Fiber Optic Sensors for Vital Signs Monitoring. A Review of Its Practicality in the Health Field
Abstract
:1. Introduction
2. Sensor Principles
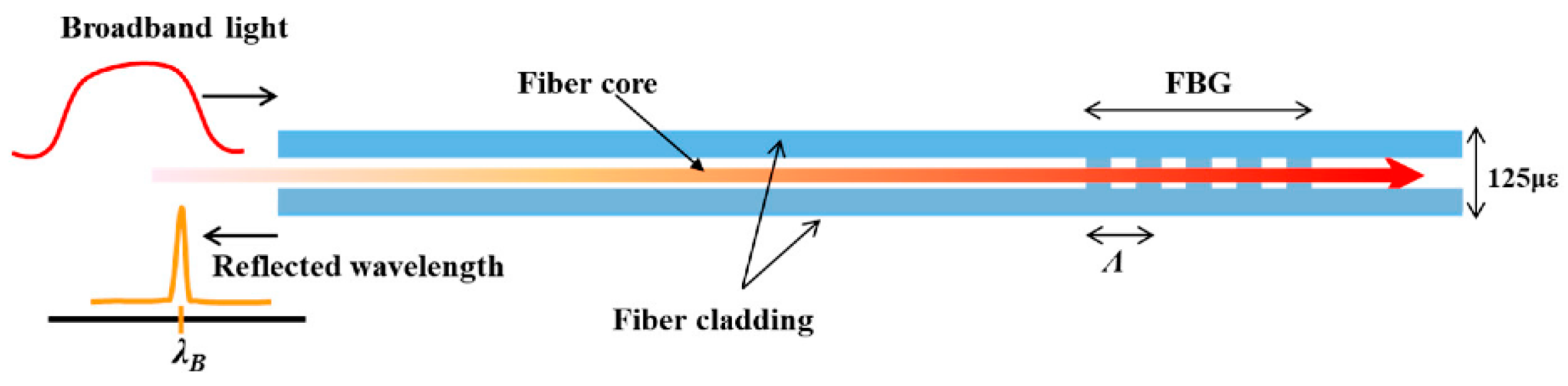
2.1. Fiber Bragg Gratings (FBGs)
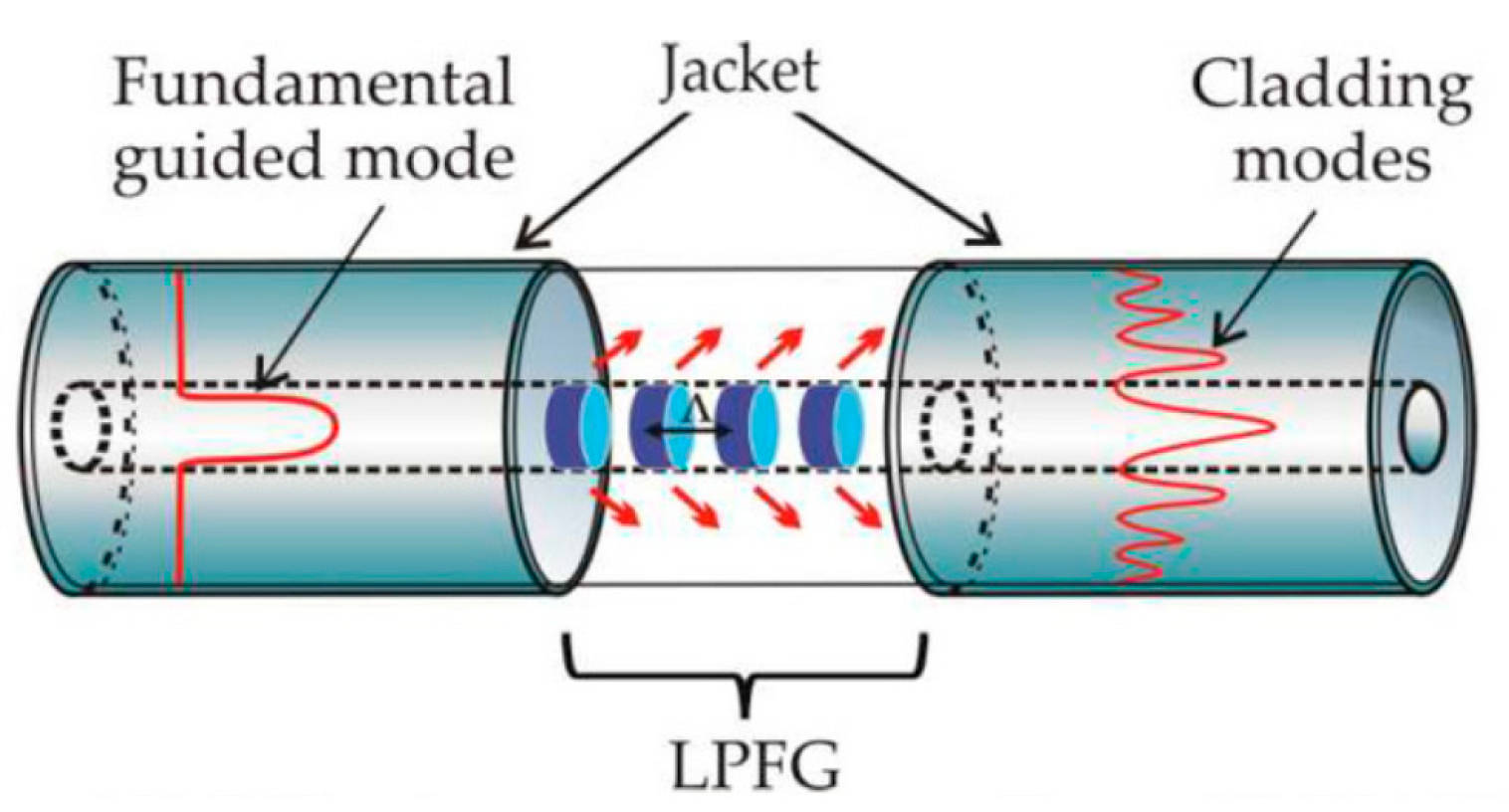
2.1.1. Long-Period Fiber Gratings (LPFGs)
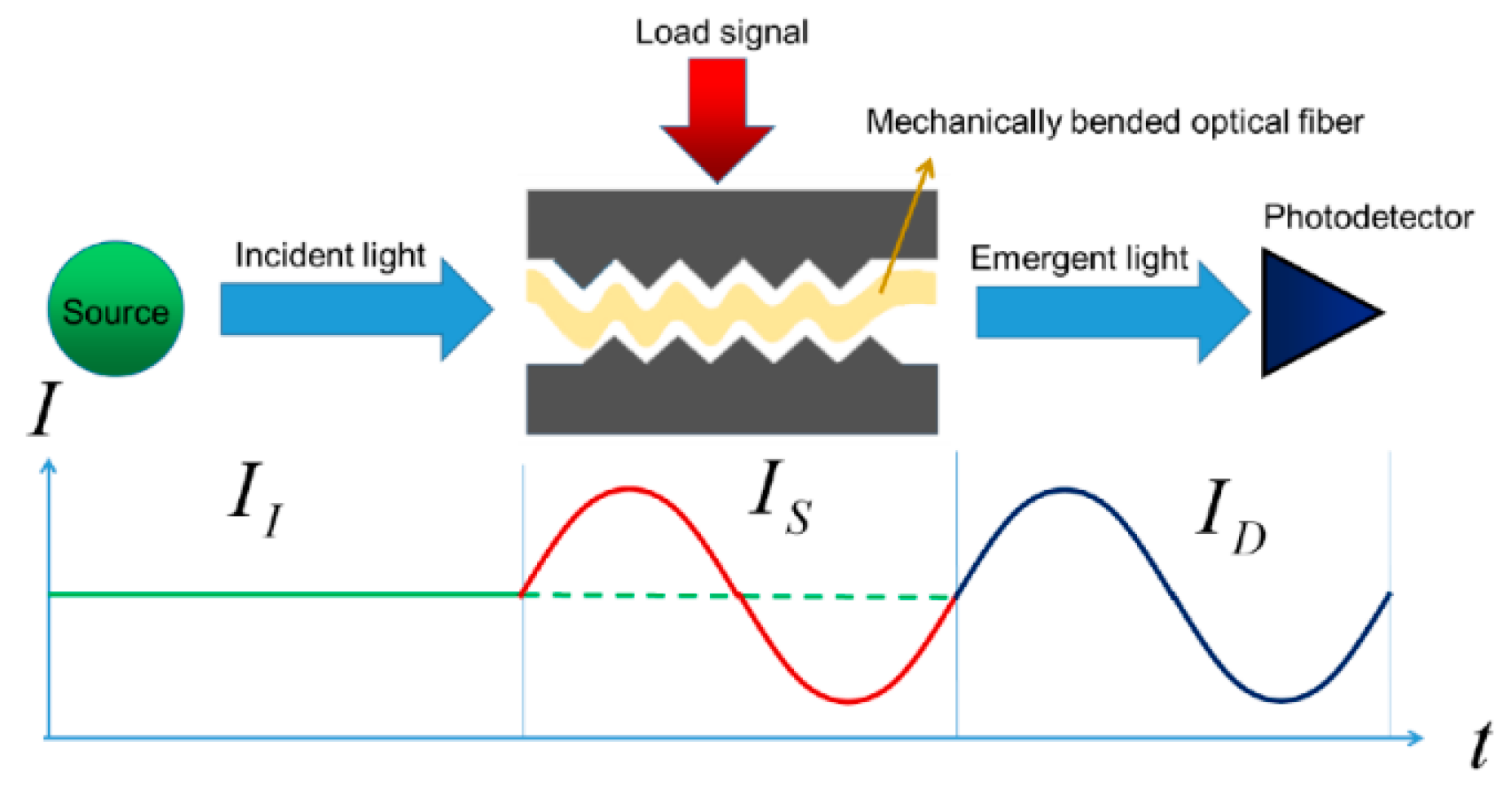
2.1.2. Mechanical Induction and Fiber Optic Sensors
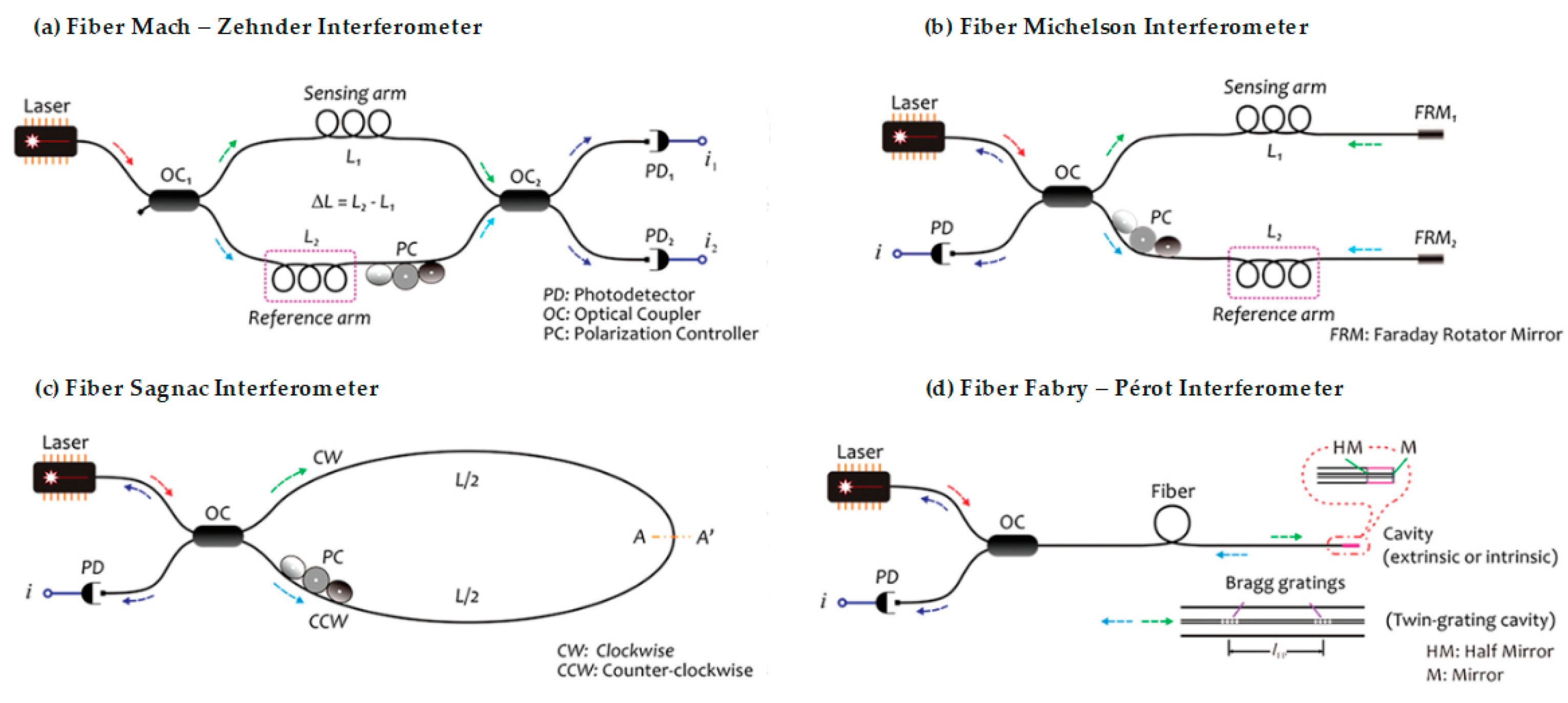
2.2. Optical Fiber Interferometers

2.2.1. Optical Fiber Mach–Zehnder Interferometer
2.2.2. Optical Fiber Michelson Interferometer
2.2.3. Optical Fiber Sagnac Interferometer
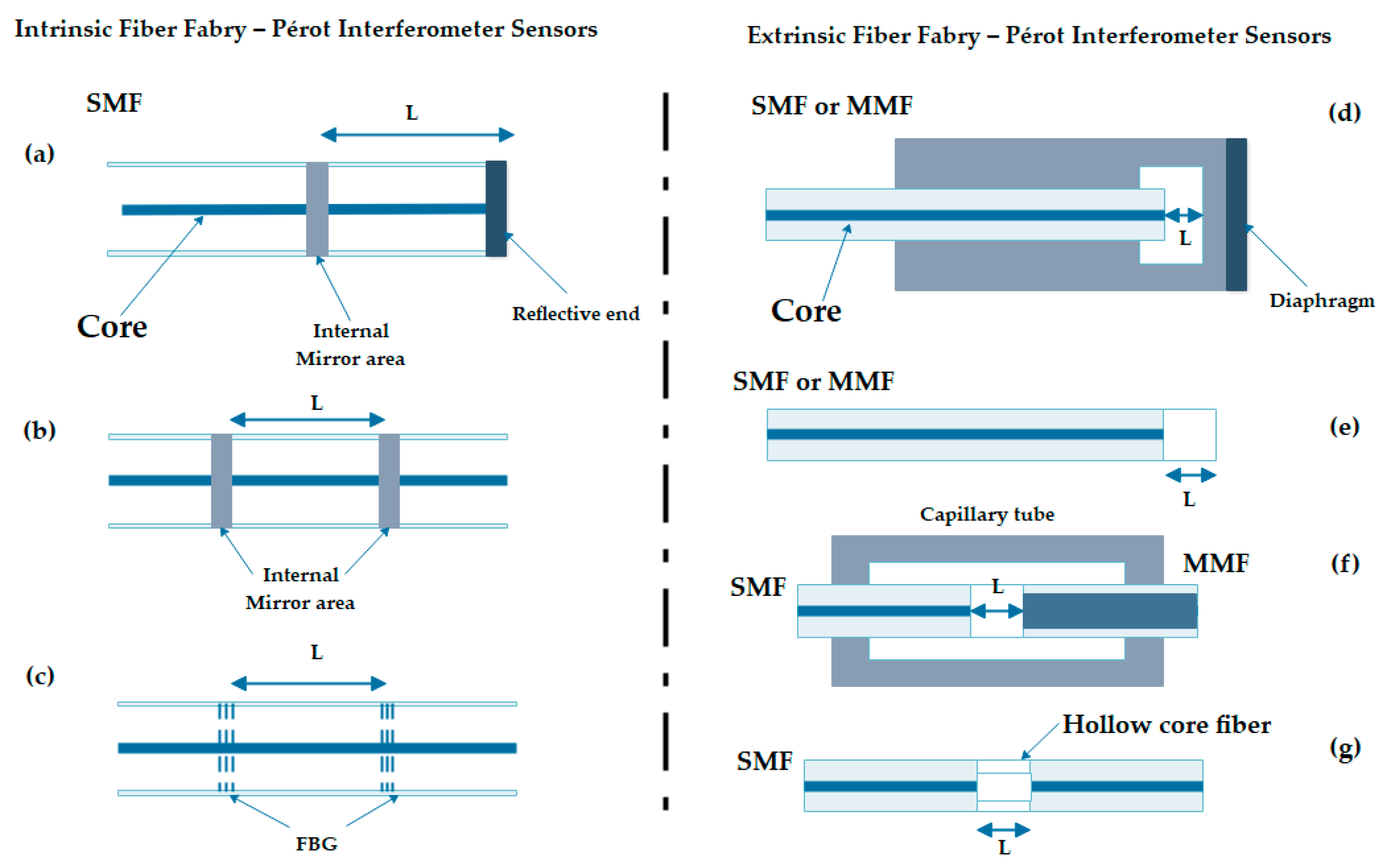
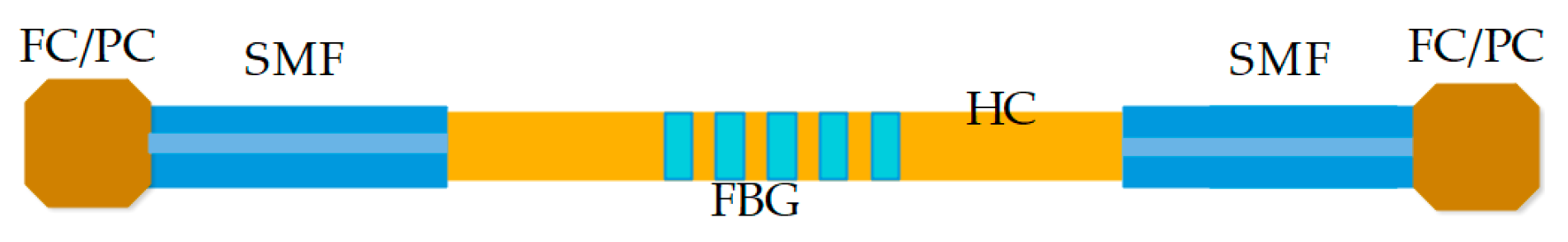
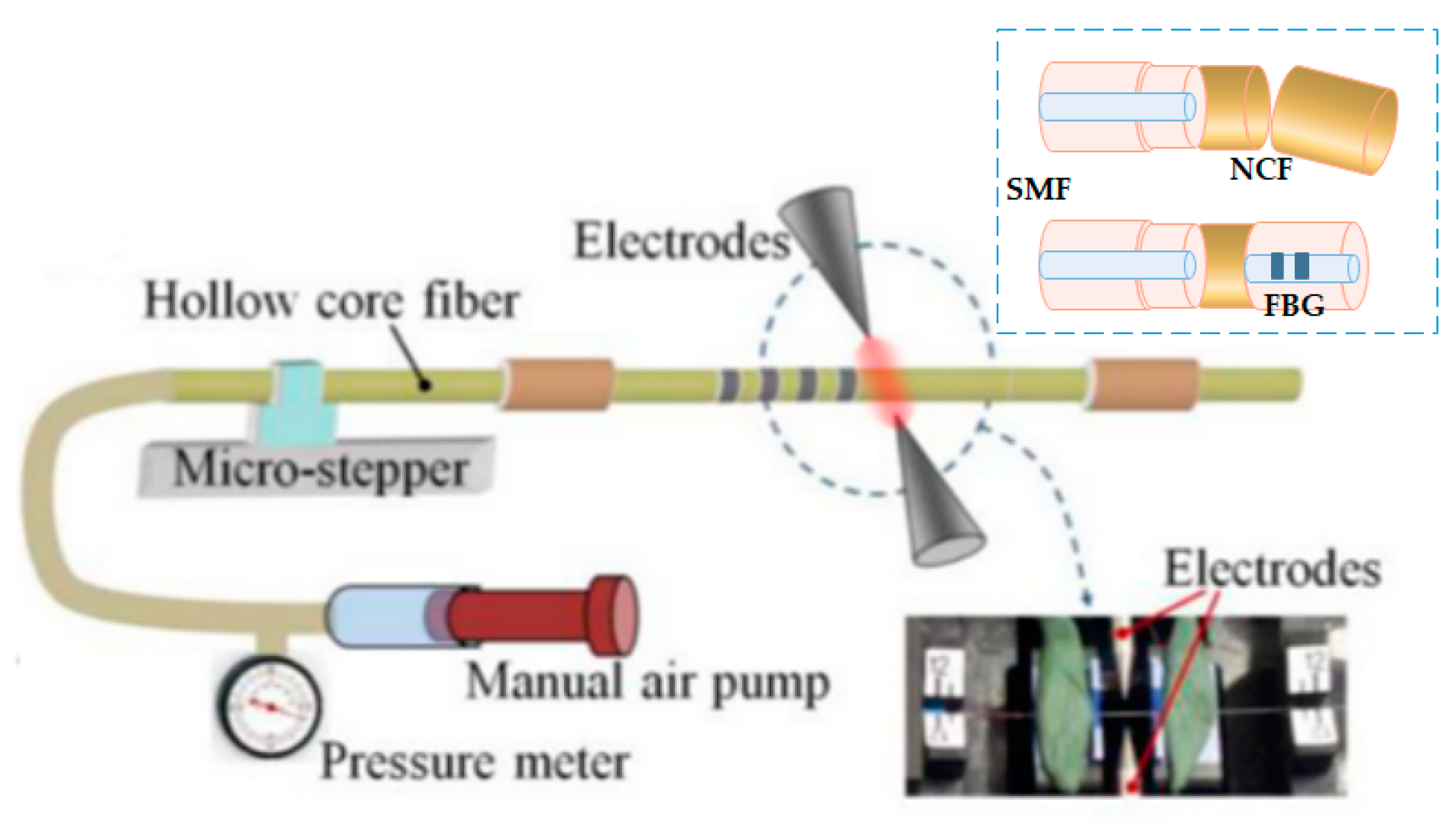
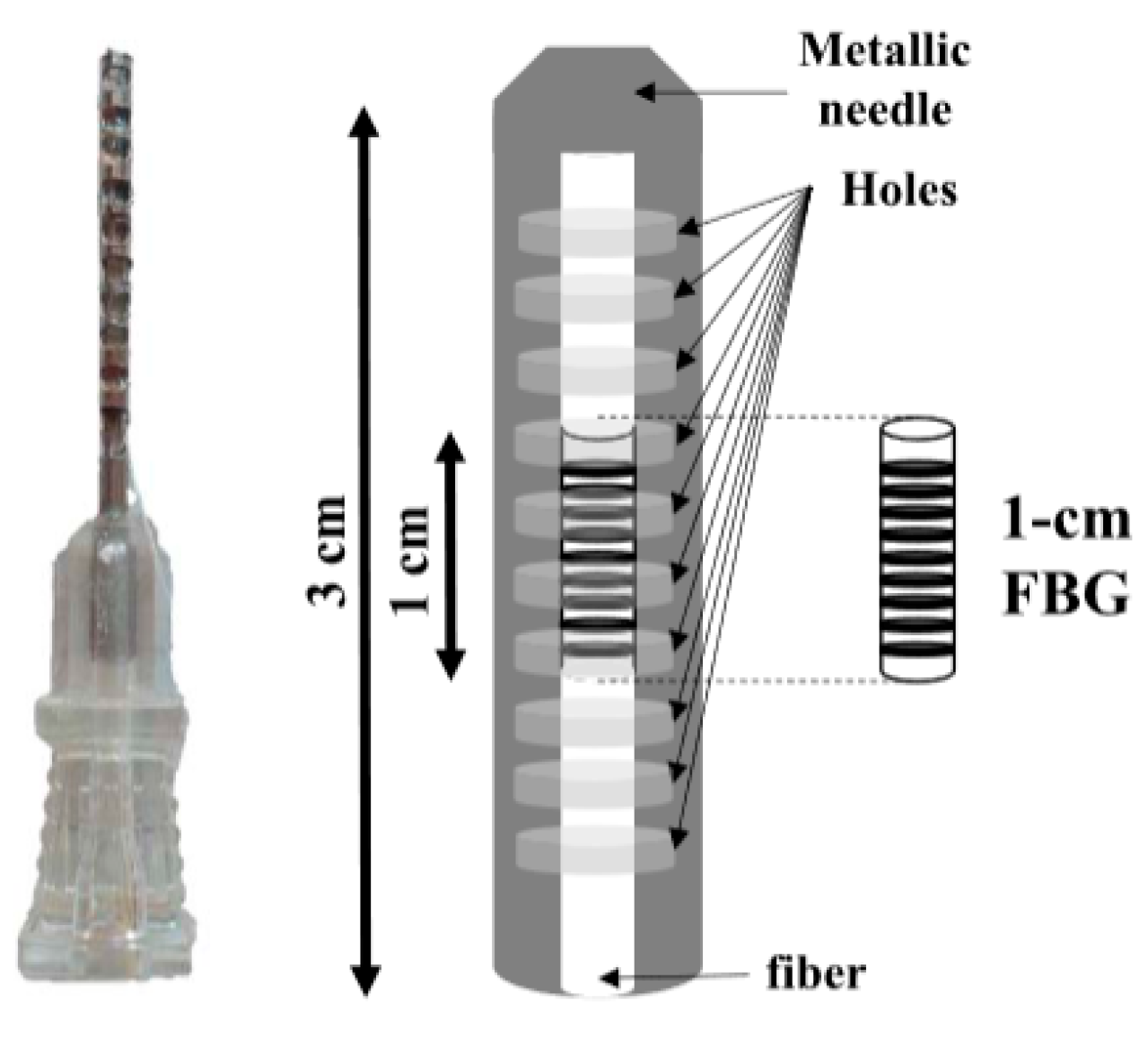
2.2.4. Optical Fiber Fabry–Pérot Interferometer
3. Health Field Sensing
3.1. Body Temperature
3.2. Respiration or Breathing Rate
3.3. Pulse or Heart Rate
3.4. Arterial or Blood Pressure
3.5. Multiparametric
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
For Accessibility to the Use of the Figures of the Scientific Journal Publishers
Conflicts of Interest
References
- Pinet, É. Medical Applications: Saving Lives. Nat. Photonics 2008, 2, 150–153. [Google Scholar] [CrossRef]
- Güemes, J.A.; Sierra-Pérez, J. Fiber Optics Sensors. In New Trends in Structural Health Monitoring; Ostachowicz, W., Güemes, J.A., Eds.; CISM International Centre for Mechanical Sciences; Springer: Vienna, Austria, 2013; Volume 542, pp. 265–316. ISBN 978-3-7091-1390-5. [Google Scholar]
- Roriz, P.; Carvalho, L.; Frazão, O.; Santos, J.L.; Simões, J.A. From Conventional Sensors to Fibre Optic Sensors for Strain and Force Measurements in Biomechanics Applications: A Review. J. Biomech. 2014, 47, 1251–1261. [Google Scholar] [CrossRef] [Green Version]
- Kanellos, G.T.; Papaioannou, G.; Tsiokos, D.; Mitrogiannis, C.; Nianios, G.; Pleros, N. Two Dimensional Polymer-Embedded Quasi-Distributed FBG Pressure Sensor for Biomedical Applications. Opt. Express 2010, 18, 179–186. [Google Scholar] [CrossRef]
- Poeggel, S.; Tosi, D.; Duraibabu, D.; Leen, G.; McGrath, D.; Lewis, E. Optical Fibre Pressure Sensors in Medical Applications. Sensors 2015, 15, 17115–17148. [Google Scholar] [CrossRef]
- Nedoma, J.; Fajkus, M.; Martinek, R.; Vasinek, V. Non-Invasive Fiber-Optic Biomedical Sensor for Basic Vital Sign Monitoring. Adv. Electr. Electron. Eng. 2017, 15, 336–342. [Google Scholar] [CrossRef]
- Ogawa, K.; Koyama, S.; Ishizawa, H.; Fujiwara, S.; Fujimoto, K. Simultaneous Measurement of Heart Sound, Pulse Wave and Respiration with Single Fiber Bragg Grating Sensor. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018. [Google Scholar] [CrossRef]
- Krebber, K.; Liehr, S.; Witt, J. Smart Technical Textiles Based on Fibre Optic Sensors. In Proceedings of the OFS2012 22nd International Conference on Optical Fiber Sensors, Beijing, China, 17 October 2012; Volume 8421, p. 84212A. [Google Scholar]
- Du, W.; Tao, X.M.; Tam, H.Y.; Choy, C.L. Fundamentals and Applications of Optical Fiber Bragg Grating Sensors to Textile Structural Composites. Compos. Struct. 1998, 42, 217–229. [Google Scholar] [CrossRef]
- Measures, R.M. Smart Composite Structures with Embedded Sensors. Compos. Eng. 1992, 2, 597–618. [Google Scholar] [CrossRef]
- Lindberg, L.-G.; Ugnell, H.; Öberg, P.Å. Monitoring of Respiratory and Heart Rates Using a Fibre-Optic Sensor. Med. Biol. Eng. Comput. 1992, 30, 533–537. [Google Scholar] [CrossRef]
- Fajkus, M.; Nedoma, J.; Martinek, R.; Novak, M.; Jargus, J.; Vasinek, V. Fiber Optic Sensor Encapsulated in Polydimethylsiloxane for Heart Rate Monitoring. In Proceedings of the Fiber Optic Sensors and Applications XIV, Anaheim, CA, USA, 27 April 2017; Volume 10208, p. 102080W. [Google Scholar]
- Fajkus, M.; Nedoma, J.; Martinek, R. Alternative Fiber Optic Sensor Based on Bragg Grating for Heart Rate Monitoring. Int. J. Biosens. Bioelectron. 2018, 4. [Google Scholar] [CrossRef]
- Manujło, A.; Osuch, T. Temperature Fiber Bragg Grating Based Sensor for Respiration Monitoring. In Proceedings of the Photonics Applications in Astronomy, Communications, Industry, and High Energy Physics Experiments 2017, Wilga, Poland, 7 August 2017; Volume 10445, p. 104451A. [Google Scholar]
- Tripathi, S.M.; Bock, W.J.; Kumar, A.; Mikulic, P. Temperature Insensitive High-Precision Refractive-Index Sensor Using Two Concatenated Dual-Resonance Long-Period Gratings. Opt. Lett. 2013, 38, 1666–1668. [Google Scholar] [CrossRef]
- Wang, G.; Pran, K.; Sagvolden, G.; Havsgård, G.B.; Jensen, A.E.; Johnson, G.A.; Vohra, S.T. Ship Hull Structure Monitoring Using Fibre Optic Sensors. Smart Mater. Struct. 2001, 10, 472–478. [Google Scholar] [CrossRef]
- Fresvig, T.; Ludvigsen, P.; Steen, H.; Reikerås, O. Fibre Optic Bragg Grating Sensors: An Alternative Method to Strain Gauges for Measuring Deformation in Bone. Med. Eng. Phys. 2008, 30, 104–108. [Google Scholar] [CrossRef]
