Label-Free and Highly-Sensitive Detection of Ochratoxin A Using One-Pot Synthesized Reduced Graphene Oxide/Gold Nanoparticles-Based Impedimetric Aptasensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Synthesis of 3D-rGO/Au NPs Nanocomposites
2.3. Preparation of the Aptasensor Surface
2.4. Electrochemical Measurement
2.5. Instrumentation
3. Results
3.1. Principles of 3D-rGO/Au NPs Nanocomposites Based Label-Free Aptasensor for OTA
3.2. Optimization and Characterization of 3D-rGO/AuNPs Nanocomposites
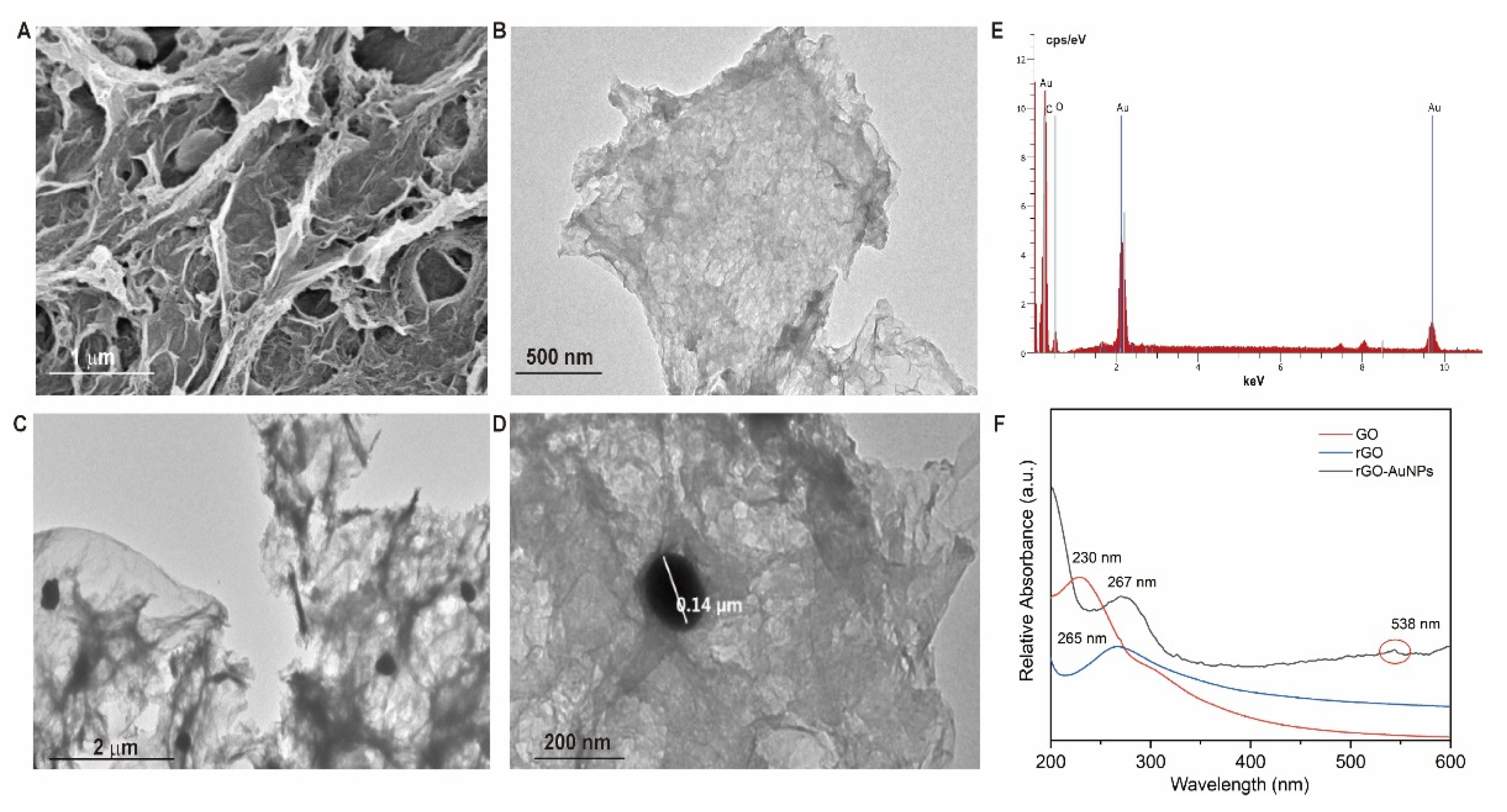
3.2.1. Morphology and Structure Characterization of 3D-rGO/AuNPs Nanocomposites
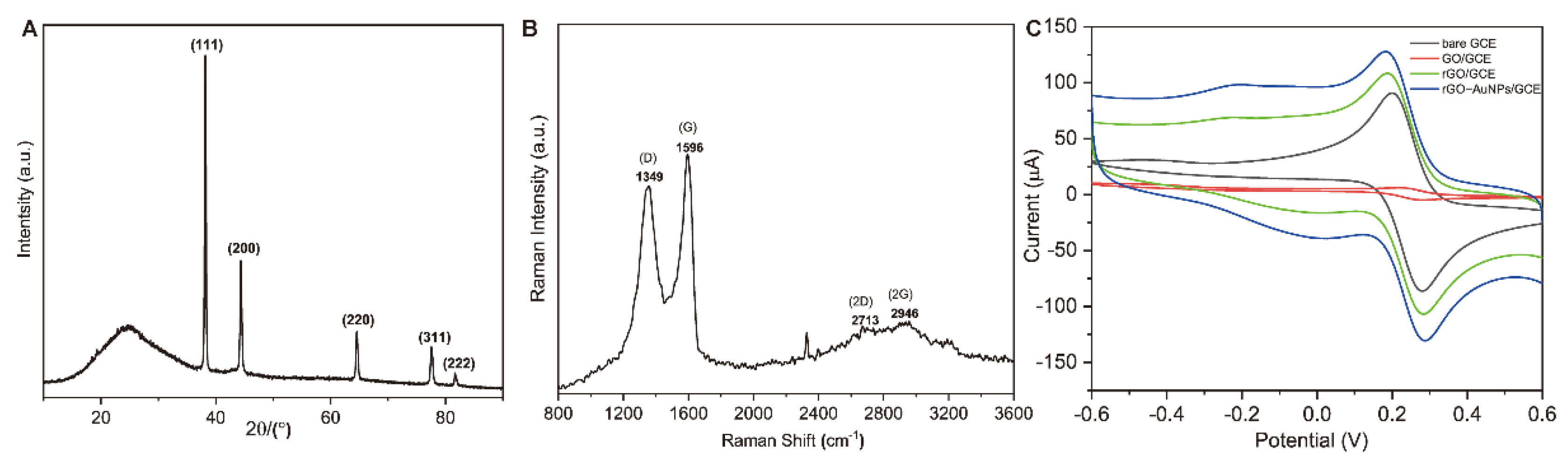
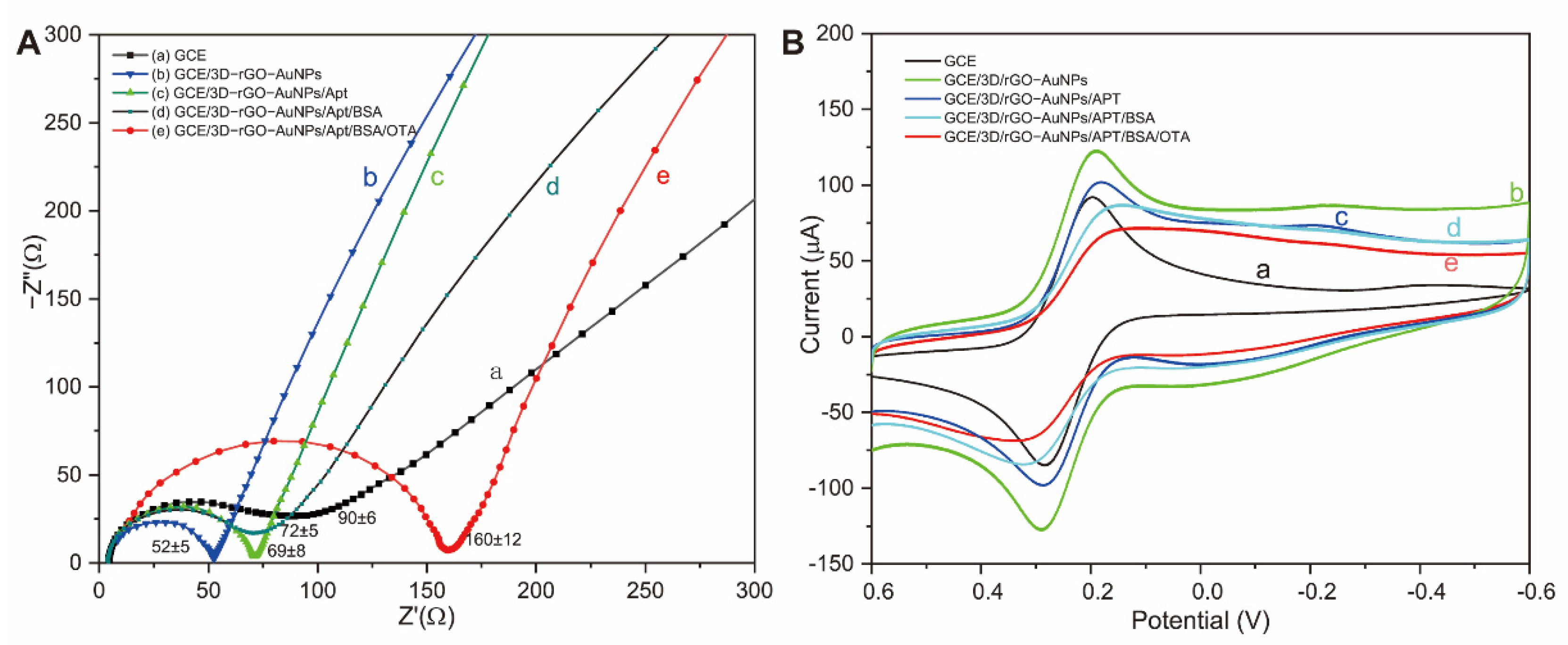
3.2.2. Electrochemical Characterization of the Aptasensor
- (1)
- EIS measurements
- (2)
- Cyclic voltammetry (CV)
3.3. Optimization of Aptasensing Parameters
3.4. Analytical Performance of Electrochemical Aptasensors for OTA
3.5. Selectivity and Reproducibility of the Electrochemical Aptasensors
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Iqbal, S.Z.; Asi, M.R.; Hanif, U.; Zuber, M.; Jinap, S. The presence of aflatoxins and ochratoxin a in rice and rice products, and evaluation of dietary intake. Food Chem. 2016, 210, 135–140. [Google Scholar] [CrossRef]
- Fernández-Baldo, M.A.; Bertolino, F.A.; Messina, G.A.; Sanz, M.I.; Raba, J. Modified magnetic nanoparticles in an electrochemical method for the ochratoxin A determination in Vitis vinifera red grapes tissues. Talanta 2010, 83, 651–657. [Google Scholar] [CrossRef]
- Yang, J.; Gao, P.; Liu, Y.; Li, R.; Ma, H.; Du, B. Label-free photoelectrochemical immunosensor for sensitive detection of Ochratoxin, A. Biosens. Bioelectron. 2015, 64, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Qi, X.; Zheng, J.; Luo, Y.; Zhao, C.; Hao, J.; Li, X.; Huang, K.; Xu, W. Lipid Rafts Disruption Increases Ochratoxin A Cytotoxicity to Hepatocytes. J. Biochem. Mol. Toxicol. 2015, 30, 71–79. [Google Scholar] [CrossRef]
