Recent Advances of Field-Effect Transistor Technology for Infectious Diseases
Abstract
:1. Introduction
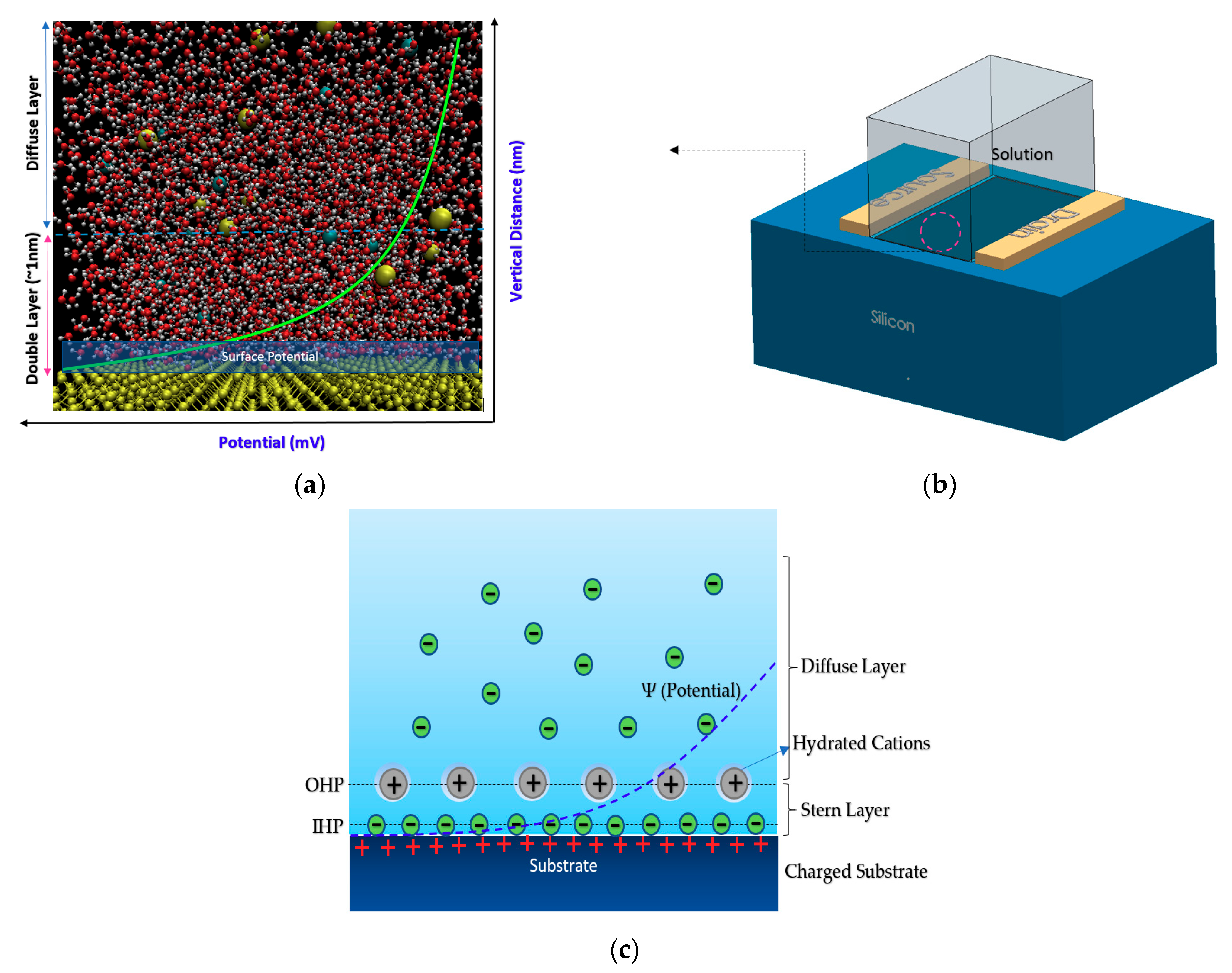
2. Sensing Mechanism and Different Structures of Chem/BioFETs
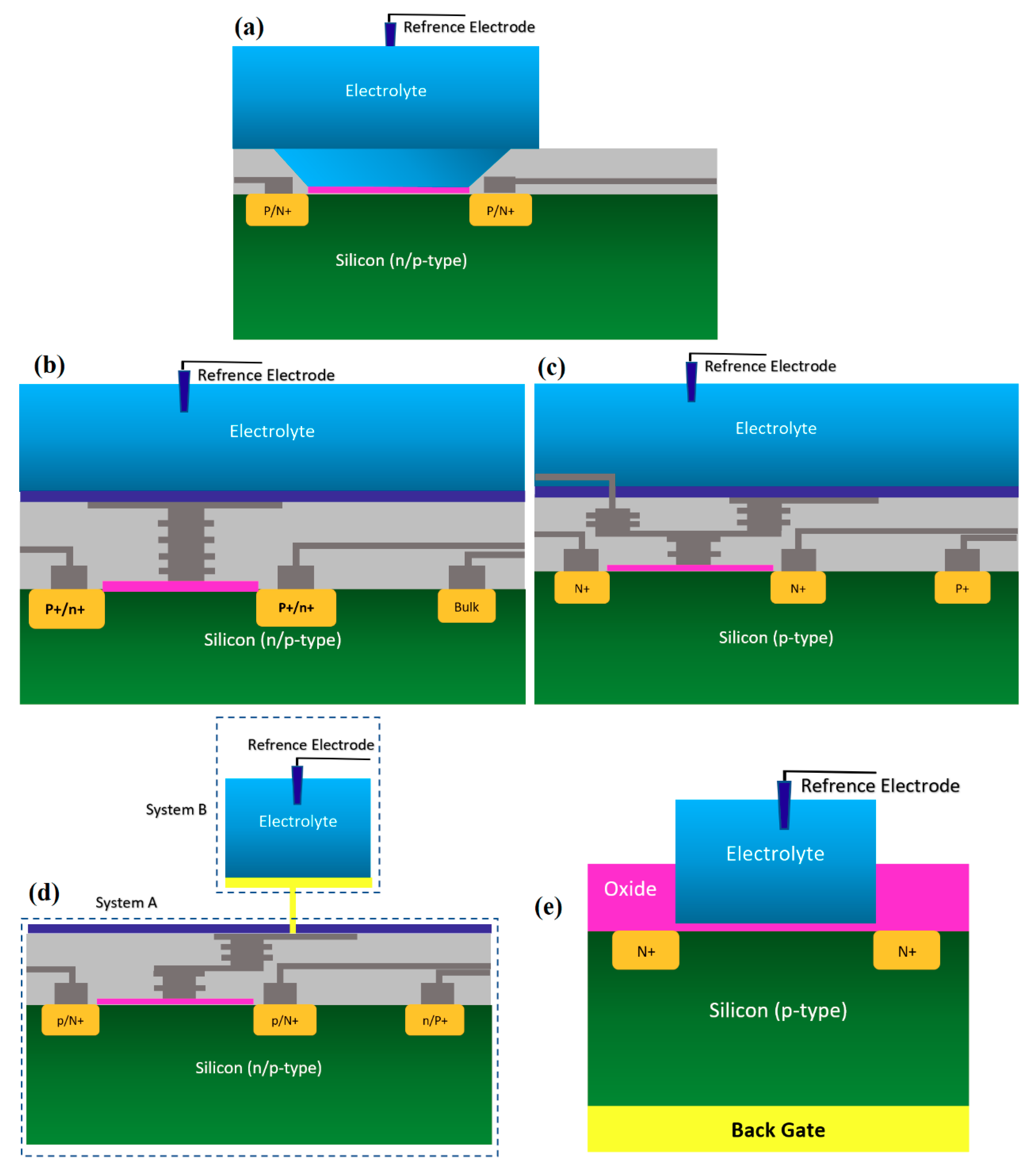
2.1. Chem/BioFETs Device Structures
2.1.1. Oxide-Electrolyte Gate Chem/BioFETs
2.1.2. Chem/BioFET Based on Standard CMOS
2.1.3. Floating Gate Chem/BioFET
2.1.4. Extended Gate Chem/BioFET
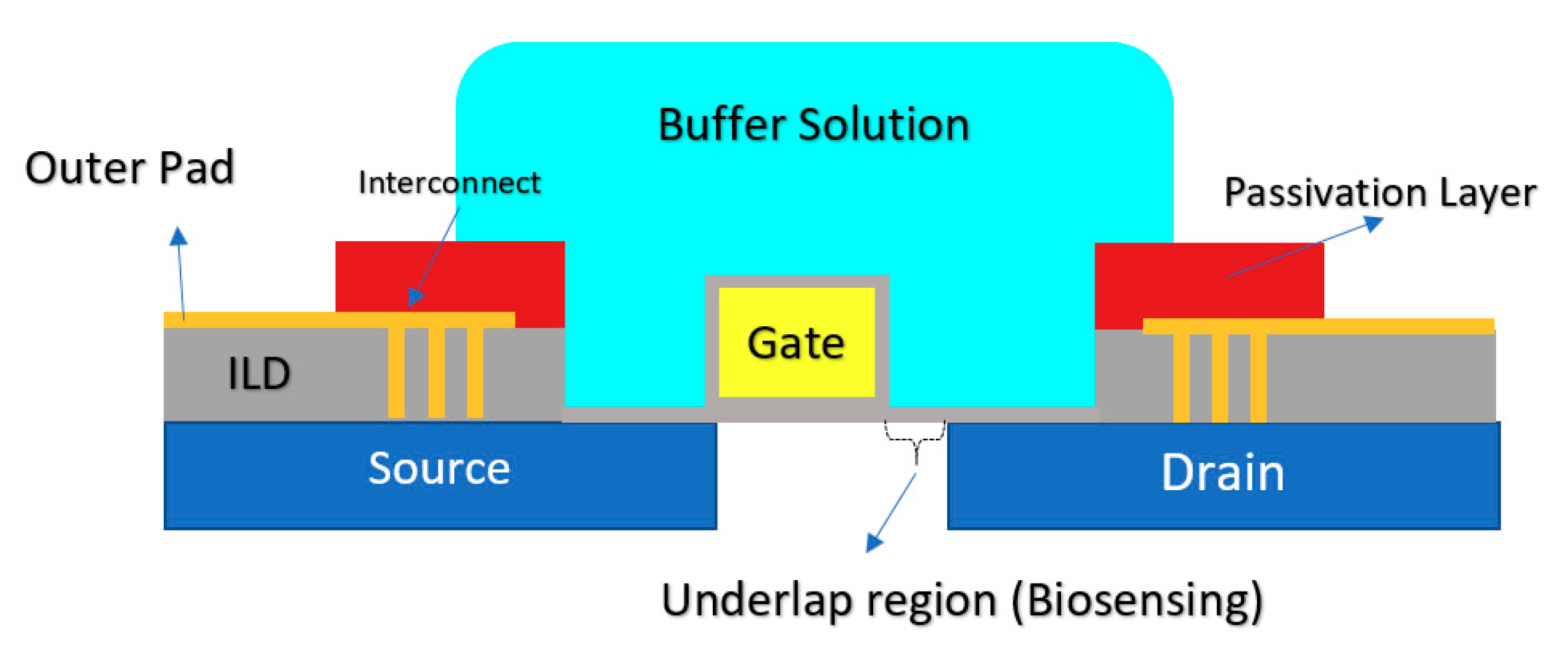
2.1.5. Double Gate Chem/BioFET
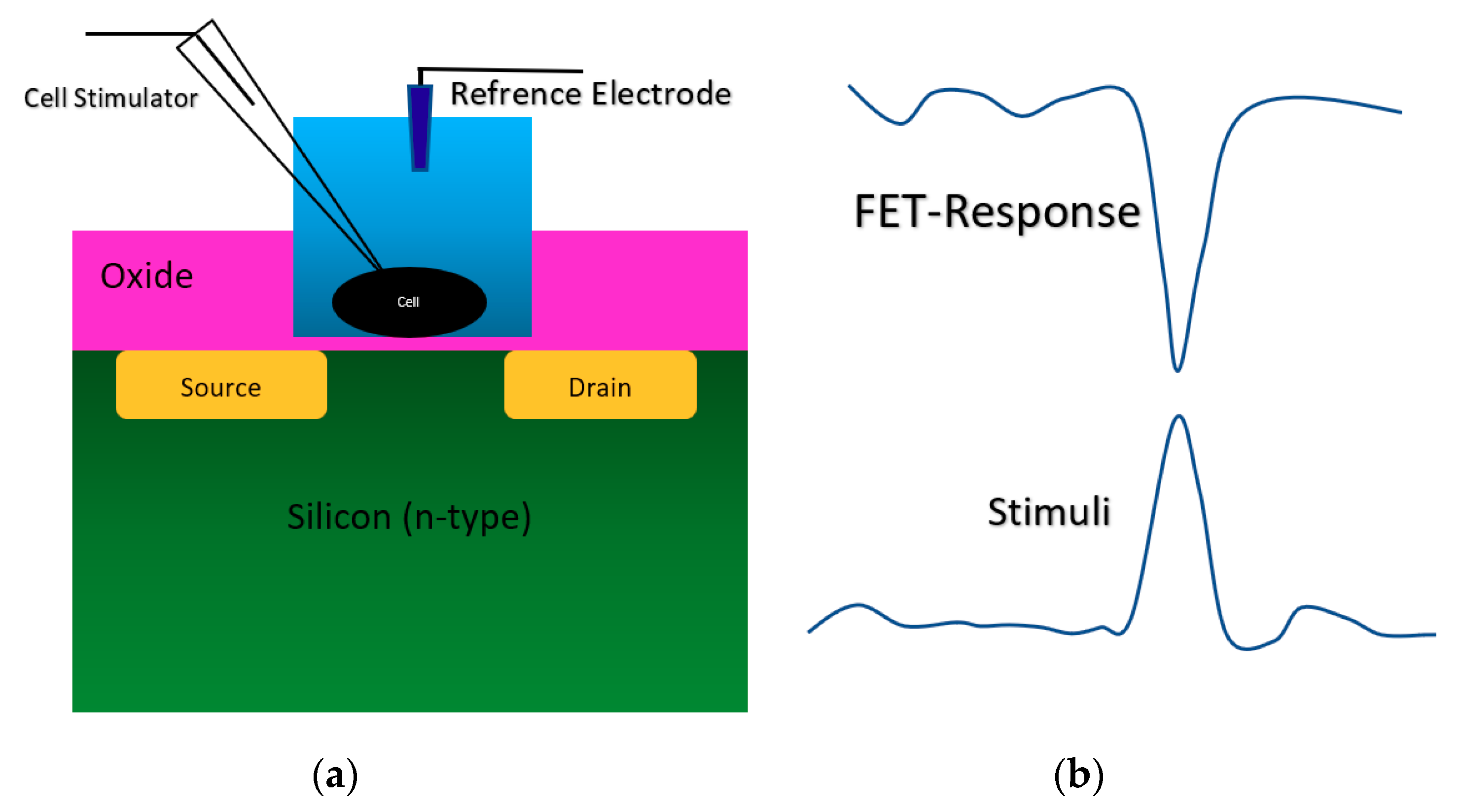
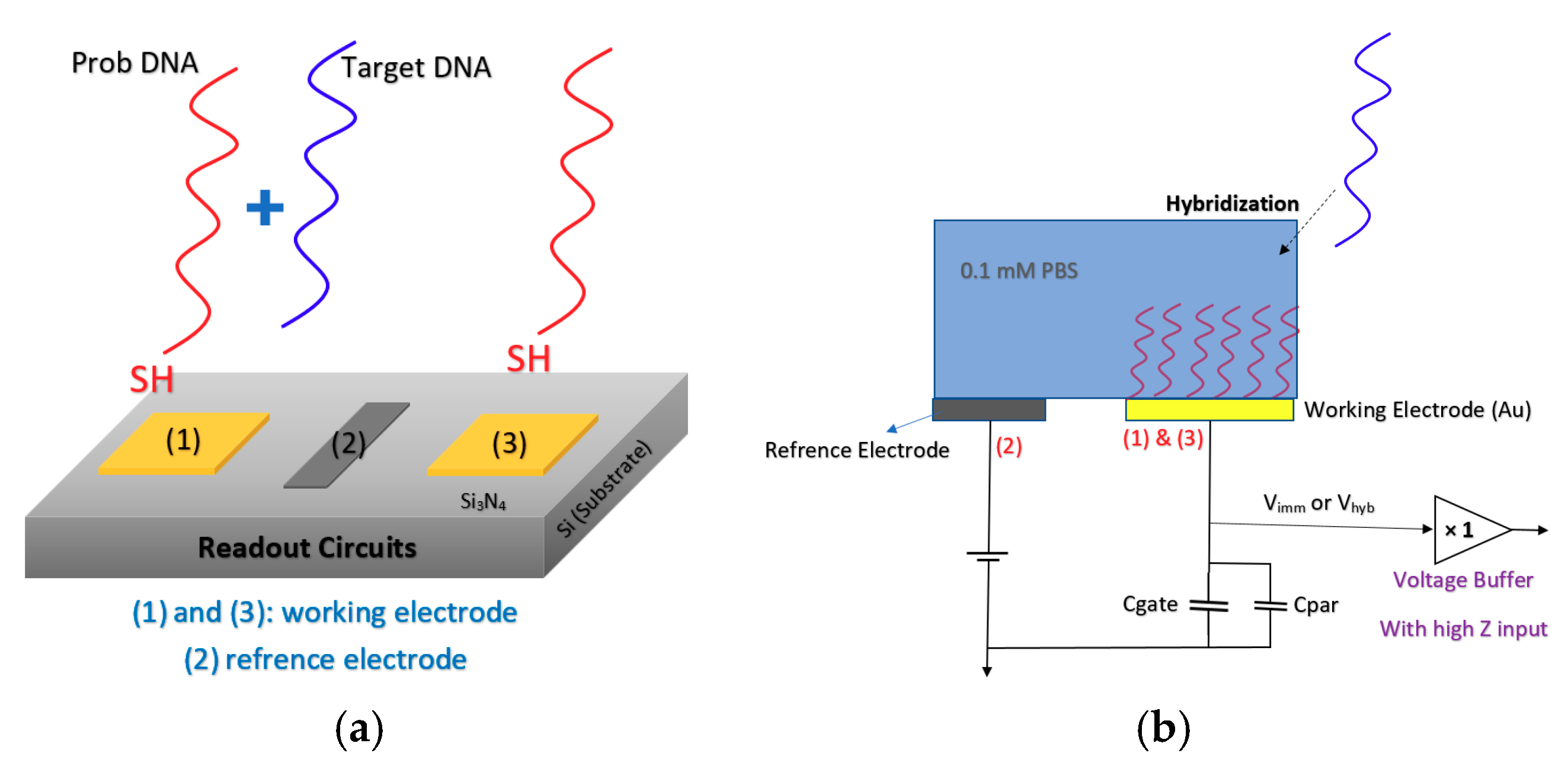
2.2. Chem/BioFET Structures Used for Infectious Disease Screening
3. Surface Modification and Functionalization of Chem/BioFETs
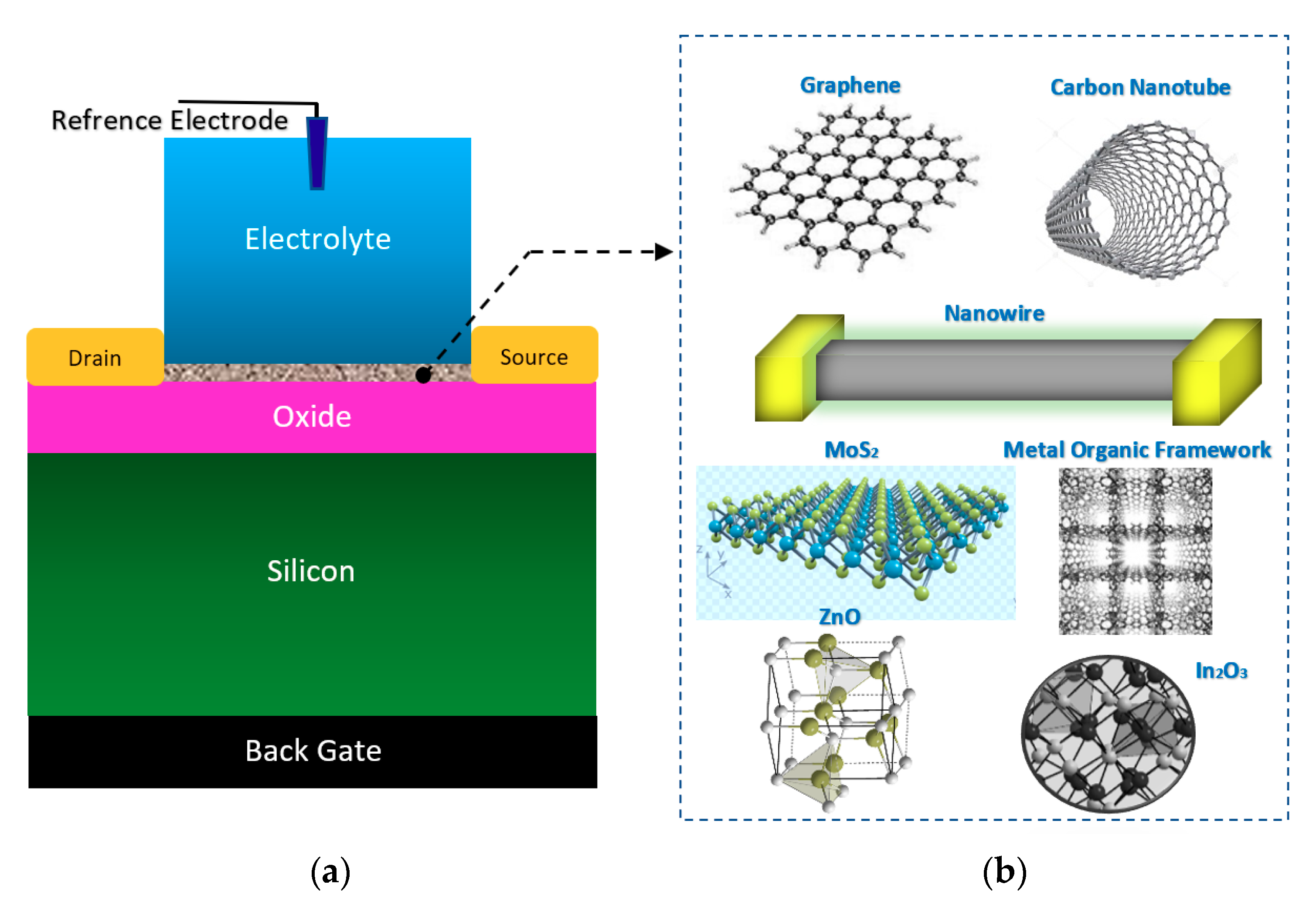
3.1. Surface Materials
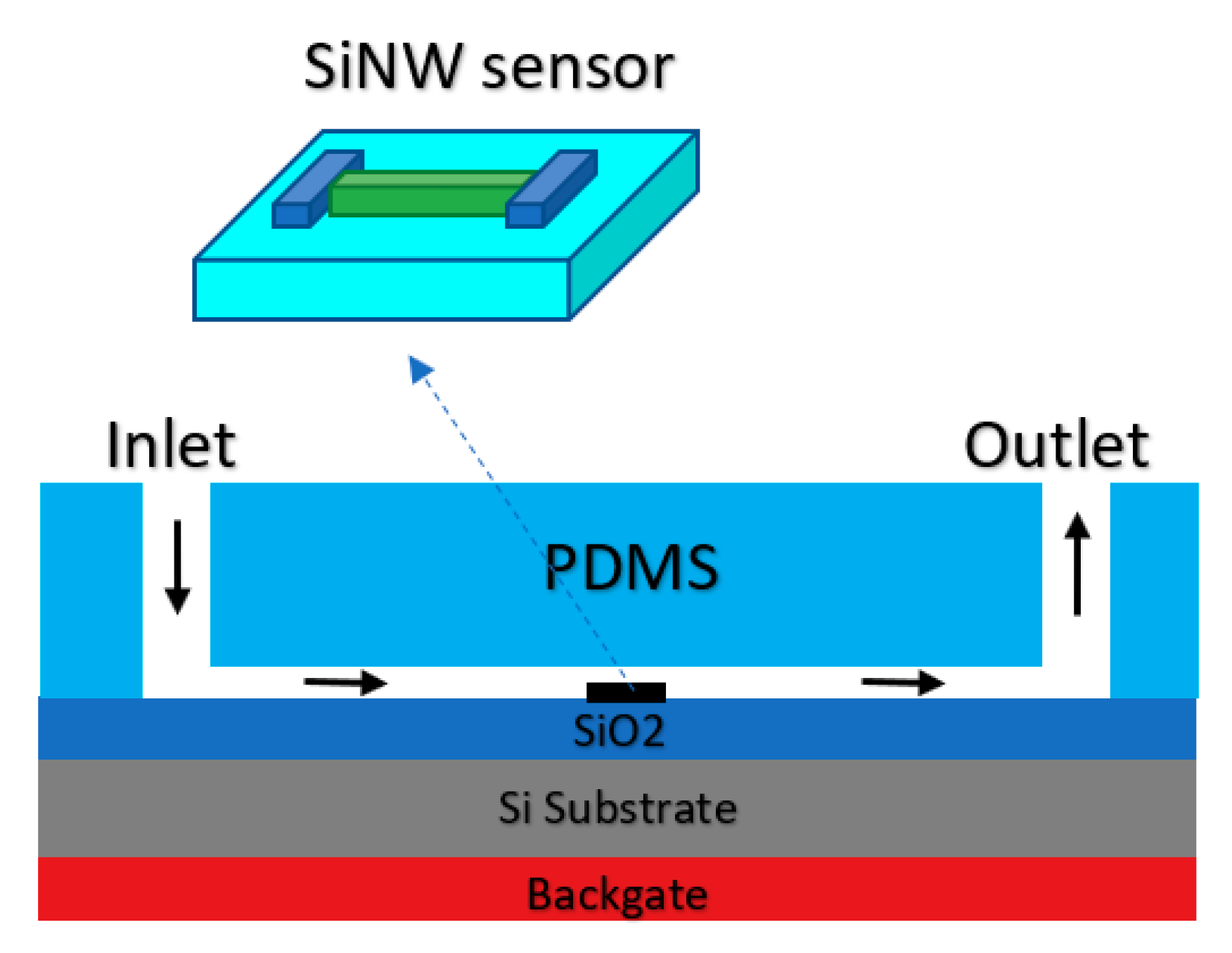
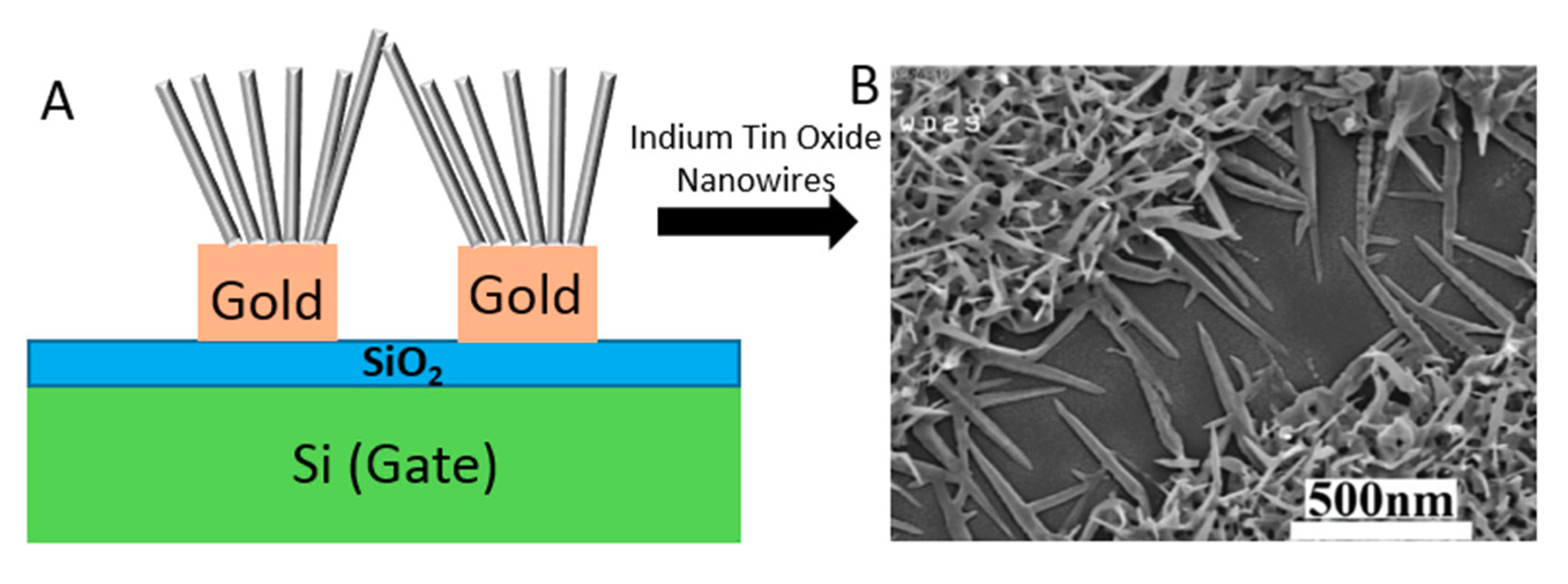
3.1.1. Nanowires
3.1.2. Gold
3.1.3. Graphene
3.1.4. Carbon Nanotubes
3.1.5. Transition Metal Dichalcogenides (TMDs)
3.1.6. Conducting Polymers (CPs)
3.2. Different Types of Bio-Recognition Elements (BREs)
3.2.1. Antibody or Antigen
3.2.2. Nucleic Acid
3.2.3. Aptamer
3.2.4. Other
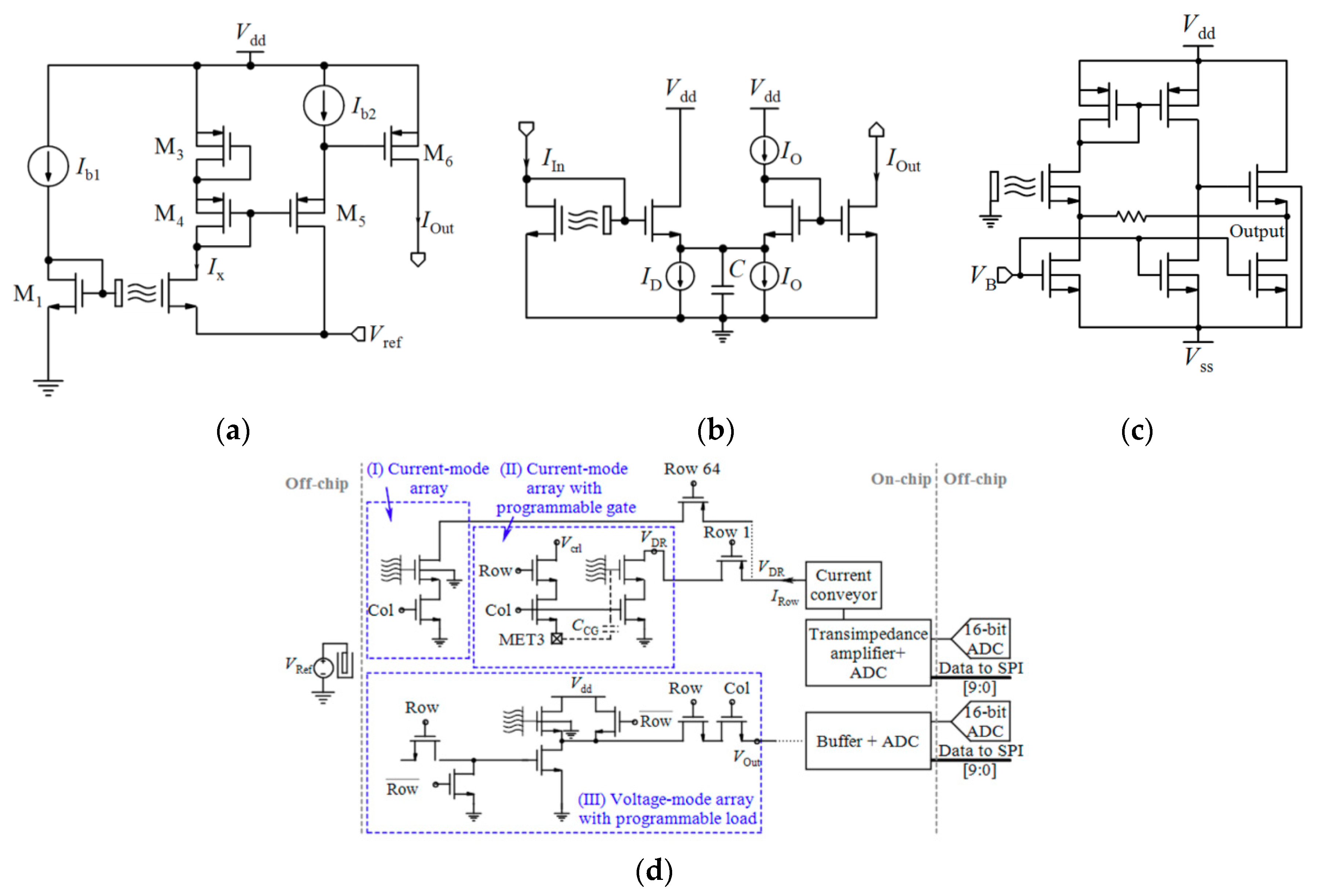
4. Readout Circuit and Systems
| CMOS Tech. | Array # | Diff. | Operational Region of FET | Configuration | Output Signal | Resolution | Sensitivity | IDR | Sensing Area (µm2) | Pixel/Active Area (µm2) | Total Area (mm2) | Power (mW) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.6 µm | 1 | No | Sat. | Wheatstone-bridge, variable VRef | V | - | 58 mV/pH | - | - | - | 4 × 4 | - | [161] |
| - | 1 | No | Tr. | CVCC | V | - | - | - | - | - | - | - | [160] |
| 0.35 µm | 1.5 M | - | - | CVCC | - | - | - | - | - | - | 10.6 × 10.9 | - | [20] * |
| 7.2 M | 17.5 × 17.5 | ||||||||||||
| 13 M | 17.5 × 17.5 | ||||||||||||
| 1.0 µm | 1 | No | - | CVCC, Feedback | V | - | 47 mV/pH | pH: 2.5 to 9.2 | - | - | - | - | [159] |
| 2.5 µm | - | No | Tr. | CVCC | V | - | 58 mV/pH | pH: 3 to 11 | - | - | <0.25 | 10 | [162] |
| 0.18 µm | 6 | No | Tr. | CVCC | V | - | - | - | - | - | 1.5 × 0.6 | - | [22] * |
| 0.35 µm | 64 × 64 | No | - | CVCC, SPT | V | - | 20 mV/pH | pH: 4 to 10 | - | 10.2 × 10.2 | 0.7158 × 0.7158 | - | [163] |
| 0.35 µm | 64 × 64 | No | Tr. | CVCC, APS | D | - | −9.23 mV/pH | - | - | 96 | 0.56 | - | [116] * |
| 5 μm | 10 × 10 | No | - | CVCC, APS | V | - | −229 mV/pH | pH: 4 to 9.1 | 2000 × 2000 (total) | 200 × 200 | 5.1 × 5.1 | - | [164] |
| 2 µm | 1024 × 1024 | No | - | CVCC, Charge transfer APS | V | - | 29.8 mV/pH | pH: 2 to 10 | - | 23.55 × 23.55 | 14.8 × 14.8 | - | [165] |
| 0.35 µm | 3 × 11 | No | Sub. | CVCC, integrate-and-fire topology, AER | D | - | −7.73 dB/pH | pH: 1 to 14 | 57.5 × 57.5 | 80 × 100 | - | (EP: 157 nW) | [166] |
| 0.35 µm | 8 × 4 | No | - | CVCC, Feedback to the gate | V | 60.3 mpH | 42.1 mV/pH | pH: 1 to 14 | - | 60 × 70 | 2 × 2.5 | SFE: 4.841 × 10−4 | [167] |
| 0.35 µm | 1 | No | Sat. | CVCC, Feedback, PG | V | - | 200 mV/pH | - | 30 × 100 | - | 0.6 × 0.5 | - | [168] |
| 0.18 µm | 8 × 8 | No | Sub. | CVCC, Current feedback | F | - | 37 mV/pH | pH: 4 to 10 | - | - | 2.6 | 0.076 | [169] |
| 0.35 µm | 1 | No | - | CVCC, VCO | F | - | 78 kHz/pH | pH: 0 to 7 | - | - | 0.045 | 0.12 | [170] |
| 0.35 µm | 64 × 128 | No | Tr. | CVCC, APS | D | - | −9.23 mV/pH | - | 9.3 × 10.3 | - | 2×4 | - | [171] |
| 64 × 200 | VS | CM | −1.033 µA/pH | 6.5 × 7.775 | |||||||||
| 64 × 200 | VS | CM, PG | −0.717 µA/pH | 6.5 × 7.775 | |||||||||
| - | 1 | No | Sub. | CM, Current feedback | V | - | −49.4 mV/pH | pH: 4 to 9 | - | - | - | - | [172] |
| 0.35 µm | 3 × 3 | No | Sub | CM, PG, RO | F | 0.008 pH | 6 to 8 kHz/pH | pH: 5 to 7 | 55 × 65 | 64 × 54 | 0.1089 | 6 × 10−3 | [173] |
| 0.35 µm | 128 × 128 | No | Tr. | CM, CC, Auto-zeroing, S/H | D | 0.24 pH (@1000 fps) 0.45 pH (@3000 fps) | 50 LSBs/pH | pH: 4 to 10 | - | 18 × 12.5 | 2.6 × 2.2 | 376 | [174] |
| 0.35 µm | 8 × 8 | No | Sub. | VM, PG, Optic., MM | D | 57 mV/pH | pH: 4 to 10 | - | - | - | - | [175] | |
| 0.18 µm | 64 × 64 | No | - | pH-TC, Optic. | D | - | −26.2 mV/pH (G = 1) −103.8 mV/pH (G = 4) | pH ~ 1 to 14 | Chem.: 22.3 Opt.: 20.1 | 10 × 10 | 2.5 × 5 | 105.6 | [176] * |
| 65 nm | 512 × 128 | No | Sub. | pH-TVC | D | 0.01 pH | 123.8 mV/pH | pH: 2.5 to 11.5 | 15 | 4.4. × 4.4 | 512 × 128 | PA: 80.6, AB: 108.4, DB: 6.5 | [23] * |
| 0.18 µm | 8 × 8 | No | Sub. | pH-TC, APS, PWM | T | 33 mpH | 6.1 μs/pH | - | - | 16.5 × 16.25 | 6.7 | 8.3 | [177] |
| 0.18 µm | 3 × 3 | No | - | pH-TC | D | 0.028 pH | 27 ns/pH | - | 10 × 10 | - | 0.036 | 0.23 | [178] |
| 0.35 µm | 1 | No | Sub. | ISFET logic | V | 0.5 pH | 3700 mV/pH | pH: 3.7 to 10.95 | 95 × 200 | - | - | - | [179] |
| 0.35 µm | 8 × 8 | No | Sat. | ISFET logic | V | 0.5 pH | 50 mV/pH | pH: 1 to 14 | - | - | - | - | [180] |
| 0.35 µm | 4 pairs | Yes | Sat. | CVCC, PG, Feedback to the gate | D | - | 100 mV/pH | pH: 5 to 9 | 30 × 100 | 120 × 120 | 0.65 × 0.5 | - | [181] |
| 0.35 µm | 2 × 2 | Yes | Tr. | ISFET/REFET diff., CVCC | D | - | 40 mV/pH | - | 11.6 × 11.6 | - | 1.4 × 2.6 | 15 | [182] |
| 0.35 µm | 16 × 16 | Yes | Tr. | ISFET/REFET diff., CVCC | D | - | 46 mV/pH | pH: 3.28 to 7.22 | 11.6 × 11.6 | 12.8 × 12.8 | - | 60 | [63] |
| 0.6 µm | 1 | Yes | Sat. | ISFET/REFET diff., CVCC | V | - | 400 mV/pH | 6 pH | - | - | 18.225 | 2.1 | [183] |
| (CMOS) | 1 | Yes | - | ISFET/REFET diff. | V | 0.01 pH | −40 to −43 mV/pH | pH: 4 to 9 | - | 1650 × 2600 | 4.9 × 3.9 | - | [184] |
| 2.5 µm | 1 | Yes. | Tr. | ISFET/REFET diff., CVCC | C | 0.15 pH | −0.3875 µA/pH | pH: 3 to 11 | - | - | - | - | [185] |
| 0.35 µm | 1 | Yes | Tr. | ISFET/ISFET diff., CM | D | 0.1 pH | 800 mV/pH | pH: 5 to 9 | - | - | - | - | [186] |
| 0.35 µm | 1 | Yes | Sub. | Diff, Gilbert gain cell, CM, Translinear | C | - | 45 mV/pH | pH: 5 to 9 | 34 × 100 | - | 2.5 × 2.81 | 1.65 × 10−4 | [187] |
| 0.35 µm | 1 | Yes | - | Diff, direct charge accumulation | D | 36 µV | - | 88.3 dB | 71 × 71 | 1000 × 1640 | 4 × 5 | - | [88] * |
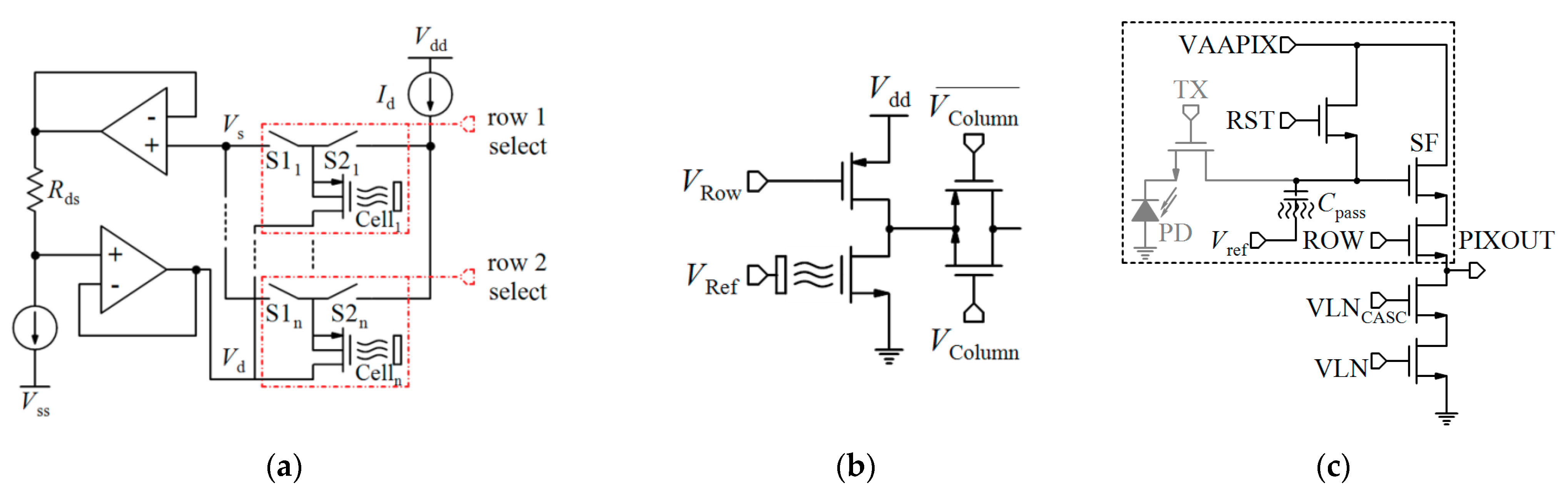
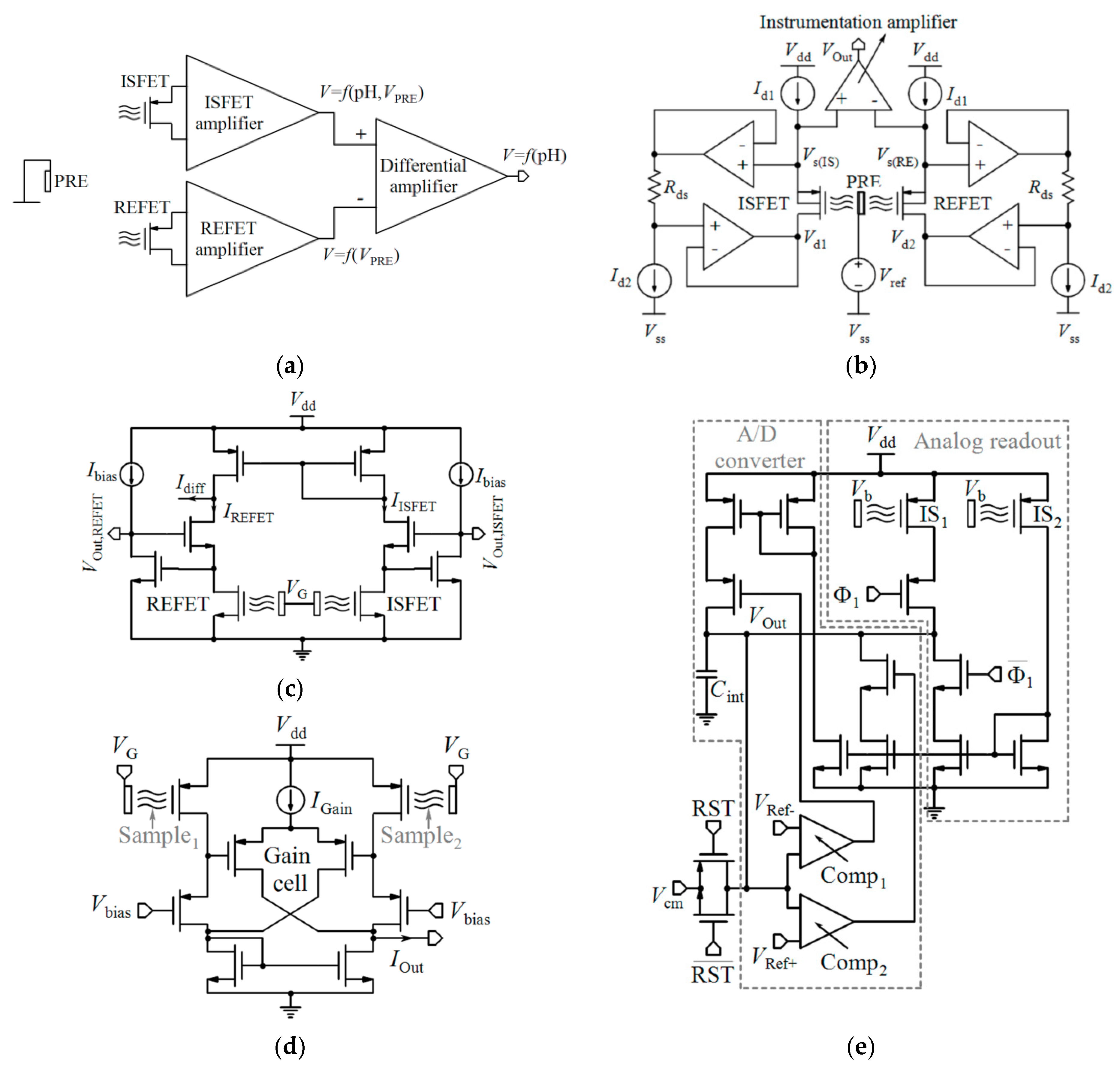
4.1. Non-Differential Measurements
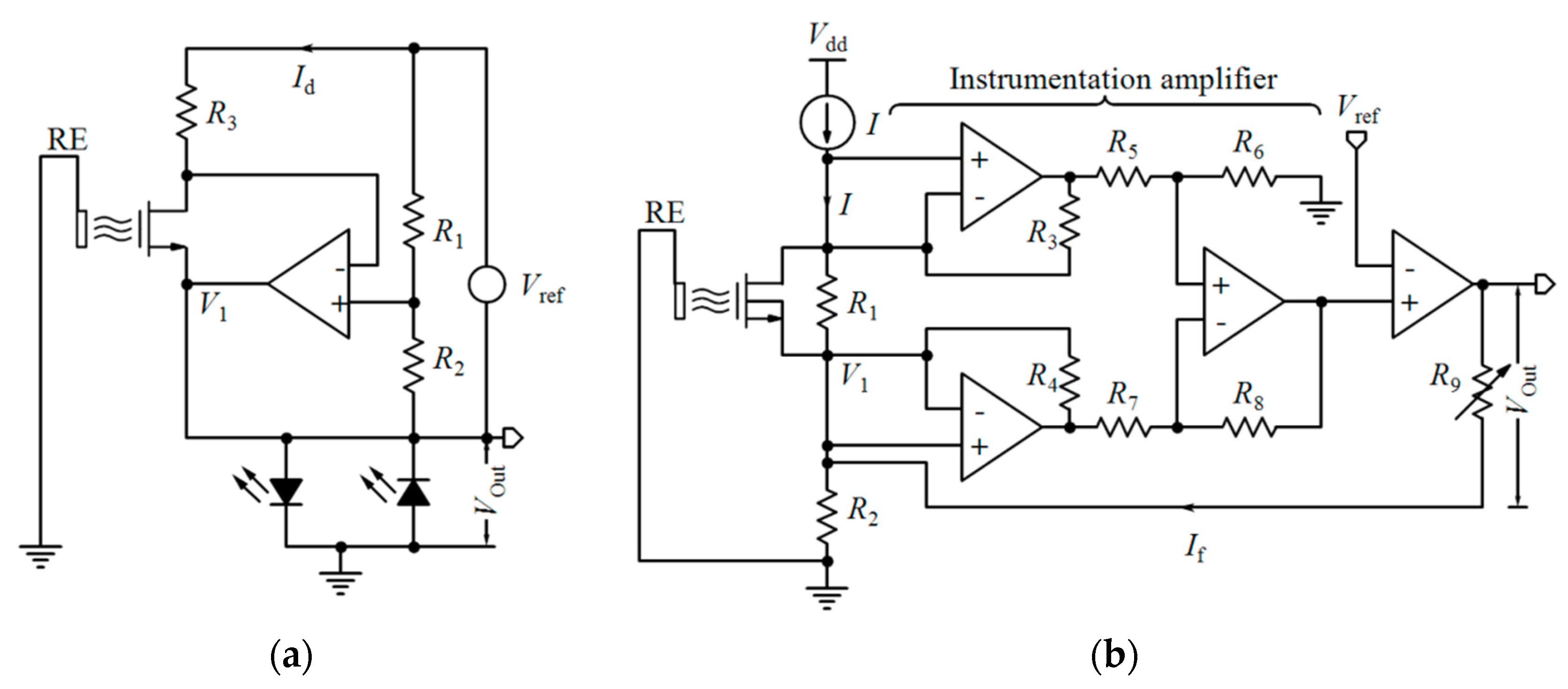
4.1.1. Variable Voltage Reference in Feedback Mode
4.1.2. Constant-Voltage Constant-Current (CVCC)
4.1.3. Current-Mode Readout Circuits
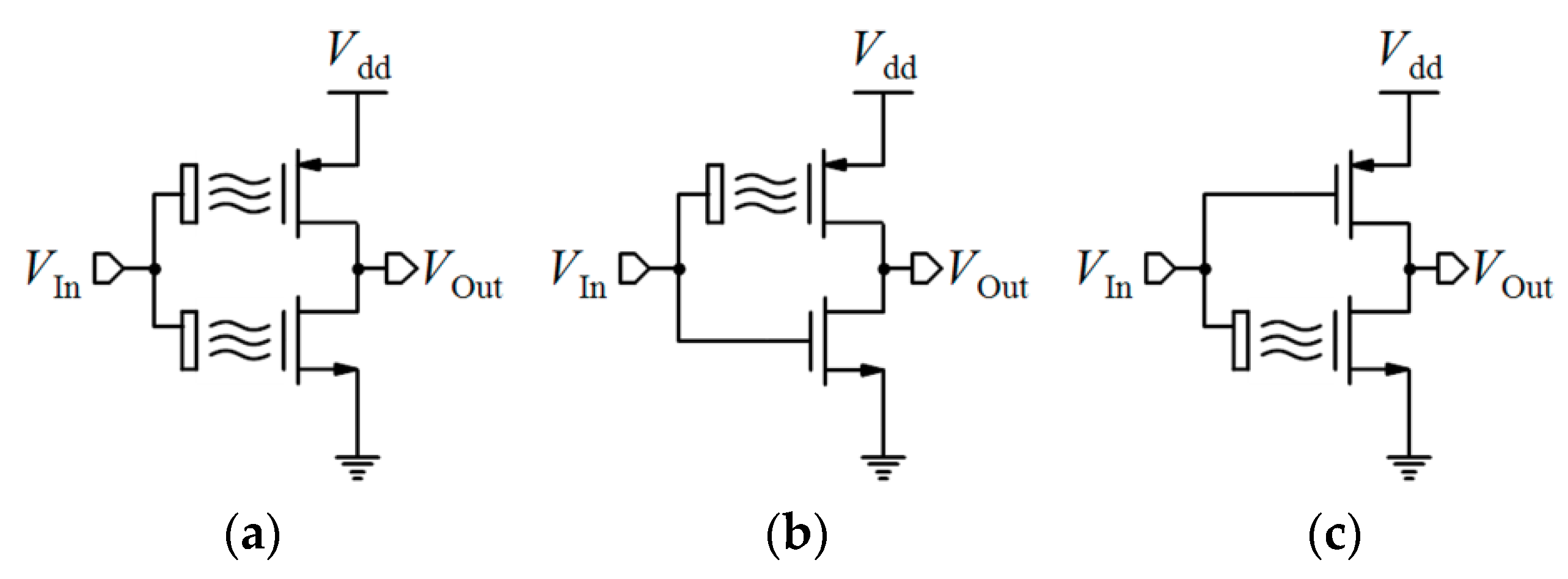
4.1.4. ISFET Logic
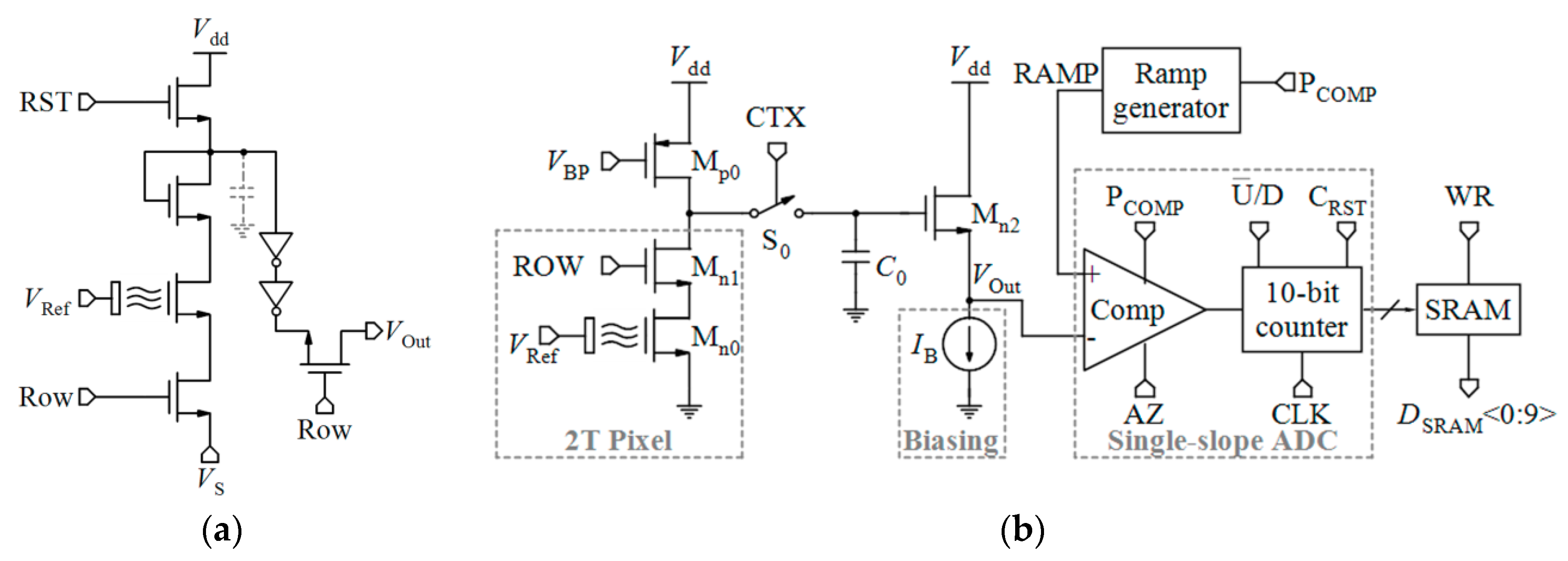
4.1.5. pH-to-Time Conversion
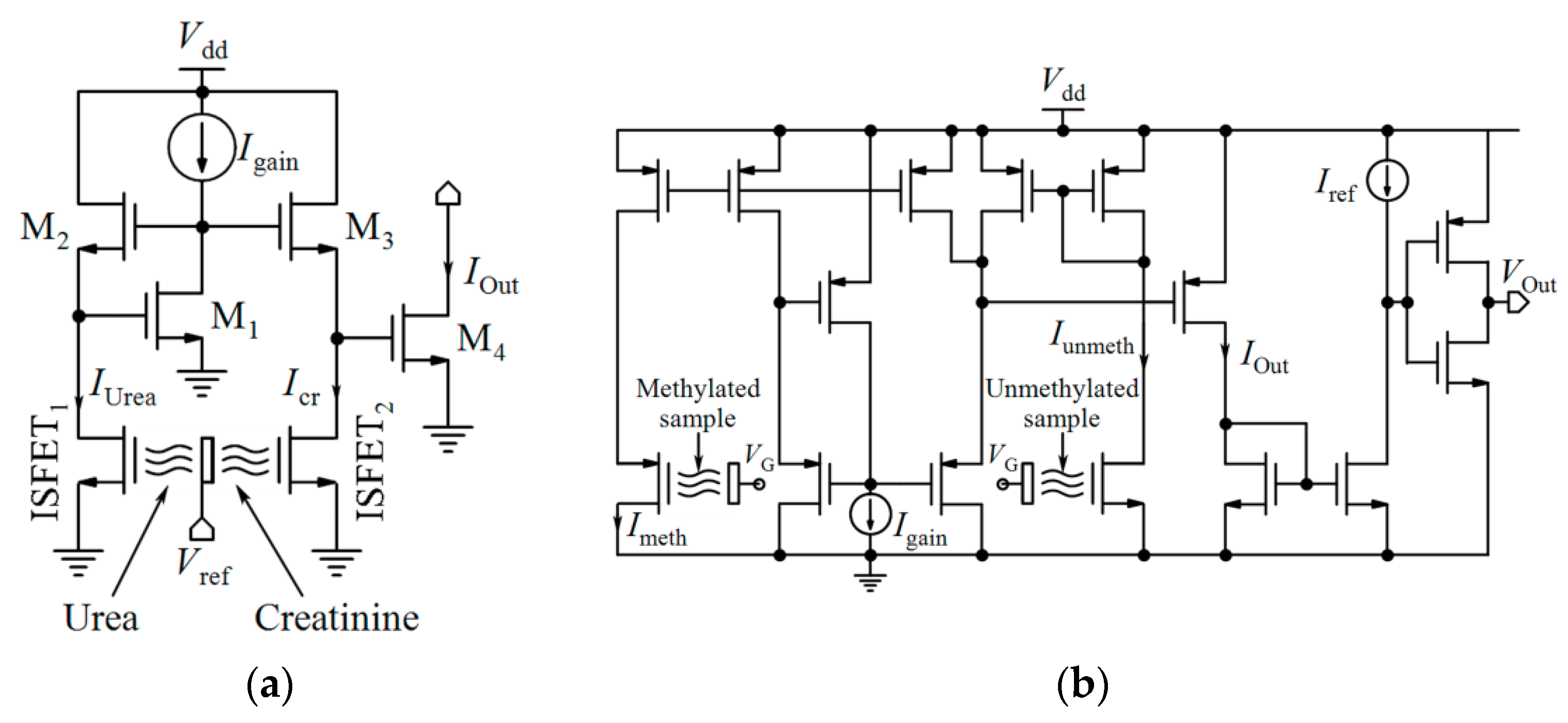
4.2. Differential Measurements
4.3. Amelioration of Some Non-Idealities
4.3.1. Trapped Charges
4.3.2. Noise
4.3.3. Temperature Sensitivity
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mojsoska, B.; Larsen, S.; Olsen, D.A.; Madsen, J.S.; Brandslund, I.; Alatraktchi, F.A. Rapid SARS-CoV-2 Detection Using Electrochemical Immunosensor. Sensors 2021, 21, 390. [Google Scholar] [CrossRef] [PubMed]
- Mahase, E. Covid-19: What Have We Learnt about the New Variant in the UK? British Medical Journal Publishing Group: London, UK, 2020. [Google Scholar]
- Al-Jighefee, H.T.; Yassine, H.M.; Nasrallah, G.K. Evaluation of Antibody Response in Symptomatic and Asymptomatic COVID-19 Patients and Diagnostic Assessment of New IgM/IgG ELISA Kits. Pathogens 2021, 10, 161. [Google Scholar] [CrossRef] [PubMed]
- Haliga, R.E.; Sorodoc, V.; Lionte, C.; Petris, O.R.; Bologa, C.; Coman, A.E.; Vata, L.G.; Puha, G.; Dumitrescu, G.; Sirbu, O. Acute Clinical Syndromes and Suspicion of SARS-CoV-2 Infection: The Experience of a Single Romanian Center in the Early Pandemic Period. Medicina 2021, 57, 121. [Google Scholar] [CrossRef]
- Oyewole, A.O.; Barrass, L.; Robertson, E.G.; Woltmann, J.; O’Keefe, H.; Sarpal, H.; Dangova, K.; Richmond, C.; Craig, D. COVID-19 Impact on Diagnostic Innovations: Emerging Trends and Implications. Diagnostics 2021, 11, 182. [Google Scholar] [CrossRef]
- Salas Rojas, M.; Silva Garcia, R.; Bini, E.; Pérez de la Cruz, V.; León Contreras, J.C.; Hernández Pando, R.; Bastida Gonzalez, F.; Davila-Gonzalez, E.; Orozco Morales, M.; Gamboa Domínguez, A. Quinacrine, an Antimalarial Drug with Strong Activity Inhibiting SARS-CoV-2 Viral Replication In Vitro. Viruses 2021, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Alipour, M.; Chodari, L.; Maleki Dizaj, S.; Ardalan, M.; Samiei, M.; Sharifi, S.; Zununi Vahed, S.; Huseynova, I.; Khalilov, R.; et al. A Comprehensive Review of Detection Methods for SARS-CoV-2. Microorganisms 2021, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Adeel, M.; Rahman, M.M.; Caligiuri, I.; Canzonieri, V.; Rizzolio, F.; Daniele, S. Recent advances of electrochemical and optical enzyme-free glucose sensors operating at physiological conditions. Biosens. Bioelectron. 2020, 165, 112331. [Google Scholar] [CrossRef]
