Quartz Crystal Microbalance with Dissipation Monitoring of Dynamic Viscoelastic Changes of Tobacco BY-2 Cells under Different Osmotic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Quartz Crystal Microbalance with Dissipation Monitoring
2.4. Optical Microscope Observation
3. Results
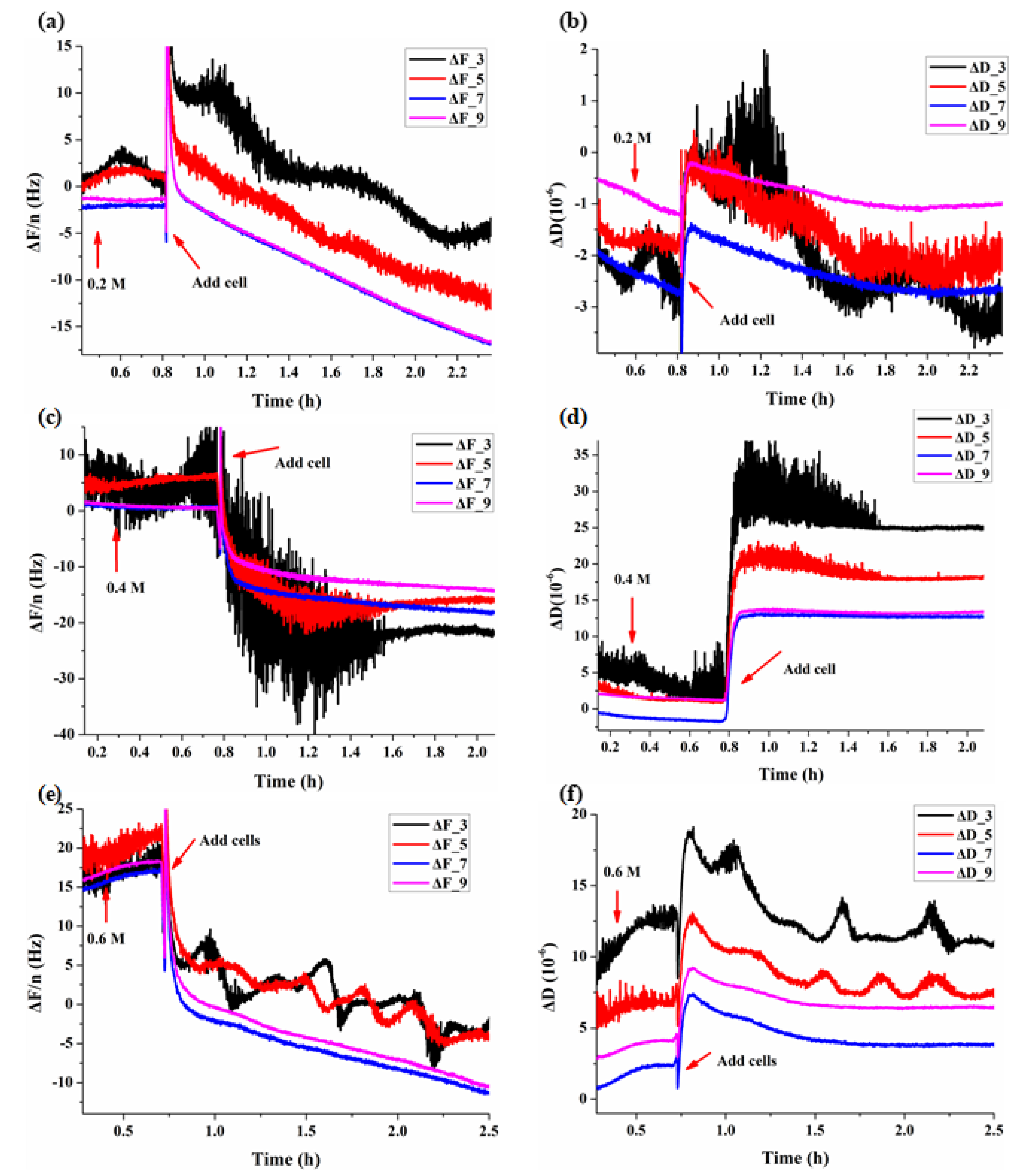
3.1. Real-Time QCM-D Responses during Adhesions of BY-2 Cells
3.2. Dynamic Viscoelastic Changes of BY-2 Cells under Osmotic Stresses
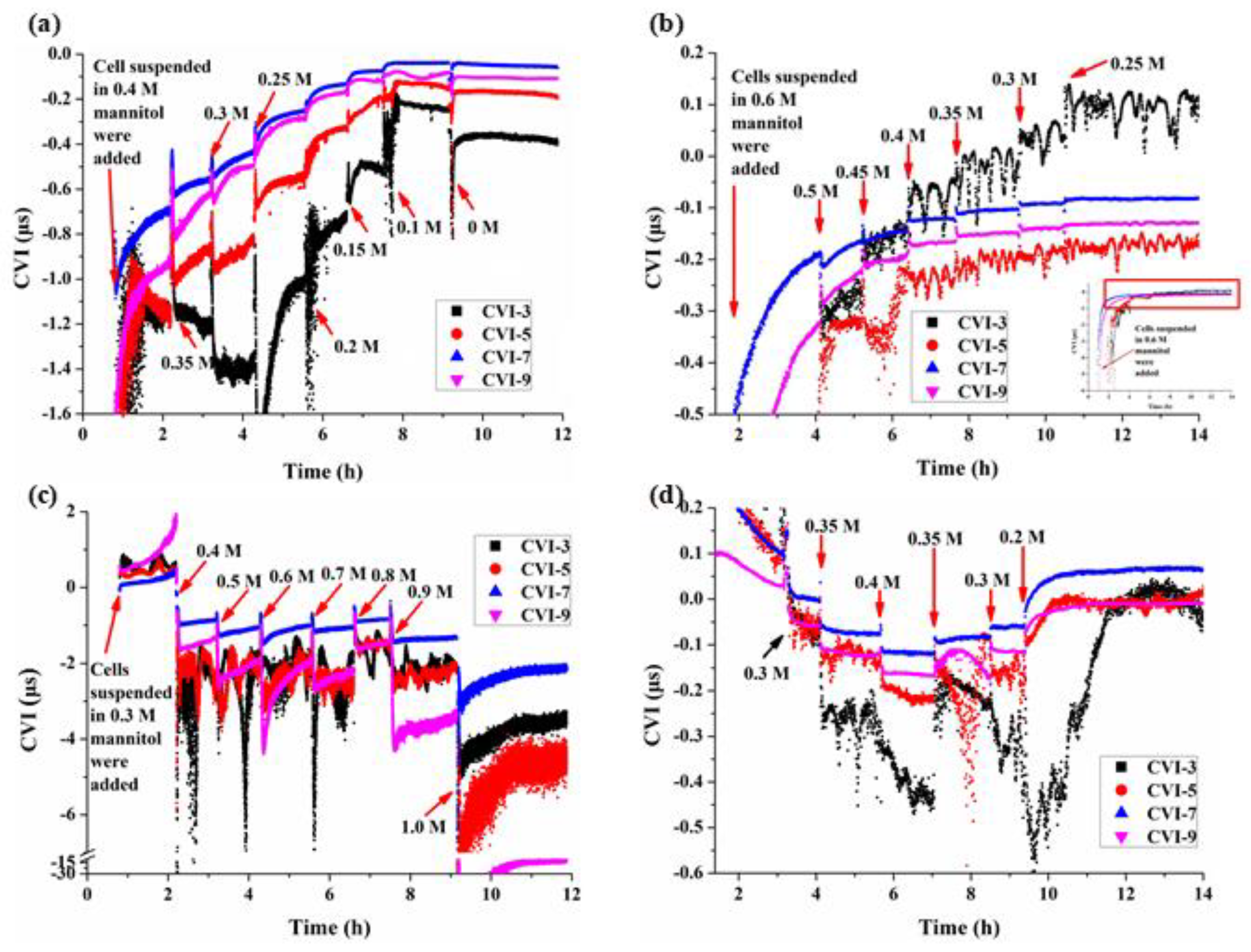
3.3. Multi-Structural Changes of BY-2 Cells under Different Mannitol Concentrations
4. Discussion
4.1. The Cell Viscoelastic Index CVI
4.2. Characteristics of Cell Viscoelasticity under Hypertonic Conditions
4.3. Characteristics of Cell Viscoelasticity under Hypotonic Conditions
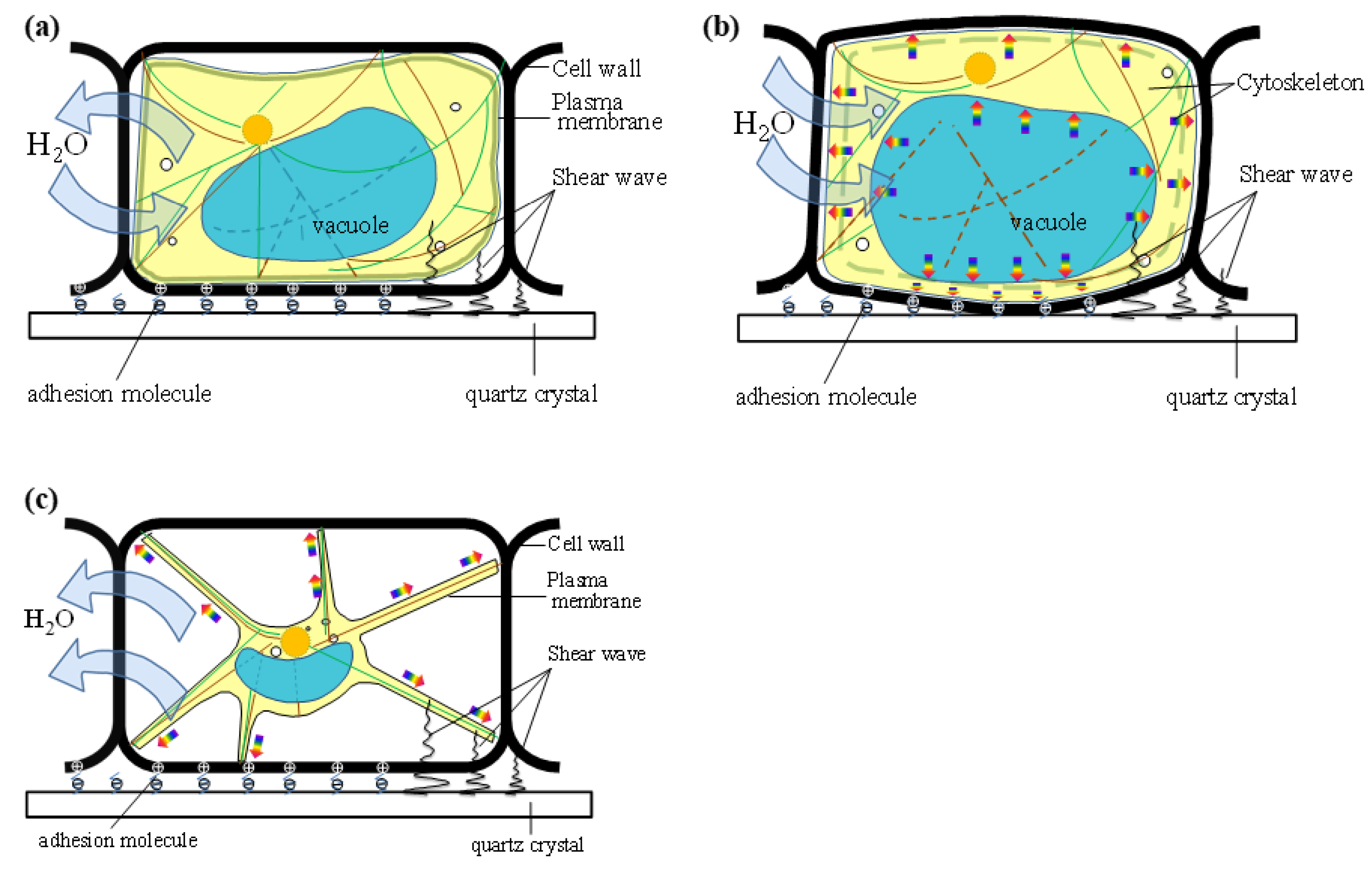
4.4. Osmotic Pressure Model of Plant Cells
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yadav, S.; Barton, M.J.; Nguyen, N.T. Biophysical properties of cells for cancer diagnosis. J. Biomech. 2019, 86, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guck, J.; Chilvers, E.R. Mechanics meets medicine. Sci. Transl. Med. 2013, 5, 212–241. [Google Scholar] [CrossRef] [PubMed]
- Ermis, M.; Sayn, E.; Antmen, E.; Hasirci, V. Mechanobiology. In Biological Soft Matter: Fundamentals, Properties, and Applications; Wiley-VCH: Weinheim, Germany, 2021; Chapter 8. [Google Scholar]
- Šamaj, J.; Menzel, D.; Wojtaszek, P.; Volkmann, D.; Baluška, F. Cytoskeleton-plasma membrane-cell wall continuum in plants emerging links revisited. Plant Physiol. 2003, 133, 482–491. [Google Scholar]
- Cosgrove, D.J. Plant cell wall extensibility: Connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 2016, 67, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Rui, Y.; Dinneny, J.R. A wall with integrity: Surveillance and maintenance of the plant cell wall under stress. New Phytol. 