- Armutcu, C.; Uzun, L.; Denizli, A. Determination of Ochratoxin A traces in foodstuffs: Comparison of an automated on-line two-dimensional high-performance liquid chromatography and off-line immunoaffinity-high-performance liquid chromatography system. J. Chromatogr. A 2018, 1569, 139–148. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, S.; Lee, J.; Kim, B. Accurate determination of ochratoxin A in Korean fermented soybean paste by isotope dilution-liquid chromatography tandem mass spectrometry. Food Chem. 2016, 190, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, G.H.; Xu, X.; Zhu, L.; Huang, R.M.; Chen, X.Q. Application of nano-ELISA in food analysis: Recent advances and challenges. Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Jiang, L.; Qian, J.; Yang, X.W.; Yan, Y.T.; Liu, Q.; Wang, K. Amplified impedimetric aptasensor based on gold nanoparticles covalently bound graphene sheet for the picomolar detection of ochratoxin A. Anal. Chim. Acta 2014, 806, 128–135. [Google Scholar] [CrossRef]
- Chen, W.; Yan, C.; Cheng, L.; Yao, L.; Xue, F.; Xu, J.G. An ultrasensitive signal-on electrochemical aptasensor for ochratoxin A determination based on DNA controlled layer-by-layer assembly of dual gold nanoparticle conjugates. Biosens. Bioelectron. 2018, 117, 845–851. [Google Scholar] [CrossRef]
- Rhouati, A.; Yang, C.; Hayat, A.; Marty, J.L. Aptamers: A promising tool for ochratoxin A detection in food analysis. Toxins 2013, 5, 1988–2008. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, W.Y. A novel impedimetric aptasensor based on AuNPs–carboxylic porous carbon for the ultrasensitive detection of ochratoxin A. RSC Adv. 2017, 7, 28655. [Google Scholar] [CrossRef] [Green Version]
- Hayat, A.; Andreescu, S.; Marty, J.L. Design of PEG-aptamer two-piece macromolecules as convenient and integrated sensing platform: Application to the label free detection of small size molecules. Biosens. Bioelectron. 2013, 45, 168–173. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, Y.; Arsalan, M.; Yang, S.; Wang, Y.; Sheng, Q.; Yue, T. A Label-free aptasensor for the detection of ochratoxin a based on competitive molecule-level interactions. J. Electrochem. Soc. 2020, 167, 147518. [Google Scholar] [CrossRef]
- de Almeida, P.R.; Murad, A.M.; Silva, L.P.; Rech, E.L.; Alves, E.S. Development of a Graphene-Based Biosensor for Detecting Recombinant Cyanovirin-N. Biosensors 2020, 10, 206. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.P.; Zhu, J.H.; Huang, L. A review of three-dimensional graphene-based materials: Synthesis and applications to energy conversion/storage and environment. Carbon 2019, 143, 610–640. [Google Scholar] [CrossRef]