- Liu, J.; Geng, Z.; Fan, Z.; Liu, J.; Chen, H. Point-of-care testing based on smartphone: The current state-of-the-art (2017–2018). Biosens. Bioelectron. 2019, 132, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef]
- Dzik, S. COVID-19 convalescent plasma: Now is the time for better science. Transfus. Med. Rev. 2020, 34, 141–144. [Google Scholar] [CrossRef]
- Ho, W.-J.; Chen, J.-S.; Ker, M.-D.; Wu, T.-K.; Wu, C.-Y.; Yang, Y.-S.; Li, Y.-K.; Yuan, C.-J. Fabrication of a miniature CMOS-based optical biosensor. Biosens. Bioelectron. 2007, 22, 3008–3013. [Google Scholar] [CrossRef] [PubMed]
- Forouhi, S.; Dehghani, R.; Ghafar-Zadeh, E. CMOS Based Capacitive Sensors for Life Science Applications: A Review. Sens. Actuators A Phys. 2019, 297, 111531. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 70–71. [Google Scholar] [CrossRef]
- Kaisti, M. Detection principles of biological and chemical FET sensors. Biosens. Bioelectron. 2017, 98, 437–448. [Google Scholar] [CrossRef]
- Xu, D.; Huang, X.; Guo, J.; Ma, X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018, 110, 78–88. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.; Choi, M.; Ku, K.B.; Lee, C.-S.; Jun, S.; Park, D.; Kim, H.G. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Qian, C.; Liu, C.; Shen, H.; Wang, Z.; Ping, J.; Wu, J.; Chen, H. Nucleic acid amplification free biosensors for pathogen detection. Biosens. Bioelectron. 2020, 153, 112049. [Google Scholar] [CrossRef]
- Rothberg, J.M.; Hinz, W.; Rearick, T.M.; Schultz, J.; Mileski, W.; Davey, M.; Leamon, J.H.; Johnson, K.; Milgrew, M.J.; Edwards, M. An integrated semiconductor device enabling non-optical genome sequencing. Nature 2011, 475, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Gurrala, R.; Lang, Z.; Shepherd, L.; Davidson, D.; Harrison, E.; McClure, M.; Kaye, S.; Toumazou, C.; Cooke, G. Novel pH sensing semiconductor for point-of-care detection of HIV-1 viremia. Sci. Rep. 2016, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nikkhoo, N.; Gulak, P.G.; Maxwell, K. Rapid detection of E. coli bacteria using potassium-sensitive FETs in CMOS. IEEE Trans. Biomed. Circuits Syst. 2013, 7, 621–630. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, X.; Dang, T.C.; Huang, X.; Feng, H.; Zhang, Q.; Yu, H. A High-Sensitivity Potentiometric 65-nm CMOS ISFET Sensor for Rapid E. coli Screening. IEEE Trans. Biomed. Circuits Syst. 2018, 12, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Whitcomb, J.; Boyd, J.; Varghese, J. Transient finite element analysis of electric double layer using Nernst–Planck–Poisson equations with a modified Stern layer. J. Colloid Interface Sci. 2007, 305, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagy, T.; Henderson, D.; Boda, D. Simulation of an electrical double layer model with a low dielectric layer between the electrode and the electrolyte. J. Phys. Chem. B 2011, 115, 11409–11419. [Google Scholar] [CrossRef] [PubMed]
- Meixner, L.; Koch, S. Simulation of ISFET operation based on the site-binding model. Sens. Actuators B Chem. 1992, 6, 315–318. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, P.G.; Stiegler, H.J.; Zhao, M.; Cantley, K.D.; Obradovic, B.; Chapman, R.A.; Wen, H.-C.; Mahmud, G.; Vogel, E.M. SPICE macromodel of silicon-on-insulator-field-effect-transistor-based biological sensors. Sens. Actuators B Chem. 2012, 161, 163–170. [Google Scholar] [CrossRef]
- Van Hal, R.; Eijkel, J.; Bergveld, P. A novel description of ISFET sensitivity with the buffer capacity and double-layer capacitance as key parameters. Sens. Actuators B Chem. 1995, 24, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Yates, D.E.; Levine, S.; Healy, T.W. Site-binding model of the electrical double layer at the oxide/water interface. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1974, 70, 1807–1818. [Google Scholar] [CrossRef]
- Bourg, I.C.; Sposito, G. Molecular dynamics simulations of the electrical double layer on smectite surfaces contacting concentrated mixed electrolyte (NaCl–CaCl2) solutions. J. Colloid Interface Sci. 2011, 360, 701–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shepherd, L.; Toumazou, C. Weak inversion ISFETs for ultra-low power biochemical sensing and real-time analysis. Sens. Actuators B Chem. 2005, 107, 468–473. [Google Scholar] [CrossRef]
- Miscourides, N.; Georgiou, P. Linear current-mode ISFET arrays. In Proceedings of the 2016 IEEE International Symposium on Circuits and Systems (ISCAS), Montreal, QC, Canada, 22–25 May 2016; pp. 2827–2830. [Google Scholar]
- Ben-Yaakov, D.; Andelman, D.; Podgornik, R.; Harries, D. Ion-specific hydration effects: Extending the Poisson-Boltzmann theory. Curr. Opin. Colloid Interface Sci. 2011, 16, 542–550. [Google Scholar] [CrossRef] [Green Version]
- Ozcelik, H.G.; Sozen, Y.; Sahin, H.; Barisik, M. Parametrizing nonbonded interactions between silica and water from first principles. Appl. Surf. Sci. 2020, 504, 144359. [Google Scholar] [CrossRef]
- Jiang, G.; Cheng, C.; Li, D.; Liu, J.Z. Molecular dynamics simulations of the electric double layer capacitance of graphene electrodes in mono-valent aqueous electrolytes. Nano Res. 2016, 9, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Fakih, I.; Durnan, O.; Mahvash, F.; Napal, I.; Centeno, A.; Zurutuza, A.; Yargeau, V.; Szkopek, T. Selective ion sensing with high resolution large area graphene field effect transistor arrays. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Molinnus, D.; Beging, S.; Lowis, C.; Schöning, M.J. Towards a multi-enzyme capacitive field-effect biosensor by comparative study of drop-coating and nano-spotting technique. Sensors 2020, 20, 4924. [Google Scholar] [CrossRef] [PubMed]
- Poghossian, A.; Schöning, M.J. Label-Free Sensing of Biomolecules with Field-Effect Devices for Clinical Applications. Electroanalysis 2014, 26, 1197–1213. [Google Scholar] [CrossRef]
- Su, S.; Sun, Q.; Gu, X.; Xu, Y.; Shen, J.; Zhu, D.; Chao, J.; Fan, C.; Wang, L. Two-dimensional nanomaterials for biosensing applications. TrAC Trends Anal. Chem. 2019, 119, 115610. [Google Scholar] [CrossRef]
- Sengupta, J.; Hussain, C.M. Graphene-based field-effect transistor biosensors for the rapid detection and analysis of viruses: A perspective in view of COVID-19. Carbon Trends 2020, 2, 100011. [Google Scholar] [CrossRef]
- Pilehvar, S.; De Wael, K. Recent advances in electrochemical biosensors based on fullerene-C60 nano-structured platforms. Biosensors 2015, 5, 712–735. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Luo, Y.; Gao, L.; Liu, B.; Duan, G. High-performance field-effect transistor glucose biosensors based on bimetallic Ni/Cu metal-organic frameworks. Biosens. Bioelectron. 2021, 171, 112736. [Google Scholar] [CrossRef]
- Campbell, M.G.; Dincă, M. Metal–organic frameworks as active materials in electronic sensor devices. Sensors 2017, 17, 1108. [Google Scholar] [CrossRef]
- Syu, Y.-C.; Hsu, W.-E.; Lin, C.-T. Field-effect transistor biosensing: Devices and clinical applications. ECS J. Solid State Sci. Technol. 2018, 7, Q3196. [Google Scholar] [CrossRef]
- Singh, N.K.; Thungon, P.D.; Estrela, P.; Goswami, P. Development of an aptamer-based field effect transistor biosensor for quantitative detection of Plasmodium falciparum glutamate dehydrogenase in serum samples. Biosens. Bioelectron. 2019, 123, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bergveld, P. Development, operation, and application of the ion-sensitive field-effect transistor as a tool for electrophysiology. IEEE Trans. Biomed. Eng. 1972, 342–351. [Google Scholar] [CrossRef]
- Chida, R.; Igarashi, K.; Kamiyama, K.; Hoshino, E.; Esashi, M. Characterization of human dental plaque formed on hydrogen-ion-sensitive field-effect transistor electrodes. J. Dent. Res. 1986, 65, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Akimenko, V.; Khomutov, S.; Obraztsova, A.Y.; Vishnivetskii, S.; Chuvilskaya, N.; Laurinavichus, K.; Reshetilov, A. A rapid method for detection of Clostridium thermocellum by field-effect transistor-based immunodetection. J. Microbiol. Methods 1996, 24, 203–209. [Google Scholar] [CrossRef]
- Weis, R.; Müller, B.; Fromherz, P. Neuron adhesion on a silicon chip probed by an array of field-effect transistors. Phys. Rev. Lett. 1996, 76, 327. [Google Scholar] [CrossRef] [PubMed]
