Applications of Microwave Energy in Medicine
Abstract
:1. Introduction
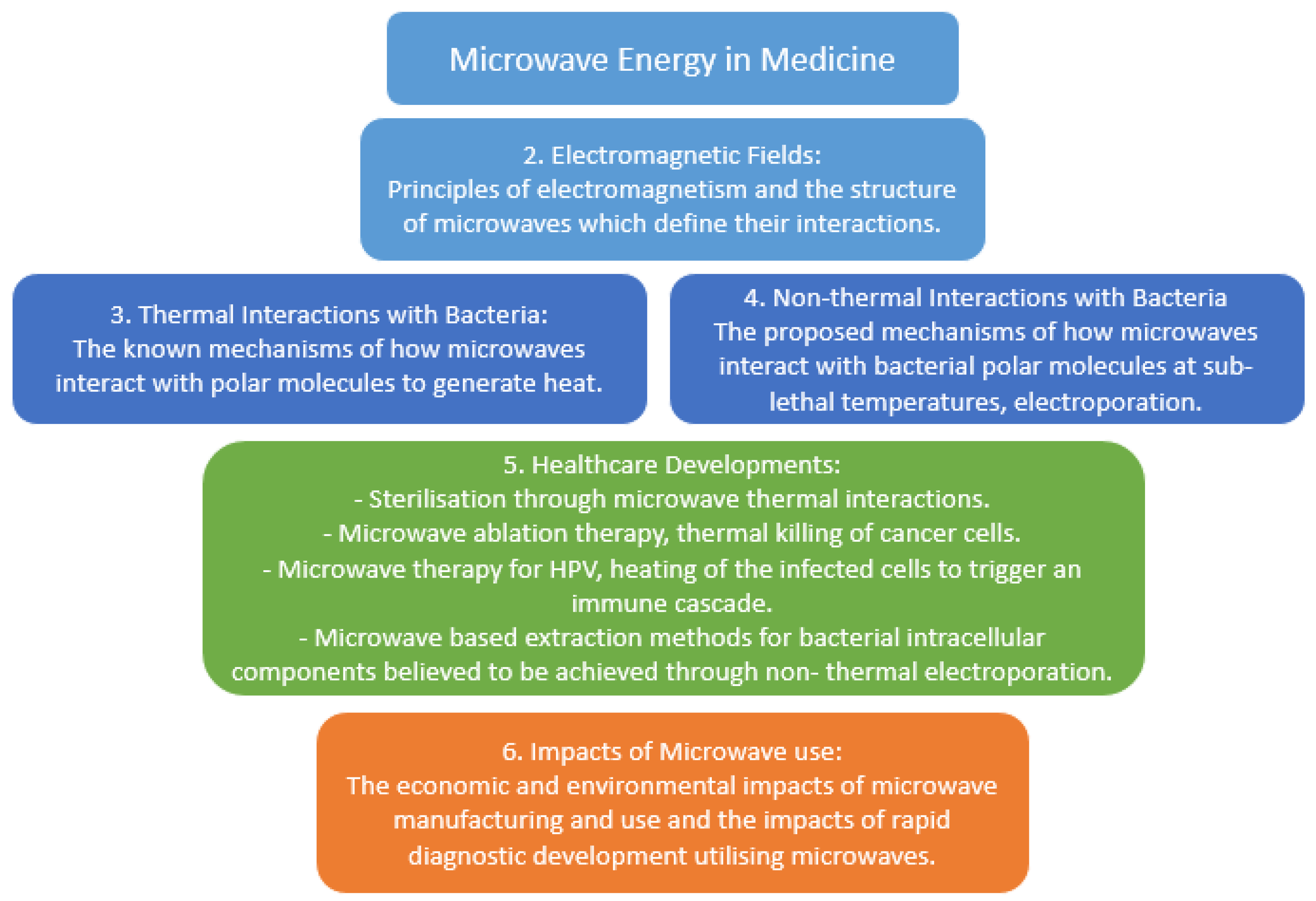
2. Electromagnetic Fields
3. Thermal Interactions with Bacteria
4. Non-Thermal Interactions with Bacteria
5. Healthcare Developments
6. Wider Impacts of Microwave Use
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sapling Learning. Electromagnetic Spectrum. Available online: https://sites.google.com/site/chempendix/em-spectrum (accessed on 13 June 2020).
