeef, M.; Dighe, K.; Moitra, P.; Pan, D. Rapid, Ultrasensitive, and Quantitative Detection of SARS-CoV-2 Using Antisense Oligonucleotides Directed Electrochemical Biosensor Chip. ACS Nano 2020, 14, 17028–17045. [Google Scholar] [CrossRef]
- Giljohann, D.A.; Mirkin, C.A. Drivers of biodiagnostic development. Nature 2009, 462, 461–464. [Google Scholar] [CrossRef]
- Iwanaga, M.; Choi, B. Heteroplasmon Hybridization in Stacked Complementary Plasmo-Photonic Crystals. Nano Lett. 2015, 15, 1904–1910. [Google Scholar] [CrossRef]
- Greffet, J.J.; Nieto-Vesperinas, M. Field theory for generalized bidirectional reflectivity: Derivation of Helmholtz’s reciprocity principle and Kirchhoff’s law. J. Opt. Soc. Am. A 1998, 15, 2735–2744. [Google Scholar] [CrossRef]
- Choi, B.; Iwanaga, M.; Miyazaki, H.T.; Sugimoto, Y.; Ohtake, A.; Sakoda, K. Overcoming metal-induced fluorescence quenching on plasmo-photonic metasurfaces coated by a self-assembled monolayer. Chem. Commun. 2015, 51, 11470–11473. [Google Scholar] [CrossRef]
- Iwanaga, M.; Choi, B.; Miyazaki, H.T.; Sugimoto, Y. The artificial control of enhanced optical processes in fluorescent molecules on high-emittance metasurfaces. Nanoscale 2016, 8, 11099–11107. [Google Scholar] [CrossRef] [Green Version]
- Iwanaga, M. All-Dielectric Metasurfaces with High-Fluorescence-Enhancing Capability. Appl. Sci. 2018, 8, 1328. [Google Scholar] [CrossRef] [Green Version]
- Iwanaga, M. All-Dielectric Metasurface Fluorescence Biosensors for High-Sensitivity Antibody/Antigen Detection. ACS Nano 2020, 14, 17458–17467. [Google Scholar] [CrossRef] [PubMed]
- Jahani, S.; Jacob, Z. All-dielectric metamaterials. Nat. Nanotechnol. 2016, 11, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Genevet, P.; Capasso, F.; Aieta, F.; Khorasaninejad, M.; Devlin, R. Recent advances in planar optics: From plasmonic to dielectric metasurfaces. Optica 2017, 4, 139–152. [Google Scholar] [CrossRef]
- Campbell, S.D.; Sell, D.; Jenkins, R.P.; Whiting, E.B.; Fan, J.A.; Werner, D.H. Review of numerical optimization techniques for meta-device design. Opt. Mater. Express 2019, 9, 1842–1863. [Google Scholar] [CrossRef]
- Khorasaninejad, M.; Aieta, F.; Kanhaiya, P.; Kats, M.A.; Genevet, P.; Rousso, D.; Capasso, F. Achromatic Metasurface Lens at Telecommunication Wavelengths. Nano Lett. 2015, 15, 5358–5362. [Google Scholar] [CrossRef]
- Khorasaninejad, M.; Chen, W.T.; Devlin, R.C.; Oh, J.; Zhu, A.Y.; Capasso, F. Metalenses at visible wavelengths: Diffraction-limited focusing and subwavelength resolution imaging. Science 2016, 352, 1190–1194. [Google Scholar] [CrossRef] [Green Version]
- She, A.; Zhang, S.; Shian, S.; Clarke, D.R.; Capasso, F. Large area metalenses: Design, characterization, and mass manufacturing. Opt. Express 2018, 26, 1573–1585. [Google Scholar] [CrossRef] [Green Version]