- Souteyrand, E.; Cloarec, J.; Martin, J.; Wilson, C.; Lawrence, I.; Mikkelsen, S.; Lawrence, M. Direct detection of the hybridization of synthetic homo-oligomer DNA sequences by field effect. J. Phys. Chem. B 1997, 101, 2980–2985. [Google Scholar] [CrossRef]
- Sprössler, C.; Richter, D.; Denyer, M.; Offenhäusser, A. Long-term recording system based on field-effect transistor arrays for monitoring electrogenic cells in culture. Biosens. Bioelectron. 1998, 13, 613–618. [Google Scholar] [CrossRef]
- Brischwein, M.; Baumann, W.; Ehret, R.; Schwinde, A.; Kraus, M.; Wolf, B. Mikrosensorische Systeme in der zellbiologischen Grundlagenforschung und der medizinischen Diagnostik. Naturwissenschaften 1996, 83, 193–200. [Google Scholar] [CrossRef]
- Wolf, B.; Brischwein, M.; Baumann, W.; Ehret, R.; Kraus, M. Monitoring of cellular signalling and metabolism with modular sensor-technique: The PhysioControl-Microsystem (PCM®). Biosens. Bioelectron. 1998, 13, 501–509. [Google Scholar] [CrossRef]
- Vassanelli, S.; Fromherz, P. Transistor records of excitable neurons from rat brain. Appl. Phys. A 1998, 66, 459–463. [Google Scholar] [CrossRef]
- Sprössler, C.; Denyer, M.; Britland, S.; Knoll, W.; Offenhäusser, A. Electrical recordings from rat cardiac muscle cells using field-effect transistors. Phys. Rev. E 1999, 60, 2171. [Google Scholar] [CrossRef] [PubMed]
- Baumann, W.; Lehmann, M.; Schwinde, A.; Ehret, R.; Brischwein, M.; Wolf, B. Microelectronic sensor system for microphysiological application on living cells. Sens. Actuators B Chem. 1999, 55, 77–89. [Google Scholar] [CrossRef]
- Eversmann, B.; Jenkner, M.; Hofmann, F.; Paulus, C.; Brederlow, R.; Holzapfl, B.; Fromherz, P.; Merz, M.; Brenner, M.; Schreiter, M. A 128 × 128 CMOS biosensor array for extracellular recording of neural activity. IEEE J. Solid State Circuits 2003, 38, 2306–2317. [Google Scholar] [CrossRef]
- Lambacher, A.; Jenkner, M.; Merz, M.; Eversmann, B.; Kaul, R.; Hofmann, F.; Thewes, R.; Fromherz, P. Electrical imaging of neuronal activity by multi-transistor-array (MTA) recording at 7.8 μm resolution. Appl. Phys. A 2004, 79, 1607–1611. [Google Scholar] [CrossRef]
- Lehmann, M.; Baumann, W. New insights into the nanometer-scaled cell-surface interspace by cell-sensor measurements. Exp. Cell Res. 2005, 305, 374–382. [Google Scholar] [CrossRef]
- Sakata, T.; Sugimoto, H.; Saito, A. Live monitoring of microenvironmental pH based on extracellular acidosis around cancer cells with cell-coupled gate ion-sensitive field-effect transistor. Anal. Chem. 2018, 90, 12731–12736. [Google Scholar] [CrossRef]
- Sakata, T. Biologically coupled gate field-effect transistors meet in vitro diagnostics. ACS Omega 2019, 4, 11852–11862. [Google Scholar] [CrossRef]
- Takshi, A.; Yaghoubi, H.; Wang, J.; Jun, D.; Beatty, J.T. Electrochemical Field-Effect Transistor Utilization to Study the Coupling Success Rate of Photosynthetic Protein Complexes to Cytochrome c. Biosensors 2017, 7, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milgrew, M.; Riehle, M.; Cumming, D. A large transistor-based sensor array chip for direct extracellular imaging. Sens. Actuators B Chem. 2005, 111, 347–353. [Google Scholar] [CrossRef]
- Errachid, A.; Zine, N.; Samitier, J.; Bausells, J. FET-Based Chemical Sensor Systems Fabricated with Standard Technologies. Electroanal. Int. J. Devoted Fundam. Pract. Asp. Electroanal. 2004, 16, 1843–1851. [Google Scholar] [CrossRef]
- Georgiou, P.; Toumazou, C. ISFET characteristics in CMOS and their application to weak inversion operation. Sens. Actuators B Chem. 2009, 143, 211–217. [Google Scholar] [CrossRef]
- Sohbati, M.; Toumazou, C. Dimension and shape effects on the ISFET performance. IEEE Sens. J. 2014, 15, 1670–1679. [Google Scholar] [CrossRef]
- Barbaro, M.; Caboni, A.; Loi, D.; Lai, S.; Homsy, A.; Van Der Wal, P.; De Rooij, N. Label-free, direct DNA detection by means of a standard CMOS electronic chip. Sens. Actuators B Chem. 2012, 171, 148–154. [Google Scholar] [CrossRef]
- Park, M.-H.; Han, D.; Chand, R.; Lee, D.-H.; Kim, Y.-S. Mechanism of label-free DNA detection using the floating electrode on pentacene thin film transistor. J. Phys. Chem. C 2016, 120, 4854–4859. [Google Scholar] [CrossRef]
- Barbaro, M.; Bonfiglio, A.; Raffo, L.; Alessandrini, A.; Facci, P.; BarakBarak, I. A CMOS, fully integrated sensor for electronic detection of DNA hybridization. IEEE Electron Device Lett. 2006, 27, 595–597. [Google Scholar] [CrossRef]
- Zhang, Q.; Majumdar, H.S.; Kaisti, M.; Prabhu, A.; Ivaska, A.; Österbacka, R.; Rahman, A.; Levon, K. Surface functionalization of ion-sensitive floating-gate field-effect transistors with organic electronics. IEEE Trans. Electron Devices 2015, 62, 1291–1298. [Google Scholar] [CrossRef]
- Jayant, K.; Auluck, K.; Funke, M.; Anwar, S.; Phelps, J.B.; Gordon, P.H.; Rajwade, S.R.; Kan, E.C. Programmable ion-sensitive transistor interfaces. I. Electrochemical gating. Phys. Rev. E 2013, 88, 012801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayant, K.; Auluck, K.; Rodriguez, S.; Cao, Y.; Kan, E.C. Programmable ion-sensitive transistor interfaces. III. Design considerations, signal generation, and sensitivity enhancement. Phys. Rev. E 2014, 89, 052817. [Google Scholar] [CrossRef]
- Cohen, A.; Spira, M.E.; Yitshaik, S.; Borghs, G.; Shwartzglass, O.; Shappir, J. Depletion type floating gate p-channel MOS transistor for recording action potentials generated by cultured neurons. Biosens. Bioelectron. 2004, 19, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Jayant, K.; Singhai, A.; Cao, Y.; Phelps, J.B.; Lindau, M.; Holowka, D.A.; Baird, B.A.; Kan, E.C. Non-Faradaic Electrochemical Detection of Exocytosis from Mast and Chromaffin Cells Using Floating-Gate MOS Transistors. Sci. Rep. 2015, 5, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pullano, S.A.; Critello, C.D.; Mahbub, I.; Tasneem, N.T.; Shamsir, S.; Islam, S.K.; Greco, M.; Fiorillo, A.S. EGFET-based sensors for bioanalytical applications: A review. Sensors 2018, 18, 4042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarasov, A.; Gray, D.W.; Tsai, M.-Y.; Shields, N.; Montrose, A.; Creedon, N.; Lovera, P.; O’Riordan, A.; Mooney, M.H.; Vogel, E.M. A potentiometric biosensor for rapid on-site disease diagnostics. Biosens. Bioelectron. 2016, 79, 669–678. [Google Scholar] [CrossRef] [Green Version]
- Baldacchini, C.; Montanarella, A.F.; Francioso, L.; Signore, M.A.; Cannistraro, S.; Bizzarri, A.R. A Reliable BioFET Immunosensor for Detection of p53 Tumour Suppressor in Physiological-Like Environment. Sensors 2020, 20, 6364. [Google Scholar] [CrossRef]
- Jang, H.-J.; Cho, W.-J. Performance enhancement of capacitive-coupling dual-gate ion-sensitive field-effect transistor in ultra-thin-body. Sci. Rep. 2014, 4, 1–8. [Google Scholar] [CrossRef]
- Spijkman, M.J.; Myny, K.; Smits, E.C.; Heremans, P.; Blom, P.W.; De Leeuw, D.M. Dual-gate thin-film transistors, integrated circuits and sensors. Adv. Mater. 2011, 23, 3231–3242. [Google Scholar] [CrossRef]
- Spijkman, M.; Smits, E.; Cillessen, J.; Biscarini, F.; Blom, P.; De Leeuw, D. Beyond the Nernst-limit with dual-gate ZnO ion-sensitive field-effect transistors. Appl. Phys. Lett. 2011, 98, 043502. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-J.; Lin, C.-C.; Huang, J.-C.; Hsieh, C.-H.; Wen, C.-H.; Chen, T.-T.; Jeng, L.-S.; Yang, C.-K.; Yang, J.-H.; Tsui, F. High performance dual-gate ISFET with non-ideal effect reduction schemes in a SOI-CMOS bioelectrical SoC. In Proceedings of the 2015 IEEE International Electron Devices Meeting (IEDM), Washington, DC, USA, 7–9 December 2015. [Google Scholar]
- Novodchuk, I.; Bajcsy, M.; Yavuz, M. Graphene-Based Field Effect Transistor Biosensors for Breast Cancer Detection: A Review on Biosensing Strategies. Carbon 2020. [Google Scholar] [CrossRef]
- Yang, N.; Chen, X.; Ren, T.; Zhang, P.; Yang, D. Carbon nanotube based biosensors. Sens. Actuators B Chem. 2015, 207, 690–715. [Google Scholar] [CrossRef]
- Shen, M.-Y.; Li, B.-R.; Li, Y.-K. Silicon nanowire field-effect-transistor based biosensors: From sensitive to ultra-sensitive. Biosens. Bioelectron. 2014, 60, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Zhao, H.; Quan, X. Two-dimensional MoS2: A promising building block for biosensors. Biosens. Bioelectron. 2017, 89, 56–71. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Ahn, M.-S.; Hahn, Y.-B. ZnO nanorods array based field-effect transistor biosensor for phosphate detection. J. Colloid Interface Sci. 2017, 498, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Hagen, J.A.; Kim, S.N.; Bayraktaroglu, B.; Leedy, K.; Chávez, J.L.; Kelley-Loughnane, N.; Naik, R.R.; Stone, M.O. Biofunctionalized Zinc Oxide Field Effect Transistors for Selective Sensing of Riboflavin with Current Modulation. Sensors 2011, 11, 6645–6655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.H.; Lee, J.O.; Choi, S.; Yoon, J.B.; Cho, G.H. A CMOS label-free DNA sensor using electrostatic induction of molecular charges. Biosens. Bioelectron. 2012, 31, 343–348. [Google Scholar] [CrossRef]