- Qiu, H.J.; Guan, Y.X.; Luo, P.; Wang, Y. Recent advance in fabricating monolithic 3D porous graphene and their applications in biosensing and biofuel cells. Biosens. Bioelectron. 2017, 89, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Zhong, H.G.; Chen, M.; Zhao, C.; Liu, Y.; Xi, F.N.; Luo, T. Functional nanostructure-loaded three-dimensional graphene foam as a non-enzymatic electrochemical sensor for reagentless glucose detection. RSC Adv. 2020, 10, 33739–33746. [Google Scholar] [CrossRef]
- Shi, L.; Wang, Y.; Ding, S.M.; Chu, Z.Y.; Yin, Y.; Jiang, D.F.; Luo, J.Y.; Jin, W.Q. A facile and green strategy for preparing newly-designed 3D graphene/gold film and its application in highly efficient electrochemical mercury assay. Biosens. Bioelectron. 2017, 89, 871–879. [Google Scholar] [CrossRef]
- You, M.; Yang, S.; Tang, W.X.; Zhang, F.; He, P.G. Molecularly imprinted polymers-based electrochemical DNA biosensor for the determination of BRCA-1 amplified by SiO2@Ag. Biosens. Bioelectron. 2018, 112, 72–78. [Google Scholar] [CrossRef]
- Perumal, V.; Saheed, M.S.M.; Mohamed, N.M.; Saheed, M.S.M.; Murthe, S.S.; Gopinath, S.C.B.; Chiu, J.M. Gold nanorod embedded novel 3D graphene nanocomposite for selective bio-capture in rapid detection of Mycobacterium tuberculosis. Biosens. Bioelectron. 2018, 116, 116–122. [Google Scholar] [CrossRef]
- Yue, H.Y.; Zhang, H.; Huang, S.; Lin, X.Y.; Gao, X.; Chang, J.; Yao, L.H.; Guo, E.J. Synthesis of ZnO nanowire arrays/3D graphene foam and application for determination of levodopa in the presence of uric acid. Biosens. Bioelectron. 2017, 89, 592–597. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Akbarian, F.; Heydari-Soureshjani, E.; Rezaei, B. A novel aptasensor based on 3D-reduced graphene oxide modified gold nanoparticles for determination of arsenite. Biosens. Bioelectron. 2018, 122, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Reiner-Rozman, C.; Hageneder, S.; Dubiak-Szepietowska, M.; Dostalek, J.; Feller, K.H. Fast and sensitive detection of ochratoxin A in red wine by nanoparticle-enhanced SPR. Anal. Chim. Acta 2016, 937, 143–150. [Google Scholar] [CrossRef]