2020, 225, 1428–1439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blume, Y.B.; Krasylenko, Y.A.; Yemets, A.I. The role of the plant cytoskeleton in phytohormone signaling under abiotic and biotic stresses. In Mechanism of Plant Hormone Signaling under Stress; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 127–185. [Google Scholar]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2015, 5, 771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Lu, X.X.; Hao, Y.; Yang, P.H. Real-time monitoring of the regulatory volume decrease of cancer cells: A model for the evaluation of cell migration. Anal. Chem. 2019, 91, 8078–8084. [Google Scholar] [CrossRef]
- Skládal, P. Piezoelectric biosensors. Trends Anal. Chem. 2016, 79, 127–133. [Google Scholar] [CrossRef]
- Chen, J.Y.; Penn, L.S.; Xi, J. Quartz crystal microbalance: Sensing cell-substrate adhesion and beyond. Biosens. Bioelectron. 2017, 99, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Migon, D.; Wasilewski, T.; Suchy, D. Application of QCM in peptide and protein-based drug product development. Molecules 2020, 25, 3950. [Google Scholar] [CrossRef] [PubMed]
- Neupane, S.; Renner, F.U.; Losada, P.P. Quartz crystal microbalance with dissipation monitoring: A versatile tool to monitor phase transitions in biomimetic membranes. Front. Mater. 2018, 5, 46. [Google Scholar] [CrossRef] [Green Version]
- Alexander, T.E.; Lozeau, L.D.; Camesano, T.A. QCM-D characterization of time-dependence of bacterial adhesion. Cell Surf. 2019, 5, 100024. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.J.; Saha, T.; Tey, B.T.; Tan, W.S.; Ooi, C.W. Quartz crystal microbalance-based biosensors as rapid diagnostic devices for infectious diseases. Biosens. Bioelectron. 2020, 112513. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.B.; Zhou, T.A.; Hu, J.J. A high-throughput qcm chip configuration for the study of living cells and cell-drug interactions. Anal. Bioanal. Chem. 2017, 409, 6463–6473. [Google Scholar] [CrossRef]
- Yongabi, D.; Khorshid, M.; Gennaro, A.; Jooken, S.; Duwé, S.; Deschaume, D.; Losada-Pérez, P.; Dedecker, P.; Bartic, C.; Wubbenhorst, M.; et al. QCM-D Study of time-resolved cell adhesion and detachment: Effect of surface free energy on eukaryotes and prokaryotes. ACS Appl. Mater. Interfaces 2020, 12, 18258–18272. [Google Scholar] [CrossRef]