- Othonos, A. Fiber Bragg Gratings. Rev. Sci. Instrum. 1997, 68, 4309–4341. [Google Scholar] [CrossRef]
- Wu, L.; Maheshwari, M.; Yang, Y.; Xiao, W. Selection and Characterization of Packaged FBG Sensors for Offshore Applications. Sensors 2018, 18, 3963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, J.Y.; Lee, K.S. A Birefringence Compensation Method for Mechanically Induced Long-Period Fiber Gratings. Opt. Commun. 2002, 213, 281–284. [Google Scholar] [CrossRef]
- Kumar, S.; Nair, B.; Pillai, V.P.M.; Nayar, U. Amplified Spontaneous Emission–Induced Thermal Nonlinearities in a Mechanically Induced Long-Period Fiber Grating. Opt. Eng. 2008, 47, 1–7. [Google Scholar] [CrossRef]
- Yan, L.S.; Yi, A.; Pan, W.; Luo, B. A Simple Demodulation Method for FBG Temperature Sensors Using a Narrow Band Wavelength Tunable DFB Laser. IEEE Photonics Technol. Lett. 2010, 22, 1391–1393. [Google Scholar] [CrossRef]
- Dong, X.; Tam, H.Y.; Shum, P. Temperature-Insensitive Strain Sensor with Polarization-Maintaining Photonic Crystal Fiber Based Sagnac Interferometer. Appl. Phys. Lett. 2007, 90, 151113. [Google Scholar] [CrossRef] [Green Version]
- Tian, K.; Farrell, G.; Wang, X.; Yang, W.; Xin, Y.; Liang, H.; Lewis, E.; Wang, P. Strain Sensor Based on Gourd-Shaped Single-Mode-Multimode-Single-Mode Hybrid Optical Fibre Structure. Opt. Express 2017, 25, 18885–18896. [Google Scholar] [CrossRef] [Green Version]
- Wright, R.F.; Lu, P.; Devkota, J.; Lu, F.; Ziomek-Moroz, M.; Ohodnicki, P.R. Corrosion Sensors for Structural Health Monitoring of Oil and Natural Gas Infrastructure: A Review. Sensors 2019, 19, 3964. [Google Scholar] [CrossRef] [Green Version]
- Allsop, T.; Reeves, R.; Webb, D.J.; Bennion, I.; Neal, R. A High Sensitivity Refractometer Based upon a Long Period Grating Mach–Zehnder Interferometer. Rev. Sci. Instrum. 2002, 73, 1702–1705. [Google Scholar] [CrossRef]
- Berthold, J.W. Historical Review of Microbend Fiber-Optic Sensors. J. Light. Technol. 1995, 13, 1193–1199. [Google Scholar] [CrossRef]
- Gong, Z.; Xiang, Z.; OuYang, X.; Zhang, J.; Lau, N.; Zhou, J.; Chan, C.C. Wearable Fiber Optic Technology Based on Smart Textile: A Review. Materials 2019, 12, 3311. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.H.; Kim, Y.H.; Park, K.S.; Eom, J.B.; Kim, M.J.; Rho, B.S.; Choi, H.Y. Interferometric Fiber Optic Sensors. Sensors 2012, 12, 2467–2486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, T.; Wu, D.; Liu, M.; Duan, D.-W. In-Line Fiber Optic Interferometric Sensors in Single-Mode Fibers. Sensors 2012, 12, 10430–10449. [Google Scholar] [CrossRef] [Green Version]
- Vali, V.; Shorthill, R.W. Fiber Ring Interferometer. Appl. Opt. 1976, 15, 1099. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-C.; Tung, P.-C.; Jywe, W.-Y.; Chang, C.-P.; Shyu, L.-H.; Hsieh, T.-H. Investigation on the Differential Quadrature Fabry–Pérot Interferometer with Variable Measurement Mirrors. Appl. Sci. 2020, 10, 6191. [Google Scholar] [CrossRef]
- Heideman, R.G.; Lambeck, P.V. Remote Opto-Chemical Sensing with Extreme Sensitivity: Design, Fabrication and Performance of a Pigtailed Integrated Optical Phase-Modulated Mach–Zehnder Interferometer System. Sens. Actuators B Chem. 1999, 61, 100–127. [Google Scholar] [CrossRef]
- Kashyap, R.; Nayar, B. An All Single-Mode Fiber Michelson Interferometer Sensor. J. Light. Technol. 1983, 1, 619–624. [Google Scholar] [CrossRef]
- Starodumov, A.N.; Zenteno, L.A.; Monzon, D.; De La Rosa, E. Fiber Sagnac Interferometer Temperature Sensor. Appl. Phys. Lett. 1997, 70, 19–21. [Google Scholar] [CrossRef]
- Flores, C.E.D. Fabricación y Caracterización de Interferómetros Fabry—Perot de Fibra Óptica Extrínsecos y su Aplicación en Sistemas de Sensado de Variables Físicas; Centro de Investigaciones en Óptica, A.C.: León, Guanajuato, Mexico, 2018. [Google Scholar]
- Wang, L.; Fang, N. Applications of Fiber-Optic Interferometry Technology in Sensor Fields. In Optical Interferometry; Banishev, A.A., Bhowmick, M., Wang, J., Eds.; InTech: Shanghai, China, 2008; ISBN 978-953-51-2955-4. [Google Scholar]
- Yin, S.; Ruffin, P.B.; Yu, F.T.S. Fiber Optic Sensors, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; ISBN 978-1-4200-5366-1. [Google Scholar]
- Liao, Y.-C.; Liu, B.; Liu, J.; Wan, S.-P.; He, X.-D.; Yuan, J.; Fan, X.; Wu, Q. High Temperature (Up to 950 °C) Sensor Based on Micro Taper In-Line Fiber Mach–Zehnder Interferometer. Appl. Sci. 2019, 9, 2394. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Li, Y.; Zhou, C.; Hu, M.; Xiong, Y.; Zeng, Z. In-Fiber Mach-Zehnder Interferometer Based on Three-Core Fiber for Measurement of Directional Bending. Sensors 2019, 19, 205. [Google Scholar] [CrossRef] [Green Version]
- Aref, S.H. Physical Measurement with In-Line Fiber Mach-Zehnder Interferometer Using Differential Phase White Light Interferometry. Opt. Fiber Technol. 2017, 38, 98–103. [Google Scholar] [CrossRef]