- Aiello, M.D.; Backer, A.S.; Sapon, A.J.; Smits, J.; Perreault, J.D.; Llull, P.; Acosta, V.M. Achromatic Varifocal Metalens for the Visible Spectrum. ACS Photonics 2019, 6, 2432–2440. [Google Scholar] [CrossRef] [Green Version]
- Brière, G.; Ni, P.; Héron, S.; Chenot, S.; Vézian, S.; Brändli, V.; Damilano, B.; Duboz, J.Y.; Iwanaga, M.; Genevet, P. An Etching-Free Approach Toward Large-Scale Light-Emitting Metasurfaces. Adv. Opt. Mater. 2019, 7, 1801271. [Google Scholar] [CrossRef]
- Meem, M.; Banerji, S.; Pies, C.; Oberbiermann, T.; Majumder, A.; Sensale-Rodriguez, B.; Menon, R. Large-area, high-numerical-aperture multi-level diffractive lens via inverse design. Optica 2020, 7, 252–253. [Google Scholar] [CrossRef] [Green Version]
- Iwanaga, M. Non-Empirical Large-Scale Search for Optical Metasurfaces. Nanomaterials 2020, 10, 1739. [Google Scholar] [CrossRef] [PubMed]
- Iwanaga, M. Ultracompact waveplates: Approach from metamaterials. Appl. Phys. Lett. 2008, 92, 153102. [Google Scholar] [CrossRef] [Green Version]
- Iwanaga, M. Polarization-selective transmission in stacked two-dimensional complementary plasmonic crystal slabs. Appl. Phys. Lett. 2010, 96, 083106. [Google Scholar] [CrossRef]
- Iwanaga, M. Subwavelength electromagnetic dynamics in stacked complementary plasmonic crystal slabs. Opt. Express 2010, 18, 15389–15398. [Google Scholar] [CrossRef]
- Yu, N.; Genevet, P.; Kats, M.A.; Aieta, F.; Tetienne, J.P.; Capasso, F.; Gaburro, Z. Light Propagation with Phase Discontinuities: Generalized Laws of Reflection and Refraction. Science 2011, 334, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Arbabi, A.; Horie, Y.; Bagheri, M.; Faraon, A. Dielectric metasurfaces for complete control of phase and polarization with subwavelength spatial resolution and high transmission. Nat. Nanotechnol. 2015, 10, 937–943. [Google Scholar] [CrossRef] [Green Version]
- Kurosawa, H.; Choi, B.; Sugimoto, Y.; Iwanaga, M. High-performance metasurface polarizers with extinction ratios exceeding 12000. Opt. Express 2017, 25, 4446–4455. [Google Scholar] [CrossRef]
- Teperik, T.V.; de Abajo, F.J.G.; Borisov, A.G.; Abdelsalam, M.; Bartlett, P.N.; Sugawara, Y.; Baumberg, J.J. Omnidirectional absorption in nanostructured metal surfaces. Nat. Photonics 2008, 2, 299–301. [Google Scholar] [CrossRef] [Green Version]
- Hendrickson, J.; Guo, J.; Zhang, B.; Buchwald, W.; Soref, R. Wideband perfect light absorber at midwave infrared using multiplexed metal structures. Opt. Lett. 2012, 37, 371–373. [Google Scholar] [CrossRef]