- Kim, J.Y.; Choi, K.; Moon, D.I.; Ahn, J.H.; Park, T.J.; Lee, S.Y.; Choi, Y.K. Surface engineering for enhancement of sensitivity in an underlap-FET biosensor by control of wettability. Biosens. Bioelectron. 2013, 41, 867–870. [Google Scholar] [CrossRef]
- Gao, A.; Zou, N.; Dai, P.; Lu, N.; Li, T.; Wang, Y.; Zhao, J.; Mao, H. Signal-to-noise ratio enhancement of silicon nanowires biosensor with rolling circle amplification. Nano Lett. 2013, 13, 4123–4130. [Google Scholar] [CrossRef]
- Park, K.-T.; Cho, D.-G.; Park, J.-W.; Hong, S.; Hwang, J. Detection of airborne viruses using electro-aerodynamic deposition and a field-effect transistor. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Choi, J.; Jeun, M.; Kim, Y.; Yuk, S.S.; Kim, S.K.; Song, C.S.; Lee, S.; Lee, K.H. Detection of Avian Influenza Virus from Cloacal Swabs Using a Disposable Well Gate FET Sensor. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, H.; Li, Y.-T.; Xiao, M.-M.; Zhang, Z.-L.; Pang, D.-W.; Wong, G.; Zhang, Z.-Y.; Zhang, G.-J. A field effect transistor modified with reduced graphene oxide for immunodetection of Ebola virus. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef]
- Shariati, M. The field effect transistor DNA biosensor based on ITO nanowires in label-free hepatitis B virus detecting compatible with CMOS technology. Biosens. Bioelectron. 2018, 105, 58–64. [Google Scholar] [CrossRef]
- Vu, C.A.; Chen, W.Y. Field-Effect Transistor Biosensors for Biomedical Applications: Recent Advances and Future Prospects. Sensors 2019, 19, 4214. [Google Scholar] [CrossRef] [Green Version]
- Dai, X.; Vo, R.; Hsu, H.H.; Deng, P.; Zhang, Y.; Jiang, X. Modularized Field-Effect Transistor Biosensors. Nano Lett. 2019, 19, 6658–6664. [Google Scholar] [CrossRef] [PubMed]
- Rajan, N.K.; Brower, K.; Duan, X.; Reed, M.A. Limit of detection of field effect transistor biosensors: Effects of surface modification and size dependence. Appl. Phys. Lett. 2014, 104, 084106. [Google Scholar] [CrossRef] [Green Version]
- Yüce, M.; Kurt, H. How to make nanobiosensors: Surface modification and characterisation of nanomaterials for biosensing applications. RSC Adv. 2017, 7, 49386–49403. [Google Scholar] [CrossRef] [Green Version]
- Noah, N.M.; Ndangili, P.M. Current Trends of Nanobiosensors for Point-of-Care Diagnostics. J. Anal. Methods Chem. 2019, 2019, 2179718. [Google Scholar] [CrossRef] [PubMed]
- Chartuprayoon, N.; Zhang, M.; Bosze, W.; Choa, Y.H.; Myung, N.V. One-dimensional nanostructures based bio-detection. Biosens. Bioelectron. 2015, 63, 432–443. [Google Scholar] [CrossRef]
- Hou, S.; Zhang, A.; Su, M. Nanomaterials for Biosensing Applications. Nanomaterials 2016, 6, 58. [Google Scholar] [CrossRef] [Green Version]
- Uhm, M.; Lee, J.M.; Lee, J.; Lee, J.H.; Choi, S.; Park, B.G.; Kim, D.M.; Choi, S.J.; Mo, H.S.; Jeong, Y.J.; et al. Ultrasensitive Electrical Detection of Hemagglutinin for Point-of-Care Detection of Influenza Virus Based on a CMP-NANA Probe and Top-Down Processed Silicon Nanowire Field-Effect Transistors. Sensors 2019, 19, 4502. [Google Scholar] [CrossRef] [Green Version]
- Chiang, P.L.; Chou, T.C.; Wu, T.H.; Li, C.C.; Liao, C.D.; Lin, J.Y.; Tsai, M.H.; Tsai, C.C.; Sun, C.J.; Wang, C.H.; et al. Nanowire transistor-based ultrasensitive virus detection with reversible surface functionalization. Chem. Asian J. 2012, 7, 2073–2079. [Google Scholar] [CrossRef]
- Kwon, J.; Lee, Y.; Lee, T.; Ahn, J.H. Aptamer-Based Field-Effect Transistor for Detection of Avian Influenza Virus in Chicken Serum. Anal. Chem. 2020, 92, 5524–5531. [Google Scholar] [CrossRef]
- Macchia, E.; Sarcina, L.; Picca, R.A.; Manoli, K.; Di Franco, C.; Scamarcio, G.; Torsi, L. Ultra-low HIV-1 p24 detection limits with a bioelectronic sensor. Anal. Bioanal. Chem. 2020, 412, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Basu, J.; RoyChaudhuri, C. Graphene Nanogrids FET Immunosensor: Signal to Noise Ratio Enhancement. Sensors 2016, 16, 1481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Kim, Y.H.; Cheon, D.S.; Seo, T.S. Micropatterned reduced graphene oxide based field-effect transistor for real-time virus detection. Sens. Actuators B Chem. 2013, 186, 252–257. [Google Scholar] [CrossRef]
- Pant, M.; Kharkwal, H.; Singh, K.P.; Joshi, H.C. Detection of Rota Virus with the Help of Nanomaterial Based Field Effect Transistor (BIO-FET). Biosens. J. 2017, 06. [Google Scholar] [CrossRef]
- Aspermair, P.; Mishyn, V.; Bintinger, J.; Happy, H.; Bagga, K.; Subramanian, P.; Knoll, W.; Boukherroub, R.; Szunerits, S. Reduced graphene oxide-based field effect transistors for the detection of E7 protein of human papillomavirus in saliva. Anal. Bioanal. Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ren, R.; Pu, H.; Guo, X.; Chang, J.; Zhou, G.; Mao, S.; Kron, M.; Chen, J. Field-Effect Transistor Biosensor for Rapid Detection of Ebola Antigen. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.W.; Kim, S.; Jang, Y.H.; Lim, K.I.; Lee, W.H. Attomolar detection of virus by liquid coplanar-gate graphene transistor on plastic. Nanotechnology 2019, 30, 345502. [Google Scholar] [CrossRef] [PubMed]
- Ono, T.; Kawata, T.; Kanai, Y.; Ohno, Y.; Maehashi, K.; Inoue, K.; Watanabe, Y.; Nakakita, S.; Suzuki, Y.; Kawahara, T.; et al. High-Sensitive and Selective Detection of Human-Infectious Influenza Virus Using Biomimetic Graphene Field-Effect Transistor. In Proceedings of the 2018 76th Device Research Conference (DRC), Santa Barbara, CA, USA, 24–27 June 2018. [Google Scholar] [CrossRef]
- Ono, T.; Kamada, K.; Hayashi, R.; Piacenti, A.R.; Gabbutt, C.; Sriwilaijaroen, N.; Hiramatsu, H.; Kanai, Y.; Inoue, K.; Nakakita, S.; et al. Lab-on-a-graphene-FET detection of key molecular events underpinning influenza virus infection and effect of antiviral drugs. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Hideshima, S.; Hayashi, H.; Hinou, H.; Nambuya, S.; Kuroiwa, S.; Nakanishi, T.; Momma, T.; Nishimura, S.I.; Sakoda, Y.; Osaka, T. Glycan-immobilized dual-channel field effect transistor biosensor for the rapid identification of pandemic influenza viral particles. Sci. Rep. 2019, 9, 11616. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Carey, P.; Ren, F.; Mastro, M.A.; Beers, K.; Pearton, S.J.; Kravchenko, I.I. Zika virus detection using antibody-immobilized disposable cover glass and AlGaN/GaN high electron mobility transistors. Appl. Phys. Lett. 2018, 113, 032101. [Google Scholar] [CrossRef] [Green Version]
- Malpartida-Cardenas, K.; Miscourides, N.; Rodriguez-Manzano, J.; Yu, L.-S.; Moser, N.; Baum, J.; Georgiou, P. Quantitative and rapid Plasmodium falciparum malaria diagnosis and artemisinin-resistance detection using a CMOS Lab-on-Chip platform. Biosens. Bioelectron. 2019, 145, 111678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Zheng, G.; Lieber, C.M. Nanowire Field-Effect Transistor Sensors; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Ambhorkar, P.; Wang, Z.; Ko, H.; Lee, S.; Koo, K.I.; Kim, K.; Cho, D.D. Nanowire-Based Biosensors: From Growth to Applications. Micromachines 2018, 9, 679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyyappan, M.; Lee, J.-S. Nanowire BioFETs: An Overview. Nanowire Field Effect Transistors Princ. Appl. 2014. [Google Scholar] [CrossRef]
- Namdari, P.; Daraee, H.; Eatemadi, A. Recent Advances in Silicon Nanowire Biosensors: Synthesis Methods, Properties, and Applications. Nanoscale Res. Lett. 2016, 11, 406. [Google Scholar] [CrossRef] [Green Version]
- Zheng, G.; Lieber, C.M. Nanowire biosensors for label-free, real-time, ultrasensitive protein detection. Methods Mol. Biol. 2011, 790, 223–237. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.Y.; Lin, C.H.; Feng, M.H.; Chen, C.H.; Su, P.C.; Yang, P.W.; Zheng, J.M.; Fu, C.W.; Yang, Y.S. Preparation of Silicon Nanowire Field-effect Transistor for Chemical and Biosensing Applications. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, B.; Morrow, T.J.; Keating, C.D. Nanowire sensors for multiplexed detection of biomolecules. Curr. Opin. Chem. Biol. 2008, 12, 522–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattarai, J.K.; Neupane, D.; Nepal, B.; Mikhaylov, V.; Demchenko, A.V.; Stine, K.J. Preparation, Modification, Characterization, and Biosensing Application of Nanoporous Gold Using Electrochemical Techniques. Nanomaterials 2018, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of Gold Nanoparticles in Non-Optical Biosensors. Nanomaterials 2018, 8, 977. [Google Scholar] [CrossRef] [Green Version]