- Tian, Z.; Yam, S.S.-H.; Loock, H.-P. Single-Mode Fiber Refractive Index Sensor Based on Core-Offset Attenuators. IEEE Photonics Technol. Lett. 2008, 20, 1387–1389. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Xu, S.; Jiang, Y.; Chen, H.; Feng, W. Multi-Parameter Sensor Based on the Fiber Bragg Grating Combined with Triangular-Lattice Four-Core Fiber. Optik 2020, 208, 164094. [Google Scholar] [CrossRef]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 15 February 2021).
- Atrial Fibrillation. Available online: https://www.ahajournals.org/doi/epub/10.1161/01.CIR.0000022730.66617.D9 (accessed on 15 February 2021).
- Weissler, A.M.; Harris, W.S.; Schoenfeld, C.D. Systolic Time Intervals in Heart Failure in Man. Circulation 1968, 37, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Ball, J.; Dains, J.; Solomon, B.; Stewart, R. Seidel’s Guide to Physical Examination, 9th ed.; Available online: https://www.elsevier.com/books/seidels-guide-to-physical-examination/ball/978-0-323-48195-3 (accessed on 28 December 2020).
- The Arterial Pulse. Available online: http://healthcaresciencesocw.wayne.edu/vs/4_2.htm (accessed on 21 February 2021).
- Introduction to Vital Signs Examination and Evaluation Study Guide. Available online: http://healthcaresciencesocw.wayne.edu/vs/start.htm (accessed on 15 February 2021).
- Blood Pressure UK. Available online: http://www.bloodpressureuk.org/your-blood-pressure/understanding-your-blood-pressure/what-do-the-numbers-mean/ (accessed on 15 February 2021).
- Gutierrez-Arroyo, A.; Sanchez-Perez, C.; Aleman-Garcia, N. Optical Sensor for Heat Conduction Measurement in Biological Tissue. J. Phys. Conf. Ser. 2013, 450, 012027. [Google Scholar] [CrossRef] [Green Version]
- Mastrapa, G.C.; Guimarães, G.G.; Inácio, P.L.; de Oliveira, V.; Kalinowski, H.J. Fibre Bragg Gratings in the Visible: Towards Low-Cost Detection. In Proceedings of the Optical Sensing and Detection V, Strasbourg, France, 9 May 2018; Volume 10680, pp. 1–4. [Google Scholar]
- Massaroni, C.; Caponero, M.A.; D’Amato, R.; Lo Presti, D.; Schena, E. Fiber Bragg Grating Measuring System for Simultaneous Monitoring of Temperature and Humidity in Mechanical Ventilation. Sensors 2017, 17, 749. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Ohashi, M.; Miyoshi, Y. Temperature-Sensitive Mechanical LPFG Using Contractive Force of Heat-Shrinkable Tube. Opt. Fiber Technol. 2013, 19, 55–59. [Google Scholar] [CrossRef]
- Tan, Y.; Sun, L.-P.; Jin, L.; Li, J.; Guan, B.-O. Microfiber Mach-Zehnder Interferometer Based on Long Period Grating for Sensing Applications. Opt. Express 2013, 21, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Iadicicco, A.; Campopiano, S. Sensing Features of Long Period Gratings in Hollow Core Fibers. Sensors 2015, 15, 8009–8019. [Google Scholar] [CrossRef] [Green Version]
- Urrutia Azcona, A.; Goicoechea Fernández, J.; Ricchiuti, A.L.; Barrera, D.; Arregui San Martín, F.J. Simultaneous Measurement of Humidity and Temperature Based on a Partially Coated Optical Fiber Long Period Grating. Sens. Actuators B Chem. 2016, 227, 135–141. [Google Scholar] [CrossRef] [Green Version]
- Geng, T.; Zhang, S.; Peng, F.; Yang, W.; Sun, C.; Chen, X.; Zhou, Y.; Hu, Q.; Yuan, L. A Temperature-Insensitive Refractive Index Sensor Based on No-Core Fiber Embedded Long Period Grating. J. Light. Technol. 2017, 35, 5391–5396. [Google Scholar] [CrossRef]
- Lu, L.; Lu, L.; Jiang, Z.; Hu, Y.; Zhou, H.; Liu, G.; Liu, G.; Chen, Y.; Chen, Y.; Luo, Y.; et al. A Portable Optical Fiber SPR Temperature Sensor Based on a Smart-Phone. Opt. Express 2019, 27, 25420–25427. [Google Scholar] [CrossRef] [PubMed]
- Bonefacino, J.; Tam, H.-Y.; Glen, T.S.; Cheng, X.; Pun, C.-F.J.; Wang, J.; Lee, P.-H.; Tse, M.-L.V.; Boles, S.T. Ultra-Fast Polymer Optical Fibre Bragg Grating Inscription for Medical Devices. Light Sci. Appl. 2018, 7, 17161. [Google Scholar] [CrossRef] [PubMed]
- Elsarnagawy, T.; Haueisen, J.; Farrag, M.; Ansari, S.G.; Fouad, H. Embedded Fiber Bragg Grating Based Strain Sensor as Smart Costume for Vital Signal Sensing. Sens. Lett. 2014, 12, 1669–1674. [Google Scholar] [CrossRef]
- Fajkus, M.; Nedoma, J.; Martinek, R.; Vasinek, V.; Nazeran, H.; Siska, P. A Non-Invasive Multichannel Hybrid Fiber-Optic Sensor System for Vital Sign Monitoring. Sensors 2017, 17, 111. [Google Scholar] [CrossRef] [PubMed]
- Nedoma, J.; Fajkus, M.; Martinek, R.; Nazeran, H. Vital Sign Monitoring and Cardiac Triggering at 1.5 Tesla: A Practical Solution by an MR-Ballistocardiography Fiber-Optic Sensor. Sensors 2019, 19, 470. [Google Scholar] [CrossRef] [Green Version]
- Lee, B. Review of the Present Status of Optical Fiber Sensors. Opt. Fiber Technol. 2003, 9, 57–79. [Google Scholar] [CrossRef]
- Fïdanboylu, K.; Efendïoğlu, H. Review of Turn around Point Long Period Fiber Gratings. J. Sens. Technol. 2009, 5, 81. [Google Scholar]
