Siphon-Controlled Automation on a Lab-on-a-Disc Using Event-Triggered Dissolvable Film Valves
Abstract
:1. Introduction
2. Materials and Methods
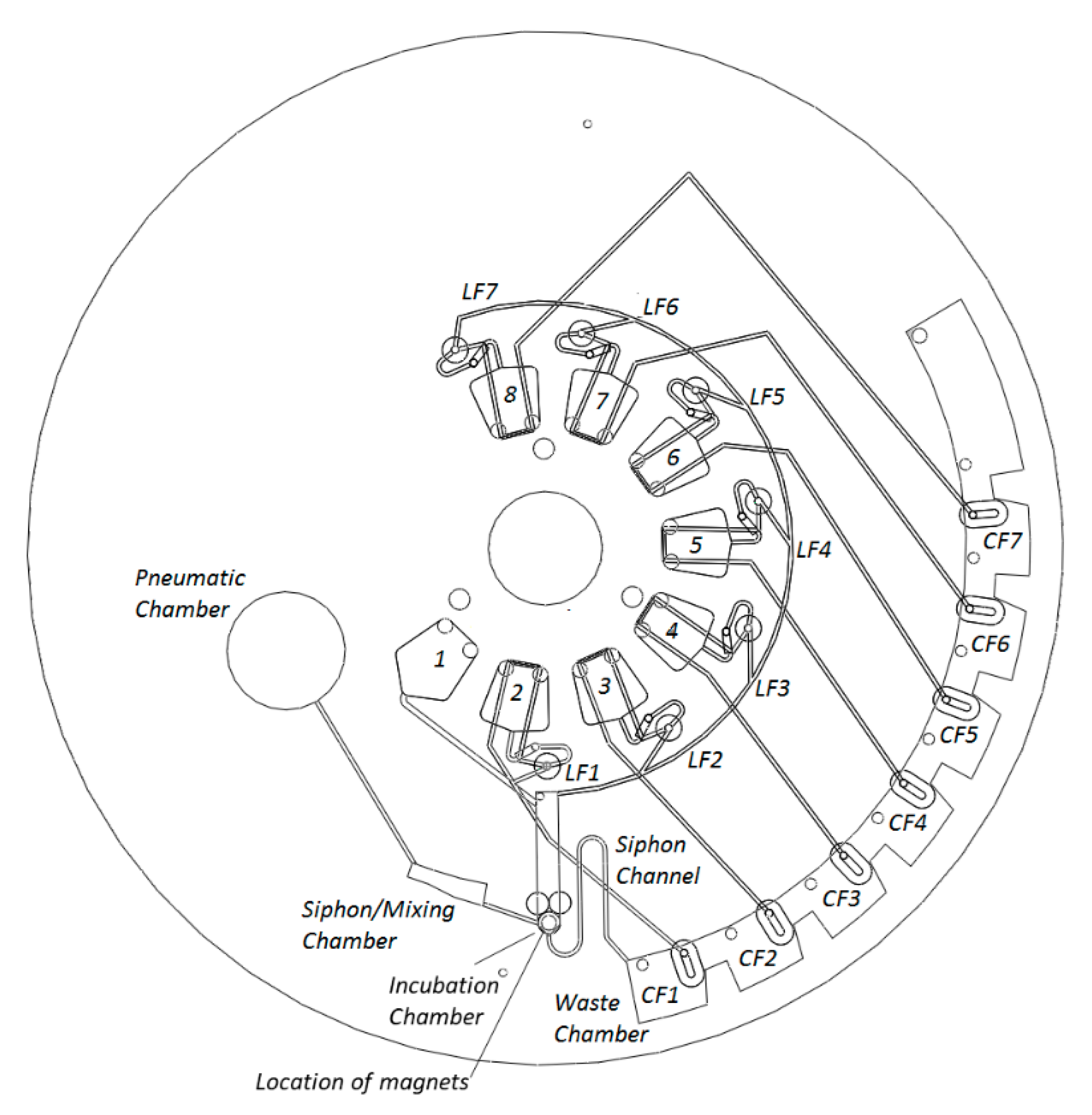
2.1. Disc Architecture
2.2. Disc Manufacture and Assembly
- (1)
- Vent layer of PMMA, containing loading ports/air vents;
- (2)
- Microchannel layer of PSA, containing microchannels for reagent and air transport;
- (3)
- Reservoir layer of PMMA, containing reagent reservoirs, waste chambers, pneumatic chambers, incubation chamber, and connecting vertical vias;
- (4)
- DF cover layer (PSA), which seals DF tabs into the disc;
- (5)
- DF support layer (PSA), which provides alignment and mechanical support for DF tabs;
- (6)
- Intermediate layer (PMMA) provides mechanical support for DFs;
- (7)
- Lower channels (PSA), containing microchannels for reagent and air transport; and
- (8)
- Base (PMMA) provides a layer to seal the lower channels. This layer also contains mechanical support for permanent magnets.