- Al-Ani, L.A.; AlSaadi, M.A.; Kadir, F.A.; Hashim, N.M.; Julkapli, N.M.; Yehye, W.A. Graphene- gold based nanocomposites applications in cancer diseases; Efficient detection and therapeutic tools. Eur. J. Med. Chem. 2017, 139, 349–366. [Google Scholar] [CrossRef] [PubMed]
- Kasturi, S.; Eom, Y.; Torati, S.R.; Kim, C.G. Highly sensitive electrochemical biosensor based on naturally reduced rGO/Au nanocomposite for the detection of miRNA-122 biomarker. J. Ind. Eng. Chem. 2021, 93, 186–195. [Google Scholar] [CrossRef]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-Based Composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef]
- Guo, W.; Wu, L.; Fan, K.; Nie, D.; He, W.; Yang, J.; Zhao, Z.; Han, Z. Reduced graphene oxide-gold nanoparticle nanoframework as a highly selective separation material for aflatoxins. Sci. Rep. 2017, 7, 14484. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Jiang, L.; Yang, X.W.; Yan, Y.T.; Mao, H.P.; Wang, K. Highly sensitive impedimetric aptasensor based on covalent binding of gold nanoparticles on reduced graphene oxide with good dispersity and high density. Analyst 2014, 139, 5587–5593. [Google Scholar] [CrossRef]
- Wu, X.; Xing, Y.; Pierce, D.; Zhao, J.X. One-Pot Synthesis of Reduced Graphene Oxide/Metal (Oxide) Composites. ACS Appl. Mater. Interfaces 2017, 9, 37962–37971. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, X.; Liu, F.; Cheng, Y.; Shi, A.D. Effective reduction of nonspecific binding by surface engineering of quantum dots with bovine serum albumin for cell-targeted imaging. Langmuir 2012, 28, 16605–16613. [Google Scholar] [CrossRef]
- Lichtenberg, J.Y.; Ling, Y.; Kim, S. Non-Specific Adsorption Reduction Methods in Biosensing. Sensors 2019, 19, 2488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Peng, L.; Yuan, C.; Wang, J.; Mo, S.; Zhao, C. Facile synthesis of 3d reduced graphene oxide and its polyaniline composite for super capacitor application. Synth. Met. 2015, 202, 140–146. [Google Scholar] [CrossRef]
- Shen, J.F.; Yan, B.; Shi, M.; Ma, H.W.; Li, N.; Ye, M.X. One step hydrothermal synthesis of TiO2-reduced graphene oxide sheets. J. Mater. Chem. 2011, 21, 3415–3421. [Google Scholar] [CrossRef]