- Shoaib, S.; Tabrizian, M. A QCM-D sensing strategy for investigating the real-time effects of oxidative stress on the viscoelastic properties of pre-osteoblast cells. Sens. Actuators B Chem. 2019, 293, 235–246. [Google Scholar] [CrossRef]
- Li, X.M.; Song, Y.Y.; Pei, Y.X.; Dong, H.; Aastrup, T.; Pei, Z.C. Direct attachment of suspension cells to PDA surface and its application in suspension-cell QCM biosensor. Sens. Actuators B Chem. 2021, 326, 128823. [Google Scholar] [CrossRef]
- Zeng, M.; Zhou, T.; Su, Z.; Pan, W. Electrochemically prepared poly (l-lysine) and 3-hydroxyphenylboronic acid composite as a conventional adhesion material for rice suspension cells. Electrochem. Commun. 2020, 115, 106737. [Google Scholar] [CrossRef]
- Noi, K.; Iwata, A.; Kato, F.; Ogi, H. Ultrahigh-frequency wireless MEMS QCM biosensor for direct label-free detection of biomarkers in a large amount of contaminants. Anal. Chem. 2019, 91, 9398–9402. [Google Scholar] [CrossRef] [PubMed]
- Milioni, D.; Mateos, G.P.; Papadakis, G.; Tsortos, A.; Gizeli, E. An acoustic methodology for selecting highly dissipative probes for ultra-sensitive DNA detection. Anal. Chem. 2020, 92, 8186–8193. [Google Scholar] [CrossRef]
- Zhou, T.A.; Marx, K.A.; Dewilde, A.H.; McIntosh, D.; Braunhut, S.J. Dynamic cell adhesion and viscoelastic signatures distin-guish normal from malignant human mammary cells using quartz crystal microbalance. Anal. Biochem. 2012, 421, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.P.; Qi, Y.L.; Yan, M.M.; Wei, Y.; Lin, Z.H.; Jiang, Z.Y. Immobilization of Plant Protoplast on Gold Electrode Surface. Chem. J. Chin. Univ. 2002, 23, 1392–1394. [Google Scholar]
- Cho, N.J.; D’Amour, J.N.; Stalgren, J.; Knoll, W.; Kanazawa, K.; Frank, C.W. Quartz resonator signatures under newtonian liquid loading for initial instrument check. J. Colloid Interface Sci. 2007, 315, 248–254. [Google Scholar] [CrossRef]