- Tang, J. Unlocking Potentials of Microwaves for Food Safety and Quality. J. Food Sci. 2015, 80, E1776–E1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- International Telecommunication Union. 19 October 2009. 1.15. Industrial, Scientific and Medical (ISM) Applications (of Radio Frequency Energy). Available online: https://www.itu.int/dms_pubrec/itu-r/rec/sm/R-REC-SM.1056-1-200704-I!!PDF-E.pdfspectrum (accessed on 13 June 2020).
- Rosen, A.; Stuchly, M.A.; Vorst, A.V. Applications of RF/Microwaves in Medicine. IEEE Trans. Microw. Theory Tech. 2002, 50, 963–974. [Google Scholar] [CrossRef]
- Lantis, J., II; Carr, K.; Grabowy, R.; Connolly, R.; Schwaitzberg, S. Microwave applications in clinical medicine. Surg. Endosc. 1998, 12, 107–176. [Google Scholar] [CrossRef]
- Yeap, K.H.; Hirasawa, K. Introductory Chapter: Electromagnetism. In Electromagnetic Fields and Waves; IntechOpen: London, UK, 2019; p. 12. [Google Scholar] [CrossRef]
- Li, Z. Physics Essay: The Nature of Charge, Principle of Charge Interaction and Coulomb’s Law. Appl. Phys. Res. 2015, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, Y.; Nitta, S.A. Study on Characteristics of Electromagnetic Waves Propagation through the Spce between Overlapped Metal Plates. IEEE Trans. Electromagn. Compat. 2016, 58, 54–65. [Google Scholar] [CrossRef]
- Pieraccini, M.; Bicci, A.; Mecatti, D.; Macaluso, G.; Atzeni, C. Propagation of large bandwidth microwave signals in water. IEEE Trans. Antennas Propag. 2009, 57, 3612–3618. [Google Scholar] [CrossRef]
- Kivshar, Y.S. Control of electromagnetic waves in metamaterials: From microwaves to optics. In Proceedings of the 2013 International Kharkov Symposium on Physics and Engineering of Microwaves, Millimeter and Submillimeter Waves, Kharkiv, Ukraine, 23–28 June 2013; Volume 23, p. 30. [Google Scholar] [CrossRef]
- Tu, Z.C.; Hu, Y.M.; Wang, H.; Huang, X.Q.; Xia, S.Q.; Niu, P.P. Microwave heating enhances antioxidant and emulsifying activities of ovalbumin glycated with glucose in solid-state. J. Food Sci. Technol. 2015, 52, 1453–1461. [Google Scholar] [CrossRef] [Green Version]
- Kimura, W.D. What Are Electromagnetic Waves? In Electromagnetic Waves and Lasers; Morgan & Claypool Publishers: San Rafael, CA, USA, 2017. [Google Scholar]
- Fung, D.Y.; Cunningham, F.E. Effect of microwaves on microorganisms in foods. J. Food Prot. 1980, 43, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, G.; Condón, S.; Mañas, P. Physiology of the inactivation of vegetative bacteria by thermal treatments: Mode of action, influence of environmental factors and inactivation kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef] [Green Version]
- Bowman, G.; Lyuksyutova, A.; Sharpiro, L. Bacterial Polarity. Curr. Opin. Cell Biol. 2011, 23, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, H.; Gutierrez, S.; Bodrenko, I.; Malloci, G.; Scorciapino, M.; Winterhalter, M.; Ceccarelli, M. Bacterial Outer Membrane Porins as Electrostatic Nanosieves: Exploring Transport Rules of Small Polar Molecules. ACS Nano 2017, 11, 5465–5473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, A. Lethal Effects of Heat on Bacterial Physiology and Structure. Sci. Prog. 2003, 86, 115–137. [Google Scholar] [CrossRef]