2.3. Centrifugal Test Stand
2.4. Biological Assay Materials
2.5. Dynabead Antibody Coupling Procedure
2.6. Benchtop Magnetic Chemiluminescence Assay
2.7. Lab-on-a-Disc Magnetic Chemiluminescent Assay
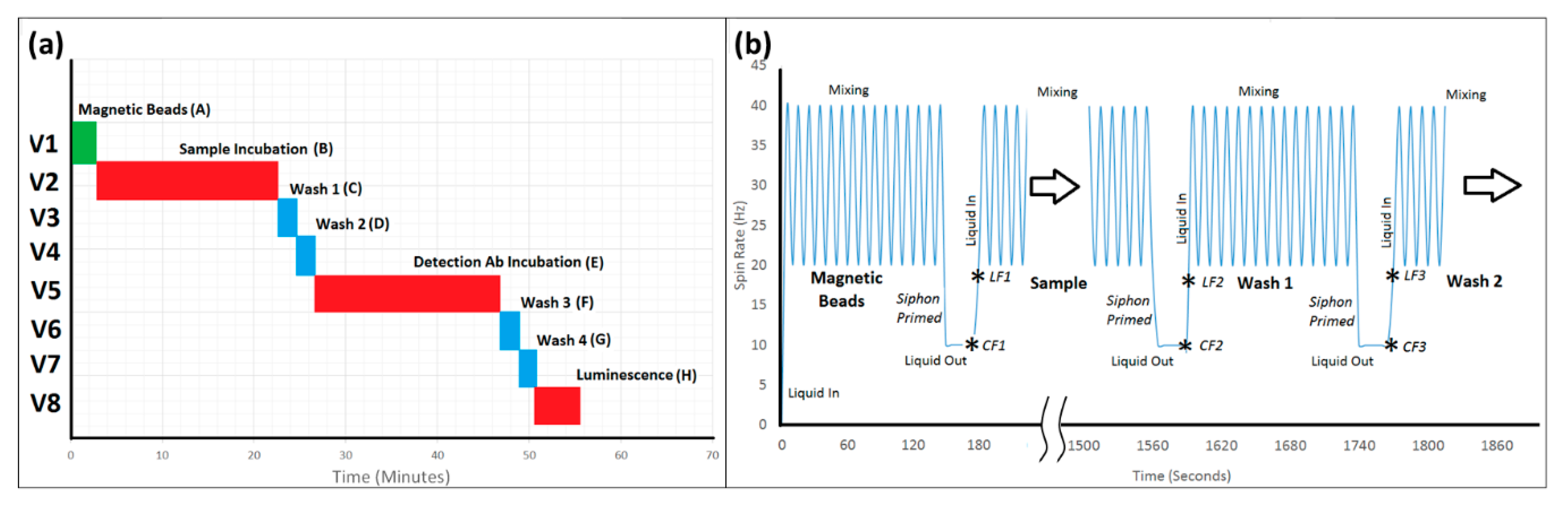
2.8. Automated Lab-on-a-Disc Protocol
3. Results
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glezeva, N.; Gallagher, J.; Ledwidge, M.; O’Donoghue, J.; McDonald, K.; Chipolombwe, J.; Watson, C. Heart failure in sub-Saharan Africa: Review of the aetiology of heart failure and the role of point-of-care biomarker diagnostics. Trop. Med. Int. Health 2015, 20, 581–588. [Google Scholar] [CrossRef]
- Damasceno, A.; Cotter, G.; Dzudie, A.; Sliwa, K.; Mayosi, B.M. Heart failure in sub-Saharan Africa: Time for action. J. Am. Coll. Cardiol. 2007, 50, 1688–1693. [Google Scholar] [CrossRef] [Green Version]
- McDonnell, B.; Hearty, S.; Leonard, P.; O’Kennedy, R. Cardiac biomarkers and the case for point-of-care testing. Clin. Biochem. 2009, 42, 549–561. [Google Scholar] [CrossRef]
- Wu, J.; Dong, M.; Santos, S.; Rigatto, C.; Liu, Y.; Lin, F. Lab-on-a-chip platforms for detection of cardiovascular disease and cancer biomarkers. Sensors 2017, 17, 2934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, C.D.; Linder, V.; Sia, S.K. Lab-on-a-chip devices for global health: Past studies and future opportunities. Lab Chip 2007, 7, 41–57. [Google Scholar] [CrossRef]
- Mohammed, M.-I.; Desmulliez, M.P.Y. Lab-on-a-chip based immunosensor principles and technologies for the detection of cardiac biomarkers: A review. Lab Chip 2011, 11, 569–595. [Google Scholar] [CrossRef]
- Christodoulides, N.; Floriano, P.N.; Acosta, S.A.; Ballard, K.L.M.; Weigum, S.E.; Mohanty, S.; Dharshan, P.; Romanovicz, D.; McDevitt, J.T. Toward the development of a lab-on-a-chip dual-function leukocyte and C-reactive protein analysis method for the assessment of inflammation and cardiac risk. Clin. Chem. 2005, 51, 2391–2395. [Google Scholar] [CrossRef] [Green Version]
- Adams, J.; Apple, F. New blood tests for detecting heart disease. Circulation 2004, 109, e12–e14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czilwik, G.; Vashist, S.K.; Klein, V.; Buderer, A.; Roth, G.; von Stetten, F.; Zengerle, R.; Mark, D. Magnetic chemiluminescent immunoassay for human C-reactive protein on the centrifugal microfluidics platform. RSC Adv. 2015, 5, 61906–61912. [Google Scholar] [CrossRef]
- Lagrand, W.K.; Visser, C.A.; Hermens, W.T.; Niessen, H.W.M.; Verheugt, F.W.A.; Wolbink, G.-J.; Hack, C.E. C-reactive protein as a cardiovascular risk factor: More than an epiphenomenon? Circulation 1999, 100, 96–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yousuf, O.; Mohanty, B.D.; Martin, S.S.; Joshi, P.H.; Blaha, M.J.; Nasir, K.; Blumenthal, R.S.; Budoff, M.J. High-sensitivity C-reactive protein and cardiovascular disease: A resolute belief or an elusive link? J. Am. Coll. Cardiol. 2013, 62, 397–408. [Google Scholar] [CrossRef] [Green Version]
- Madou, M.; Zoval, J.; Jia, G.; Kido, H.; Kim, J.; Kim, N. Lab on a CD. Annu. Rev. Biomed. Eng. 2006, 8, 601–628. [Google Scholar] [CrossRef] [Green Version]
- Strohmeier, O.; Keller, M.; Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugal microfluidic platforms: Advanced unit operations and applications. Chem. Soc. Rev. 2015, 44, 6187–6229. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.X.; Perebikovsky, A.; Moebius, J.; Kulinsky, L.; Madou, M. Lab-on-a-CD: A fully integrated molecular diagnostic system. J. Lab. Autom. 2016, 21, 323–355. [Google Scholar] [CrossRef] [Green Version]
- Gorkin, R.; Park, J.; Siegrist, J.; Amasia, M.; Lee, B.S.; Park, J.-M.; Kim, J.; Kim, H.; Madou, M.; Cho, Y.-K. Centrifugal microfluidics for biomedical applications. Lab Chip 2010, 10, 1758–1773. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Wang, G.; Kong, S.-K.; Ho, H.-P. A review of biomedical centrifugal microfluidic platforms. Micromachines 2016, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, C.M.; Carthy, E.; Kinahan, D.J. Biosensing on the Centrifugal Microfluidic Lab-on-a-Disc Platform. Processes 2020, 8, 1360. [Google Scholar] [CrossRef]