- Haswell, E.S.; Verslues, P.E. The ongoing search for the molecular basis of plant osmosensing. J. Gen. Physiol. 2015, 145, 389–394. [Google Scholar] [CrossRef]
- Ingber, D.E. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 2008, 97, 163–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasprowicz, A; Smolarkiewicz, M; Wierzchowiec, M; Michalak, M; Wojtaszek P. Introduction: Tensegral World of Plants. In Mechanical Integration of Plant Cells and Plants; Springer-Verlag: Berlin/Heidelberg, Germany, 2011; pp. 1–25. [Google Scholar]
- Zhang, Q.; Song, X.; Bartels, D. Sugar metabolism in the desiccation tolerant grass Oropetium thomaeum in response to environmental stresses. Plant Sci. 2018, 270, 30–36. [Google Scholar] [CrossRef]
- Blum, A.; Tuberosa, R. Dehydration survival of crop plants and its measurement. J. Exp. Bot. 2018, 69, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Macedo, F.D.C.O.; Daneluzzi, G.S.; Capelin, D.; Barbosa, F.D.S.; Oliveira, R.F.D. Equipment and protocol for measurement of extracellular electrical signals, gas exchange and turgor pressure in plants. MethodsX 2021, 8, 101214. [Google Scholar] [CrossRef]
- Coussement, J.R.; Swaef, T.D.; Lootens, P.; Steppe, K. Turgor-driven plant growth applied in a soybean functional-structural plant model. Ann. Bot. 2020, 126, 729–744. [Google Scholar] [CrossRef]
- Tolmie, A.; Poulet, A.; Mckenna, J.; Sassmann, S.; Graumann, K.; Deeks, M.; Runions, J. The cell wall of arabidopsis thaliana influences actin network dynamics. J. Exp. Bot. 2017, 68, 4517–4527. [Google Scholar] [CrossRef] [PubMed]
- Karnik, R.; Waghmare, S.; Zhang, B.; Larson, E.; Lefoulon, C.; Gonzalez, W.; Blatt, M.R. Commandeering channel voltage sensors for secretion, cell turgor, and volume control. Trends Plant Sci. 2017, 22, 81–95. [Google Scholar] [CrossRef] [Green Version]
- Bitsikas, V.; Karachaliou, M.; Gournas, C.; Diallinas, G. Hypertonic conditions trigger transientplasmolysis, growth arrest and blockage of transporter endocytosis in Aspergillus nidulans and Saccharomyces cerevisiae. Mol. Membr. Biol. 2011, 28, 54–68. [Google Scholar] [CrossRef]