- Mitsuzawa, S.; Deguchi, S.; Horikoshi, K. Cell structure degradation in Escherichia coli and Thermococcus sp. Strain Tc-1-95 associated with thermal death resulting from brief heat treatment. Fems Microbiol. Lett. 2006, 260, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimi, A.; Csonka, L.; Alam, M. Analyzing Thermal Stability of Cell Membrane of Salmonella Using Time-Multiplexed Impedance Sensing. Biophys. J. 2018, 114, 609–618. [Google Scholar] [CrossRef] [Green Version]
- Chipley, J.R. Effects of microwave irradiation on microorganisms. In Advances in Applied Microbiology; Academic Press: Cambridge, MA, USA, 1980; Volume 26, pp. 129–145. [Google Scholar] [CrossRef]
- Dreyfuss, M.S.; Chipley, J.R. Comparison of effects of sublethal microwave radiation and conventional heating on the metabolic activity of Staphylococcus aureus. Appl. Environ. Microbiol. 1980, 39, 13–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamis, Y.; Taube, A.; Mitik-Dineva, N.; Croft, R.; Crawford, R.J.; Ivanova, E.P. Specific electromagnetic effects of microwave radiation on Escherichia Coli. Appl. Environ. Microbiol. 2011, 77, 3017–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, E.; Rosenheck, K. Permeability changes induced by electric impulses in vesicular membranes. J. Membr. Biol. 1972, 10, 279–290. [Google Scholar] [CrossRef] [Green Version]
- Sustarsic, M.; Plochowietz, A.; Aigrain, L.; Yuzenkova, Y.; Zenkin, N.; Kapanidis, A. Optimized delivery of fluorescently labeled proteins in live bacteria using electroporation. Histochem. Cell Biol. 2014, 142, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Calvin, N.; Hanawalt, P. High-efficiency transformation of bacterial cells by electroporation. J. Bacteriol. 1988, 170, 2796–2801. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, D.; Sorg, J. Factors and Conditions that Impact Electroporation of Clostridium difficile Strains. Am. Soc. Microbiol. 2020, 5, e00941-19. [Google Scholar]
- Rougier, C.; Prorot, A.; Chazal, P.; Leveque, P.; Leprat, P. Thermal and nonthermal effects of discontinuous microwave exposure (2.45 gigahertz) on the cell membrane of Escherichia coli. Appl. Environ. Microbiol. 2014, 80, 4832–4841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, J. Microwave & Wireless Communications Technology; Newnes: Boston, Brazil, 1997; ISBN 0750697075. [Google Scholar]
- Lassiter, E. Navstar Global Positioning System: A Satellite Based Microwave Navigation System. In Proceedings of the 1975 IEEE-MTT-S International Microwave Symposium, Palo Alton, CA, USA, 12–14 May 1975. [Google Scholar]
- Porcelli, M.; Cacciapuoti, G.; Fusco, S.; Massa, R.; d’Ambrosio, G.; Bertoldo, C.; Rosa, M.; Zappia, V. Non-thermal effects of microwaves on proteins: Thermophilic enzymes as model systems. Febs Lett. 1997, 402, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Jacob, J.; Chia, L.; Boey, F. Thermal and non-thermal interaction of microwave radiation with materials. J. Mater. Sci. 1995, 30, 5321–5327. [Google Scholar] [CrossRef]
- Jeng, D.K.; Kaczmarek, K.A.; Woodworth, A.G.; Balasky, G.L. Mechanism of microwave sterilization in the dry state. Appl. Environ. Microbiol. 1987, 53, 2133–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanborn, M.R.; Wan, S.K.; Bulard, R. Microwave sterilization of plastic tissue culture vessels for reuse. Appl. Environ. Microbiol. 1982, 44, 960–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yezdani, A.; Mahalakshmi, K.; Padmavathy, K. Orthodontic instrument sterilization with microwave irradiation. J. Pharm. Bioallied Sci. 2015, 7, s111–s115. [Google Scholar] [CrossRef]
