Electrochemical Biosensing of Dopamine Neurotransmitter: A Review
Abstract
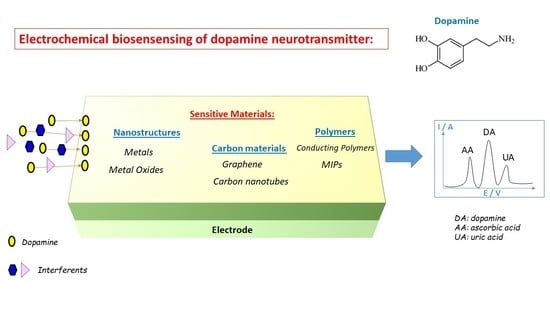
:1. Introduction
2. Electroanalytical Methods
2.1. Amperometry
2.2. Cyclic Voltammetry (CV)
2.3. Differential Pulse Voltammetry (DPV)
3. Electrodes and Microelectrodes
4. General Overview of Modification Materials for Dopamine Electrochemical Sensing
4.1. Metal and Metal Oxide Nanomaterials
4.1.1. Metal Nanostructures
| Active Layer | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| Au nanostructures (spikes) | 1–100 µM | 5 µM | [59] |
| Au nanostructures | 1–10 µM | 0.57 µM | [60] |
| Au nanostructures (cones) | 1–43 µM | 0.184 µM | [61] |
| Au nanopillars | 1–100 µM | 5.83 µM | [62] |
| Au nanopyramids | 10 nm–500 µM | 0.5 nM | [63] |
| Pd NPs | 0.5–160 µM | 0.2 µM | [65] |
| Au–Pt nanoflowers | 0.5 µM–0.18 mM | 0.11 µM | [66] |
| Au–Pt NPs | 1 µM–1 mM | 6 nM | [67] |
| Au nanostars—dopamine aptamer | 1–100 ng/L | 0.29 ng/L | [68] |
| Au nanostructures—dopamine aptamer | 25 ng/L–3 µg/L | 2 ng/L | [69] |
4.1.2. Metal Oxide Nanostructures
4.2. Carbon Materials
4.2.1. Carbon Nanotubes
4.2.2. Graphene
| Active Layer | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| Graphene | 10 nM–100 µM | 1 nM | [100] |
| Graphene | 4–100 μM | 2.6 µM | [101] |
| Reduced graphene | 1–80 μM | 0.46 µM | [102] |
| PEDOT–Graphene | 1–150 µM | 0.33 µM | [103] |
| PPy–Graphene | 0.5–10 µM | 0.1 µM | [104] |
| PPy–Graphene oxide | 1–150 µM | 0.02 µM | [105] |
| PVP–Graphene | 0.5 pM–1.13 mM | 0.2 nM | [106] |
| Fe3O4–Graphene | 0.02–130 μM | 7 nM | [107] |
| Ni(OH)2–Graphene | 0.44–3.3 μM | 120 nM | [111] |
| Cu2O–Graphene oxide | 0.01–1 µM | 6 nM | [108] |
| Zn–NiAl–Graphene oxide | 1 nM–1 µM | 0.1 nM | [112] |
| Au NPs–Graphene oxide | 0.1–100 μM | 0.098 μM | [113] |
| Ionic liquid–Au NPs-Graphene oxide | 7 nM–5 μM | 2.3 nM | [114] |
| Pt NPs–Graphene oxide | 87 nM–100 μM | 5 nM | [116] |
| Pt NPs–Graphene oxide (FET) | 1 pM–0.1 µM | 10−4 pM | [115] |
4.3. Polymer Materials
4.3.1. Conducting Polymers
| Active Layer | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| Polypyrrole | 1–1000 µM | 7 nM | [122] |
| Poly(pyrrole-3-carboxylic acid) | 0.025–7.5 μM | 2.5 nM | [123] |
| Poly(2-naphtol) | 0.6–250 μM | 95 nM | [130] |
| poly-4-amino-6-hydroxy–2-mercaptopyrimidine | 2.5–25 μM | 0.2 µM | [124] |
| Poly(eriochrome black T) | 0.1–200 μM | 20 nM | [127] |
| poly(safranine O) | 0.3–10 µM | 0.05 µM | [128] |
| poly(trypan blue) | 1–40 µM | 0.36 µM | [129] |
| poly(1,5-diaminonaphthalene)–SO3- | 5–100 µM | 0.1 µM | [131] |
| PEDOT–ferrocene | 0.01–0.9 mM | 1 µM | [133] |
| PANI–Au NPs | 20–100 µM | 16 µM | [135] |
| PEDOT–sodium dodecyl sulfate | 0.5–140 µM | 0.39 nM | [134] |
| PANI–Au NPs | 10–1700 µM | 5 µM | [136] |
| PEDOT–Graphene oxide | 0.1–175 µM | 39 nM | [137] |
| PANI–Graphene–aptamer | 0.007–90 nM | 1.98 pM | [138] |
| Polypyrrole–Graphene | 0.8–10 µM | 4 nM | [139] |
| PEDOT–CNT | 0.1–20 µM | 20 nM | [140] |
| Polycystine–CNT | 10–200 µM | 2.8 µM | [141] |
| Poly(anilineboronic acid) –CNT | 1–10 nM | 0.0.16 nM | [142] |
| PEDOT–nanoceria-MWCNT | 0.1–400 µM | 0.03 µM | [143] |
| PEDOT:PSS (FET) | 50 nM–3 µM | 5 nM | [146] |
| PEDOT:PSS (FET) | 5–100 µM | 6 µM | [147] |
| Carboxylated polypyrrole–CNT–aptamer (FET) | 0.1 nM–10 µM | 100 pM | [149] |
| 3-carboxylate polypyrrole–Pt NPs | 0.1 pM–1 nM | 0.1 pM | [150] |
| cysteamine and 4-formylphenyl boronic acid (FET) | 1 pM–1 mM | 1 pM | [151] |
4.3.2. Molecularly Imprinted Polymers
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bucolo, C.; Leggio, G.M.; Drago, F.; Salomone, S. Dopamine outside the brain: The eye, cardiovascular system and endocrine pancreas. Pharmacol. Ther. 2019, 203, 107392. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fernandez, V.; Harman, D.G.; Morley, J.W.; Cameron, M.A. Optimized Method to Quantify Dopamine Turnover in the Mammalian Retina. Anal. Chem. 2017, 89, 12276–12283. [Google Scholar] [CrossRef]
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC-MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Baig, N.; Alhooshani, K. Chemically modified electrodes for electrochemical detection of dopamine: Challenges and opportunities. Trends Anal. Chem. 2019, 118, 368–385. [Google Scholar] [CrossRef]
- Sajid, M.; Nazal, M.K.; Mansha, M.; Alsharaa, A.; Jillani, S.M.S.; Basheer, C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: A review. Trends Anal. Chem. 2016, 76, 15–29. [Google Scholar] [CrossRef]
- Ferapontova, E.F. Electrochemical Analysis of Dopamine: Perspectives of Specific In Vivo Detection. Electrochim. Acta 2017, 245, 664–671. [Google Scholar] [CrossRef]
- Segular-Aguilar, J.; Paris, I.; Munoz, P.; Ferrari, E.; Zecca, L.; Zucca, F.A. Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 2014, 129, 898–915. [Google Scholar] [CrossRef]
- Lakard, B. Electrochemical Biosensors Based on Conducting Polymers: A Review. Appl. Sci. 2020, 10, 6614. [Google Scholar] [CrossRef]
- Pan, H.M.; Gonuguntla, S.; Li, S.; Trau, D. 3.33 Conjugated Polymers for Biosensor Devices. Compr. Biomater. II 2017, 3, 716–754. [Google Scholar]