- Lan, S.; Veiseh, M.; Zhang, M. Surface modification of silicon and gold-patterned silicon surfaces for improved biocompatibility and cell patterning selectivity. Biosens. Bioelectron. 2005, 20, 1697–1708. [Google Scholar] [CrossRef]
- Forsyth, R.; Devadoss, A.; Guy, O.J. Graphene Field Effect Transistors for Biomedical Applications: Current Status and Future Prospects. Diagnostics 2017, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, A.N. Graphene and its derivatives as biomedical materials: Future prospects and challenges. Interface Focus 2018, 8, 20170056. [Google Scholar] [CrossRef]
- Sturala, J.; Luxa, J.; Pumera, M.; Sofer, Z. Chemistry of Graphene Derivatives: Synthesis, Applications, and Perspectives. Chemistry 2018, 24, 5992–6006. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xu, T.; Zhang, X. Graphene-Based Biosensors for Detection of Biomarkers. Micromachines 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szunerits, S.; Boukherroub, R. Graphene-based biosensors. Interface Focus 2018, 8, 20160132. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-N.; Hu, P.-A. Carbon nanomaterials: Controlled growth and field-effect transistor biosensors. Front. Mater. Sci. 2012, 6, 26–46. [Google Scholar] [CrossRef]
- Giordano, C.; Filatrella, G.; Sarno, M.; Di Bartolomeo, A. Multi-walled carbon nanotube films for the measurement of the alcoholic concentration. Micro Nano Lett. 2019, 14, 304–308. [Google Scholar] [CrossRef] [Green Version]
- Tilmaciu, C.M.; Morris, M.C. Carbon nanotube biosensors. Front. Chem. 2015, 3, 59. [Google Scholar] [CrossRef] [Green Version]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [Green Version]
- Eatemadi, A.; Daraee, H.; Karimkhanloo, H.; Kouhi, M.; Zarghami, N.; Akbarzadeh, A.; Abasi, M.; Hanifehpour, Y.; Joo, S.W. Carbon nanotubes: Properties, synthesis, purification, and medical applications. Nanoscale Res. Lett. 2014, 9, 393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilli, L.; Passacantando, M. Advances on Sensors Based on Carbon Nanotubes. Chemosensors 2018, 6, 62. [Google Scholar] [CrossRef] [Green Version]
- Sedki, M.; Chen, Y.; Mulchandani, A. Non-Carbon 2D Materials-Based Field-Effect Transistor Biosensors: Recent Advances, Challenges, and Future Perspectives. Sensors 2020, 20, 4811. [Google Scholar] [CrossRef] [PubMed]
- Manzeli, S.; Ovchinnikov, D.; Pasquier, D.; Yazyev, O.V.; Kis, A. 2D transition metal dichalcogenides. Nat. Rev. Mater. 2017, 2. [Google Scholar] [CrossRef]
- Zhou, X.; Sun, H.; Bai, X. Two-Dimensional Transition Metal Dichalcogenides: Synthesis, Biomedical Applications and Biosafety Evaluation. Front. Bioeng. Biotechnol. 2020, 8, 236. [Google Scholar] [CrossRef]
- Pumera, M.; Loo, A.H. Layered transition-metal dichalcogenides (MoS2 and WS2) for sensing and biosensing. Trac Trends Anal. Chem. 2014, 61, 49–53. [Google Scholar] [CrossRef]
- Singh, A.K.; Kumar, P.; Late, D.J.; Kumar, A.; Patel, S.; Singh, J. 2D layered transition metal dichalcogenides (MoS2): Synthesis, applications and theoretical aspects. Appl. Mater. Today 2018, 13, 242–270. [Google Scholar] [CrossRef]
- Hu, Y.; Huang, Y.; Tan, C.; Zhang, X.; Lu, Q.; Sindoro, M.; Huang, X.; Huang, W.; Wang, L.; Zhang, H. Two-dimensional transition metal dichalcogenide nanomaterials for biosensing applications. Mater. Chem. Front. 2017, 1, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Chen, X.; Wang, Q.; Xiao, M.; Zhong, D.; Sun, W.; Zhang, G.; Zhang, Z. Ultrasensitive monolayer MoS2 field-effect transistor based DNA sensors for screening of down syndrome. Nano Lett. 2019, 19, 1437–1444. [Google Scholar] [CrossRef]
- Gong, X.; Liu, Y.; Xiang, H.; Liu, H.; Liu, Z.; Zhao, X.; Li, J.; Li, H.; Hong, G.; Hu, T.S.; et al. Membraneless reproducible MoS2 field-effect transistor biosensor for high sensitive and selective detection of FGF21. Sci. China Mater. 2019, 62, 1479–1487. [Google Scholar] [CrossRef] [Green Version]
- Mei, J.; Li, Y.T.; Zhang, H.; Xiao, M.M.; Ning, Y.; Zhang, Z.Y.; Zhang, G.J. Molybdenum disulfide field-effect transistor biosensor for ultrasensitive detection of DNA by employing morpholino as probe. Biosens. Bioelectron. 2018, 110, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Dak, P.; Lee, Y.; Park, H.; Choi, W.; Alam, M.A.; Kim, S. Two-dimensional Layered MoS2 Biosensors Enable Highly Sensitive Detection of Biomolecules. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nam, H.; Oh, B.-R.; Chen, M.; Wi, S.; Li, D.; Kurabayashi, K.; Liang, X. Fabrication and comparison of MoS2 and WSe2 field-effect transistor biosensors. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2015, 33, 06FG01. [Google Scholar] [CrossRef]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS(2) field-effect transistor for next-generation label-free biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, P.; Zeng, X.; Young, C.D.; Hu, W. High-stability pH sensing with a few-layer MoS2 field-effect transistor. Nanotechnology 2019, 30, 375203. [Google Scholar] [CrossRef]
- Borole, D.D.; Kapadi, U.R.; Mahulikar, P.P.; Hundiwale, D.G. Conducting polymers: An emerging field of biosensors. Des. Monomers Polym. 2012, 9, 1–11. [Google Scholar] [CrossRef]
- Gerard, M. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Arshak, K.; Velusamy, V.; Korostynska, O.; Oliwa-Stasiak, K.; Adley, C. Conducting Polymers and Their Applications to Biosensors: Emphasizing on Foodborne Pathogen Detection. IEEE Sens. J. 2009, 9, 1942–1951. [Google Scholar] [CrossRef]
- Hangarter, C.M.; Bangar, M.; Mulchandani, A.; Myung, N.V. Conducting polymer nanowires for chemiresistive and FET-based bio/chemical sensors. J. Mater. Chem. 2010, 20, 3131. [Google Scholar] [CrossRef]
- Park, C.S.; Lee, C.; Kwon, O.S. Conducting Polymer Based Nanobiosensors. Polymers 2016, 8, 249. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.; Seo, S.E.; Kim, K.H.; Park, C.S.; Lee, S.H.; Ban, H.S.; Lee, B.D.; Song, H.S.; Kim, J.; et al. High-Performance Conducting Polymer Nanotube-based Liquid-Ion Gated Field-Effect Transistor Aptasensor for Dopamine Exocytosis. Sci. Rep. 2020, 10, 3772. [Google Scholar] [CrossRef] [Green Version]
- Su, P.-C.; Chen, B.-H.; Lee, Y.-C.; Yang, Y.-S. Silicon Nanowire Field-Effect Transistor as Biosensing Platforms for Post-Translational Modification. Biosensors 2020, 10, 213. [Google Scholar] [CrossRef] [PubMed]
- Dastagir, T.; Forzani, E.S.; Zhang, R.; Amlani, I.; Nagahara, L.A.; Tsui, R.; Tao, N. Electrical detection of hepatitis C virus RNA on single wall carbon nanotube-field effect transistors. Analyst 2007, 132, 738–740. [Google Scholar] [CrossRef] [PubMed]
- Bausells, J.; Carrabina, J.; Errachid, A.; Merlos, A. Ion-sensitive field-effect transistors fabricated in a commercial CMOS technology. Sens. Actuators B Chem. 1999, 57, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Bergveld, P. The operation of an ISFET as an electronic device. Sens. Actuators 1981, 1, 17–29. [Google Scholar] [CrossRef] [Green Version]
- Morgenshtein, A.; Sudakov-Boreysha, L.; Dinnar, U.; Jakobson, C.G.; Nemirovsky, Y. Wheatstone-Bridge readout interface for ISFET/REFET applications. Sens. Actuators B Chem. 2004, 98, 18–27. [Google Scholar] [CrossRef]
- Ravezzi, L.; Conci, P. ISFET sensor coupled with CMOS read-out circuit microsystem. Electron. Lett. 1998, 34, 2234–2235. [Google Scholar] [CrossRef]
- Nemeth, B.; Piechocinski, M.S.; Cumming, D.R. High-resolution real-time ion-camera system using a CMOS-based chemical sensor array for proton imaging. Sens. Actuators B Chem. 2012, 171, 747–752. [Google Scholar] [CrossRef]
- Hizawa, T.; Sawada, K.; Takao, H.; Ishida, M. Fabrication of a two-dimensional pH image sensor using a charge transfer technique. Sens. Actuators B Chem. 2006, 117, 509–515. [Google Scholar] [CrossRef]
- Futagawa, M.; Otake, R.; Dasai, F.; Ishida, M.; Sawada, K. Realization of 1 million pixel charge transfer type ion image sensor with 12 µm pixel pitch. In Proceedings of the 2015 Transducers-2015 18th International Conference on Solid-State Sensors, Actuators and Microsystems (TRANSDUCERS), Anchorage, AK, USA, 21–25 June 2015; pp. 236–239. [Google Scholar]