- Sharma, A.K.; Gupta, J.; Basu, R. Simulation and Performance Evaluation of Fiber Optic Sensor for Detection of Hepatic Malignancies in Human Liver Tissues. Opt. Laser Technol. 2018, 98, 291–297. [Google Scholar] [CrossRef]
- Hunsperger, R.G.; Mentzer, M.A. Photonic Devices and Systems; Routledge: Boca Raton, FL, USA, 2017; ISBN 978-0-203-74351-5. [Google Scholar]
- Dziuda, L.; Skibniewski, F.W.; Krej, M.; Lewandowski, J. Monitoring Respiration and Cardiac Activity Using Fiber Bragg Grating-Based Sensor. IEEE Trans. Biomed. Eng. 2012, 59, 1934–1942. [Google Scholar] [CrossRef]
- Yu, C.; Xu, W.; Shen, Y.; Bian, S.; Yu, C.; You, S. Non-Invasive Vital Signs Monitoring System Based on Smart Sensor Mat Embedded with Optical Fiber Interferometer. In Proceedings of the 2018 27th Wireless and Optical Communication Conference (WOCC), Hualien, Taiwan, 30 April–1 May 2018; pp. 1–3. [Google Scholar]
- Zazula, D.; Šprager, S. Monitoring Respiration by Using Fiber-Optic Interferomtery and Maximum A Posteriori Estimation. In Proceedings of the 2013 Africon, Pointe aux Piments, Mauritius, 9–12 September 2013. [Google Scholar]
- Krebber, K.; Lenke, P.; Liehr, S.; Witt, J.; Schukar, M. Smart Technical Textiles with Integrated POF Sensors. In Proceedings of the Smart Sensor Phenomena, Technology, Networks, and Systems 2008, San Diego, CA, USA, 7 April 2008; Volume 6933, p. 69330V. [Google Scholar]
- Massaroni, C.; Presti, D.L.; Saccomandi, P.; Caponero, M.A.; D’Amato, R.; Schena, E. Fiber Bragg Grating Probe for Relative Humidity and Respiratory Frequency Estimation: Assessment During Mechanical Ventilation. IEEE Sens. J. 2018, 18, 2125–2130. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Hee, H.I. Noninvasive Measurement of Heart Rate and Respiratory Rate for Perioperative Infants. J. Light. Technol. 2019, 37, 2807–2814. [Google Scholar] [CrossRef]
- Hariyanti Devara, K.; Aisyah, F.N.; Nadia, K.V.A.W.; Purnamaningsih, R.W. Design of a Wearable Fiber Optic Respiration Sensor for Application in NICU Incubators. AIP Conf. Proc. 2019, 2092, 020002. [Google Scholar] [CrossRef]
- Allsop, T.; Carroll, K.; Lloyd, G.; Webb, D.J.; Miller, M.; Bennion, I. Application of Long-Period-Grating Sensors to Respiratory Plethysmography. J. Biomed. Opt. 2007, 12, 064003. [Google Scholar] [CrossRef]
- Allsop, T.D.P.; Earthrowl, T.; Revees, R.; Webb, D.J.; Miller, M.; Jones, B.W.; Bennion, I. Application of Long-Period Grating Sensors to Respiratory Function Monitoring. In Proceedings of the Smart Medical and Biomedical Sensor Technology II, Philadelphia, PA, USA, 7 December 2004; Volume 5588, pp. 148–156. [Google Scholar]
- Witt, J.; Narbonneau, F.; Schukar, M.; Krebber, K.; Jonckheere, J.D.; Jeanne, M.; Kinet, D.; Paquet, B.; Depre, A.; D’Angelo, L.T.; et al. Medical Textiles With Embedded Fiber Optic Sensors for Monitoring of Respiratory Movement. IEEE Sens. J. 2012, 12, 246–254. [Google Scholar] [CrossRef]
- Petrović, M.D.; Petrovic, J.; Daničić, A.; Vukčević, M.; Bojović, B.; Hadžievski, L.; Allsop, T.; Lloyd, G.; Webb, D.J. Non-Invasive Respiratory Monitoring Using Long-Period Fiber Grating Sensors. Biomed. Opt. Express 2014, 5, 1136–1144. [Google Scholar] [CrossRef] [Green Version]
- Allsop, T.D.P.; Earthrowl-Gould, T.; Webb, D.J.; Bennion, I. Embedded Progressive-Three-Layered Fiber Long-Period Gratings for Respiratory Monitoring. J. Biomed. Opt. 2003, 8, 552–558. [Google Scholar] [CrossRef] [PubMed]
- Koyama, Y.; Nishiyama, M.; Watanabe, K. Smart Textile Using Hetero-Core Optical Fiber for Heartbeat and Respiration Monitoring. IEEE Sens. J. 2018, 18, 6175–6180. [Google Scholar] [CrossRef]
- Ciocchetti, M.; Massaroni, C.; Saccomandi, P.; Caponero, M.A.; Polimadei, A.; Formica, D.; Schena, E. Smart Textile Based on Fiber Bragg Grating Sensors for Respiratory Monitoring: Design and Preliminary Trials. Biosensors 2015, 5, 602–615. [Google Scholar] [CrossRef] [Green Version]
- Presti, D.L.; Massaroni, C.; Formica, D.; Saccomandi, P.; Giurazza, F.; Caponero, M.A.; Schena, E. Smart Textile Based on 12 Fiber Bragg Gratings Array for Vital Signs Monitoring. IEEE Sens. J. 2017, 17, 6037–6043. [Google Scholar] [CrossRef]
- Lo Presti, D.; Massaroni, C.; Saccomandi, P.; Caponero, M.A.; Formica, D.; Schena, E. A Wearable Textile for Respiratory Monitoring: Feasibility Assessment and Analysis of Sensors Position on System Response. Annu. Int. Conf. 2017, 2017, 4423–4426. [Google Scholar] [CrossRef]
- Chen, Z.; Lau, D.; Teo, J.T.; Ng, S.H.; Yang, X.; Kei, P.L. Simultaneous Measurement of Breathing Rate and Heart Rate Using a Microbend Multimode Fiber Optic Sensor. J. Biomed. Opt. 2014, 19, 057001. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Tabor, J.; Ghosh, T.K. Electrically Conductive Coatings for Fiber-Based E-Textiles. Fibers 2019, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Chethana, K.; Prasad, A.S.G.; Omkar, S.N.; Asokan, S. Fiber Bragg Grating Sensor Based Device for Simultaneous Measurement of Respiratory and Cardiac Activities. J. Biophotonics 2017, 10, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Ishizawa, H. Vital Sign Measurement Using FBG Sensor for New Wearable Sensor Development. Fiber Opt. Sens. Princ. Meas. Appl. 2019, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Tan, F.; Chen, S.; Lyu, W.; Liu, Z.; Liu, Z.; Yu, C.; Yu, C.; Lu, C.; Tam, H.-Y. Non-Invasive Human Vital Signs Monitoring Based on Twin-Core Optical Fiber Sensors. Biomed. Opt. Express 2019, 10, 5940–5952. [Google Scholar] [CrossRef]