- Yang, S.; Yue, W.; Huang, D.; Chen, C.; Lin, H.; Yang, X. A facile green strategy for rapid reduction of graphene oxide by metallic zinc. RSC Adv. 2012, 2, 8827–8832. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Aepuru, R.; Panda, H.S.; Bahadur, D. Ice-templated synthesis of multifunctional three-dimensional graphene/noble metal nanocomposites and their mechanical, electrical, catalytic, and electromagnetic shielding properties. Sci. Rep. 2015, 5, 17726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, X.L.; Li, Z.Q.; Yin, L.W. Metal-organic frameworks derived porous core/shellCoP@C polyhedrons anchored on 3D reduced graphene oxide networks as anode for sodium- ion battery. Nano Energy 2017, 32, 117–124. [Google Scholar] [CrossRef]
- Guo, D.M.; Cai, P.J.; Sun, J.; He, W.N.; Wu, X.H.; Zhang, T.; Wang, X.; Zhang, X.T. Reduced-graphene-oxide/metal-oxide p-n heterojunction aerogels as efficient 3D sensing frameworks for phenol detection. Carbon 2016, 99, 571–578. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; An, S.; Choe, S.R.; Kwak, C.H.; Huh, Y.S.; Lee, J.; Han, Y.K. Fabrication of 3D honeycomb-like porous polyurethane-functionalized reduced graphene oxide for detection of dopamine. Biosens. Bioelectron. 2016, 86, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Rashid, J.I.A.; Vayithiswary, K.; Muhammad, H.A.; Aye, A.M.; Safura, T.; Azizi, M.; Keat, K.O.; Nor Azah, Y. An electrochemical sensor based on gold nanoparticles-functionalized reduced graphene oxide screen printed electrode for the detection of pyocyanin biomarker in Pseudomonas aeruginosa infection. Mat. Sci. Eng. C 2021, 120, 111625. [Google Scholar] [CrossRef]
- Zamfir, L.G.; Puiu, M.; Bala, C. Advances in Electrochemical Impedance Spectroscopy Detection of Endocrine Disruptors. Sensors 2020, 20, 6443. [Google Scholar] [CrossRef]