- Okorie, A.; Entwistle, J.; Dean, J. The optimization of microwave digestion procedures and application to an evaluation of potentially toxic element contamination on a former industrial site. Talanta 2010, 88, 1421–1425. [Google Scholar] [CrossRef]
- Sahuquillo, A.; Rubio, R.; Ribo, J.; Ros, E.; Vela, M. Application of focused-microwave wet digestion to the determination of trace metals in human gallstones by ICP/AES. J. Trace Elem. Med. Biol. 2000, 14, 96–99. [Google Scholar] [CrossRef]
- Ishak, I.; Rosli, F.D.; Mohamed, J.; Mohd Ismail, M.F. Comparison of Digestion Methods for the Determination of Trace Elements and Heavy Metals in Human Hair and Nails. Malays. J. Med. Sci. 2015, 22, 11–20. [Google Scholar]
- Irving, J.; Mario, C.; Francisco, V.; Geshel, G. Microwave ablation: State-of-the-art review. OncoTargets Ther. 2015, 8, 1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubner, M.G.; Brace, C.L.; Hinshaw, J.L.; Lee, F.T. Microwave Tumor Ablation: Mechanism of Action, Clinical Results and Devices. J. Vasc. Interv. Radiol. 2010, 21, S192–S203. [Google Scholar] [CrossRef] [Green Version]
- Masoud, H.; Tehrani, M.; Soltani, M.; Kashkooli, F.; Raahemifar, K. Use of microwave ablation for thermal treatment of solid tumors with different shapes and sizes—A computational approach. PLoS ONE 2020, 15, e0233219. [Google Scholar] [CrossRef]
- Wang, T.; Lu, X.J.; Chi, J.C.; Ding, M.; Zhang, Y.; Tang, X.Y.; Li, P.; Zhang, L.; Zhang, X.Y.; Zhai, B. Microwave ablation of hepatocellular carcinoma as first-line treatment: Long term outcomes and prognostic factors in 221 patients. Sci. Rep. 2016, 6, 32728. [Google Scholar] [CrossRef] [PubMed]
- Aldhaeebi, M.A.; Alzoubi, K.; Almoneef, T.S.; Bamatraf, S.M.; Attia, H.; Ramahi, O.M. Review of Microwaves Techniques for Breast Cancer Detection. Sensors 2020, 20, 2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulou, L.; Bosta, E.; Thanou, I.; Ziakas, P.; Thanos, L. Percutaneous microwave ablation vs. radiofrequency ablation in the treatment of hepatocellular carcinoma. World J. Hepatol. 2015, 18, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Wong, J.; Hui, J.; Cheung, Y.; Chong, C.; Fong, A.; Yu, S.; Lai, P. Long-term outcomes of microwave versus radiofrequency ablation for hepatocellular carcinoma by surgical approach: A retrospective comparative study. Asian J. Surg. 2017, 40, 301–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, W.; Deng, Q.; Lin, S.; Wand, Y.; Xu, G. Comparison of microwave ablation and radiofrequency ablation for hepatocellular carcinoma: A systematic review and meta-analysis. Int. J. Hyperth. 2019, 36, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Glassberg, M.; Ghosh, S.; Clymer, J.; Qadeer, R.; Ferko, N.; Sadeghirad, B.; Wright, G.; Amaral, J. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: A systematic review and meta-analysis. Oncotargets Ther. 2019, 12, 6407–6438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Shao, N.; Xi, X.; Hao, X. Use of microwave ablation in the treatment of patients with multiple primary malignant tumors. Thorac. Cancer 2017, 8, 365–371. [Google Scholar] [CrossRef] [Green Version]