- Bouchon, P.; Koechlin, C.; Pardo, F.; Haïdar, R.; Pelouard, J.L. Wideband omnidirectional infrared absorber with a patchwork of plasmonic nanoantennas. Opt. Lett. 2012, 37, 1038–1040. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.D.; Chen, K.; Ishii, S.; Ohi, A.; Nabatame, T.; Kitajima, M.; Nagao, T. Infrared Perfect Absorbers Fabricated by Colloidal Mask Etching of Al-Al2O3-Al Trilayers. ACS Photonics 2015, 2, 964–970. [Google Scholar] [CrossRef]
- Iwanaga, M. Perfect Light Absorbers Made of Tungsten-Ceramic Membranes. Appl. Sci. 2017, 7, 458. [Google Scholar] [CrossRef]
- Rana, A.S.; Mehmood, M.Q.; Jeong, H.; Kim, I.; Rho, J. Tungsten-based Ultrathin Absorber for Visible Regime. Sci. Rep. 2018, 8, 2443. [Google Scholar] [CrossRef] [PubMed]
- Kinkhabwala, A.; Yu, Z.; Fan, S.; Avlasevich, Y.; Müllen, K.; Moerner, W.E. Large single-molecule fluorescence enhancements produced by a bowtie nanoantenna. Nat. Photonics 2009, 3, 654–657. [Google Scholar] [CrossRef]
- Schmelzeisen, M.; Zhao, Y.; Klapper, M.; Müllen, K.; Kreiter, M. Fluorescence Enhancement from Individual Plasmonic Gap Resonances. ACS Nano 2010, 4, 3309–3317. [Google Scholar] [CrossRef]
- Fu, C.C.; Ossato, G.; Long, M.; Digman, M.A.; Gopinathan, A.; Lee, L.P.; Gratton, E.; Khine, M. Bimetallic nanopetals for thousand-fold fluorescence enhancements. Appl. Phys. Lett. 2010, 97, 203101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Ding, F.; Li, W.D.; Wang, Y.; Hu, J.; Chou, S.Y. Giant and uniform fluorescence enhancement over large areas using plasmonic nanodots in 3D resonant cavity nanoantenna by nanoimprinting. Nanotechnology 2012, 23, 225301. [Google Scholar] [CrossRef]
- Zhou, L.; Ding, F.; Chen, H.; Ding, W.; Zhang, W.; Chou, S.Y. Enhancement of Immunoassay’s Fluorescence and Detection Sensitivity Using Three-Dimensional Plasmonic Nano-Antenna-Dots Array. Anal. Chem. 2012, 84, 4489–4495. [Google Scholar] [CrossRef]
- Punj, D.; Mivelle, M.; Moparthi, S.B.; van Zanten, T.S.; Rigneault, H.; van Hulst, N.F.; García-Parajó, M.F.; Wenger, J. A plasmonic ‘antenna-in-box’ platform for enhanced single-molecule analysis at micromolar concentrations. Nat. Nanotechnol. 2013, 8, 512–516. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, A.I.; Miroshnichenko, A.E.; Brongersma, M.L.; Kivshar, Y.S.; Luk’yanchuk, B. Optically resonant dielectric nanostructures. Science 2017, 354, aag2472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wetmur, J.G. DNA Probes: Applications of the Principles of Nucleic Acid Hybridization. Crit. Rev. Biochem. Mol. Biol. 1991, 26, 227–259. [Google Scholar] [CrossRef]
- FL Probes. Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/Brochure/fluorescent_dna_probes.pdf (accessed on 2 December 2020).
- OligoDNA Modification. Available online: https://www.eurofinsgenomics.jp/jp/product/oligo-dna/standard-oligo-overview/modification (accessed on 2 December 2020).