- Rivas, L.; Mayorga-Martinez, C.C.; Quesada-González, D.; Zamora-Gálvez, A.; de la Escosura-Muñiz, A.; Merkoçi, A. Label-free impedimetric aptasensor for ochratoxin-A detection using iridium oxide nanoparticles. Anal. Chem. 2015, 87, 5167–5172. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Rong, X.J.; Wang, Y.; Ding, S.M.; Tang, W.Y. High-performance and versatile electrochemical aptasensor based on self-supported nanoporous gold microelectrode and enzyme-induced signal amplification. Biosens. Bioelectron. 2018, 102, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Suea-Ngam, A.; Howes, P.D.; Stanley, C.E.; de Mello, A.J. An exonuclease I-assisted silver-metallized electrochemical aptasensor for ochratoxin a detection. ACS Sens. 2019, 4, 1560–1568. [Google Scholar] [CrossRef]
- Nan, M.; Bi, Y.; Xue, H.L.; Xue, S.L.; Long, H.T.; Pu, L.M.; Fu, G. Rapid determination of ochratoxin A in grape and its commodities based on a label-free impedimetric aptasensor constructed by layer-by-layer self-assembly. Toxins 2019, 11, 71. [Google Scholar] [CrossRef] [Green Version]
- Tozlovanu, M.; Pfohl-Leszkowicz, A. Ochratoxin A in roasted coffee from French supermarkets and transfer in coffee beverages: Comparison of analysis methods. Toxins 2010, 2, 1928–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, A.L.; Zhang, Y.F.; Sun, G.P.; Wang, X.N.; Tang, D.P. Homogeneous electrochemical detection of ochratoxin A in foodstuff using aptamer–graphene oxide nanosheets and DNaseI-based target recycling reaction. Biosens. Bioelectron. 2018, 89, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Alhamoud, Y.; Yang, D.; Kenston, S.S.F.; Liu, G.; Liu, L.; Zhou, H.; Ahmed, F.; Zhao, J. Advances in biosensors for the detection of ochratoxin A: Bio-receptors, nanomaterials, and their applications. Biosens. Bioelectron. 2019, 141, 111418. [Google Scholar] [CrossRef] [PubMed]
- Lyu, H.; Sun, H.; Zhu, Y.; Wang, J.; Xie, Z.; Li, J. A double-recognized aptamer-molecularly imprinted monolithic column for high-specificity recognition of ochratoxin A. Anal. Chim. Acta 2020, 1103, 97–105. [Google Scholar] [CrossRef]
- Chi, J.; Chen, M.; Deng, L.; Lin, X.; Xie, Z. A facile AuNPs@ aptamer-modified mercaptosiloxane-based hybrid affinity monolith with an unusually high coverage density of aptamer for on-column selective extraction of ochratoxin A. Analyst 2018, 143, 5210–5217. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, X.; Zhu, D.; Lin, X.; Xie, Z. Preparation and evaluation of highly hydrophilic aptamer-based hybrid affinity monolith for on-column specific discrimination of ochratoxin A. Talanta 2019, 200, 193–202. [Google Scholar] [CrossRef]