- Loveman, E.; Jones, J.; Clegg, A.; Picot, J.; Colquitt, J.; Mendes, D.; Breen, D.; Moore, E.; George, S.; Poston, G.; et al. The clinical effectiveness and cost-effectiveness of ablative therapies in the management of liver metastases: Systematic review and economic evaluation. Health Technol. Assess. 2014, 18, 1–283. [Google Scholar] [CrossRef]
- De Cobelli, F.; Papa, M.; Panzeri, M.; Colombo, M.; Steidler, S.; Ambrosi, A.; Cao, R.; Gusmini, S.; Marra, P.; Capitanio, U.; et al. Percutaneous microwave ablation versus cryoablation in the treatment of T1a renal tumors. Cardiovascular and interventional radiology. 2020, 43, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Moloney, B.; O’Loughlin, D.; Elwahab, S.; Kerin, M. Breast Cancer Detection—A Synopsis of Conventional Modalities and the Potential Role of Microwave Imaging. Diagnostics 2020, 10, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Modiri, A.; Goudreau, S.; Rahhimi, A.; Kiasaleh, K. Review of breast screening: Toward clinical realization of microwave imaging. Am. Assoc. Phys. Med. 2017, 44, 446–458. [Google Scholar] [CrossRef]
- Yu, M.; Pan, H.; Che, N.; Li, L.; Wang, C.; Wang, Y.; Ma, G.; Qian, M.; Liu, J.; Zheng, M.; et al. Microwave ablation of primary breast cancer inhibits metastatic progression in model mice via activation of natural killer cells. Cell Mol. Immunol. 2020. [Google Scholar] [CrossRef]
- Dooley, W.C.; Vargas, H.I.; Fenn, A.J.; Tomaselli, M.B.; Harness, J.K. Focused Microwave Thermotherapy for Preoperative Treatment of Invasive Breast Cancer: A Review of Clinical Studies. Ann. Surg. Oncol. 2010, 17, 1076–1093. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zha, X.; Liu, X.; Ding, Q.; Chen, L.; Ni, Y.; Zhang, Y.; Xu, Y.; Chen, L.; Zhao, Y.; et al. US-guided percutaneous microwave coagulation of small breast cancers: A clinical study. Radiology 2012, 263, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Bristow, I.; Lim, W.C.; Lee, A.; Holbrook, D.; Savelyeva, N.; Thomson, P.; Webb, C.; Polak, M.; Ardern-Jones, M.R. Microwave therapy for cutaneous human papilloma virus infection. Eur. J. Derm. 2017, 27, 511–518. [Google Scholar] [CrossRef]
- Bristow, I.R.; Webb, C.; Ardern-Jones, M.R. The successful use of a novel microwave device in the treatment of a plantar wart. Case Rep. Derm. 2017, 9, 102–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, D.N.; Hogarth, F.J.; Sutherland, D.; Holmes, E.M.; Donnan, P.T.; Proby, C.M. A feasibility study of microwave therapy for precancerous actinic keratosis. Br. J. Derm. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epifano, I.; Conley, M.J.; Stevenson, A.; Doorbar, J.; Graham, S.V. Microwaves can reverse the tumour phenotype of human papillomavirus type 16 (HPV16)-positive keratinocytes in 3D cell culture models: A novel therapy for HPV-associated disease? Access Microbiol. 2020, 2, 593. [Google Scholar] [CrossRef]
- Melendez, J.; Huppert, J.; Jett-Goheen, M.; Hesse, E.; Quinn, N.; Gaydos, C.; Geddes, C. Blind evaluation of the microwave-accelerated metal-enhanced fluorescence ultrarapid and sensitive Chlamydia trachomatis test by use of clinical samples. J. Clin. Microbiol. 2013, 51, 2913–2920. [Google Scholar] [CrossRef] [Green Version]