- Sadeghi, S.J. Amperometric Biosensors. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Clark, L.C. Monitor and control of blood and tissue oxygen tensions. Trans. Am. Soc. Artif. Intern. Organs 1956, 2, 41–48. [Google Scholar]
- Lupu, S.; Lete, C.; Balaure, P.C.; Caval, D.I.; Mihailciuc, C.; Lakard, B.; Hihn, J.Y.; del Campo, F.J. Development of Amperometric Biosensors Based on Nanostructured Tyrosinase-Conducting Polymer Composite Electrodes. Sensors 2013, 13, 6759–6774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Njagi, J.; Chernov, M.M.; Leiter, J.C.; Andreescu, S. Amperometric Detection of Dopamine in Vivo with an Enzyme Based Carbon Fiber Microbiosensor. Anal. Chem. 2010, 82, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Yoo, Y.J. Amperometric detection of dopamine based on tyrosinase–SWNTs–Ppy composite electrode. Talanta 2009, 80, 1007–1011. [Google Scholar] [CrossRef]
- Ding, J.; Qin, W. Recent advances in potentiometric biosensors. TrAC 2020, 124, 115803. [Google Scholar] [CrossRef]
- Yunus, S.; Jonas, A.M.; Lakard, B. Potentiometric Biosensors. In Encyclopedia of Biophysics; Roberts, G.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Robinson, D.L.; Hermans, A.; Seipel, A.T.; Wightman, R.M. Monitoring rapid chemical communication in the brain. Chem. Rev. 2008, 108, 2554–2584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesana, M.; Lee, S.T.; Wang, Y.; Venton, B.J. Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods. Anal. Chem. 2017, 89, 314–341. [Google Scholar] [CrossRef] [Green Version]
- Venton, B.J.; Wightman, R.M. Psychoanalytical Electrochemistry: Dopamine and Behavior. Anal. Chem. 2003, 75, 414A–421A. [Google Scholar] [CrossRef] [Green Version]
- Wightman, R.M. Microvoltammetric electrodes. Anal. Chem. 1981, 53, 1125A–1134A. [Google Scholar] [CrossRef]
- Huffman, M.L.; Venton, B.J. Carbon-Fiber Microelectrodes for In Vivo Applications. Analyst 2009, 134, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Puthongkham, P.; Venton, B.J. Recent advances in fast-scan cyclic voltammetry. Analyst 2020, 145, 1087–1102. [Google Scholar] [CrossRef]
- Venton, B.J.; Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 2020, 145, 1158–1168. [Google Scholar] [CrossRef] [PubMed]
- Simoes, F.R.; Xavier, M.G. 6—Electrochemical Sensors. In Micro and Nano Technologies, Nanoscience and Its Applications; da Roz, A.L., Ferreira, M., de Lima Leite, F., Oliveira, O.N., Eds.; William Andrew Publishing: Norwich, NY, USA, 2017; pp. 155–178. [Google Scholar]
- Westbroek, P. 2—Electrochemical methods. In Woodhead Publishing Series in Textiles, Analytical Electrochemistry in Textiles; Westbroek, P., Priniotakis, G., Kiekens, P., Eds.; Woodhead Publishing: Sawston, UK, 2005; pp. 37–69. [Google Scholar]
- Baranowska, I.; Markowski, P.; Gerle, A.; Baranowski, J. Determination of selected drugs in human urine by differential pulse voltammetry technique. Bioelectrochemistry 2008, 73, 5–10. [Google Scholar] [CrossRef]
- Jackowska, K.; Krysinski, P. New trends in the electrochemical sensing of dopamine. Anal. Bioanal. Chem. 2013, 405, 3753–3771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apollo, N.V.; Maturana, M.I.; Tong, W.; Nayagam, D.A.X.; Shivdasani, M.N.; Foroughi, J.; Wallace, G.G.; Prawer, S.; Ibbotson, M.R.; Garrett, D.J. Soft, Flexible Freestanding Neural Stimulation and Recording Electrodes Fabricated from Reduced Graphene Oxide. Adv. Funct. Mater. 2015, 25, 3551–3559. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Song, Y.J.; Boo, H.; Chung, T.D. Nanoporous Pt Microelectrode for Neural Stimulation and Recording: In Vitro Characterization. J. Phys. Chem. C 2010, 114, 8721–8726. [Google Scholar] [CrossRef]
- Cogan, S.F. Neural Stimulation and Recording Electrodes. Ann. Rev. Biomed. Eng. 2008, 10, 275–309. [Google Scholar] [CrossRef] [Green Version]
- Tykocinski, M.; Duan, Y.; Tabor, B.; Cowan, R.S. Chronic electrical stimulation of the auditory nerve using high surface area (HiQ) platinum electrodes. Hear. Res. 2001, 159, 53–68. [Google Scholar] [CrossRef]
- Ucar, A.; Gonzalez-Fernadez, E.; Staderini, M.; Avlonitis, N.; Murray, A.F.; Bradley, M.; Mount, A.R. Miniaturisation of a peptide-based electrochemical protease activity sensor using platinum microelectrodes. Analyst 2020, 145, 975–982. [Google Scholar] [CrossRef] [Green Version]
- Ibau, C.; Arshad, M.K.M.; Gopinath, S.C.B.; Nuzaihan, M.N.M.; Fathil, M.F.M.; Estrela, P. Gold interdigitated triple-microelectrodes for label-free prognosticative aptasensing of prostate cancer biomarker in serum. Biosens. Bioelectron. 2019, 136, 118–127. [Google Scholar] [CrossRef]
- Miyata, M.; Kitazumi, Y.; Shirai, O.; Kataoka, K.; Kano, K. Diffusion-limited biosensing of dissolved oxygen by direct electron transfer-type bioelectrocatalysis of multi-copper oxidases immobilized on porous gold microelectrodes. J. Electroanal. Chem. 2020, 860, 113895. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.H.; Chu, J.; Chen, Y.P.; Li, W.W.; Yu, H.Q.; Liu, G.; Tian, Y.C.; Xiong, Y. A nano-sized Au electrode fabricated using lithographic technology for electrochemical detection of dopamine. Biosens. Bioelectron. 2012, 35, 115–122. [Google Scholar] [CrossRef]