- Georgiou, P.; Toumazou, C. An adaptive ISFET chemical imager chip. In Proceedings of the 2008 IEEE International Symposium on Circuits and Systems, Seattle, WA, USA, 18–21 May 2008; pp. 2078–2081. [Google Scholar]
- Hu, Y.; Georgiou, P. A robust ISFET pH-measuring front-end for chemical reaction monitoring. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 177–185. [Google Scholar]
- Yan, L.; Georgiou, P.; Constandinou, T.G.; Garner, D.; Toumazou, C. An auto-offset-removal circuit for chemical sensing based on the PG-ISFET. In Proceedings of the 2009 IEEE International Symposium on Circuits and Systems, Taipei, Taiwan, 24–27 May 2009; pp. 1165–1168. [Google Scholar]
- Chan, W.P.; Premanode, B.; Toumazou, C. An integrated ISFETs instrumentation system in standard CMOS technology. IEEE J. Solid State Circuits 2010, 45, 1923–1934. [Google Scholar] [CrossRef] [Green Version]
- Croce, R.A.; Vaddiraju, S.; Legassey, A.; Zhu, K.; Islam, S.K.; Papadimitrakopoulos, F.; Jain, F.C. A highly miniaturized low-power CMOS-based pH monitoring platform. IEEE Sens. J. 2014, 15, 895–901. [Google Scholar] [CrossRef]
- Miscourides, N.; Georgiou, P. ISFET arrays in CMOS: A head-to-head comparison between voltage and current mode. IEEE Sens. J. 2018, 19, 1224–1238. [Google Scholar] [CrossRef]
- Premanode, B.; Silawan, N.; Chan, W.P.; Toumazou, C. A composite ISFET readout circuit employing current feedback. Sens. Actuators B Chem. 2007, 127, 486–490. [Google Scholar] [CrossRef]
- Douthwaite, M.; Koutsos, E.; Yates, D.C.; Mitcheson, P.D.; Georgiou, P. A thermally powered ISFET array for on-body pH measurement. IEEE Trans. Biomed. Circuits Syst. 2017, 11, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Kuang, L.; Miscourides, N.; Georgiou, P. A 128 × 128 current-mode ultra-high frame rate ISFET array with in-pixel calibration for real-time ion imaging. IEEE Trans. Biomed. Circuits Syst. 2020, 14, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Goh, Z.D.C.; Georgiou, P.; Constandinou, T.G.; Prodromakis, T.; Toumazou, C. Live demonstration: A CMOS-based lab-on-chip array for combined magnetic manipulation and opto-chemical sensing. In Proceedings of the 2011 IEEE International Symposium of Circuits and Systems (ISCAS), Rio de Janeiro, Brazi, 15–18 May 2011; pp. 1997–2001. [Google Scholar]
- Huang, X.; Yu, H.; Liu, X.; Jiang, Y.; Yan, M.; Wu, D. A dual-mode large-arrayed CMOS ISFET sensor for accurate and high-throughput pH sensing in biomedical diagnosis. IEEE Trans. Biomed. Eng. 2015, 62, 2224–2233. [Google Scholar] [CrossRef]
- Moser, N.; Lande, T.S.; Georgiou, P. A robust ISFET array with in-pixel quantisation and automatic offset calibration. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS), Shanghai, China, 17–19 October 2016; pp. 50–53. [Google Scholar]
- Wang, K.; Liu, Y.; Toumazou, C.; Georgiou, P. A TDC based ISFET readout for large-scale chemical sensing systems. In Proceedings of the 2012 IEEE Biomedical Circuits and Systems Conference (BioCAS), Hsinchu, Taiwan, 28–30 November 2012; pp. 176–179. [Google Scholar]
- Al-Ahdal, A.; Toumazou, C. High gain ISFET based νMOS chemical inverter. Sens. Actuators B Chem. 2012, 171, 110–117. [Google Scholar] [CrossRef]
- Nabovati, G.; Ghafar-Zadeh, E.; Sawan, M. A 64 pixel ISFET-based biosensor for extracellular pH gradient monitoring. In Proceedings of the 2015 IEEE International Symposium on Circuits and Systems (ISCAS), Lisbon, Portugal, 24–27 May 2015; pp. 1762–1765. [Google Scholar]
- Liu, Y.; Toumazou, C. An ISFET based sensing array with sensor offset compensation and pH sensitivity enhancement. In Proceedings of the Proceedings of 2010 IEEE International Symposium on Circuits and Systems, Paris, France, 30 May–2 June 2010; pp. 2283–2286. [Google Scholar]
- Milgrew, M.; Hammond, P.; Cumming, D. The development of scalable sensor arrays using standard CMOS technology. Sens. Actuators B Chem. 2004, 103, 37–42. [Google Scholar] [CrossRef]
- Hammond, P.A.; Ali, D.; Cumming, D.R. Design of a single-chip pH sensor using a conventional 0.6 µm CMOS process. IEEE Sens. J. 2004, 4, 706–712. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.-S.; White, M.H. A CMOS-integrated’ISFET-operational amplifier’chemical sensor employing differential sensing. IEEE Trans. Electron Devices 1989, 36, 479–487. [Google Scholar] [CrossRef]
- Palan, B.; Santos, F.; Karam, J.; Courtois, B.; Husak, M. New ISFET sensor interface circuit for biomedical applications. Sens. Actuators B Chem. 1999, 57, 63–68. [Google Scholar] [CrossRef]
- Nabovati, G.; Ghafarzadeh, E.; Awwad, F.; Sawan, M. A sigma delta ISFET readout circuit for Lab-on-Chip applications. In Proceedings of the New Circuits and Systems Conference (NEWCAS), Paris, France, 16–19 June 2013; pp. 1–4. [Google Scholar]
- Kalofonou, M.; Toumazou, C. A Low Power Sub-µW Chemical Gilbert Cell for ISFET Differential Reaction Monitoring. IEEE Trans. Biomed. Circuits Syst. 2013, 8, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Bergveld, P. Thirty years of ISFETOLOGY: What happened in the past 30 years and what may happen in the next 30 years. Sens. Actuators B Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Georgiou, P.; Toumazou, C. An adaptive CMOS-based PG-ISFET for pH sensing. In Proceedings of the 2009 IEEE International Symposium on Circuits and Systems, Taipei, Taiwan, 24–27 May 2009; pp. 557–560. [Google Scholar]
- Jamasb, S. An analytical technique for counteracting drift in ion-selective field effect transistors (ISFETs). IEEE Sens. J. 2004, 4, 795–801. [Google Scholar] [CrossRef]
- Moser, N.; Lande, T.S.; Toumazou, C.; Georgiou, P. ISFETs in CMOS and emergent trends in instrumentation: A review. IEEE Sens. J. 2016, 16, 6496–6514. [Google Scholar] [CrossRef]
- Hammond, P.; Cumming, D. Performance and system-on-chip integration of an unmodified CMOS ISFET. Sens. Actuators B Chem. 2005, 111, 254–258. [Google Scholar] [CrossRef]
- Hammond, P.A.; Ali, D.; Cumming, D.R. A system-on-chip digital pH meter for use in a wireless diagnostic capsule. IEEE Trans. Biomed. Eng. 2005, 52, 687–694. [Google Scholar] [CrossRef]
- Rothberg, J.M.; Hinz, W.; Johnson, K.L.; Bustillo, J. Methods and Apparatus for Measuring Analytes Using Large Scale FET Arrays. Google Patents GB2461127B, 18 September 2011. [Google Scholar]
- Hu, Y.; Georgiou, P. 3-T ISFET front-end utilising parasitic device capacitance. Electron. Lett. 2014, 50, 1507–1509. [Google Scholar] [CrossRef]
- Uzzal, M.M.; Zarkesh-Ha, P.; Edwards, J.S.; Coelho, E.; Rawat, P. A highly sensitive ISFET using pH-to-current conversion for real-time DNA sequencing. In Proceedings of the 2014 27th IEEE International System-on-Chip Conference (SOCC), Las Vegas, NV, USA, 2–5 September 2014; pp. 410–414. [Google Scholar]
- Georgiou, P.; Toumazou, C. Chemical log-domain filter. Electron. Lett. 2009, 45, 391–392. [Google Scholar] [CrossRef]
- Shepherd, L.M.; Toumazou, C. A biochemical translinear principle with weak inversion ISFETs. IEEE Trans. Circuits Syst. I Regul. Pap. 2005, 52, 2614–2619. [Google Scholar] [CrossRef]
- Pookaiyaudom, P.; Toumazou, C.; Lidgey, F. The chemical current-conveyor: A new microchip biosensor. In Proceedings of the 2008 IEEE International Symposium on Circuits and Systems, Seattle, WA, USA, 18–21 May 2008; pp. 3166–3169. [Google Scholar]
- Miscourides, N.; Georgiou, P. A linear programmable-gate ISFET array operating in velocity saturation. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS), Shanghai, China, 17–19 October 2016; pp. 292–295. [Google Scholar]
- Premanode, B.; Chan, W.; Toumazou, C. Ultra-low power precision ISFET readout using global current feedback. Electron. Lett. 2006, 42, 1264–1265. [Google Scholar] [CrossRef]
- Chan, W.; Premanode, B.; Toumazou, C. 64 pH-ISFET averaging array employing global negative current feedback. Electron. Lett. 2009, 45, 536–537. [Google Scholar] [CrossRef]
- Juffali, W.; Georgiou, P.; Toumazou, C. ISFET based Urea: Creatinine translinear sensor. Electron. Lett. 2010, 46, 746–748. [Google Scholar] [CrossRef]
- Kalofonou, M.; Georgiou, P.; Ou, C.-P.; Toumazou, C. An ISFET based translinear sensor for DNA methylation detection. Sens. Actuators B Chem. 2012, 161, 156–162. [Google Scholar] [CrossRef]
- Wong, W.; Shepherd, L.; Georgiou, P.; Toumazou, C. Towards ISFET based DNA logic for rapid nucleic acid detection. In Proceedings of the SENSORS, Christchurch, New Zealand, 25–28 October 200; pp. 1451–1454.