- Cleary, A.L. Plasma membrane-cell wall connections: Roles in mitosis and cytokinesis revealed by plasmolysis of Tradescantia virginiana leaf epidermal cells. Protoplasma 2001, 215, 21–34. [Google Scholar] [CrossRef]
- Komis, G.; Quader, H.; Galatis, B.; Apostolakos, P. Macrotubule-dependent protoplast volume regulation in plasmolysed root-tip cells of Triticum turgidum: Involvement of phospholipase D. New Phytol. 2006, 171, 737–750. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.L.; Wang, B.C.; Gong, W.; Zhang, Y.G.; Zhu, L.Q.; Yang, X.Y. Actin filaments and microtubules of arabidopsis suspension cells show different responses to changing turgor pressure. Biochem. Biophys. Res. Commun. 2011, 405, 632–637. [Google Scholar] [CrossRef]
- Terasaki, M.; Loew, L.; Lippincott-Schwartz, J.; Zaal, K. Fluorescent staining of subcellular organelles: ER, Golgi complex, and mitochondria. Curr. Protoc. Cell Biol. 2001, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, T.A.; Chen, Z.X.; Pan, W.S.; Tan, C.F. Real-time monitoring of viscoelastic responses of tobacco (Nicotiana tabacum) cells under peg6000 stress by wide frequency quartz crystal microbalance with dissipation. J. Agric. Biotechnol. 2020, 28, 2270–2280. [Google Scholar]
- Guerriero, G.; Hausman, J.F.; Cai, G. No stress! Relax! Mechanisms governing growth and shape in plant cells. Int. J. Mol. Sci. 2014, 15, 5094–5114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volgger, M.; Lang, I.; Ovecka, M.; Lichtscheidl, I. Plasmolysis and cell wall deposition in wheat root hairs under osmotic stress. Protoplasma 2010, 243, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Miroslaw, K.; Agata, D.G.; Agnieszka, J.; Karolina, C.; Urszula, N.; Gaurav, S.; Iwona, S. Transcriptome analysis reveals the role of the root hairs as environmental sensors to maintain plant functions underwater-deficiency conditions. J. Exp. Bot. 2016, 67, 1079–1094. [Google Scholar]