| Transducer | Linear Range (ng·mL−1) | LOD (ng·mL−1) | Reference |
|---|---|---|---|
| Thionine and IrO2 NPs modified SPCE | 0.004~40 | 5.6 × 10−3 | [41] |
| Au-ATP-rGO composite modified Au Electrode | 0.1~200 | 0.03 | [28] |
| Self-supported np-Au microelectrode | 0.01~5 | 5 × 10−3 | [42] |
| Layer-by-layer self-assembly modified Au electrode | 0.1~10.0 | 0.03 | [43] |
| Silver metallization of aptamers on disposable screenprinted Au electrodes | 0.001~100 | 7 × 10−4 | [44] |
| 3D-rGO/AuNPs modified GCE | 0.001~10 | 3.4 × 10−4 | This work |
| Sample | Spike (ng/mL) | Determined (ng/mL) | Recovery (%) |
|---|---|---|---|
| 1 | 0.05 | 0.050 ± 0.002 | 100.4 |
| 2 | 0.1 | 0.099 ± 0.013 | 98.81 |
| 3 | 0.5 | 0.53 ± 0.053 | 105.55 |
| 4 | 1 | 0.93 ± 0.072 | 93.14 |
| 5 | 2 | 2.26 ± 0.253 | 112.75 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhamoud, Y.; Li, Y.; Zhou, H.; Al-Wazer, R.; Gong, Y.; Zhi, S.; Yang, D. Label-Free and Highly-Sensitive Detection of Ochratoxin A Using One-Pot Synthesized Reduced Graphene Oxide/Gold Nanoparticles-Based Impedimetric Aptasensor. Biosensors 2021, 11, 87. https://doi.org/10.3390/bios11030087
Alhamoud Y, Li Y, Zhou H, Al-Wazer R, Gong Y, Zhi S, Yang D. Label-Free and Highly-Sensitive Detection of Ochratoxin A Using One-Pot Synthesized Reduced Graphene Oxide/Gold Nanoparticles-Based Impedimetric Aptasensor. Biosensors. 2021; 11(3):87. https://doi.org/10.3390/bios11030087
Chicago/Turabian StyleAlhamoud, Yasmin, Yingying Li, Haibo Zhou, Ragwa Al-Wazer, Yiying Gong, Shuai Zhi, and Danting Yang. 2021. "Label-Free and Highly-Sensitive Detection of Ochratoxin A Using One-Pot Synthesized Reduced Graphene Oxide/Gold Nanoparticles-Based Impedimetric Aptasensor" Biosensors 11, no. 3: 87. https://doi.org/10.3390/bios11030087
APA StyleAlhamoud, Y., Li, Y., Zhou, H., Al-Wazer, R., Gong, Y., Zhi, S., & Yang, D. (2021). Label-Free and Highly-Sensitive Detection of Ochratoxin A Using One-Pot Synthesized Reduced Graphene Oxide/Gold Nanoparticles-Based Impedimetric Aptasensor. Biosensors, 11(3), 87. https://doi.org/10.3390/bios11030087