- Al-Ahdal, A.; Toumazou, C. ISFET-based chemical switch. IEEE Sens. J. 2011, 12, 1140–1146. [Google Scholar] [CrossRef]
- AlAhdal, A.; Toumazou, C. ISFET-based chemical Schmitt trigger. Electron. Lett. 2012, 48, 549–551. [Google Scholar] [CrossRef]
- Liu, Y.; Al-Ahdal, A.; Georgiou, P.; Toumazou, C. Minimal readout scheme for ISFET sensing arrays based on pulse width modulation. Electron. Lett. 2012, 48, 548–549. [Google Scholar] [CrossRef]
- Do, A.T.; Minkyu, J.; Yeo, K.S. Improved inverter-based read-out scheme for low-power ISFET sensing array. Electron. Lett. 2013, 49, 1517–1518. [Google Scholar] [CrossRef]
- Moser, N.; Lande, T.S.; Georgiou, P. A novel pH-to-time ISFET pixel architecture with offset compensation. In Proceedings of the 2015 IEEE International Symposium on Circuits and Systems (ISCAS), Lisbon, Portugal, 24–27 May 2015; pp. 481–484. [Google Scholar]
- Jiang, Y.; Coquet, P.; Yu, H. Fast food safety screening with CMOS high-sensitivity large-arrayed ISFET sensor. In Proceedings of the 2017 IEEE Biomedical Circuits and Systems Conference (BioCAS), Turin, Italy, 19–21 October 2017; pp. 1–4. [Google Scholar]
- Nakajima, H.; Esashi, M.; Matsuo, T. The cation concentration response of polymer gate ISFET. J. Electrochem. Soc. 1982, 129, 141. [Google Scholar] [CrossRef]
- Matsuo, T.; Nakajima, H. Characteristics of reference electrodes using a polymer gate ISFET. Sens. Actuators 1984, 5, 293–305. [Google Scholar] [CrossRef]
- Chovelon, J.; Fombon, J.; Clechet, P.; Jaffrezic-Renault, N.; Martelet, C.; Nyamsi, A.; Cros, Y. Sensitization of dielectric surfaces by chemical grafting: Application to pH ISFETs and REFETs. Sens. Actuators B Chem. 1992, 8, 221–225. [Google Scholar] [CrossRef]
- Errachid, A.; Bausells, J.; Jaffrezic-Renault, N. A simple REFET for pH detection in differential mode. Sens. Actuators B Chem. 1999, 60, 43–48. [Google Scholar] [CrossRef]
- Bergveld, P.; Van Den Berg, A.; Van Der Wal, P.; Skowronska-Ptasinska, M.; Sudhölter, E.; Reinhoudt, D. How electrical and chemical requirements for REFETs may coincide. Sens. Actuators 1989, 18, 309–327. [Google Scholar] [CrossRef] [Green Version]
- Chudy, M.; Wróblewski, W.; Brzózka, Z. Towards REFET. Sens. Actuators B Chem. 1999, 57, 47–50. [Google Scholar] [CrossRef]
- Chodavarapu, V.; Titus, A.; Cartwright, A. Differential read-out architecture for CMOS ISFET microsystems. Electron. Lett. 2005, 41, 698–699. [Google Scholar] [CrossRef] [Green Version]
- Shepherd, L.; Georgiou, P.; Toumazou, C. A novel voltage-clamped CMOS ISFET sensor interface. In Proceedings of the 2007 IEEE International Symposium on Circuits and Systems, New Orleans, LA, USA, 27–30 May 2007; pp. 3331–3334. [Google Scholar]
- Sohbati, M.; Georgiou, P.; Toumazou, C. REFET replication for ISFET-based SNP detection arrays. In Proceedings of the 2013 IEEE International Symposium on Circuits and Systems (ISCAS), Beijing, China, 19–23 May 2013; pp. 185–188. [Google Scholar]
- Sohbati, M.; Toumazou, C. A temperature insensitive continuous time ΔpH to digital converter. In Proceedings of the 2014 IEEE International Symposium on Circuits and Systems (ISCAS), Melbourne, VIC, Australia, 1–5 June 2014; pp. 37–40. [Google Scholar]
- Milgrew, M.J.; Cumming, D.R. Matching the transconductance characteristics of CMOS ISFET arrays by removing trapped charge. IEEE Trans. Electron Devices 2008, 55, 1074–1079. [Google Scholar] [CrossRef]
- Georgiou, P.; Toumazou, C. ISFET threshold voltage programming in CMOS using hot-electron injection. Electron. Lett. 2009, 45, 1112–1113. [Google Scholar] [CrossRef]
- Al-Ahdal, A.; Toumazou, C. ISFET threshold voltage programming in CMOS using electron tunnelling. Electron. Lett. 2011, 47, 1398–1399. [Google Scholar] [CrossRef]
- Georgiou, P.; Toumazou, C. CMOS-based programmable gate ISFET. Electron. Lett. 2008, 44, 1289–1291. [Google Scholar] [CrossRef]
- Jakobson, C.; Feinsod, M.; Nemirovsky, Y. Low frequency noise and drift in ion sensitive field effect transistors. Sens. Actuators B Chem. 2000, 68, 134–139. [Google Scholar] [CrossRef]
- Jakobson, C.; Nemirovsky, Y. 1/f noise in ion sensitive field effect transistors from subthreshold to saturation. IEEE Trans. Electron Devices 1999, 46, 259–261. [Google Scholar] [CrossRef] [Green Version]
- Aw, C.-Y.; Cheung, P.W. A pH-ISFET sensor with on-chip temperature sensing. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, New Orleans, LA, USA, 4–7 November 1988; pp. 772–773. [Google Scholar]