- Nanjareddy, K.; Arthikala, M.K.; Blanco, L.; Arellano, E.S.; Lara, M. Protoplast isolation, transient transformation of leaf mesophyll protoplasts and improved Agrobacterium-mediated leaf disc infiltration of Phaseolus vulgaris: Tools for rapid geneexpression analysis. BMC Biotechnol. 2016, 16, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michael, E.; Nitzan, Y.; Langzam, Y.; Luboshits, G.; Cahan, R. Effect of toluene on Pseudomonas stutzeri ST-9 morphology: Plasmolysis, cell size and formation of outer membrane vesicles. Can. J. Microbiol. 2016, 62, 682–691. [Google Scholar] [CrossRef] [PubMed]




| Application Examples | Technical Feature | Technical Characteristics |
|---|---|---|
| High-throughput QCM (HQCM) chip for the study of cell-drug interactions | Configuration of interference-free, negligible installation-induced stress | Fabricated using independent yet same-batch quartz crystal resonators within a common glass substrate through the rigid (quartz)-soft (silicone)-rigid (glass) structure [17] |
| Cell−material interactions | Tuning the surface free energy of materials | The results demonstrated that tuning the surface free energy of materials is a useful strategy for selectively promoting eukaryotic cell adhesion and preventing bacterial adhesion [18] |
| Oxidative stress on the viscoelastic properties of pre-osteoblast cells | Cell recovery from oxidative stress indicated by viscoelasticity | While a return to baseline values of the energy dissipation (ΔD) response was obtained at 325 min (recovery point) after the incubation of cells with 25 μM H2O2, higher concentrations (50 μM–10 mM) exhibited no recovery [19] |
| Evaluation of cell migration | Real time monitoring of cell regulatory volume decrease (RVD) | RVD was tracked via analyzing frequency shifts during the cell swelling and shrinkage and the results showed that the level of RVD for MCF-7 cells and MDA-MB-231 cells was 32.8% and 49.7% indicating their difference in migration [9] |
| Attachment of suspension cells | Mussel-inspired polydopamine (PDA) as the coating to attach suspension cells | The PDA-based suspension-cell QCM biosensor showed high degree of repeatability and stability as well as low nonspecific binding to the irrelevant protein [20] |