- Johnson, M.D.; Franklin, R.K.; Gibson, M.D.; Brown, R.B.; Kipke, D.R. Implantable microelectrode arrays for simultaneous electrophysiological and neurochemical recordings. J. Neurosci. Methods 2008, 174, 62–70. [Google Scholar] [CrossRef] [Green Version]
- Day, B.K.; Pomerleau, F.; Burmeister, J.J.; Huettl, P.; Gerhardt, G.A. Microelectrode array studies of basal and potassium-evoked release of L-glutamate in the anesthetized rat brain. J. Neurochem. 2006, 96, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.C.; Guo, C.X.; Han, H.Z.; Li, Y.T.; Huang, Y.Z.; Li, C.M.; Chen, J.J.J. Microelectrodes with gold nanoparticles and self-assembled monolayers for in vivo recording of striatal dopamine. Analyst 2012, 137, 2813–2820. [Google Scholar] [CrossRef]
- Robinson, D.L.; Venton, B.J.; Heien, M.L.A.V.; Wightman, R.M. Detecting subsecond dopamine release with fast-scan cyclic voltammetry in vivo. Clin. Chem. 2003, 49, 1763–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keithley, R.B.; Takmakov, P.; Bucher, E.S.; Belle, A.M.; Owesson-White, C.A.; Park, J.; Wightman, R.M. Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry. Anal. Chem. 2011, 83, 3563–3571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, R. Carbon Fiber Biocompatibility for Implants. Fibers 2016, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Ewing, A.G.; Dayton, M.A.; Wightman, R.M. Pulse voltammetry with microvoltammetric electrodes. Anal. Chem. 1981, 53, 1842–1847. [Google Scholar] [CrossRef]
- Michael, A.C.; Wightman, R.M. Laboratory Techniques in Electroanalytical Chemistry; Kissinger, P., Heineman, W.R., Eds.; Marcel Dekker: New York, NY, USA, 1996; pp. 367–402. [Google Scholar]
- Nagy, G.; Gerhardt, G.A.; Oke, A.F.; Rice, M.E.; Adams, R.N.; Moore, R.B.; Szentirmay, M.N.; Martin, C.R. Ion exchange and transport of neurotransmitters in nation films on conventional and microelectrode surfaces. J. Electroanal. Chem. 1985, 188, 85–94. [Google Scholar] [CrossRef]
- Hermans, A.; Seipel, A.T.; Miller, C.E.; Wightman, R.M. Carbon-fiber microelectrodes modified with 4-sulfobenzene have increased sensitivity and selectivity for catecholamines. Langmuir 2006, 22, 1964–1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musameh, M.; Wang, J.; Merkoci, A.; Lin, Y. Low-potential stable NADH detection at carbon-nanotube-modified glassy carbon electrodes. Electrochem. Commun. 2002, 4, 743–746. [Google Scholar] [CrossRef]
- Jacobs, C.B.; Vickerey, T.L.; Venton, B.J. Functional groups modulate the sensitivity and electron transfer kinetics of neurochemicals at carbon nanotube modified microelectrodes. Analyst 2011, 136, 3557–3565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cogan, S.F.; Troyk, P.R.; Ehrlich, J.; Plante, T.D.; Detlefsen, D.E. Potential-biased, asymmetric waveforms for charge-injection with activated iridium oxide (AIROF) neural stimulation electrodes. IEEE Trans. Biomed. Eng. 2006, 53, 327–332. [Google Scholar] [CrossRef]
- Cogan, S.F.; Plante, T.D.; Ehrlich, J. Sputtered iridium oxide films (SIROFs) for low-impedance neural stimulation and recording electrodes. Proc. IEEE. Eng. Med. Biol. Soc. 2004, 6, 4153–4156. [Google Scholar]
- Vetter, R.J.; Williams, J.C.; Hetke, J.F.; Nunamaker, E.A.; Kipke, D.R. Silicon-substrate intracortical microelectrode arrays for long-term recording of neuronal spike activity in cerebral cortex. IEEE Trans. Neural Syst. Rehabil. Eng. 2003, 11, 151–155. [Google Scholar]
- Vetter, R.J.; Williams, J.C.; Hetke, J.F.; Nunamaker, E.A.; Kipke, D.R. Chronic neural recording using silicon-substrate microelectrode arrays implanted in cerebral cortex. IEEE Trans. Biomed. Eng. 2004, 51, 896–905. [Google Scholar] [CrossRef] [PubMed]
- Dunevall, J.; Soodabeh, M.; Larsson, A.; Ewing, A. Vesicle impact electrochemical cytometry compared to amperometric exocytosis measurements. Curr. Opin. Electrochem. 2017, 5, 85–91. [Google Scholar] [CrossRef]
- Guille-Collignon, M.; Lemaître, F. Recent Developments Concerning the Investigation of Exocytosis with Amperometry. Curr. Opin. Electrochem. 2021, 29, 100751. [Google Scholar] [CrossRef]
- Zhang, X.W.; Hatamie, A.; Ewing, A.G. Simultaneous Quantification of Vesicle Size and Catecholamine Content by Resistive Pulses in Nanopores and Vesicle Impact Electrochemical Cytometry. JACS 2020, 142, 4093–4097. [Google Scholar] [CrossRef] [Green Version]
- Hu, K.; Jia, R.; Hatamie, A.; Le Vo, K.L.; Mirkin, M.V.; Ewing, A.G. Correlating molecule count and release kinetics with vesicular size using open carbon nanopipettes. JACS 2020, 142, 16910–16914. [Google Scholar] [CrossRef]
- Pingarron, J.M.; Yanez-Sedeno, P.; Gonzalez-Cortes, A.J.E.A. Gold nanoparticle-based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Angeline, N.; Suhito, I.R.; Kim, C.H.; Hong, G.P.; Park, C.G.; Bhang, S.H.; Luo, Z.; Kim, T.H. A fibronectin-coated gold nanostructure composite for electrochemical detection of effects of curcumin-carrying nanoliposomes on human stomach cancer cells. R. Soc. Chem. 2020, 145, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Plowman, B.J.; Mahajan, M.; O’Mullane, A.P.; Bhargava, S.K. Electrochemical detection of dopamine and cytochrome c at a nanostructured gold electrode. Electrochim. Acta 2010, 55, 8953–8959. [Google Scholar] [CrossRef]