- Hill, A.V. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, 4–7. [Google Scholar]
- Gesztelyi, R.; Zsuga, J.; Kemeny-Beke, A.; Varga, B.; Juhasz, B.; Tosaki, A. The Hill equation and the origin of quantitative pharmacology. Arch. Hist. Exact Sci. 2012, 66, 427–438. [Google Scholar] [CrossRef]
- Neubig, R.R.; Spedding, M.; Kenakin, T.; Christopoulos, A. International Union of Pharmacology Committee on Receptor Nomenclature and Drug Classification. XXXVIII. Update on Terms and Symbols in Quantitative Pharmacology. Pharmacol. Rev. 2003, 55, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Young, B.D.; Anderson, M.L.M. Quantitative Analysis of Solution Hybridisation. In Nucleic Acid Hybridization—A Practical Approach; IRL Press: Oxford, UK, 1985. [Google Scholar]
- Irrera, A.; Leonardi, A.A.; Di Franco, C.; Lo Faro, M.J.; Palazzo, G.; D’Andrea, C.; Manoli, K.; Franzò, G.; Musumeci, P.; Fazio, B.; et al. New Generation of Ultrasensitive Label-Free Optical Si Nanowire-Based Biosensors. ACS Photonics 2018, 5, 471–479. [Google Scholar] [CrossRef]
- Pan, H.M.; Gonuguntla, S.; Li, S.; Trau, D. Conjugated Polymers for Biosensor Devices. In Comprehensive Biomaterials II, Volume 3; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Hong, K.L. An overview of DNA/RNA-based monitoring tools and biosensors: Benefits and applications in the environmental toxicology. In Tools, Techniques and Protocols for