- Nwankire, C.E.; Czugala, M.; Burger, R.; Fraser, K.J.; Glennon, T.; Onwuliri, B.E.; Nduaguibe, I.E.; Diamond, D.; Ducrée, J. A portable centrifugal analyser for liver function screening. Biosens. Bioelectron. 2014, 56, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Van Nguyen, H.; Nguyen, V.D.; Nguyen, H.Q.; Chau, T.H.T.; Lee, E.Y.; Seo, T.S. Nucleic acid diagnostics on the total integrated lab-on-a-disc for point-of-care testing. Biosens. Bioelectron. 2019, 141, 111466. [Google Scholar] [CrossRef]
- Brennan, D.; Coughlan, H.; Clancy, E.; Dimov, N.; Barry, T.; Kinahan, D.; Ducrée, J.; Smith, T.J.; Galvin, P. Development of an on-disc isothermal in vitro amplification and detection of bacterial RNA. Sens. Actuators B Chem. 2017, 239, 235–242. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.S.; Lee, J.-N.; Park, J.-M.; Lee, J.-G.; Kim, S.; Cho, Y.-K.; Ko, C. A fully automated immunoassay from whole blood on a disc. Lab Chip 2009, 9, 1548–1555. [Google Scholar] [CrossRef]
- Zhao, Y.; Czilwik, G.; Klein, V.; Mitsakakis, K.; Zengerle, R.; Paust, N. C-reactive protein and interleukin 6 microfluidic immunoassays with on-chip pre-stored reagents and centrifugo-pneumatic liquid control. Lab Chip 2017, 17, 1666–1677. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.-J.; Park, J.; Sunkara, V.; Kim, T.-H.; Lee, Y.; Lee, K.; Kim, M.-H.; Cho, Y.-K. Fully automated, on-site isolation of cfDNA from whole blood for cancer therapy monitoring. Lab Chip 2018, 18, 1320–1329. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, M.S.; Moon, H.-S.; Yoo, C.E.; Park, D.; Kim, Y.J.; Han, K.-Y.; Lee, J.-Y.; Oh, J.H.; Kim, S.S.; et al. Fully automated circulating tumor cell isolation platform with large-volume capacity based on lab-on-a-disc. Anal. Chem. 2014, 86, 3735–3742. [Google Scholar] [CrossRef] [PubMed]
- Kirby, D.; Glynn, M.; Kijanka, G.; Ducrée, J. Rapid and cost-efficient enumeration of rare cancer cells from whole blood by low-loss centrifugo-magnetophoretic purification under stopped-flow conditions. Cytom. Part A 2015, 87, 74–80. [Google Scholar] [CrossRef]
- Kong, M.C.R.; Salin, E.D. Spectrophotometric determination of aqueous sulfide on a pneumatically enhanced centrifugal microfluidic platform. Anal. Chem. 2012, 84, 10038–10043. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, Y.; Cho, J.; Lee, J.; Choi, M.-S.; Cho, Y.-K. Lab-on-a-Disc for Simultaneous Determination of Nutrients in Water. Anal. Chem. 2013, 85, 2954–2960. [Google Scholar] [CrossRef]
- Czugala, M.; Gorkin, R., III; Phelan, T.; Gaughran, J.; Curto, V.F.; Ducrée, J.; Diamond, D.; Benito-Lopez, F. Optical sensing system based on wireless paired emitter detector diode device and ionogels for lab-on-a-disc water quality analysis. Lab Chip 2012, 12, 5069–5078. [Google Scholar] [CrossRef] [Green Version]
- Torres Delgado, S.M.; Kinahan, D.J.; Nirupa Julius, L.A.; Mallette, A.; Ardila, D.S.; Mishra, R.; Miyazaki, C.M.; Korvink, J.G.; Ducrée, J.; Mager, D. Wirelessly powered and remotely controlled valve-array for highly multiplexed analytical assay automation on a centrifugal microfluidic platform. Biosens. Bioelectron. 2018, 109, 214–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Cordero, J.L.; Barrett, L.M.; O’Kennedy, R.; Ricco, A.J. Microfluidic sedimentation cytometer for milk quality and bovine mastitis monitoring. Biomed. Microdevices 2010, 12, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhu, Y.; Shen, M.; Lu, Y.; Cheng, J.; Xu, Y. Rapid and automated detection of six contaminants in milk using a centrifugal microfluidic platform with two rotation axes. Anal. Chem. 2019, 91, 7958–7964. [Google Scholar] [CrossRef]