- Fajkus, M.; Nedoma, J.; Siska, P.; Vasinek, V. FBG Sensor of Breathing Encapsulated into Polydimethylsiloxane. In Proceedings of the Optical Materials and Biomaterials in Security and Defence Systems Technology XIII, Edinburgh, UK, 24 October 2016; Volume 9994, p. 99940N. [Google Scholar]
- Hu, H.; Sun, S.; Lv, R.; Zhao, Y. Design and Experiment of an Optical Fiber Micro Bend Sensor for Respiration Monitoring. Sens. Actuators Phys. 2016, 251, 126–133. [Google Scholar] [CrossRef]
- Nedoma, J.; Fajkus, M.; Novak, M.; Strbikova, N.; Vasinek, V.; Nazeran, H.; Vanus, J.; Perecar, F.; Martinek, R. Validation of a Novel Fiber-Optic Sensor System for Monitoring Cardiorespiratory Activities During MRI Examinations. Adv. Electr. Electron. Eng. 2017, 15, 536–543. [Google Scholar] [CrossRef]
- Pant, S.; Umesh, S.; Asokan, S. Fiber Bragg Grating Respiratory Measurement Device. In Proceedings of the 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Rome, Italy, 11–13 June 2018; pp. 1–5. [Google Scholar]
- Nedoma, J.; Fajkus, M.; Siska, P.; Martinek, R.; Vasinek, V. Non-Invasive Fiber Optic Probe Encapsulated Into PolyDiMethylSiloxane for Measuring Respiratory and Heart Rate of the Human Body. Adv. Electr. Electron. Eng. 2017, 15. [Google Scholar] [CrossRef]
- Lo Presti, D.; Romano, C.; Massaroni, C.; D’Abbraccio, J.; Massari, L.; Caponero, M.A.; Oddo, C.M.; Formica, D.; Schena, E. Cardio-Respiratory Monitoring in Archery Using a Smart Textile Based on Flexible Fiber Bragg Grating Sensors. Sensors 2019, 19, 3581. [Google Scholar] [CrossRef] [Green Version]
- Jia, J.; Xu, C.; Pan, S.; Xia, S.; Wei, P.; Noh, H.Y.; Zhang, P.; Jiang, X. Conductive Thread-Based Textile Sensor for Continuous Perspiration Level Monitoring. Sensors 2018, 18, 3775. [Google Scholar] [CrossRef] [Green Version]
- Massaroni, C.; Saccomandi, P.; Schena, E. Medical Smart Textiles Based on Fiber Optic Technology: An Overview. J. Funct. Biomater. 2015, 6, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Anwar Zawawi, M.; O’Keffe, S.; Lewis, E. Intensity-modulated Fiber Optic Sensor for Health Monitoring Applications: A Comparative Review. Sens. Rev. 2013, 33, 57–67. [Google Scholar] [CrossRef]
- Ho, S.C.M.; Razavi, M.; Nazeri, A.; Song, G. FBG Sensor for Contact Level Monitoring and Prediction of Perforation in Cardiac Ablation. Sensors 2012, 12, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Yunianto, M.; Marzuki, A.; Riyanto, R.; Lestari, D. Fiber Optic Sensor Based on Reflectivity Configurations to Detect Heart Rate. J. Phys. Conf. Ser. 2016, 776, 012110. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Teo, J.T.; Ng, S.H.; Yim, H. Smart Pillow for Heart-Rate Monitoring Using a Fiber Optic Sensor. In Proceedings of the Optical Fibers, Sensors, and Devices for Biomedical Diagnostics and Treatment XI, Wuhan, China, 16 February 2011; Volume 7894, p. 789402. [Google Scholar]
- Krej, M.; Dziuda, L.; Skibniewski, F.W. A Method of Detecting Heartbeat Locations in the Ballistocardiographic Signal from the Fiber-Optic Vital Signs Sensor. IEEE J. Biomed. Health Inform. 2015, 19, 1443–1450. [Google Scholar] [CrossRef]
- Chino, S.; Ishizawa, H.; Hosoya, S.; Koyama, S.; Fujimoto, K.; Kawamura, T. Research for Wearable Multiple Vital Sign Sensor Using Fiber Bragg Grating -Verification of Several Pulsate Points in Human Body Surface. In Proceedings of the 2017 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Turin, Italy, 22–25 May 2017. [Google Scholar]
- Sadek, I.; Biswas, J.; Abdulrazak, B.; Haihong, Z.; Mokhtari, M. Continuous and Unconstrained Vital Signs Monitoring with Ballistocardiogram Sensors in Headrest Position. In Proceedings of the 2017 IEEE EMBS International Conference on Biomedical Health Informatics (BHI), Orlando, FL, USA, 16–19 February 2017; pp. 289–292. [Google Scholar]
- Arifin, A.; Lebang, A.K.; Yunus, M.; Dewang, S.; Idris, I.; Tahir, D. Measurement Heart Rate Based on Plastic Optical Fiber Sensor. J. Phys. Conf. Ser. 2019, 1170, 012074. [Google Scholar] [CrossRef]
- Wang, J.; Liu, K.; Sun, Q.; Ni, X.; Ai, F.; Wang, S.; Yan, Z.; Liu, D. Diaphragm-Based Optical Fiber Sensor for Pulse Wave Monitoring and Cardiovascular Diseases Diagnosis. J. Biophotonics 2019, 12, e201900084. [Google Scholar] [CrossRef]
- Sensors/Free Full-Text/Continuous Vital Monitoring During Sleep and Light Activity Using Carbon-Black Elastomer Sensors/HTML. Available online: https://www.mdpi.com/1424-8220/20/6/1583/htm (accessed on 12 February 2021).