Monitoring Environmental Contaminants; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Touahira, L.; Galopinb, E.; Boukherroubb, R.; Gouget-Laemmela, A.C.; Chazalviela, J.N.; Ozanama, F.; Szunerits, S. Localized surface plasmon-enhanced fluorescence spectroscopy for highly-sensitive real-time detection of DNA hybridization. Biosens. Bioelectron. 2010, 25, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Myszka, D.G.; He, X.; Dembo, M.; Morton, T.A.; Goldstein, B. Extending the Range of Rate Constants Available from BIACORE: Interpreting Mass Transport-Influenced Binding Data. Biophys. J. 1998, 75, 583–594. [Google Scholar] [CrossRef] [Green Version]
- Joshi, G.K.; Deitz-McElyea, S.; Liyanage, T.; Lawrence, K.; Mali, S.; Sardar, R.; Korc, M. Label-Free Nanoplasmonic-Based Short Noncoding RNA Sensing at Attomolar Concentrations Allows for Quantitative and Highly Specific Assay of MicroRNA-10b in Biological Fluids and Circulating Exosomes. ACS Nano 2015, 9, 11075–11089. [Google Scholar] [CrossRef] [Green Version]
- Miti, A.; Thamm, S.; Muller, P.; Csaki, A.; Fritzsche, W.; Zuccheri, G. A miRNA biosensor based on localized surface plasmon resonance enhanced by surface-bound hybridization chain reaction. Biosens. Bioelectron. 2020, 167, 112465. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Lo Faro, M.J.; Petralia, S.; Fazio, B.; Musumeci, P.; Conoci, S.; Irrera, A.; Priolo, F. Ultrasensitive Label- and PCR-Free Genome Detection Based on Cooperative Hybridization of Silicon Nanowires Optical Biosensors. ACS Sens. 2018, 3, 1690–1697. [Google Scholar] [CrossRef] [PubMed]