- Steigert, J.; Brenner, T.; Grumann, M.; Riegger, L.; Lutz, S.; Zengerle, R.; Ducrée, J. Integrated siphon-based metering and sedimentation of whole blood on a hydrophilic lab-on-a-disk. Biomed. Microdevices 2007, 9, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.; Weber, P.; Lutz, S.; Focke, M.; Zengerle, R.; von Stetten, F. Aliquoting on the centrifugal microfluidic platform based on centrifugo-pneumatic valves. Microfluid. Nanofluid. 2011, 10, 1279–1288. [Google Scholar] [CrossRef]
- Burger, R.; Kinahan, D.J.; Cayron, H.; Reis, N.; Fonseca, J.; Ducrée, J. Siphon-Induced Droplet Break-Off for Enhanced Mixing on a Centrifugal Platform. Inventions 2020, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Dimov, N.; Clancy, E.; Gaughran, J.; Boyle, D.; Mc Auley, D.; Glynn, M.T.; Dwyer, R.M.; Coughlan, H.; Barry, T.; Barrett, L.M.; et al. Solvent-selective routing for centrifugally automated solid-phase purification of RNA. Microfluid. Nanofluid. 2015, 18, 859–871. [Google Scholar] [CrossRef] [Green Version]
- Cox, J.O.; DeCarmen, T.S.; Ouyang, Y.; Strachan, B.; Sloane, H.; Connon, C.; Gibson, K.; Jackson, K.; Landers, J.P.; Cruz, T.D. A novel, integrated forensic microdevice on a rotation-driven platform: Buccal swab to STR product in less than 2 h. Electrophoresis 2016, 37, 3046–3058. [Google Scholar] [CrossRef] [PubMed]
- Clime, L.; Daoud, J.; Brassard, D.; Malic, L.; Geissler, M.; Veres, T. Active pumping and control of flows in centrifugal microfluidics. Microfluid. Nanofluid. 2019, 23, 29. [Google Scholar] [CrossRef]
- Ukita, Y.; Takamura, Y.; Utsumi, Y. Water-clock-based autonomous flow sequencing in steadily rotating centrifugal microfluidic device. Sens. Actuators B Chem. 2015, 220, 180–183. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Kearney, S.M.; Dimov, N.; Glynn, T.; Ducrée, J. Event-triggered logical flow control for comprehensive process integration of multi-step assays on centrifugal microfluidic platforms. Lab Chip 2014, 14, 2249–2258. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.M.; Huang, P.-C.; Lin, M.-G. Analysis and experiment of capillary valves for microfluidics on a rotating disk. Microfluid. Nanofluid. 2008, 4, 427–437. [Google Scholar] [CrossRef]
- Moore, J.L.; McCuiston, A.; Mittendorf, I.; Ottway, R.; Johnson, R.D. Behavior of capillary valves in centrifugal microfluidic devices prepared by three-dimensional printing. Microfluid. Nanofluid. 2011, 10, 877–888. [Google Scholar] [CrossRef]
- Kitsara, M.; Nwankire, C.E.; Walsh, L.; Hughes, G.; Somers, M.; Kurzbuch, D.; Zhang, X.; Donohoe, G.G.; O’Kennedy, R.; Ducrée, J. Spin coating of hydrophilic polymeric films for enhanced centrifugal flow control by serial siphoning. Microfluid. Nanofluid. 2014, 16, 691–699. [Google Scholar] [CrossRef]
- Siegrist, J.; Gorkin, R.; Clime, L.; Roy, E.; Peytavi, R.; Kido, H.; Bergeron, M.; Veres, T.; Madou, M. Serial siphon valving for centrifugal microfluidic platforms. Microfluid. Nanofluid. 2010, 9, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Nwankire, C.E.; Donohoe, G.G.; Zhang, X.; Siegrist, J.; Somers, M.; Kurzbuch, D.; Monaghan, R.; Kitsara, M.; Burger, R.; Hearty, S. At-line bioprocess monitoring by immunoassay with rotationally controlled serial siphoning and integrated supercritical angle fluorescence optics. Anal. Chim. Acta 2013, 781, 54–62. [Google Scholar] [CrossRef]
- McArdle, H.; Jimenez-Mateos, E.M.; Raoof, R.; Carthy, E.; Boyle, D.; ElNaggar, H.; Delanty, N.; Hamer, H.; Dogan, M.; Huchtemann, T.; et al. “TORNADO”—Theranostic One-Step RNA Detector; microfluidic disc for the direct detection of microRNA-134 in plasma and cerebrospinal fluid. Sci. Rep. 2017, 7, 1750. [Google Scholar] [CrossRef]