- Cevallos-Morillo, C.A.; Hernandez-Vargas, S.G.; Aguilar-Cordero, J.C. Electrochemical Formation of Nanostructured Gold Surfaces on Glassy Carbon for the Determination of Dopamine. Electroanalysis 2018, 30, 1627–1633. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, W.; Xu, C.H.; Xu, J.J.; Chen, H.Y. Amperometric monitoring of vesicular dopamine release using a gold nanocone electrode. Chem. Commun. 2019, 55, 3461–3464. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Kang, E.S.; Baek, S.; Choo, S.S.; Chung, Y.H.; Lee, D.; Min, J.; Kim, T.H. Electrochemical detection of dopamine using periodic cylindrical gold nanoelectrode arrays. Sci. Rep. 2018, 8, 140490. [Google Scholar] [CrossRef] [Green Version]
- Senel, S.; Dervisevic, M.; Alhassen, S.; Alachkar, A.; Voelcker, N.H. Electrochemical micropyramid array-based sensor for in situ monitoring of dopamine released from neuroblastoma cells. Anal. Chem. 2020, 92, 7746–7753. [Google Scholar] [CrossRef]
- Barlow, S.T.; Louie, M.; Hai, R.; Defnet, P.A.; Zhang, B. Electrodeposited gold on carbon-fiber microelectrodes for enhancing amperometric detection of dopamine release from pheochromocytoma cells. Anal. Chem. 2018, 90, 10049–10055. [Google Scholar] [CrossRef]
- Huang, J.; Liu, Y.; Hou, H.; You, T. Simultaneous electrochemical determination of dopamine, uric acid and ascorbic acid using palladium nanoparticle-loaded carbon nanofibers modified electrode. Biosens. Bioelectron. 2008, 24, 632–637. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, X.; Zheng, X.; Zheng, J. Synthesis of Au@Pt nanoflowers supported on graphene oxide for enhanced electrochemical sensing of dopamine. J. Electroanal. Chem. 2018, 817, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Chen, X.; Li, Z.; Wang, Y.; Sun, S.; Wang, L.; Guo, T.; Zhang, D.; Xue, Z.; Zhou, X.; et al. Au-Pt bimetallic nanoparticles decorated on sulfonated nitrogen sulfur co-doped graphene for simultaneous determination of dopamine and uric acid. Talanta 2018, 178, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Talemi, R.P.; Mousavi, S.M.; Afruzi, H. Using gold nanostars modified pencil graphite electrode as a novel substrate for design a sensitive and selective Dopamine aptasensor. Mater. Sci. Eng. C 2017, 73, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Taheri, R.A.; Eskandari, K.; Negahdary, M. An electrochemical dopamine aptasensor using the modified Au electrode with spindle-shaped gold nanostructure. Microchem. J. 2018, 143, 243–251. [Google Scholar] [CrossRef]
- Pillarisetti, S.; Uthaman, S.; Huh, K.M.; Koh, Y.S.; Lee, S.; Park, I.K. Multimodal composite iron oxide nanoparticles for biomedical applications. Tissue Eng. Regen. Med. 2019, 16, 451–465. [Google Scholar] [CrossRef]
- Yang, W.; Yu, Y.; Tang, Y.; Li, K.; Zhao, Z.; Li, M.; Yin, G.; Li, H.; Sun, S. Enhancing electrochemical detection of dopamine via dumbbell-like FePt-Fe3O4 nanoparticles. Nanoscale 2017, 9, 1022–1027. [Google Scholar] [CrossRef] [PubMed]
- Emran, M.Y.; Shenashen, M.A.; Mekawy, M.; Azzam, A.M.; Akhtar, N.; Gomaa, H.; Selim, M.M.; Faheem, A.; El-Safty, S.A. Ultrasensitive in-vitro monitoring of monoamine neurotransmitters from dopaminergic cells. Sens. Actuators B 2018, 259, 114–124. [Google Scholar] [CrossRef]
- Sakthivel, R.; Dhanalakshmi, S.; Chen, S.M.; Chen, T.W.; Selvam, V.; Ramaraj, S.K.; Weng, W.H.; Leung, W.H. A novel flakes-like structure of molybdenum disulphide modified glassy carbon electrode for the efficient electrochemical detection of dopamine. Int. J. Electrochem. Sci. 2017, 12, 9288–9300. [Google Scholar] [CrossRef]
- Hun, X.; Wang, S.; Wang, S.; Zhao, J.; Luo, X. A photoelectrochemical sensor for ultrasensitive dopamine detection based on single layer NanoMoS2 modified gold electrode. Sens. Actuators B 2017, 249, 83–89. [Google Scholar] [CrossRef]
- Xia, C.; Ning, W.; Long, W.; Lin, G. Synthesis of nanochain assembled ZnO flowers and their application to dopamine sensing. Sens Actuators B 2010, 147, 629–634. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Liu, D.; Li, M.; Yu, Y.; Yang, W.; Li, H. Facile synthesis of highly ordered mesoporous Fe3O4 with ultrasensitive detection of dopamine. Talanta 2019, 201, 511–518. [Google Scholar] [CrossRef]
- Huang, Y.; Tang, Y.; Xu, S.; Feng, M.; Yu, Y.; Yang, W.; Li, H. A highly sensitive sensor based on ordered mesoporous ZnFe2O4 for electrochemical detection of dopamine. Anal. Chim. Acta 2020, 1096, 26–33. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S.; Geim, A.K. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Zou, G.; Zhang, D.; Dong, C.; Li, H.; Xiong, K.; Fei, L.; Qian, Y. Carbon nanofibers: Synthesis, characterization, and electrochemical properties. Carbon 2006, 44, 828–832. [Google Scholar] [CrossRef]
- Jariwala, D.; Sangwan, V.K.; Lauhon, L.J.; Marks, T.J.; Hersam, M.C. Carbon nanomaterials for electronics, optoelectronics, photovoltaics, and sensing. Chem. Soc. Rev. 2013, 42, 2824–2860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schipper, M.L.; Nakayama-Ratchford, N.; Davis, C.R.; Kam, N.W.S.; Chu, P.; Liu, Z.; Sun, X.; Dai, H.; Gambhir, S.S. A pilot toxicology study of single-walled carbon nanotubes in a small sample of mice. Nat. Nanotechnol. 2008, 3, 216–221. [Google Scholar] [CrossRef]