- Aslan, K.; Geddes, C. Microwave-accelerated and metal-enhanced fluorescence Myoglobin detection on silvered surfaced: Potential application to myocardial infarction diagnosis. Plasmon. 2006, 1, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Tennant, S.M.; Zhang, Y.; Galen, J.E.; Geddes, C.D.; Levine, M.M. Ultra-fast and sensitive detection of non-typhoidal Salmonella using microwave-accelerated metal-enhanced fluorescence (“MAMEF”). PLoS ONE 2011, 6, e18700. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Agreda, P.; Kelley, S.; Gaydos, C.; Geddes, C.D. Development of a microwave—accelerated metal-enhanced fluorescence 40 Second, <100 cfu/mL point of care assay for the detection of Chlamydia Trachomatis. IEEE Trans. Biomed. Eng. 2010, 58, 781–784. [Google Scholar] [CrossRef] [Green Version]
- Joshi, L.T.; Mali, B.L.; Geddes, C.D.; Baillie, L. Extraction and sensitive detection of toxins A and B from the human pathogen Clostridium difficile in 40 seconds using microwave-accelerated metal-enhanced fluorescence. PLoS ONE 2014, 9, e104334. [Google Scholar] [CrossRef] [PubMed]
- Santaus, T.M.; Li, S.; Ladd, P.; Harvey, A.; Cole, S.; Stine, O.C.; Geddes, C.D. Rapid sample preparation with Lyse-It® for Listeria monocytogenes and Vibrio cholerae. PLoS ONE 2018, 13, e0201070. [Google Scholar] [CrossRef] [PubMed]
- Santaus, T.; Zhang, F.; Li, S.; Stine, O.; Geddes, C. Efects of Lyse-It on endonuclease fragmentation, function and activity. PLoS ONE 2019, 14, e0223008. [Google Scholar] [CrossRef] [PubMed]
- Rao, R.G.; Ravichandran, A.; Dhali, A.; Kolte, A.P.; Giridhar, K.; Manpal, S. A rapid microwave method for isolation of genomic DNA and identification of white rot fungi. bioRxiv 2018, 1, 307066. [Google Scholar] [CrossRef] [Green Version]
- Imtiaz, A.; Lees, J.; Choi, H.; Joshi, L.T. An Integrated Continuous Class-F−1Mode Power Amplifier Design Approach for Microwave Enhanced Portable Diagnostic Applications. IEEE Trans. Microw. Theory Tech. 2015, 63, 3007–3015. [Google Scholar] [CrossRef]
- Orsini, M.; Romano-Spica, V. A microwave-based method for nucleic acid isolation from environmental samples. Lett. Appl. Microbiol. 2001, 33, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Camel, V. Microwave-assisted solvent extraction of environmental samples. Trends Anal. Chem. 2000, 19, 229–248. [Google Scholar] [CrossRef]
- Giersig, M.; Firkowska, I.; Trosczcunsky, J.; Correa Duarte, M.A.; Rojas-Chapana, J.A. Novel electroporation System for both Gram-negative and Gram-positive Bacteria Assisted by Multi-Walled Carbon Nanotubes. Mrs Online Proc. Libr. 2004, 854, 145–150. [Google Scholar] [CrossRef]
- Gao, J.; Li, H.; Torab, P.; Mach, K.E.; Craft, D.W.; Thomas, N.J.; Puleo, C.M.; Liao, J.C.; Wang, T.H.; Wong, P.K. Nanotube assisted microwave electroporation for single cell pathogen identification and antimicrobial susceptibility testing. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hao, Z.; Qian, Y.; Huang, Y.; Bi, Z.; Yang, Z.; Wu, Q. Simulation and measurement of optimized microwave reflectivity for carbon nanotube absorber by controlling electromagnetic factors. Sci. Rep. 2017, 7, 479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Port, J.; Nguetse, C.; Adukpo, S.; Velevan, T. A reliable and rapid method for molecular detection of malarial parasites using microwave irradiation and loop mediated isothermal amplification. Malar. J. 2014, 13, 454. [Google Scholar] [CrossRef] [Green Version]
- O’Neil, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. 2014. Available online: https://amr-review.org/ (accessed on 19 August 2020).