- Gorkin, R.; Clime, L.; Madou, M.; Kido, H. Pneumatic pumping in centrifugal microfluidic platforms. Microfluid. Nanofluid. 2010, 9, 541–549. [Google Scholar] [CrossRef] [Green Version]
- Godino, N.; Gorkin, R., III; Linares, A.V.; Burger, R.; Ducrée, J. Comprehensive integration of homogeneous bioassays via centrifugo-pneumatic cascading. Lab Chip 2013, 13, 685–694. [Google Scholar] [CrossRef]
- Zhao, Y.; Schwemmer, F.; Zehnle, S.; von Stetten, F.; Zengerle, R.; Paust, N. Centrifugo-pneumatic sedimentation, re-suspension and transport of microparticles. Lab Chip 2015, 15, 4133–4137. [Google Scholar] [CrossRef]
- Schwemmer, F.; Zehnle, S.; Mark, D.; von Stetten, F.; Zengerle, R.; Paust, N. A microfluidic timer for timed valving and pumping in centrifugal microfluidics. Lab Chip 2015, 15, 1545–1553. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, C.M.; Kinahan, D.J.; Mishra, R.; Mangwanya, F.; Kilcawley, N.; Ferreira, M.; Ducrée, J. Label-free, spatially multiplexed SPR detection of immunoassays on a highly integrated centrifugal Lab-on-a-Disc platform. Biosens. Bioelectron. 2018, 119, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kinahan, D.J.; Mangwanya, F.; Garvey, R.; Chung, D.W.Y.; Lipinski, A.; Julius, L.A.N.; King, D.; Mohammadi, M.; Mishra, R.; Al-Ofi, M.; et al. Automation of silica bead-based nucleic acid extraction on a centrifugal lab-on-a-disc platform. Proc. J. Phys. Conf. Ser. 2016, 757, 12013. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Kearney, S.M.; Kilcawley, N.A.; Early, P.L.; Glynn, M.T.; Ducrée, J. Density-gradient mediated band extraction of leukocytes from whole blood using centrifugo-pneumatic siphon valving on centrifugal microfluidic discs. PLoS ONE 2016, 11, e0155545. [Google Scholar] [CrossRef]
- Deng, Y.; Fan, J.; Zhou, S.; Zhou, T.; Wu, J.; Li, Y.; Liu, Z.; Xuan, M.; Wu, Y. Euler force actuation mechanism for siphon valving in compact disk-like microfluidic chips. Biomicrofluidics 2014, 8, 24101. [Google Scholar] [CrossRef] [Green Version]
- Pishbin, E.; Eghbal, M.; Fakhari, S.; Kazemzadeh, A.; Navidbakhsh, M. The Effect of Moment of Inertia on the Liquids in Centrifugal Microfluidics. Micromachines 2016, 7, 215. [Google Scholar] [CrossRef] [Green Version]
- Arjmand, E.M.; Saadatmand, M.; Bakhtiari, M.R.; Eghbal, M. Design and fabrication of a centrifugal microfluidic disc including septum valve for measuring hemoglobin A1c in human whole blood using immunoturbidimetry method. Talanta 2018, 190, 134–139. [Google Scholar] [CrossRef]
- Van Oordt, T.; Barb, Y.; Smetana, J.; Zengerle, R.; von Stetten, F. Miniature stick-packaging–an industrial technology for pre-storage and release of reagents in lab-on-a-chip systems. Lab Chip 2013, 13, 2888–2892. [Google Scholar] [CrossRef]
- Hwang, H.; Kim, H.-H.; Cho, Y.-K. Elastomeric membrane valves in a disc. Lab Chip 2011, 11, 1434–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorkin, R., III; Nwankire, C.E.; Gaughran, J.; Zhang, X.; Donohoe, G.G.; Rook, M.; O’Kennedy, R.; Ducrée, J. Centrifugo-pneumatic valving utilizing dissolvable films. Lab Chip 2012, 12, 2894–2902. [Google Scholar] [CrossRef]
- Mishra, R.; Zapatero-Rodriguez, J.; Sharma, S.; Kelly, D.; McAuley, D.; Gilgunn, S.; O’Kennedy, R.; Ducrée, J. Automation of multi-analyte prostate cancer biomarker immunoassay panel from whole blood by minimum-instrumentation rotational flow control. Sens. Actuators B Chem. 2018, 263, 668–675. [Google Scholar] [CrossRef]
- Kinahan, D.J.; Kearney, S.M.; Faneuil, O.P.; Glynn, M.T.; Dimov, N.; Ducrée, J. Paper imbibition for timing of multi-step liquid handling protocols on event-triggered centrifugal microfluidic lab-on-a-disc platforms. RSC Adv. 2015, 5, 1818–1826. [Google Scholar] [CrossRef] [Green Version]
- Kinahan, D.J.; Early, P.L.; Vembadi, A.; MacNamara, E.; Kilcawley, N.A.; Glennon, T.; Diamond, D.; Brabazon, D.; Ducrée, J. Xurography actuated valving for centrifugal flow control. Lab Chip 2016, 16, 3454–3459. [Google Scholar] [CrossRef] [PubMed]