- Mao, H.Y.; Laurent, S.; Chen, W.; Akhavan, O.; Imani, M.; Ashkarran, A.A.; Mahmoudi, M. Graphene: Promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013, 113, 3407–3424. [Google Scholar] [CrossRef] [PubMed]
- Alim, S.; Vejayan, J.; Yusoff, M.M.; Kafi, A.K.M. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron. 2018, 121, 125–136. [Google Scholar] [PubMed]
- Wang, L.; Pumera, M. Electrochemical catalysis at low dimensional carbons: Graphene, carbon nanotubes and beyond—A review. Appl. Mater. Today 2016, 5, 134–141. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Gao, X.; Xu, D. A review of the interfacial characteristics of polymer nanocomposites containing carbon nanotubes. RSC Adv. 2018, 8, 28048–28085. [Google Scholar] [CrossRef] [Green Version]
- Beitollahi, H.; Mohadesi, A.; Mohammadi, S.; Pahlavan, A.; Karimi-Maleh, H.; Akbari, A. New voltammetric strategy for determination of dopamine in the presence of high concentrations of acetaminophen, folic acid and N-acetylcysteine. J. Mol. Liq. 2012, 169, 130–135. [Google Scholar] [CrossRef]
- Beitollahi, H.; Mohadesi, A.; Khalilizadeh Mahani, S.; Karimi-Maleh, H.; Akbari, A. Simultaneous determination of dopamine, uric acid, and tryptophan using an MWCNT modified carbon paste electrode by square wave voltammetry. Turk. J. Chem. 2012, 36, 526–536. [Google Scholar]
- Alwarappan, S.; Liu, G.; Li, C.Z. Simultaneous detection of dopamine, ascorbic acid, and uric acid at electrochemically pretreated carbon nanotube biosensors. Nanomedicine 2010, 6, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, J.; Yang, J.; Liu, D.; Lu, X. Electrocatalytic detection of dopamine in the presence of ascorbic acid and uric acid using single-walled carbon nanotubes modified electrode. Colloids Surf. B 2012, 97, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Quan, D.P.; Tuyen, D.P.; Lam, T.D.; Tram, P.T.N.; Binh, N.H.; Viet, P.H. Electrochemically selective determination of dopamine in the presence of ascorbic and uric acids on the surface of the modified Nafion/single wall carbon nanotube/poly(3-methylthiophene) glassy carbon. Colloids Surf. B 2011, 88, 764–770. [Google Scholar] [CrossRef]
- Nagles, E.; Garcia-Beltran, O.; Calderon, J.A. Evaluation of the usefulness of a novel electrochemical sensor in detecting uric acid and dopamine in the presence of ascorbic acid using a screen-printed carbon electrode modified with single walled carbon nanotubes and ionic liquids. Electrochim. Acta 2017, 258, 512–523. [Google Scholar] [CrossRef]
- Eom, G.; Oh, C.; Moon, J.; Kim, H.; Kyung, M.; Kim, K.; Seo, J.W.; Kang, T.; Lee, H.J. Highly sensitive and selective detection of dopamine using overoxidized polypyrrole/sodium dodecyl sulfate-modified carbon nanotube electrodes. J. Electroanal. Chem. 2019, 848, 113295. [Google Scholar] [CrossRef]
- Balasubramanian, P.; He, S.B.; Jansirani, A.; Peng, H.P.; Huang, L.L.; Deng, H.H.; Chen, W. Bimetallic AgAu decorated MWCNTs enable robust nonenzyme electrochemical sensors for in-situ quantification of dopamine and H2O2 biomarkers expelled from PC-12 cells. J. Electroanal. Chem. 2020, 878, 114554. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Tong, L.; Tong, Q.X. Graphene quantum dots/multiwalled carbon nanotubes composite-based electrochemical sensor for detecting dopamine release from living cells. ACS Sustain. Chem. Eng. 2020, 8, 1644–1650. [Google Scholar] [CrossRef]
- Sakthivel, K.; Govindasamy, M.; Chen, S.M.; Muthumariappan, A.; Mani, V.; Chen, T.W.; Selvaraj, S. MWCNTs/MoS2 Decorated Cobalt Oxide Polyhedrons Composite Film Modified Electrode for Electrochemical Determination of Dopamine in Rat Brain and Human Blood Serum Samples. Int. J. Electrochem. Sci. 2017, 12, 7435–7445. [Google Scholar] [CrossRef]
- Hou, Z.L.; Song, W.L.; Wang, P.; Meziani, M.J.; Kong, C.Y.; Anderson, A.; Maimaiti, H.; LeCroy, G.E.; Qian, H.; Sun, Y.P. Flexible Graphene-Graphene Composites of Superior Thermal and Electrical Transport properties. ACS Appl. Mater. Interfaces 2014, 6, 15026–15032. [Google Scholar] [CrossRef] [PubMed]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Mechanical properties of graphene and graphene-based nanocomposites. Prog. Mater. Sci. 2017, 90, 75–127. [Google Scholar] [CrossRef]
- Wang, Y.; Li, S.; Yang, H.; Luo, J. Progress in the functional modification of graphene/graphene oxide: A review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar]
- Pumera, M.; Ambrosi, A.; Bonnani, A.; Chng, E.L.; Poh, H.L. Graphene for electrochemical sensing and biosensing. Trends Anal. Chem. 2010, 29, 954–965. [Google Scholar] [CrossRef]
- Zhu, M.; Zeng, C.; Ye, J. Graphene-modified carbon fiber microelectrode for the detection of dopamine in mice hippocampus tissue. Electroanalysis 2011, 23, 907–914. [Google Scholar] [CrossRef]
- Kim, Y.R.; Bong, S.; Kang, Y.J.; Yang, Y.; Mahajan, R.K.; Kim, J.S.; Kim, H. Electrochemical detection of dopamine in the presence of ascorbic acid using graphene modified electrodes. Biosens. Bioelectron. 2010, 25, 2366–2369. [Google Scholar] [CrossRef]