| Conventional adhesion material for plant cells | Poly-L-lysine (PLL)-3-hydroxyphenyl-boronic acid (3-PBA) composite was prepared via cyclic voltammetry | Synergistic effect of 3-PBA’s interactions with glycosyl compounds on the cell wall and PLL’s electrostatic interactions with the negatively charged cell wall promoted the adhesion of plant cells [21] |
| Direct, label-free detection of biomarkers in a large amount of contaminants | Ultrahigh-frequency, wireless MEMS QCM biosensor | Nonspecific adsorption of contaminants in analyte solution was avoided at ultrahigh-frequency (∼576 MHz) [22] |
| Selecting highly dissipative probes for ultrasensitive DNA detection | Liposomes as probes for energy-dissipation enhancement | The parameter of dissipation capacity, the ability to dissipate acoustic energy at the level of a single molecule/particle was introduced [23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhou, T.; Hu, J.; Duan, H. Quartz Crystal Microbalance with Dissipation Monitoring of Dynamic Viscoelastic Changes of Tobacco BY-2 Cells under Different Osmotic Conditions. Biosensors 2021, 11, 136. https://doi.org/10.3390/bios11050136
Chen Z, Zhou T, Hu J, Duan H. Quartz Crystal Microbalance with Dissipation Monitoring of Dynamic Viscoelastic Changes of Tobacco BY-2 Cells under Different Osmotic Conditions. Biosensors. 2021; 11(5):136. https://doi.org/10.3390/bios11050136
Chicago/Turabian StyleChen, Zongxing, Tiean Zhou, Jiajin Hu, and Haifeng Duan. 2021. "Quartz Crystal Microbalance with Dissipation Monitoring of Dynamic Viscoelastic Changes of Tobacco BY-2 Cells under Different Osmotic Conditions" Biosensors 11, no. 5: 136. https://doi.org/10.3390/bios11050136
APA StyleChen, Z., Zhou, T., Hu, J., & Duan, H. (2021). Quartz Crystal Microbalance with Dissipation Monitoring of Dynamic Viscoelastic Changes of Tobacco BY-2 Cells under Different Osmotic Conditions. Biosensors, 11(5), 136. https://doi.org/10.3390/bios11050136