- Lenk, G.; Stemme, G.; Roxhed, N. Delay valving in capillary driven devices based on dissolvable thin films. Present. Micro TAS 2014, 2014, 216–218. [Google Scholar]
- Lutz, B.; Liang, T.; Fu, E.; Ramachandran, S.; Kauffman, P.; Yager, P. Dissolvable fluidic time delays for programming multi-step assays in instrument-free paper diagnostics. Lab Chip 2013, 13, 2840–2847. [Google Scholar] [CrossRef] [PubMed]
- Houghtaling, J.; Liang, T.; Thiessen, G.; Fu, E. Dissolvable bridges for manipulating fluid volumes in paper networks. Anal. Chem. 2013, 85, 11201–11204. [Google Scholar] [CrossRef] [Green Version]
- Dai, M.; Senecal, A.; Nugen, S.R. Electrospun water-soluble polymer nanofibers for the dehydration and storage of sensitive reagents. Nanotechnology 2014, 25, 225101. [Google Scholar] [CrossRef]
- Lenk, G.; Stemme, G.; Roxhed, N. Dry reagent storage in dissolvable films and liquid triggered release for programmed multi-step lab-on-chip diagnostics. In Proceedings of the 2015 28th IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Estoril, Portugal, 18–22 January 2015; pp. 451–454. [Google Scholar]
- Berger, M.; Müller, T.; Voebel, T.; Baum, C.; Glennon, T.; Mishra, R.; Kinahan, D.; King, D.; Ducrée, J.; Brecher, C. Automated assembly of microfluidic “lab-on-a-disc”. In Proceedings of the Microfluidics, BioMEMS, and Medical Microsystems XVI, San Francisco, CA, USA, 21 February 2018; Volume 10491, p. 1049109. [Google Scholar]
- Al Mughairy, B.; Al-Lawati, H.A.J. Recent analytical advancements in microfluidics using chemiluminescence detection systems for food analysis. TrAC Trends Anal. Chem. 2020, 124, 115802. [Google Scholar] [CrossRef]
- Qiu, X.; Yang, S.; Wu, D.; Wang, D.; Qiao, S.; Ge, S.; Xia, N.; Yu, D.; Qian, S. Rapid enumeration of CD4+ T lymphocytes using an integrated microfluidic system based on Chemiluminescence image detection at point-of-care testing. Biomed. Microdevices 2018, 20, 15. [Google Scholar] [CrossRef]
- Min, X.; Fu, D.; Zhang, J.; Zeng, J.; Weng, Z.; Chen, W.; Zhang, S.; Zhang, D.; Ge, S.; Zhang, J.; et al. An automated microfluidic chemiluminescence immunoassay platform for quantitative detection of biomarkers. Biomed. Microdevices 2018, 20, 91. [Google Scholar] [CrossRef]
- Wang, Z.; Chin, S.Y.; Chin, C.D.; Sarik, J.; Harper, M.; Justman, J.; Sia, S.K. Microfluidic CD4+ T-cell counting device using chemiluminescence-based detection. Anal. Chem. 2009, 82, 36–40. [Google Scholar] [CrossRef]
- Honrado, C.; Dong, T. Development and optimization of an integrated capillary-based opto-microfluidic device for chemiluminescence quantitative detection. J. Micromech. Microeng. 2014, 24, 125023. [Google Scholar] [CrossRef]
- Hu, B.; Li, J.; Mou, L.; Liu, Y.; Deng, J.; Qian, W.; Sun, J.; Cha, R.; Jiang, X. An automated and portable microfluidic chemiluminescence immunoassay for quantitative detection of biomarkers. Lab Chip 2017, 17, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Delgado, S.M.T.; Kinahan, D.J.; Sandoval, F.S.; Julius, L.A.N.; Kilcawley, N.A.; Ducrée, J.; Mager, D. Fully automated chemiluminescence detection using an electrified-Lab-on-a-Disc (eLoaD) platform. Lab Chip 2016, 16, 4002–4011. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Xu, H.; Li, Z.; Wang, G. A Centrifugal Chemiluminescence Detection Platform and Its Application for Nucleic Acid Virus (H7N9). In Proceedings of the 2019 IEEE 4th Optoelectronics Global Conference (OGC), Shenzhen, China, 3–6 September 2019; pp. 140–143. [Google Scholar]