- Chen, C.; Li, Z.; Zhang, Y.; Zhang, S.; Hou, J.; Zhang, H. A 3D Wrist Pulse Signal Acquisition System for Width Information of Pulse Wave. Sensors 2019, 20, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reesink, K.D.; van der Nagel, T.; Bovelander, J.; Jansen, J.R.C.; van der Veen, F.H.; Schreuder, J.J. Feasibility Study of a Fiber-Optic System for Invasive Blood Pressure Measurements. Catheter. Cardiovasc. Interv. 2002, 57, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Krasovskii, V.I.; Feofanov, I.N.; Ivashkin, P.I.; Kazaryan, M.A. A Fiber-Optic Doppler Blood Flow-Velocity Sensor. St Petersburg Polytech. Univ. J. Phys. Math. 2017, 3, 35–38. [Google Scholar] [CrossRef]
- Totsu, K.; Haga, Y.; Esashi, M. Ultra-Miniature Fiber-Optic Pressure Sensor Using White Light Interferometry. J. Micromech. Microeng. 2004, 15, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Wu, N.; Tian, Y.; Zou, X.; Zhai, Y.; Barringhaus, K.; Wang, X. A Miniature Fiber Optic Blood Pressure Sensor and Its Application in in Vivo Blood Pressure Measurements of a Swine Model. Sens. Actuators B Chem. 2013, 181, 172–178. [Google Scholar] [CrossRef]
- Miyauchi, Y.; Koyama, S.; Ishizawa, H. Basic Experiment of Blood-Pressure Measurement Which Uses FBG Sensors. In Proceedings of the 2013 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Minneapolis, MN, USA, 6–9 May 2013. [Google Scholar] [CrossRef]
- Tian, Y.; Wu, N.; Zou, X.; Zhang, Y.; Barringhaus, K.; Wang, X. A Study on Packaging of Miniature Fiber Optic Sensors for In-Vivo Blood Pressure Measurements in a Swine Model. IEEE Sens. J. 2014, 14, 629–635. [Google Scholar] [CrossRef]
- Koyama, S.; Ishizawa, H.; Sakaguchi, A.; Hosoya, S.; Kawamura, T. Influence on Calculated Blood Pressure of Measurement Posture for the Development of Wearable Vital Sign Sensors. J. Sens. 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Koyama, S.; Sakaguchi, A.; Ishizawa, H.; Yasue, K.; Oshiro, H.; Kimura, H. Vital Sign Measurement Using Covered FBG Sensor Embedded into Knitted Fabric for Smart Textile. J. Fiber Sci. Technol. 2017, 73, 300–308. [Google Scholar] [CrossRef] [Green Version]
- Haseda, Y.; Bonefacino, J.; Tam, H.-Y.; Chino, S.; Koyama, S.; Ishizawa, H. Measurement of Pulse Wave Signals and Blood Pressure by a Plastic Optical Fiber FBG Sensor. Sensors 2019, 19, 5088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.-K.; Kang, D.-H.; Bang, H.-J.; Hong, C.-S.; Kim, C.-G. Cure Monitoring of Composite Laminates Using Fiber Optic Sensors. Smart Mater. Struct. 2002, 11, 279–287. [Google Scholar] [CrossRef]
- Życzkowski, M.; Szustakowski, M.; Ciurapiński, W.; Uziębło-Życzkowska, B. Interferometric Fiber Optics Based Sensor for Monitoring of the Heart Activity. Acta Phys. Pol. A 2011, 120, 782–784. [Google Scholar] [CrossRef]
- Życzkowski, M.; Uziebło-Zyczkowska, B.; Dziuda, L.; Rozanowski, K. Using Modalmetric Fiber Optic Sensors to Monitor the Activity of the Heart. In Proceedings of the Optical Fibers, Sensors, and Devices for Biomedical Diagnostics and Treatment XI, San Francisco, CA, USA, 16 February 2011; Volume 7894, pp. 1–7. [Google Scholar]
- Mishra, V.; Singh, N.; Tiwari, U.; Kapur, P. Fiber Grating Sensors in Medicine: Current and Emerging Applications. Sens. Actuators Phys. 2011, 167, 279–290. [Google Scholar] [CrossRef]
- Silvestri, S.; Schena, E. Optical-Fiber Measurement Systems for Medical Applications; Optoelectronics—Devices and Applications; IntechOpen: Rome, Italy, 2011; Volume 11. [Google Scholar]
- Tok, S.; de Haan, K.; Tseng, D.; Usanmaz, C.F.; Ceylan Koydemir, H.; Ozcan, A. Early Detection of E. Coli and Total Coliform Using an Automated, Colorimetric and Fluorometric Fiber Optics-Based Device. Lab. Chip 2019, 19, 2925–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Zhao, X.-L.; Li, Z.-H.; Zhu, Z.-G.; Qian, S.-H.; Flewitt, A. Current and Emerging Technology for Continuous Glucose Monitoring. Sensors 2017, 17, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Najafi, B.; Mohseni, H.; Grewal, G.S.; Talal, T.K.; Menzies, R.A.; Armstrong, D.G. An Optical-Fiber-Based Smart Textile (Smart Socks) to Manage Biomechanical Risk Factors Associated With Diabetic Foot Amputation. J. Diabetes Sci. Technol. 2017, 11, 668–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rifat, A.A.; Ahmed, R.; Yetisen, A.K.; Butt, H.; Sabouri, A.; Mahdiraji, G.A.; Yun, S.H.; Adikan, F.R.M. Photonic Crystal Fiber Based Plasmonic Sensors. Sens. Actuators B Chem. 2017, 243, 311–325. [Google Scholar] [CrossRef]
- Dass, S.; Jha, R. Microfiber-Wrapped Bi-Conical-Tapered SMF for Curvature Sensing. IEEE Sens. J. 2016, 16, 3649–3652. [Google Scholar] [CrossRef]
- Dash, J.N.; Dass, S.; Jha, R. Photonic Crystal Fiber Microcavity Based Bend and Temperature Sensor Using Micro Fiber. Sens. Actuators Phys. 2016, 244, 24–29. [Google Scholar] [CrossRef]