- Yu, J.; Kim, T.H. A Facile Electrochemical Fabrication of Reduced Graphene Oxide-Modified Glassy Carbon Electrode for Simultaneous Detection of Dopamine, Ascorbic Acid, and Uric Acid. J. Electrochem. Sci. Technol. 2017, 8, 274–281. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Jarjes, Z.A.; Desprez, V.; Kilmartin, P.A.; Travas-Sejdic, J. Sensitive, selective, disposable electrochemical dopamine sensor based on PEDOT-modified laser scribed graphene. Biosens. Bioelectron. 2018, 107, 184–191. [Google Scholar] [CrossRef]
- Zhuang, Z.; Li, J.; Xu, R.; Xiao, D. Electrochemical detection of dopamine in the presence of ascorbic acid using overoxidized polypyrole/graphene modified electrode. Int. J. Electrochem. Sci. 2011, 6, 2149–2161. [Google Scholar]
- Si, P.; Chen, H.; Kannan, P.; Kim, D.H. Selective and sensitive determination of dopamine by composites of polypyrrole and graphene modified electrodes. Analyst 2011, 136, 5134–5138. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, X.; Huo, Z.; He, X.; Liang, Y.; Xu, M. Electrochemical detection of dopamine in the presence of ascorbic acid using PVP/graphene modified electrodes. Talanta 2012, 97, 557–562. [Google Scholar] [CrossRef]
- Zhang, W.; Zheng, J.; Shi, J.; Lin, Z.; Huang, Q.; Zhang, H.; Wei, C.; Chen, J.; Hu, S.; Hao, A. Nafion covered core-shell structured Fe3O4@graphene nanospheres modified electrode for highly selective detection of dopamine. Anal. Chim. Acta 2015, 853, 285–290. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-reduced graphene nanocomposite modified electrodes towards ultrasensitive dopamine detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, Z.; Yang, X.; Niu, Y.; Wu, F.; Hu, Y.; Yang, Y. Voltammetric dopamine sensor based on a gold electrode modified with reduced graphene oxide and Mn3O4 on gold nanoparticles. Microchim. Acta 2017, 184, 2081–2088. [Google Scholar] [CrossRef]
- Jahani, S.; Beitollahi, H. Selective Detection of Dopamine in the Presence of Uric Acid Using NiO Nanoparticles Decorated on Graphene Nanosheets Modified Screen-printed Electrodes. Electroanalysis 2016, 28, 2022–2028. [Google Scholar] [CrossRef]
- Nancy, T.E.M.; Kumary, V.A. Synergistic electrocatalytic effect of graphene/nickel hydroxide composite for the simultaneous electrochemical determination of ascorbic acid, dopamine and uric acid. Electrochim. Acta 2014, 133, 233–240. [Google Scholar] [CrossRef]
- Asif, M.; Aziz, A.; Wang, H.; Wang, Z.; Wang, W.; Ajmal, M.; Xiao, F.; Chen, X.; Liu, H. Superlattice stacking by hybridizing layered double hydroxide nanosheets with layers of reduced graphene oxide for electrochemical simultaneous determination of dopamine, uric acid and ascorbic acid. Microchim. Acta 2019, 186, 61. [Google Scholar] [CrossRef]
- Park, D.J.; Choi, J.H.; Lee, W.J.; Um, S.H.; Oh, B.K. Selective electrochemical detection of dopamine using reduced graphene oxide sheets-gold nanoparticles modified electrode. J. Nanosci. Nanotechnol. 2017, 17, 8012–8018. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Sun, Y.; Ding, C.; Lin, Y.; Sun, W.; Luo, C. A novel ionic liquid functionalized graphene oxide supported gold nanoparticle composite film for sensitive electrochemical detection of dopamine. RSC Adv. 2017, 7, 2315–2322. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.; Lee, J.S.; Jun, J.; Kim, S.G.; Jang, J. Ultrasensitive and selective organic FET type nonenzymatic dopamine sensor based on platinum nanoparticles-decorated reduced graphene oxide. ACS Appl. Mater. Interfaces 2017, 9, 39526–39533. [Google Scholar] [CrossRef]
- Zan, X.; Bai, H.; Wang, C.; Zhao, F.; Duan, H. Graphene paper decorated with a 2D array of dendritic platinum nanoparticles for ultrasensitive electrochemical detection of dopamine secreted by live cells. Chem. Eur. J. 2016, 22, 5204–5210. [Google Scholar] [CrossRef]
- Dakshayini, B.S.; Reddy, K.R.; Mishra, A.; Shetti, N.P.; Malode, S.J.; Basu, S.; Naveen, S.; Raghu, A.V. Role of conducting polymer and metal oxide-based hybrids for applications in amperometric sensors and biosensors. Microchem. J. 2019, 147, 7–24. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Zare, E.N.; Makvandi, P.; Rossi, F.; Motahari, A.; Perale, G. Progress in Conductive Polyaniline-Based Nanocomposites for Biomedical Applications: A Review. J. Med. Chem. 2020, 63, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Naveen, M.H.; Gurudatt, N.G.; Shim, Y.B. Applications of conducting-polymer composites to electrochemical sensors: A review. Appl. Mater. Today 2017, 9, 419–433. [Google Scholar] [CrossRef]
- Moon, J.M.; Thapliyal, N.; Hussain, K.K.; Goyal, R.N.; Shim, Y.B. Conducting polymer-based electrochemical biosensors for neurotransmitters: A review. Biosens. Bioelectron. 2018, 102, 540–552. [Google Scholar] [CrossRef]
- Koyun, O.; Gursu, H.; Gorduk, S.; Sahin, Y. Highly Sensitive Electrochemical Determination of Dopamine with an Overoxidized Polypyrrole Nanofiber Pencil Graphite Electrode. Int. J. Electrochem. Sci. 2017, 12, 6428–6444. [Google Scholar] [CrossRef]
- Ozcan, A.; Ilkbas, S.; Ozcan, A.A. Development of a disposable and low-cost electrochemical sensor for dopamine detection based on poly(pyrrole-3-carboxylic acid)-modified electrochemically over-oxidized pencil graphite electrode. Talanta 2017, 165, 489–495. [Google Scholar] [CrossRef]