- Lee, W.S.; Sunkara, V.; Han, J.-R.; Park, Y.-S.; Cho, Y.-K. Electrospun TiO2 nanofiber integrated lab-on-a-disc for ultrasensitive protein detection from whole blood. Lab Chip 2015, 15, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Riegger, L.; Steigert, J.; Grumann, M.; Lutz, S.; Olofsson, G.; Khayyami, M.; Bessler, W.; Mittenbuehler, K.; Zengerle, R.; Ducrée, J. Disk-based parallel chemiluminescent detection of diagnostic markers for acute myocardial infarction. In Proceedings of the 10th International Conference on Miniaturized Systems for Chemistry and Life Sciences (µTAS2006), Tokyo, Japan, 5–9 November 2006; Volume 1, pp. 819–821. [Google Scholar]
- Gijs, M.A.M. Magnetic bead handling on-chip: New opportunities for analytical applications. Microfluid. Nanofluid. 2004, 1, 22–40. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.-W.; Oh, K.W.; Thomas, J.H.; Heineman, W.R.; Halsall, H.B.; Nevin, J.H.; Helmicki, A.J.; Henderson, H.T.; Ahn, C.H. An integrated microfluidic biochemical detection system for protein analysis with magnetic bead-based sampling capabilities. Lab Chip 2002, 2, 27–30. [Google Scholar] [CrossRef]
- Miyazaki, C.M.; Mishra, R.; Kinahan, D.J.; Ferreira, M.; Ducrée, J. Polyethylene imine/graphene oxide layer-by-layer surface functionalization for significantly improved limit of detection and binding kinetics of immunoassays on acrylate surfaces. Colloids Surf. B Biointerfaces 2017, 158, 167–174. [Google Scholar] [CrossRef]




| Name and Operation | Advantages | Disadvantages | Refs |
|---|---|---|---|
| Capillary Valves are actuated by increasing the disc spin-rate. They function based on the balance of body forces (governed by relative centrifugal force) and interfacial tension holding the liquid in place. | Simple operation and ease of manufacture. | Cannot operate at high disc speeds. Highly dependent on manufacturing fidelity. Low number of assay steps. | [40,41] |
| Capillary-action primed siphon valves are low-pass valves. Thy are triggered by reducing the disc spin-rate which allows capillary priming of a siphon. They can be combined in series (with capillary valves) to enable actuation by sequentially increasing and decreasing disc spin speed. | Simple operation and ease of manufacture. Can enable sample incubation. | Highly dependent on manufacturing fidelity. Low number of assay steps. Can use significant disc real-estate. | [32,42,43,44,45] |
| Centrifugo-pneumatic siphon valves (CPSV) function in a manner similar to siphon valves except the release of compressed air (trapped during loading of a reservoir) primes the siphon rather than the capillary force. | Simple operation and ease of manufacture. Can enable sample incubation. Reliable and tolerant to low-fidelity manufacture. | Can use up significant disc real-estate. | [46,47,48,49,50,51,52] |
| Acceleration Actuated valves incorporate disc features (over-flow structures, siphon valves) which are activated when the disc is rapidly accelerated or decelerated. | Highly reliable. No external instrumentation required—rotational control only. | Can require a powerful motor to generate necessary acceleration (Euler Force). Can use significant disc real-estate. | [53,54,55] |
| Deformable membranes/burstable foils are integrated into the disc during manufacture and can be tuned to open at a predetermined disc spin-rate (liquid body-forces overcomes the seal created by the membrane/foil). | Highly reliable. No external instrumentation required–rotational control only. Timing of valve actuations. | Requires additional components (integration of foils or stick-packs etc.) Complex assays limited by available motor speeds. Single use valves. Difficult to implement long incubations. | [56,57] |
| Dissolvable Film (DF) (Rotational Pulse) use integrated water-dissolvable films which are recessed into trapped gas pockets. The disc spin speed at which the liquid can be forced into the gas pocket (to wet and dissolve the DF) in inversely proportional to the size of the gas pocket. This relationship permits precise design of valve opening frequencies. | Highly reliable. No external instrumentation required–rotational control only. Timing of valve actuations. | Requires multilayer architecture. Requires embedded DF valves. Complex assays limited by available motor speeds. Single use valves. Difficult to implement long incubations. | [58,59] |