- Dass, S.; Jha, R. Micron Wire Assisted Inline Mach-Zehnder Interferometric Curvature Sensor. IEEE Photonics Technol. Lett. 2015, 28, 1. [Google Scholar] [CrossRef]
- Yhuwana, Y.G.Y.; Apsari, R.; Yasin, M. Fiber Optic Sensor for Heart Rate Detection. Optik 2017, 134, 28–32. [Google Scholar] [CrossRef]
- Fiber Optic Sensors: An Introduction for Engineers and Scientists, 2nd ed.; Available online: https://www.wiley.com/en-cl/Fiber+Optic+Sensors%3A+An+Introduction+for+Engineers+and+Scientists%2C+2nd+Edition-p-9780470126844 (accessed on 10 February 2021).




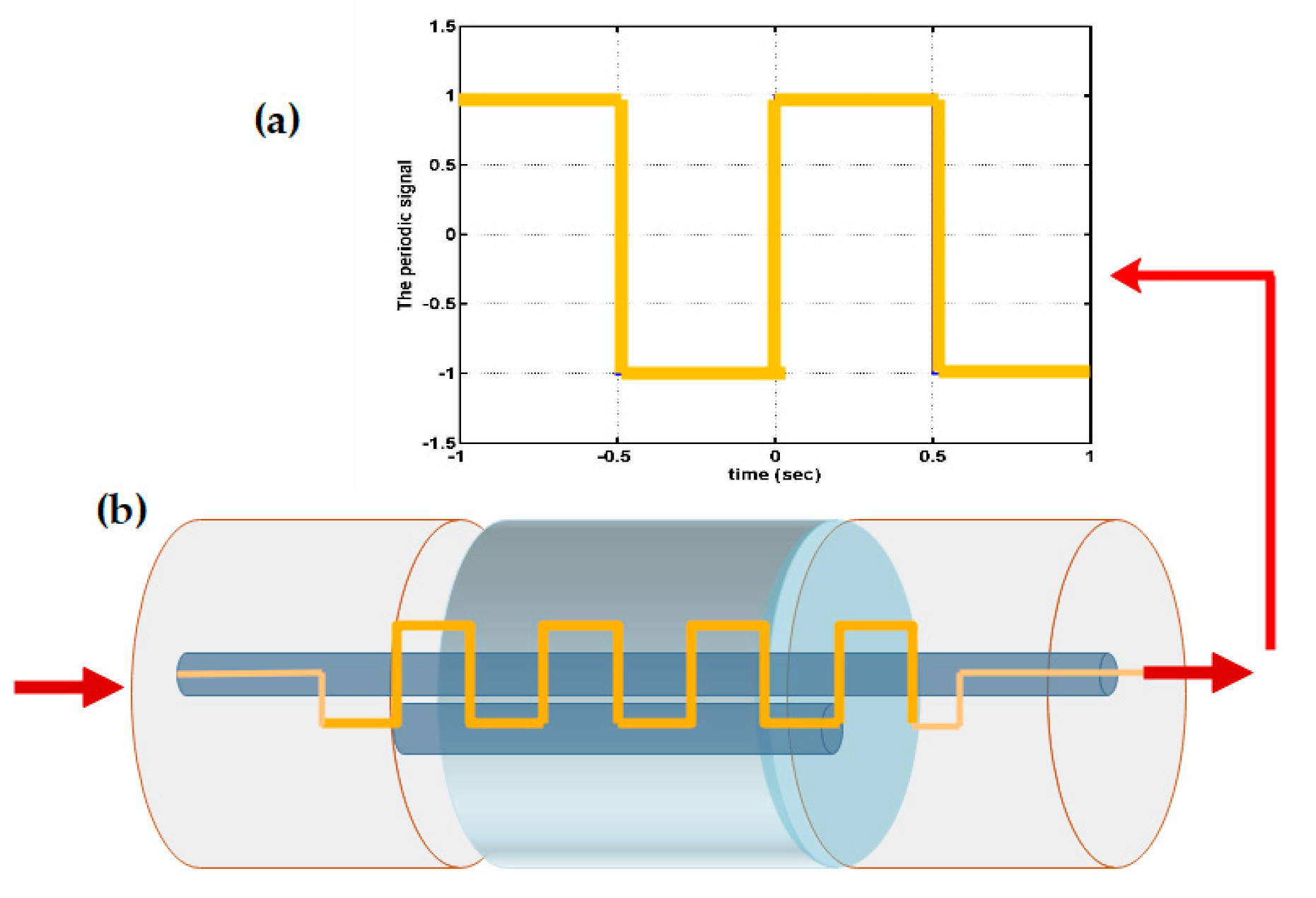

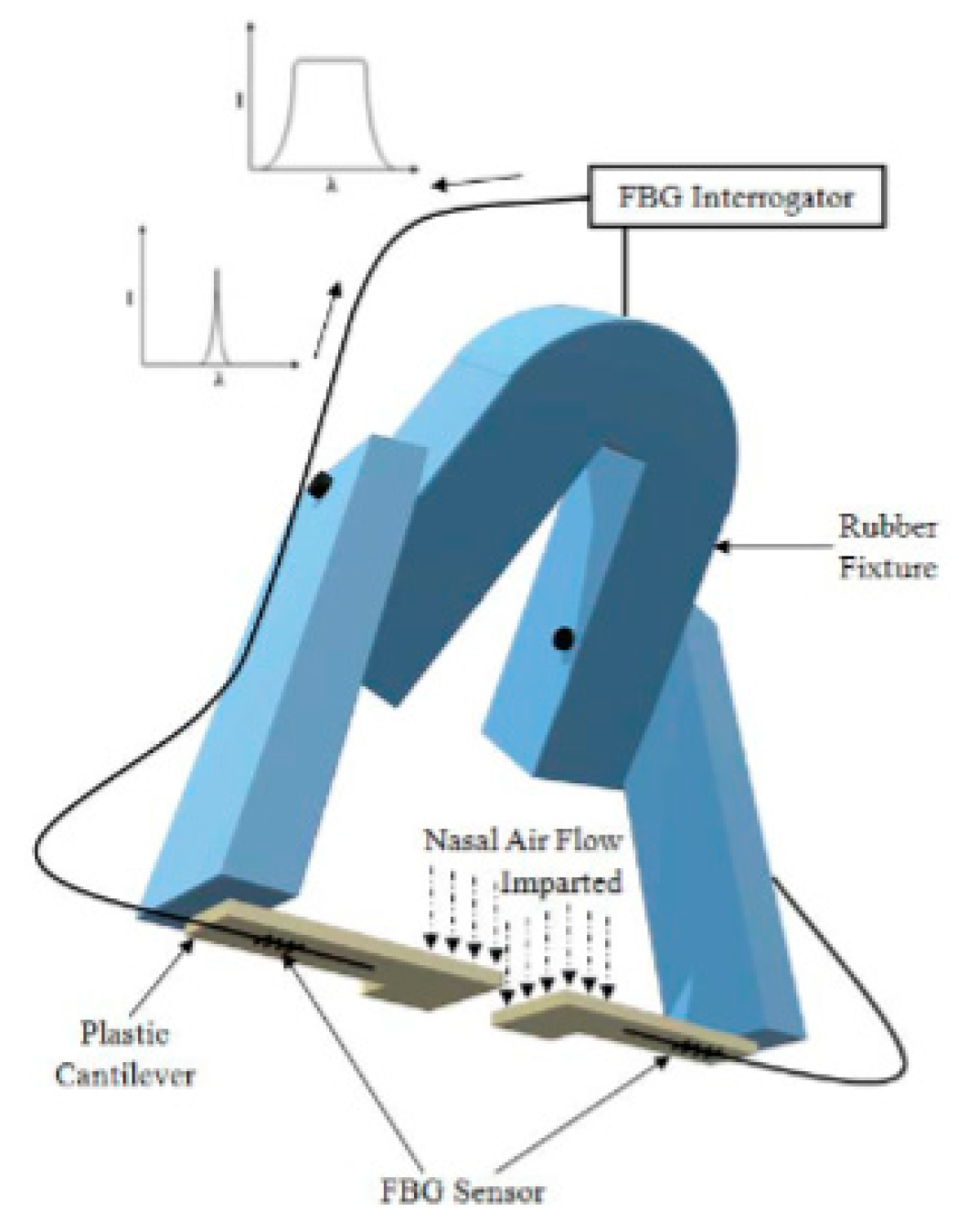
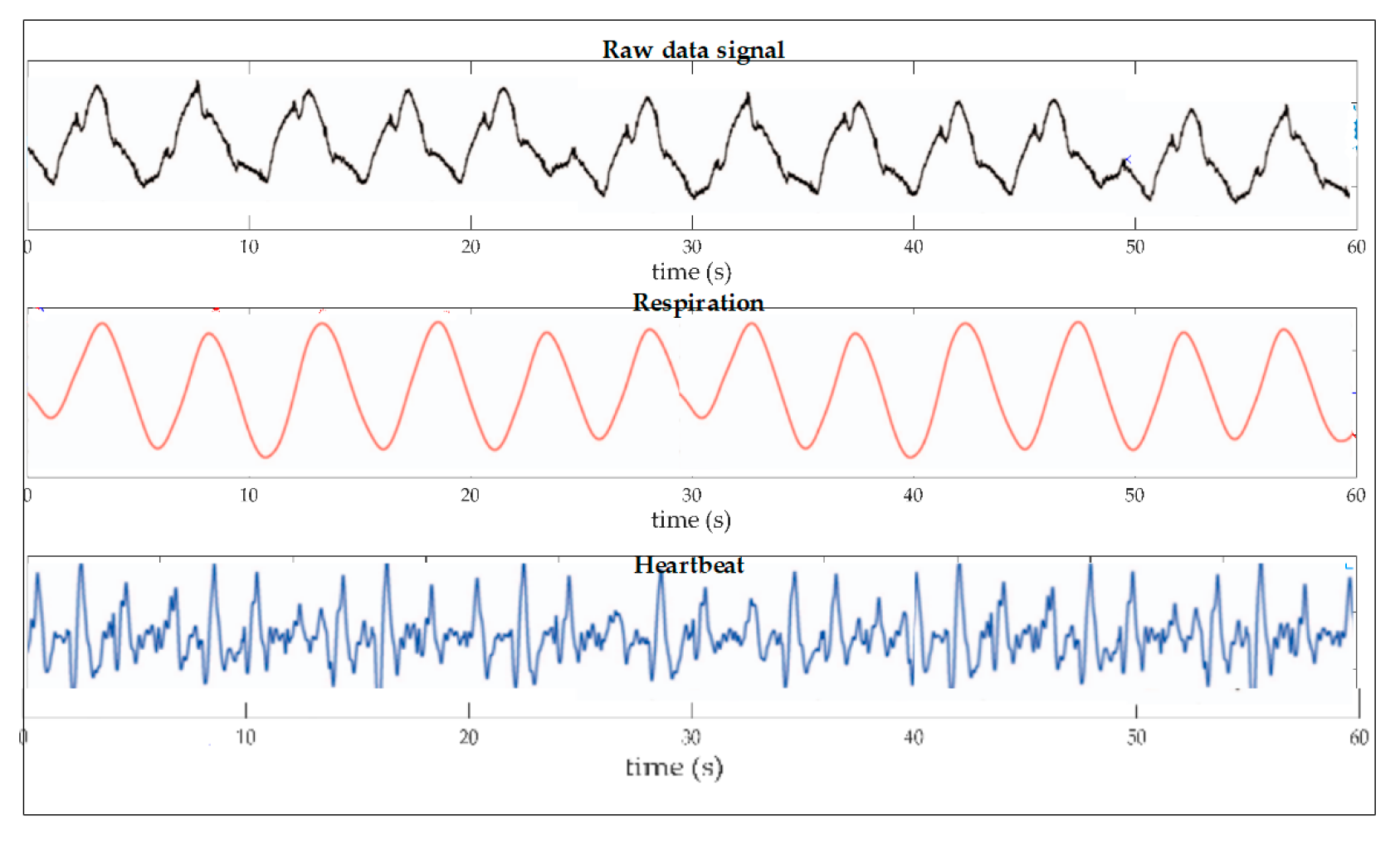
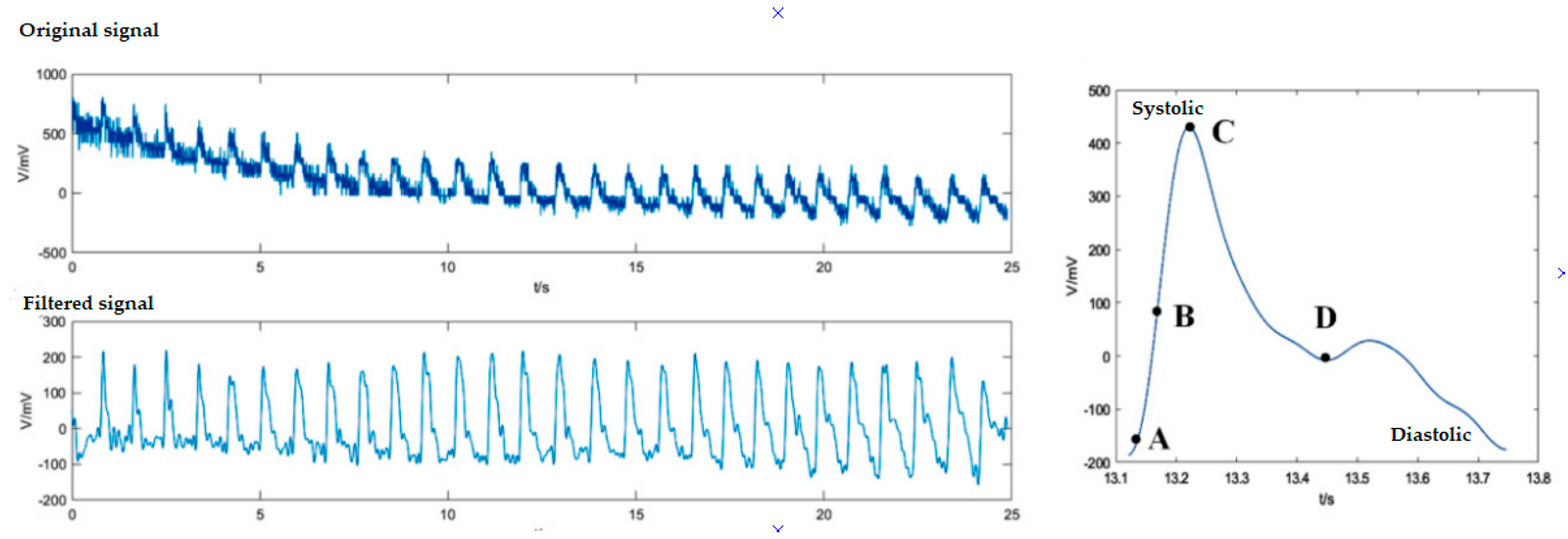

| Fiber Type and Sensor Operating Wavelength (nm) | Sensor Tested on | Sensitivity (nm/°C) | Sensor Technology | Detection Ranges (°C) | Ref. |
|---|---|---|---|---|---|
| SMF 1550 | Thorax, chest wall | 0.31 | FBG Encapsulated in PDMS | 33 to 37 | [6] |
| SMF 1561.07 to 1561.467 | Nose | 0.0114 | FBG | 10 to 44.8 | [14] |
| SMF 1557 and 1627 | Heating tube filled with water | 0.95 to 1.03 | LPFG H and KrF excimer laser | 20 to 100 | [15] |
| SMF and Photonic hollow core 1495.4 and 1520.3 | Temperature chamber | 0.0119 to 0.0138 | LPFG Electric arc discharges | 30 to 80 | [56] |
| SMF 1515 and 1547 | Climatic chamber | 0.41066 and 0.40509 | LPFG NaOH, KOH, H2SO4, H2O, C3H4O2, NaCl, C3H8ClN. argon-ion laser (244 nm—UV) | 25 to 85 | [57] |
| SMF and Non-core 1588 | Thermostatic furnace | -0.00643 | LPFG | 34 to 154 | [58] |
| MMF 630 | distilled water | -9.52519E-05 | SPR sensor Au film | 30 to 70 | [59] |
| POF 1552.45–1552.65 | Chest | −0.055 | He–Cd laser for inscription, taped fiber, DPDS dopant and UV irradiation | 20 to 50 | [60] |
| SMF 1550 | Thorax and neck | - | FBG | 35.5 to 39.5 | [61] |
| SMF 1554.1207 1512.5 to 1587.5 | Chest | 0.010378 to 0.03844 | FBG, encapsulated on PDMS, Sylgard 184 | 35.5 to 37 | [62] |
| 1550.218 and 1550.208 | Thorax | 10.3 to 11.3 | FBG, acrylic and fiberglass encapsulated | 20 to 90 | [63] |
| Fiber Type and Sensor Operating Wavelength (nm) | Sensor Test Position | Respiratory Rate—Breaths per Minute BR—(bpm) | Sensor Technology | Error or Variation (%) | Ref. |
|---|---|---|---|---|---|
| SMF 1550 | Thorax, chest wall | 13.4 to 19.5 men 14.5 to 19 women | FBG Encapsulated in PDMS | ±1.96 | [6] |
| SMF | Tricuspid area and carotid artery | 18 | MZI, FBG | - | [7] |
| SMF 1550 | Baby upper abdominal area | 10 to 100 | Curves on the fiber | 0.25 | [55] |
| SMF 1510 | Thorax, torso, and abdomen | 10 | LPFG | 6 to 8 | [56] |
| SMF 1547.77 | Thorax and abdomen (precordium area) | 9 to 11 | FBG Bending effects OTDR | 10 | [58] |
| SMF, hetero-core 1310 | Smart textile for the upper abdominal area | 16 | Macro-bending on the fiber | 1 | [60] |
| SMF 1550 | Thorax and neck | 12 to 24 | FBG | 2 | [61] |
| SMF 1533 and 1557 | Thoraco-abdominal and chest wall surface | 6 to 11 on standing, 5 to 11 on supine | FBG | 0.38 | [62] |
| SMF 1299 and 1548 | Thoraco-abdominal on resuscitation manikin | - | LPFG, FBG | 0.4 and 0.8 | [76] |
| SMF 1470.73 | Human chest wall on supine | 5 to 11 on natural, 6 to 12 on shallow | LPFG | 4.4 to 8.7 and 5.8 to 10.1 | [78] |
| SMF 1532 and 1541 | Upper thorax | 14 | FBG | 8.3 | [81] |
| MMF 1310 nm | Body back | 6 to 14 Average 12.31 | Microbendings on the fiber | 9.8 | [84] |
| Fiber Type and Sensor Operating Wavelength (nm) | Sensor Test Position | Respiratory Rate—Breaths per Minute BR—(bpm) | Sensor Technology | Error or Variation (%) | Ref. |
|---|---|---|---|---|---|
| SMF 1561.07 to 1561.467 | Nose | 12 to 18 Average 15 | FBG created with H | - | [14] |
| POF 1552.45–1552.65 | Chest | 18 | He–Cd laser for inscription, taped fiber, DPDS dopant and UV irradiation | - | [60] |
| SMF 1554.1203 1513.444–1585.787 | Thorax | 16.22 | FBG, PDMS, Sylgard 184 | 3.9 | [62] |
| SMF 1550.218 and 1550.208 | Thorax | 16 | FBG, acrylic and fiberglass for protection | 4.64 | [63] |
| SMF, MMF, TMF | – | 18 | MZI | [69] | |
| SMF 1440 to 1550, best on 1519.95 | Chest of respiratory manikin | – | LPFG, FBG UV and argon-ion laser induced Er doped and core of GeO2/SiO2, inner cladding of SiO2, outer cladding of SiO2/F/P2O5 | - | [79] |
| SMF 1533 to 1553 | Chest wall | 11 to 12 | FBG polymeric glue and POF | 0.3 | [83] |
| SMF | Chest | Average of 17 | FBG, Ge doped fiber | [86] | |
| SMF 1554.1204 nm | Chest | 15.4925 standing, 15.5119 supine, 15.7638 sitting | FBG PDMS for encapsulation, Sylgard 184, CH3Cl as dopant | 4.4 | [89] |
| MMF, ECF 1550 | Back of the body seated | 16 to 20 | FBG Microbending, He-Ne laser, mechanical induction | 15 | [90] |
| SMF 1550 | Back of the body on supine | 14 to 19 | FBG, PDMS | 4.41 | [91] |
| SMF 1536 and 1548 | Nose bridge | 9.64 to 10.76 | FBG Ge doped, strain variations | 1.3 | [92] |