- Kannan, A.; Sevvel, R. A highly selective and simultaneous determination of paracetamol and dopamine using poly-4-amino-6-hydroxy-2-mercaptopyrimidine (Poly-AHMP) film modified glassy carbon electrode. J. Electroanal. Chem. 2017, 791, 8–16. [Google Scholar] [CrossRef]
- Hsieh, M.T.; Whang, T.J. Electrical polymerization of a tetrazole polymer-modified electrode and its catalytic reaction toward dopamine. Appl. Surf. Sci. 2017, 396, 1589–1595. [Google Scholar] [CrossRef]
- Rao, V.; Reddy, Y. Simultaneous determination of dopamine, ascorbic acid and uric acid at poly (cinnamic acid) modified carbon paste electrode: A voltametric investigation. Anal. Bioanal. Electrochem. 2017, 9, 874–887. [Google Scholar]
- Yao, H.; Sun, Y.; Lin, X.; Tang, Y.; Huang, L. Electrochemical characterization of poly(eriochrome black T) modified glassy carbon electrode and its application to simultaneous determination of dopamine, ascorbic acid and uric acid. Electrochim. Acta 2007, 52, 6165–6171. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A.; Aydar, S.; Arpaci, R.B. Simultaneous electrochemical preconcentration and determination of dopamine and uric acid by square-wave adsorptive stripping voltammetry using a poly (Safranine O)—modified glassy carbon electrode. Int. J. Electrochem. Sci. 2014, 9, 2775–2789. [Google Scholar]
- Li, X.B.; Rahman, M.M.; Ge, C.Y.; Xu, G.R.; Lee, J.J. A Poly(trypan blue)-Modified Anodized Glassy Carbon Electrode for the Sensitive Detection of Dopamine in the Presence of Uric Acid and Ascorbic Acid. J. Electrochem. Soc. 2017, 164, B34. [Google Scholar] [CrossRef]
- Manikandan, R.; Deepa, P.N.; Narayanan, S.S. Fabrication and characterization of poly 2-napthol orange film modified electrode and its application to selective detection of dopamine. J. Solid State Electrochem. 2017, 21, 3567–3578. [Google Scholar] [CrossRef]
- Chandra, P.; Son, N.X.; Noh, H.B.; Goyal, R.N.; Shim, Y.B. Investigation on the downregulation of dopamine by acetaminophen administration based on their simultaneous determination in urine. Biosens. Bioelectron. 2013, 39, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Sasso, L.; Heiskanen, A.; Diazzi, F.; Dimaki, M.; Castillo-Leon, J.; Vergani, M.; Landini, E.; Raiteri, R.; Ferrari, G.; Carminati, M.; et al. Doped overoxidized polypyrrole microelectrodes as sensors for the detection of dopamine released from cell populations. Analyst 2013, 138, 3651–3659. [Google Scholar] [CrossRef]
- Scavetta, E.; Mazzoni, R.; Mariani, F.; Margutta, R.G.; Bonfiglio, A.; Demelas, M.; Fiorilli, S.; Marzocchi, M.; Fraboni, B. Dopamine amperometric detection at a ferrocene clicked PEDOT:PSS coated electrode. J. Mater. Chem. B 2014, 2, 2861–2867. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; Ekram, A.G.; El-Ads, H. Gold nanoparticles-coated poly(3,4-ethylene-dioxythiophene) for the selective determination of sub-nano concentrations of dopamine in presence of sodium dodecyl sulfate. Electrochim. Acta 2012, 69, 102–111. [Google Scholar] [CrossRef]
- Mahalakshmi, S.; Sridevi, V. In Situ Electrodeposited Gold Nanoparticles on Polyaniline-Modified Electrode Surface for the Detection of Dopamine in Presence of Ascorbic Acid and Uric Acid. Electroanalysis 2021, in press. [Google Scholar]
- Yang, L.; Liu, S.; Zhang, Q.; Li, F. Simultaneous electrochemical determination of dopamine and ascorbic acid using AuNPs@polyaniline core–shell nanocomposites modified electrode. Talanta 2012, 89, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, G.; Cui, X.T.; Sheng, G.; Luo, X. Enhanced catalytic and dopamine sensing properties of electrochemically reduced conducting polymer nanocomposite doped with pure graphene oxide. Biosens. Bioelectron. 2014, 58, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Xing, X.; Yu, J.; Lian, W.; Li, J.; Cui, M.; Huang, J. A novel label-free electrochemical aptasensor based on graphene–polyaniline composite film for dopamine determination. Biosens. Bielectron. 2012, 36, 186–191. [Google Scholar] [CrossRef]
- Wang, L.; Xu, H.; Song, Y.; Luo, J.; Wei, W.; Xu, S.; Cai, X. Highly Sensitive Detection of Quantal Dopamine Secretion from Pheochromocytoma Cells Using Neural Microelectrode Array Electrodeposited with Polypyrrole Graphene. ACS Appl. Mater. Interfaces 2015, 7, 7619–7626. [Google Scholar] [CrossRef]
- Xu, G.; Li, B.; Cui, X.T.; Ling, L.; Luo, X. Electrodeposited conducting polymer PEDOT doped with pure carbon nanotubes for the detection of dopamine in the presence of ascorbic acid. Sens. Actuators B 2013, 188, 405–410. [Google Scholar] [CrossRef]
- Shaikshavali, P.; Madhusudana Reddy, T.; Venkataprasad, G.; Gopal, P. Fabrication, Characterization and Application of Poly (L-Cystine)/multi Walled Carbon Nanotubes Modified Glassy Carbon Electrode towards the Simultaneous Determination of Dopamine in presence of Uric Acid and Folic Acid. Anal. Bioanal. Electrochem. 2017, 9, 940–955. [Google Scholar]