| Water-clock valves use liquid movement to sequentially vent channels which release air-locks intentionally designed into the disc architecture. This allows liquid rellease in a sequential pre-determined cascade while the disc rotates at a constant disc speed. | Sequential Valve Opening. Ease of Manufacture. | Operates only at low to medium disc speeds. Can take significant disc space. No timing of valve release. No long incubations/washes. | [39] |
| Dissolvable Film (Event-triggered) use a network of pneumatic channels which are blocked by dissolvable films. Dissolving a film at one point on the disc can trigger release of liquid through a DF located elsewhere on the disc. This allows liquid rellease in a sequential pre-determined cascade while the disc rotates at a constant disc speed. | Permits complex multi-step assays (20+ steps). Suitable for high disc spin-speeds. | Requires multilayer architecture. Requires embedded DF valves. No timing of valve release. No long incubations/washes. Single use valves. | [38,60] |
| Dissolvable Film (Event-triggered with instrumentation) incorporates the event-triggered architecture except the actuation of valves is through external actions such as piercing a tape or melting a wax film. | Permits complex multi-step assays (60+ steps). Suitable for high disc spin-speeds. Feedback control possible. | Requires multilayer architecture. Requires embedded DF valves. No long incubations/washes. Single use valves. Requires support instrumentation, | [29,61] |
| Dissolvable Film (Event-triggered with Siphon Control) are described in Figure 1 | Permits complex multi-step assays. Suitable for high disc spin-speeds. Timing of valve opening/incubations using only rotational control. | Requires multilayer architecture. Requires embedded DF valves. Single use valves (except siphon). | - |
| Assay Step | Reagent | Incubation Time (mins) | Mixing Cycles |
|---|---|---|---|
| Bead Capture (1) | 0.5 mg Pre-blocked magnetic beads in Incubation Buffer | 2.5 | 15 |
| CRP Incubation (2) | C-Reactive protein Standard/Sample suspended in Incubation Buffer | 20 | 120 |
| Wash 1 (3) | Wash Solution | 2.5 | 15 |
| Wash 2 (4) | Wash Solution | 2.5 | 15 |
| Detection Antibody (5) | 1:10,000 dilution in Incubation Buffer | 20 | 120 |
| Wash 3 (6) | Wash Solution | 2.5 | 15 |
| Wash 4 (7) | Wash Solution | 2.5 | 15 |
| Chemiluminescent Substrate (8) | N/A | N/A | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henderson, B.D.; Kinahan, D.J.; Rio, J.; Mishra, R.; King, D.; Torres-Delgado, S.M.; Mager, D.; Korvink, J.G.; Ducrée, J. Siphon-Controlled Automation on a Lab-on-a-Disc Using Event-Triggered Dissolvable Film Valves. Biosensors 2021, 11, 73. https://doi.org/10.3390/bios11030073
Henderson BD, Kinahan DJ, Rio J, Mishra R, King D, Torres-Delgado SM, Mager D, Korvink JG, Ducrée J. Siphon-Controlled Automation on a Lab-on-a-Disc Using Event-Triggered Dissolvable Film Valves. Biosensors. 2021; 11(3):73. https://doi.org/10.3390/bios11030073
Chicago/Turabian StyleHenderson, Brian D., David J. Kinahan, Jeanne Rio, Rohit Mishra, Damien King, Sarai M. Torres-Delgado, Dario Mager, Jan G. Korvink, and Jens Ducrée. 2021. "Siphon-Controlled Automation on a Lab-on-a-Disc Using Event-Triggered Dissolvable Film Valves" Biosensors 11, no. 3: 73. https://doi.org/10.3390/bios11030073
APA StyleHenderson, B. D., Kinahan, D. J., Rio, J., Mishra, R., King, D., Torres-Delgado, S. M., Mager, D., Korvink, J. G., & Ducrée, J. (2021). Siphon-Controlled Automation on a Lab-on-a-Disc Using Event-Triggered Dissolvable Film Valves. Biosensors, 11(3), 73. https://doi.org/10.3390/bios11030073