- Chin, Y.-L.; Chou, J.-C.; Sun, T.-P.; Chung, W.-Y.; Hsiung, S.-K. A novel pH sensitive ISFET with on chip temperature sensing using CMOS standard process. Sens. Actuators B Chem. 2001, 76, 582–593. [Google Scholar] [CrossRef]
- Chung, W.-Y.; Lin, Y.-T.; Pijanowska, D.G.; Yang, C.-H.; Wang, M.-C.; Krzyskow, A.; Torbicz, W. New ISFET interface circuit design with temperature compensation. Microelectron. J. 2006, 37, 1105–1114. [Google Scholar] [CrossRef]

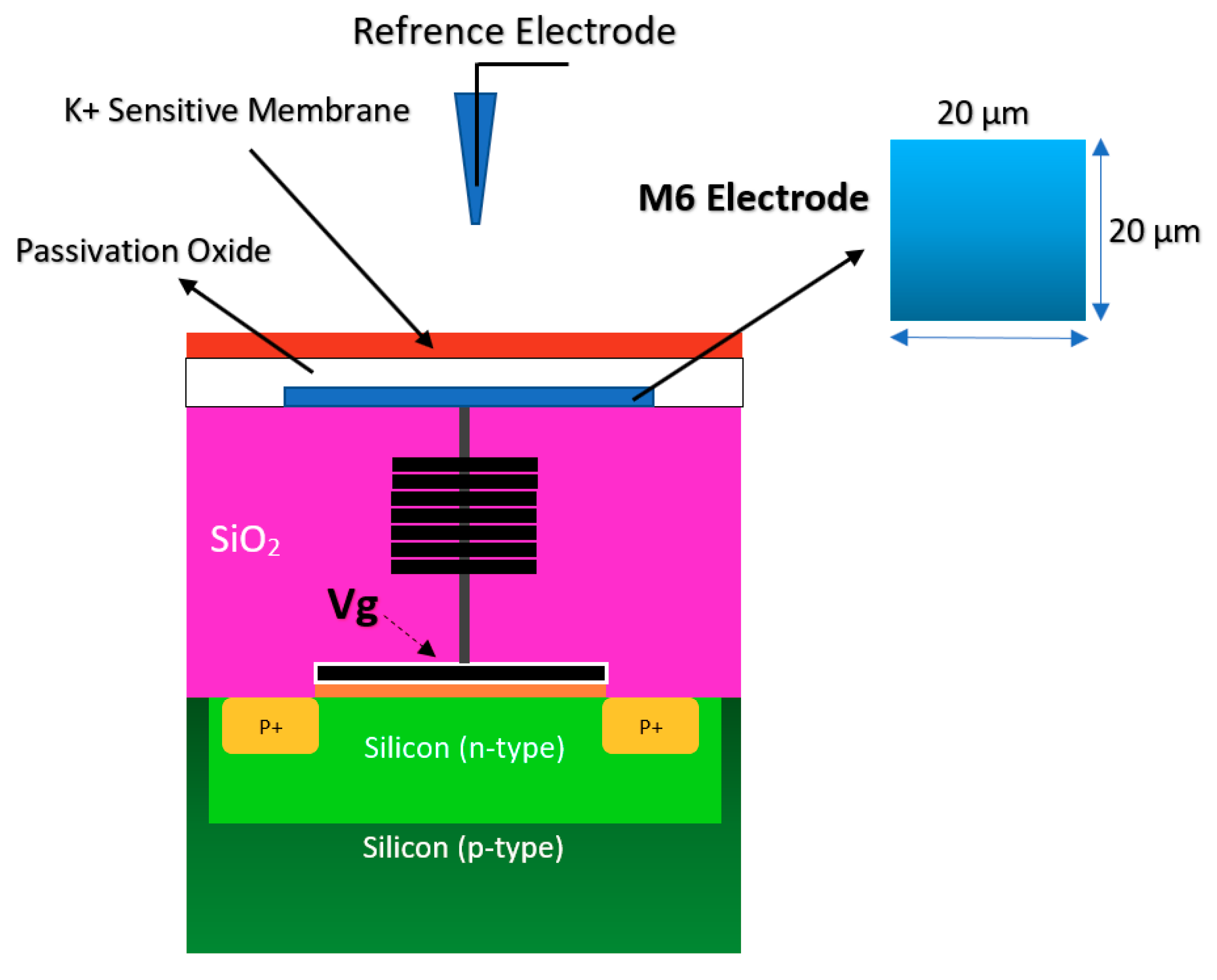
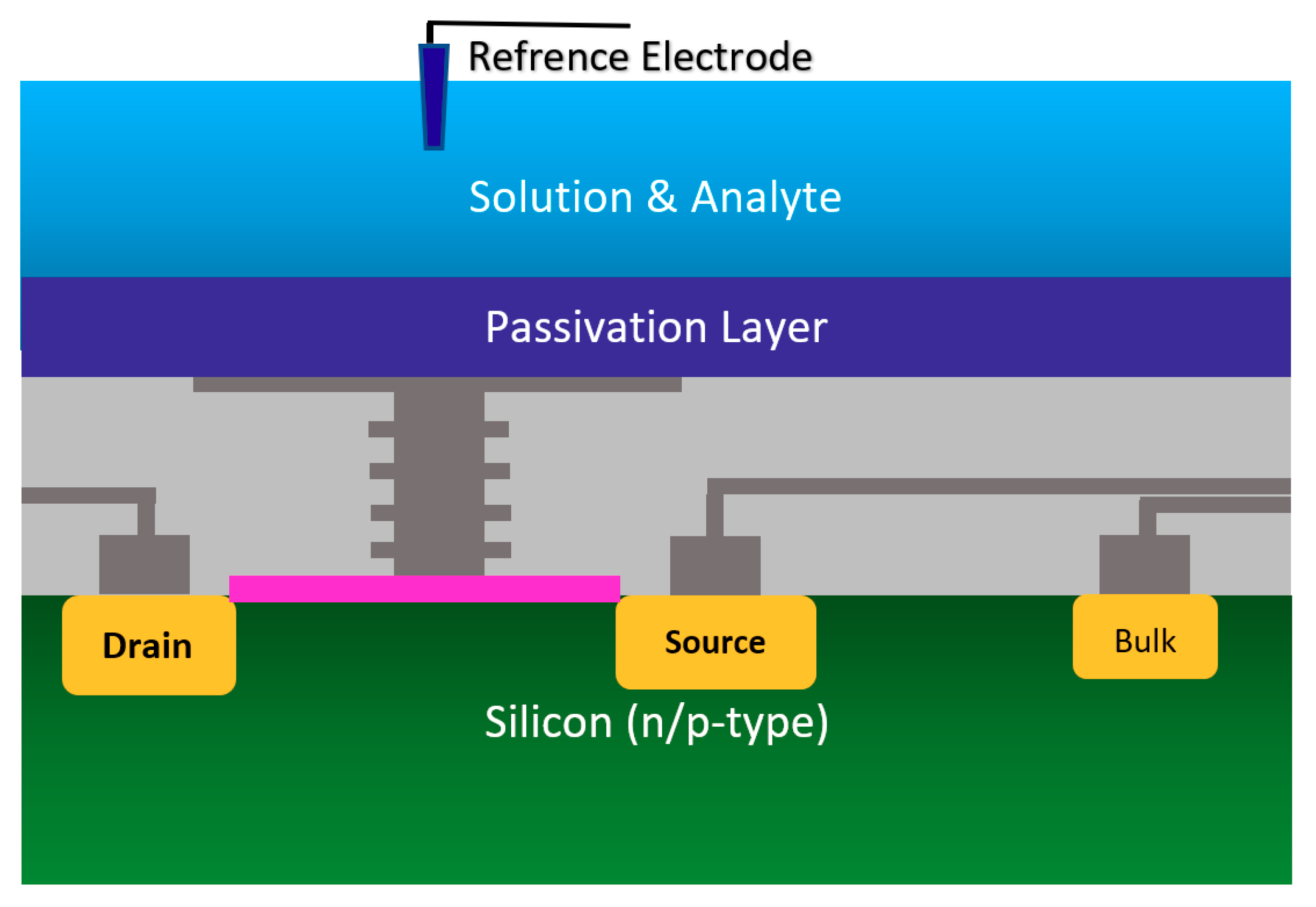




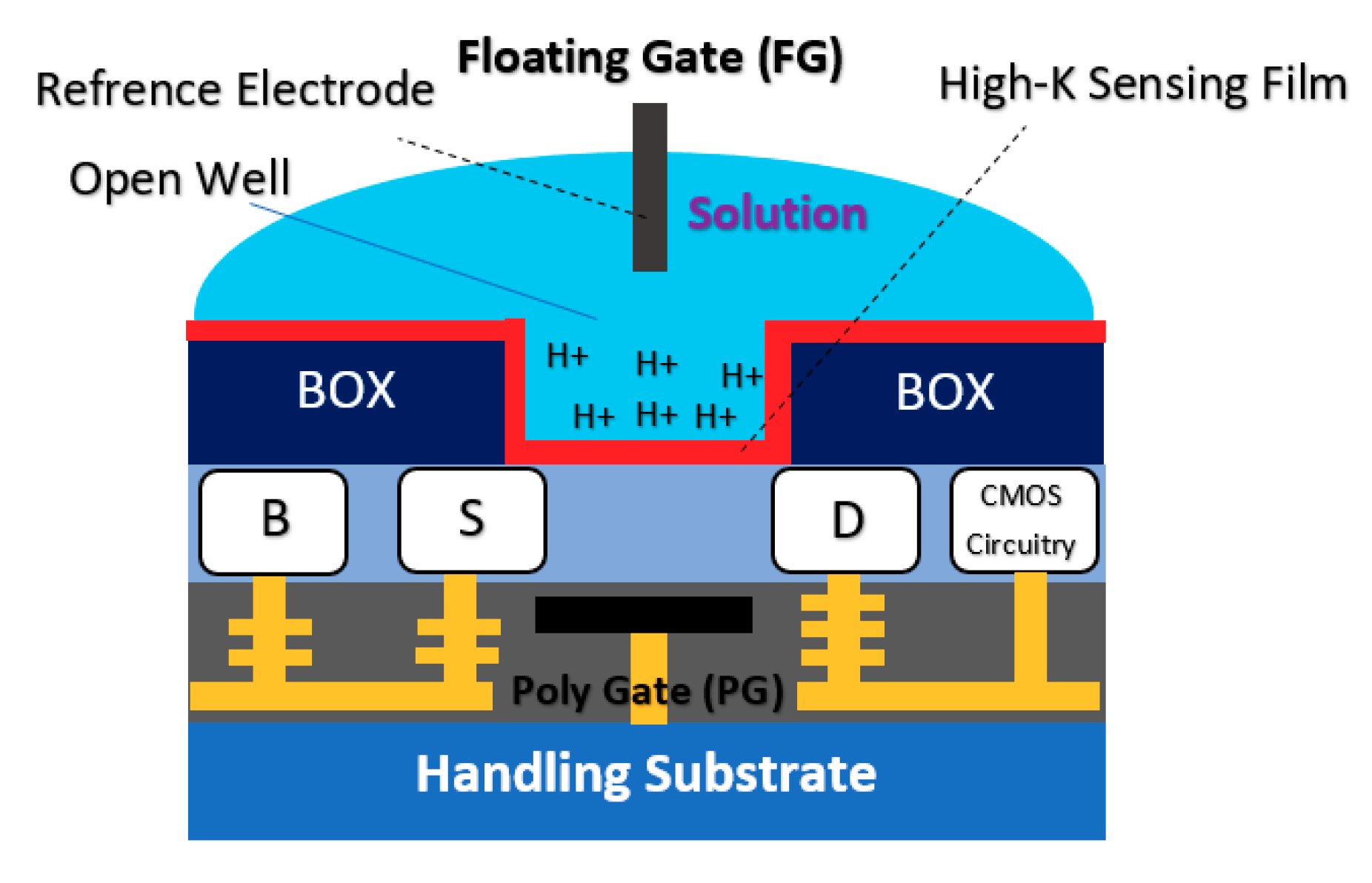
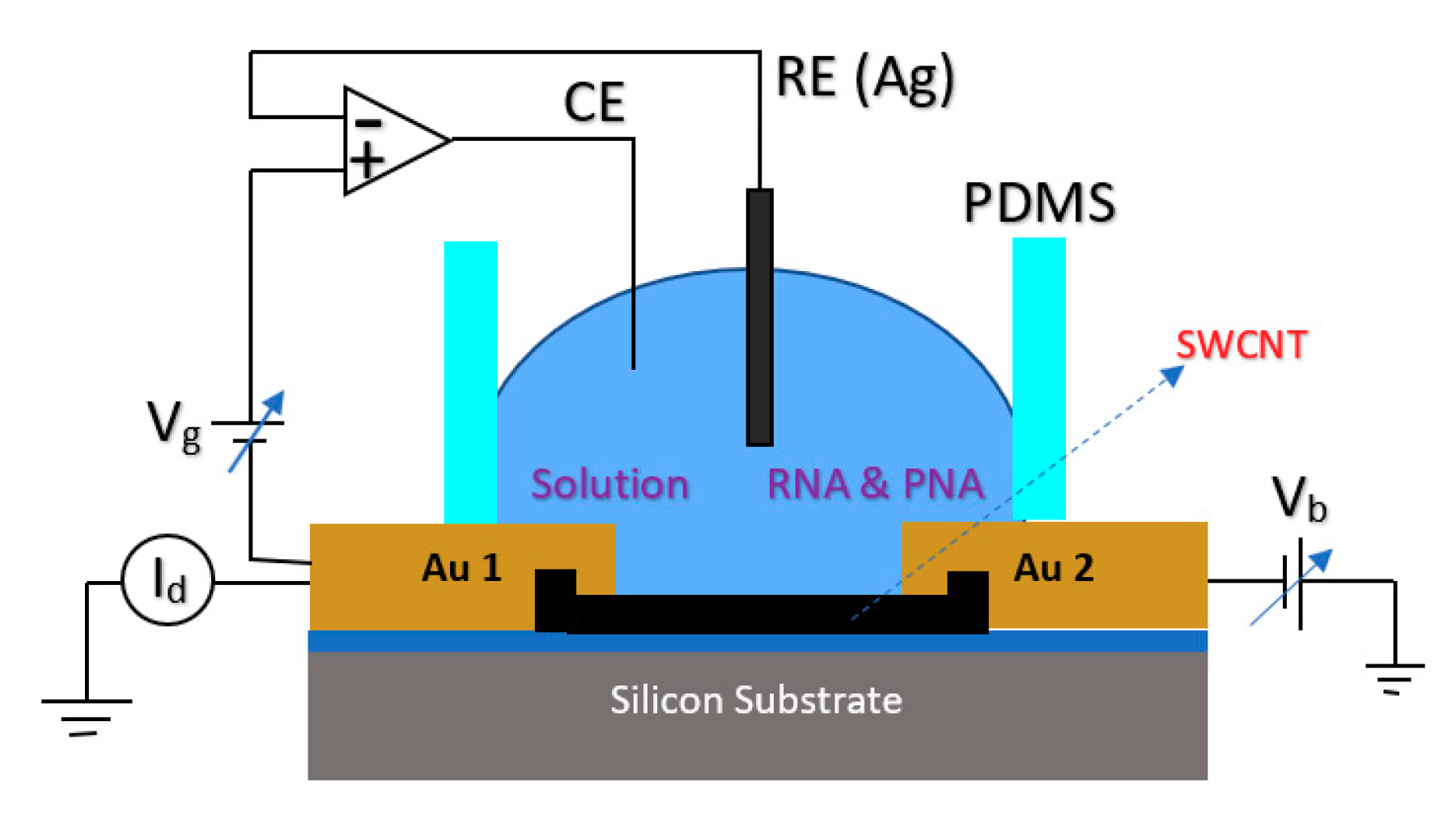




| Application | Target | BRE | Linker | Surface | Detection Range | LoD | Sample | Ref. |
|---|---|---|---|---|---|---|---|---|
| IV diagnosis | GST-tagged-HA | CMP-NANA | APTES GA | SiNW | - | 1 fM | Buffer | [102] |
| AIV diagnosis | Whole virus | Ab | MPTMS DTT biotin-HPDP | SiNW | - | 10−17 M | Buffer | [103] |
| HBV diagnosis | HBV | ssDNA | Au | ITONWs | 1 fM–10 μM | 1 fM | Buffer | [94] |
| HBV diagnosis | DNA | DNA | APTES EDC/NHS | SiNW | 1 fM | Buffer | [90] | |
| AIV diagnosis | oligonucleotide | ssDNA | Thiol chain mercaptohexanol | Al/Au | 0.1–100 nM | 100 pM | Buffer | [88] |
| AIV diagnosis | HA protein | DNA Aptamer | - | Au microelectrode | 10 pM–10 nM | 5.9 pM | Chicken serum | [104] |
| HIV-1 diagnosis | capsid protein | Ab | EDC/NHS | Au | - | 30 × 10−21 M | Serum | [105] |
| HBV diagnosis | Whole virus | Ab | GA | GNR | 0.05–0.055 fM | 0.05 fM | Buffer | [106] |
| Rotavirus diagnosis | Whole virus | Ab | PSE | MrGO | 10–105 pfu/mL | 102 pfu | Buffer | [107] |
| Rotavirus diagnosis | Whole virus | Ab | pyrene-NHS | rGO | 101–106 particle/mL | 1 nm | Buffer | [108] |
| HPV diagnosis | E7 protein | RNA aptamer | EDC/NHS pyrene | rGO | 30–1000 nM | 1.75 nM | saliva | [109] |
| EVD diagnosis | glycoprotein | Ab | GA | rGO | - | 1 ng·mL−1 | Buffer, human serum, and plasma | [110] |
| EVD diagnosis | glycoprotein | Ab | PASE | rGO | 2.4 × 10−12–1.2 × 10−7 g·mL−1 | 2.4 pg·mL−1 | Spiked serum | [93] |
| VSV, MLV, HIV diagnosis | Whole virus | Ab | PASE | PET/PS/Graphene | 47.8 aM–10.55 nM | 47.8 aM | Buffer | [111] |
| Detection of HIV-1 viremia | RNA | ? | - | SiO2/Si3N4 | >1000 copies·mL−1 | 10 copies per reaction | Plasma samples | [21] |
| IV diagnosis | Whole virus | Sialoglycan | - | Graphene | - | 2.56 HAU | Saliva | [112] |
| IV diagnosis | HA | SGP | PBASE | Graphene | - | 200 nM | Buffer | [113] |
| COVID-19 diagnosis | S protein and whole virus | Ab | PBASE | Si/SiO2/Graphene | 1.6 × 101–1.6 × 104 pfu/mL | 2.42 × 102 copies·mL−1 | Clinical samples | [18] |
| HCV diagnosis | RNA | PNA | - | SWCNT | - | 0.5 pM | buffer | [87] |
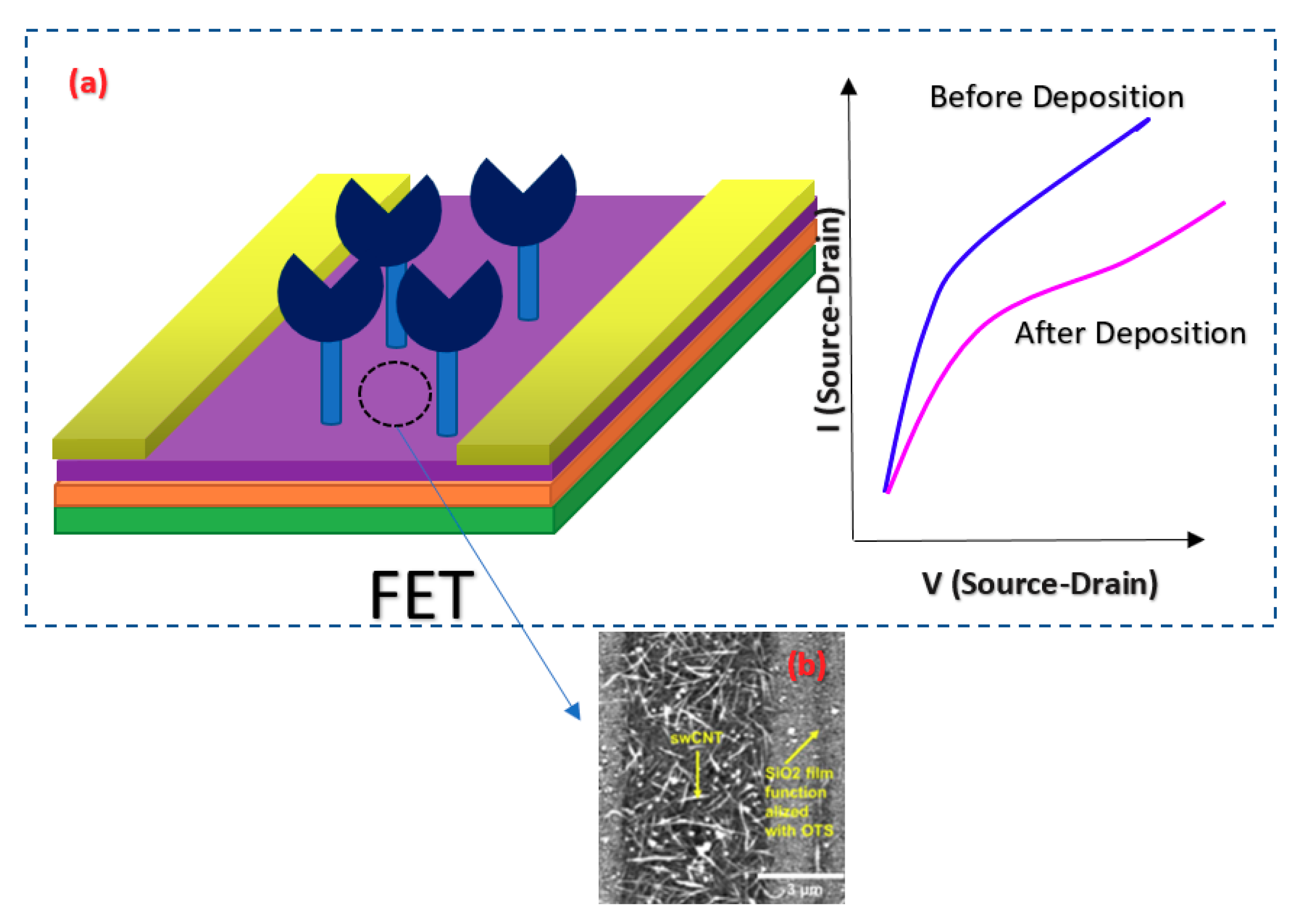
| virus detection | aerosolized bacteriophage MS2 and IV (H1N1) | Ab | - | OTS SAM SWCNT | - | Buffer | [91] | |
| AIV diagnosis | Whole virus | sialic-acid-containing glycans | APTES LCEE | sialyllactose | 100.5–108.5 TCID50/mL | 100.5 TCID50.mL−1 | nasal mucus | [114] |
| AIV diagnosis | nucleoprotein | Ab | APTES EDC/NHS | SnO2 | 102–105 EID50/mL | 103 EID50 mL−1 | cloacal swab | [92] |
| Zika virus diagnosis | Whole virus | Ab | - | AlGaN/GaN/disposable cover glass | 0.1–100 ng·mL−1 | 0.1 ng·mL−1 | Buffer | [115] |
| AIV diagnosis | AIa | Ab | SBP | CYTOPTM and Si3N4 | 10 fg·mL−1–100 pg·mL−1 | 1.9 fM 0.19 pM | Buffer | [89] |
| Plasmodium falciparum diagnosis | Nucleotide | DNA | - | SiO2/Si3N4 | - | 1 copy per reaction | Buffer | [116] |
| Plasmodium falciparum diagnosis | PfGDH | Aptamer | MCH | IDμE | 100 fM–10 nM | Serum | [45] | |
| E. coli diagnosis | K+ | Bacteriophage | - | PVC-based potassium-sensitive membrane | - | 48.6 pM | Buffer | [22] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panahi, A.; Sadighbayan, D.; Forouhi, S.; Ghafar-Zadeh, E. Recent Advances of Field-Effect Transistor Technology for Infectious Diseases. Biosensors 2021, 11, 103. https://doi.org/10.3390/bios11040103
Panahi A, Sadighbayan D, Forouhi S, Ghafar-Zadeh E. Recent Advances of Field-Effect Transistor Technology for Infectious Diseases. Biosensors. 2021; 11(4):103. https://doi.org/10.3390/bios11040103
Chicago/Turabian StylePanahi, Abbas, Deniz Sadighbayan, Saghi Forouhi, and Ebrahim Ghafar-Zadeh. 2021. "Recent Advances of Field-Effect Transistor Technology for Infectious Diseases" Biosensors 11, no. 4: 103. https://doi.org/10.3390/bios11040103
APA StylePanahi, A., Sadighbayan, D., Forouhi, S., & Ghafar-Zadeh, E. (2021). Recent Advances of Field-Effect Transistor Technology for Infectious Diseases. Biosensors, 11(4), 103. https://doi.org/10.3390/bios11040103