- Yu, J.; Liang, P. Status and advancement of microwave ablation in China. Iternational J. Hyperth. 2016, 33, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Mays, O.; Neira, L.; Luyen, H.; Wilke, L.; Behdad, N.; Hagness, S. Advances in microwave ablatioin antennas for breast tumour treatment. In Proceedings of the 10th European Conference on Antennas and Propagation (EuCAP), Davos, Switzerland, 10–14 April 2016; pp. 1–3. [Google Scholar]
- Kwon, S.; Lee, S. Recent Advances in Microwave Imaging for Breast Cancer Detection. Int. J. Biomed. Imaging 2016, 2016, 5054912. [Google Scholar] [CrossRef] [PubMed]
- Astani, S.A.; Brown, M.L.; Steusloff, K. Comparison of procedure costs of various percutaneous tumor ablation modalities. Radiol. Manag. 2014, 36, 12–17. [Google Scholar]
- Prabhakar, H. Essentials of Neuroanesthesia; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Goel, K.; Gupta, R.; Solanki, J.; Nayak, M. A comparative Study between Microwave Irradiation and Sodium Hypochlorite Chemical Disinfection: A Prosthodontic View. J. Clin. Diagn. Res. 2014, 8, 42–46. [Google Scholar]
- Gallego-Schmid, A.; Mendoza, J.M.; Azapagic, A. Environmental assessment of microwaves and the effect of European energy efficiency and waste management legislation. Sci. Total Environ. 2018, 618, 487–499. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zeng, X.; Stevels, A. Ecodesign in consumer electronics: Past, present, and future. Crit. Rev. Environ. Sci. Technol. 2015, 45, 840–860. [Google Scholar] [CrossRef]





| Microwave Energy Application | Method | Example(s) | Ref. |
|---|---|---|---|
| Sterilisation | Thermal Energy. 225 MHz to 100 GHz; 2.45 GHz | Food, Glass, Plastics | [32,33,34] |
| Heavy Metal Digestion | Thermal Energy 2.45 GHz | Metals, Gallstones | [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37] |
| Ablation Therapy | Thermal Energy 8 GHz, ~2.45 GHz | Oncogenic Tumors, Keratinised cell, Plantar Warts (HPV) | [39,40,41,43,44,45,54,55,56,57] |
| Diagnostics | Non-Thermal Energy Microwave Accelerated Metal Enhanced Fluorescence (MAMEF) 2.45 GHz | Bacterial pathogens | [59,60,61,62,63] |
| Lysis | Thermal Energy 2.45 GHz | Bacterial Pathogens | [64,65] |
| Electroporation | Non-Thermal Energy 2.45 GHz | Bacteria at single-cell level | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gartshore, A.; Kidd, M.; Joshi, L.T. Applications of Microwave Energy in Medicine. Biosensors 2021, 11, 96. https://doi.org/10.3390/bios11040096
Gartshore A, Kidd M, Joshi LT. Applications of Microwave Energy in Medicine. Biosensors. 2021; 11(4):96. https://doi.org/10.3390/bios11040096
Chicago/Turabian StyleGartshore, Alexandra, Matt Kidd, and Lovleen Tina Joshi. 2021. "Applications of Microwave Energy in Medicine" Biosensors 11, no. 4: 96. https://doi.org/10.3390/bios11040096
APA StyleGartshore, A., Kidd, M., & Joshi, L. T. (2021). Applications of Microwave Energy in Medicine. Biosensors, 11(4), 96. https://doi.org/10.3390/bios11040096