| SMF 1554.1207 nm | Chest | 21.6676 standing 20.6386 seated 14.8741 back | FBG, encapsulated on PDMS, Sylgard 184 | - | [93] |
| Fiber Type and Sensor Operating Wavelength (nm) | Sensor Test Position | Heart Rate—Beats per Minute HR—(BPM) | Sensor Technology | Error or Variation (%) | Ref. |
|---|---|---|---|---|---|
| SMF 1550 | Thorax, chest wall | 64 to 81 men 67 to 98 women | FBG Encapsulated in PDMS | ±1.96 | [6] |
| SMF | Tricuspid area and carotid artery | 61 | MZI, FBG | - | [7] |
| SMF 1550 | Chest | 57.5 | FBG Encapsulated in PDMS | 1.96 | [12] |
| POF 1552.45 - 1552.65 | Chest | 150 | He–Cd laser for inscription, taped fiber, DPDS dopant and UV irradiation | ±2 | [60] |
| SMF 1550 | Thorax and neck | 60 to 120 | FBG | 2 | [61] |
| SMF 1554.1203 1513.444–1585.787 | Thorax | 78.54 | FBG, encapsulated on PDMS, Sylgard 184 | 1.96 | [62] |
| SMF 1550.218 and 1550.208 | Thorax | 74.3 | FBG, acrylic and fiberglass encapsulated | 4.87 | [63] |
| SMF, MMF, TMF | - | 66 | MZI | - | [69] |
| MMF 1310 nm | Body back | 77 to 83 Average 66.55 | Microbendings on the fiber | 0.6 | [84] |
| SMF | Chest | Average of 107 | FBG, Ge doped | - | [86] |
| SMF 1545 to 1555 | Radial artery at the wrist | 51 | MZI, FBG, InGaAs | - | [87] |
| SMF 1554.1207 nm | Chest | 62,8363 standing 61,9159 seated 76,8499 back | FBG, PDMS, Sylgard 184 | - | [93] |
| MMF | Back of the head | 58 to 74 | Microbendings on fiber | 2.7 to 3.44 | [100] |
| SMF 1538.4 to 1538.6 | Back of the body | 76.8 | FBG | < 7.4 | [101] |
| SMF 1549.5 to 1550.5 | Temple (best), finger, ankle (worst) and dorsum pedis | Average of 66.33, 60.33, 60 and 57.66 | FBG, MZI | 1.47 (best) 28.33 (worst) | [102] |
| MMF | Back of the body | 84 | Mechanical induction | 7.31 | [103] |
| POF 950 | Neck and chest | 68 (best) and 52 (worst) | PDMS and plastic polymer macro-bending and strain | - | [104] |
| SMF 1550 nm | Wrist | 66 | Er 12µm thick Al diaphragm | 5 | [105] |
| Fiber Type and Sensor Operating Wavelength (nm) | Sensor Test Position | Blood Pressure (mmHg) | Sensor Technology | Error or Variation (mmHg) | Ref. |
|---|---|---|---|---|---|
| SMF 1549.5 to 1550.5 | Temple (best), finger, ankle(worst) and dorsum pedis | 131/73.5 average | MZI, FBG | ± 3 | [102] |
| SMF 1550 | Wrist | 116.5/71.75 average | Er, Al diaphragm | - | [105] |
| SMF 550 to 700 | On a goat left ventricle, left atrium, right atrium, and aorta | –100 to 400 | FPI, PI, C8H20O4Si | ± 4 | [110] |
| SMF, MMF 1547.5 | In-vivo coronary artery of a swine | 54 to 88 in aortic arch 60 to 100 in right coronary artery | FPI cavity with HF, SiO2 diaphragm | - | [111] |
| SMF 1549.5 to 1550.5 1559.5 to 1560.5 | Neck and ankle | 106 to 119 and 109 to 122 | MZI, FBG | 7 and 5 on systolic | [112] |
| SMF, MMF | Tortuous vessels of a swine model in-vivo | 54 to 88 | FPI, stretched core MMF, SiO2 diaphragm | - | [113] |
| SMF 1549.5 to 1550.5 | Right wrist | 110.7 supine 107.3 sitting 103.8 standing | MZI, FBG, SiO2, InGaAs | 3 supine 2.8 sitting 3.8 standing | [114] |
| SMF 1525 to 1575 | Wrists | 123.6 average with no cover 119.2 average with cover | FBG | 2 no covered 6 covered | [115] |
| POF 15543 and 1553 | Left arm | 106.5/65 average | FBG, glue NORLAND 78 | 5/3 | [116] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perezcampos Mayoral, C.; Gutiérrez Gutiérrez, J.; Cano Pérez, J.L.; Vargas Treviño, M.; Gallegos Velasco, I.B.; Hernández Cruz, P.A.; Torres Rosas, R.; Tepech Carrillo, L.; Arnaud Ríos, J.; Apreza, E.L.; et al. Fiber Optic Sensors for Vital Signs Monitoring. A Review of Its Practicality in the Health Field. Biosensors 2021, 11, 58. https://doi.org/10.3390/bios11020058
Perezcampos Mayoral C, Gutiérrez Gutiérrez J, Cano Pérez JL, Vargas Treviño M, Gallegos Velasco IB, Hernández Cruz PA, Torres Rosas R, Tepech Carrillo L, Arnaud Ríos J, Apreza EL, et al. Fiber Optic Sensors for Vital Signs Monitoring. A Review of Its Practicality in the Health Field. Biosensors. 2021; 11(2):58. https://doi.org/10.3390/bios11020058
Chicago/Turabian StylePerezcampos Mayoral, Christian, Jaime Gutiérrez Gutiérrez, José Luis Cano Pérez, Marciano Vargas Treviño, Itandehui Belem Gallegos Velasco, Pedro António Hernández Cruz, Rafael Torres Rosas, Lorenzo Tepech Carrillo, Judith Arnaud Ríos, Edmundo López Apreza, and et al. 2021. "Fiber Optic Sensors for Vital Signs Monitoring. A Review of Its Practicality in the Health Field" Biosensors 11, no. 2: 58. https://doi.org/10.3390/bios11020058
APA StylePerezcampos Mayoral, C., Gutiérrez Gutiérrez, J., Cano Pérez, J. L., Vargas Treviño, M., Gallegos Velasco, I. B., Hernández Cruz, P. A., Torres Rosas, R., Tepech Carrillo, L., Arnaud Ríos, J., Apreza, E. L., & Rojas Laguna, R. (2021). Fiber Optic Sensors for Vital Signs Monitoring. A Review of Its Practicality in the Health Field. Biosensors, 11(2), 58. https://doi.org/10.3390/bios11020058