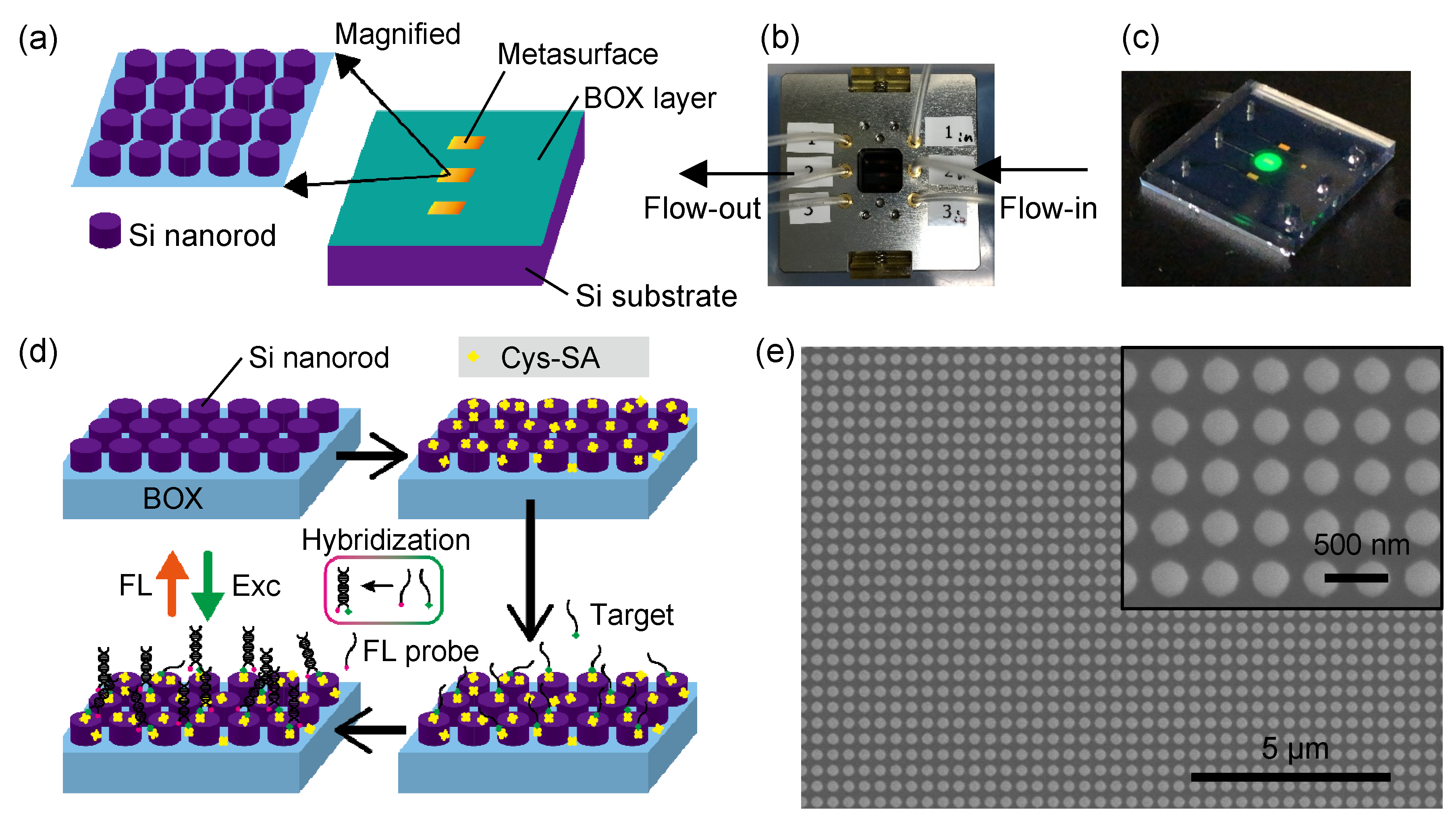
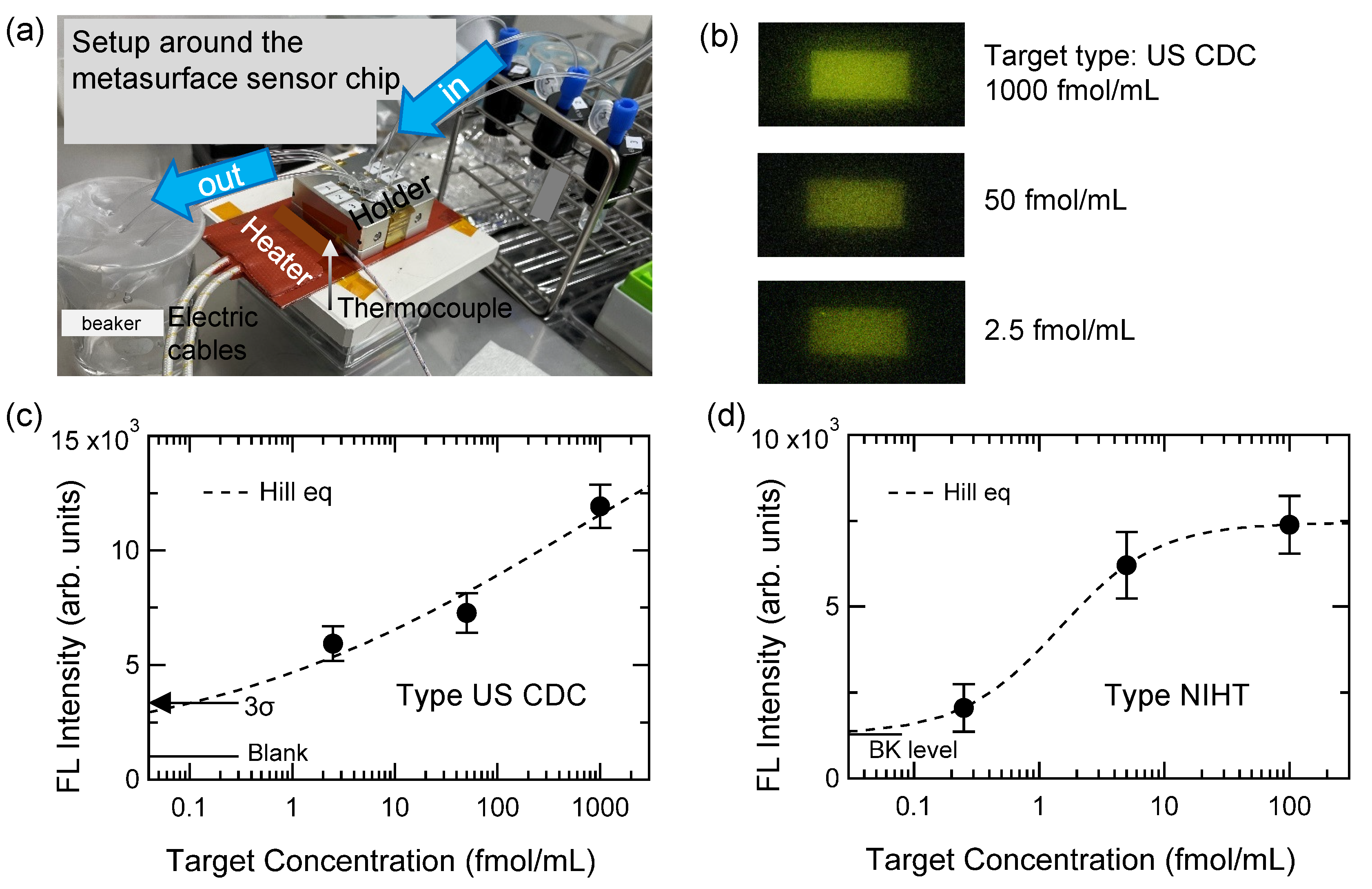

| Type | Role in Sensing | ssDNA Sequences | (°C) |
|---|---|---|---|
| US CDC | FL-labeled probe | 5-[HEX]GGTCCACCAAACGTAATGCGGGGT-3 | 63 |
| US CDC | Target | 5-TTTTTACCCCGCATTACGTTTGGTGGACCTTTTT[BIO]-3 | 62 |
| NIHT | FL-labeled probe | 5-[HEX]TGGTTACTGCCAGTTG-3 | 49 |
| NIHT | Target | 5-TTTTTCAACTGGCAGTAACCATTTTT[BIO]-3 | 53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwanaga, M. High-Sensitivity High-Throughput Detection of Nucleic Acid Targets on Metasurface Fluorescence Biosensors. Biosensors 2021, 11, 33. https://doi.org/10.3390/bios11020033
Iwanaga M. High-Sensitivity High-Throughput Detection of Nucleic Acid Targets on Metasurface Fluorescence Biosensors. Biosensors. 2021; 11(2):33. https://doi.org/10.3390/bios11020033
Chicago/Turabian StyleIwanaga, Masanobu. 2021. "High-Sensitivity High-Throughput Detection of Nucleic Acid Targets on Metasurface Fluorescence Biosensors" Biosensors 11, no. 2: 33. https://doi.org/10.3390/bios11020033
APA StyleIwanaga, M. (2021). High-Sensitivity High-Throughput Detection of Nucleic Acid Targets on Metasurface Fluorescence Biosensors. Biosensors, 11(2), 33. https://doi.org/10.3390/bios11020033