- Ali, S.R.; Parajuli, R.R.; Balogun, Y.; Ma, Y.; He, H. A Nonoxidative Electrochemical Sensor Based on a Self-Doped Polyaniline/Carbon Nanotube Composite for Sensitive and Selective Detection of the Neurotransmitter Dopamine: A Review. Sensors 2008, 8, 8423–8452. [Google Scholar] [CrossRef] [Green Version]
- Üge, A.; Zeybek, D.K.; Zeybek, B. An electrochemical sensor for sensitive detection of dopamine based on MWCNTs/CeO2-PEDOT composite. J. Electroanal. Chem. 2018, 813, 134–142. [Google Scholar] [CrossRef]
- Wadhera, T.; Kakkar, D.; Wadhwa, G.; Raj, B. Recent Advances and Progress in Development of the Field Effect Transistor Biosensor: A Review. J. Electron. Mater. 2019, 48, 7635–7646. [Google Scholar] [CrossRef] [Green Version]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Tang, H.; Lin, P.; Chan, H.L.W.; Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 2011, 26, 4559–4563. [Google Scholar] [CrossRef] [PubMed]
- Gualandi, I.; Tonelli, D.; Mariani, F.; Scavetta, E.; Marzocchi, M.; Fraboni, B. Selective detection of dopamine with an all PEDOT:PSS Organic Electrochemical Transistor. Sci. Rep. 2016, 6, 35419. [Google Scholar]
- Giordani, M.; Sensi, M.; Berto, M.; Di Lauro, M.; Bortolotti, C.A.; Gomes, H.L.; Zoli, M.; Zerbetto, F.; Fadiga, L.; Biscarini, F. Neuromorphic Organic Devices that Specifically Discriminate Dopamine from Its Metabolites by Nonspecific Interactions. Adv. Funct. Mater. 2020, 30, 2002141. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, J.; Seo, S.E.; Kim, K.H.; Park, C.S.; Lee, S.H.; Ban, H.S.; Lee, B.D.; Song, H.S.; Kim, J.; et al. High-performance conducting polymer nanotube-based liquid-ion gated field-effect transistor aptasensor for dopamine exocytosis. Sci. Rep. 2020, 10, 3772. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Oh, J.; Kim, S.G.; Jang, J. Highly Sensitive and Selective Field-Effect-Transistor Non Enzyme Dopamine Sensors Based on Pt/Conducting Polymer Hybrid Nanoparticles. Small 2015, 11, 2399–2406. [Google Scholar] [CrossRef]
- Casalini, S.; Leonardi, F.; Cramer, T.; Biscarini, F. Organic field-effect transistor for label-free dopamine sensing. Org. Electron. 2013, 14, 156–163. [Google Scholar] [CrossRef]
- BelBruno, J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Cieplak, M.; Szwabinska, K.; Sosnowska, M.; Chandra, B.K.C.; Borowski, P.; Noworyta, K.; D’Souza, F.; Kutner, W. Selective electrochemical sensing of human serum albumin by semi-covalent molecular imprinting. Biosens. Bioelectron. 2015, 74, 960–966. [Google Scholar] [CrossRef] [Green Version]
- Xia, J.; Cao, X.; Wang, Z.; Yang, M.; Zhang, F.; Lu, B.; Li, F.; Xia, L.; Li, Y.; Xia, Y. Molecularly imprinted electrochemical biosensor based on chitosan/ionic liquid–graphene composites modified electrode for determination of bovine serum albumin. Sens. Actuators B 2016, 225, 305–311. [Google Scholar] [CrossRef]
- Lopes, F.; Pacheco, J.G.; Rebelo, P.; Delerue-Matos, C. Molecularly imprinted electrochemical sensor prepared on a screen-printed carbon electrode for naloxone detection. Sens. Actuators B 2017, 243, 745–752. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Zhao, F. Electrochemical sensor for chloramphenicol based on novel multiwalled carbon nanotubes@molecularly imprinted polymer. Biosens. Bioelectron. 2015, 64, 416–422. [Google Scholar] [CrossRef]
- Han, Q.; Shen, X.; Zhu, W.; Zhu, C.; Zhou, X.; Jiang, H. Magnetic sensing film based on Fe3O4@Au-GSH molecularly imprinted polymers for the electrochemical detection of estradiol. Biosens. Bioelectron. 2016, 79, 180–186. [Google Scholar] [CrossRef]
- Liu, W.; Ma, Y.; Sun, G.; Wang, S.; Deng, J.; Wei, H. Molecularly imprinted polymers on graphene oxide surface for EIS sensing of testosterone. Biosens. Bioelectron. 2017, 92, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kan, X.; Li, C.; Zhou, H.; Zhu, A.; Xing, Z.; Zhao, Z.; Xu, G. Three-dimensional ordered microporous electrochemical sensor for dopamine recognition and detection. Am. J. Biomed. Sci. 2012, 4, 184–193. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Liu, J.; Gan, W.; Ye, B.; Li, Y. Highly sensitive and selective voltammetric determination of dopamine using a gold electrode modified with a molecularly imprinted polymeric film immobilized on flaked hollow nickel nanospheres. Microchim. Acta 2017, 184, 1285–1294. [Google Scholar] [CrossRef]
- Teng, Y.; Liu, F.; Kan, X. Voltammetric dopamine sensor based on three-dimensional electrosynthesized molecularly imprinted polymers and polypyrrole nanowires. Microchim. Acta 2017, 184, 2515–2522. [Google Scholar] [CrossRef]
- Wang, H.H.; Chen, X.J.; Li, W.T.; Zhou, W.H.; Guo, X.C.; Kang, W.Y.; Kou, D.X.; Zhou, Z.J.; Meng, Y.N.; Tian, Q.W.; et al. ZnO nanotubes supported molecularly imprinted polymers arrays as sensing materials for electrochemical detection of dopamine. Talanta 2018, 176, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Tertis, M.; Florea, A.; Adumitrachioaie, A.; Cernat, A.; Bogdan, D.; Barbu-Tudoran, L.; Jaffrezic-Renault, N.; Sandulescu, R.; Cristea, C. Detection of Dopamine by a Biomimetic Electrochemical Sensor Based on Polythioaniline-Bridged Gold Nanoparticles. ChemPlusChem 2017, 82, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Xue, C.; Han, Q.; Wang, Y.; Wu, J.; Wen, T.; Wang, R.; Hong, J.; Zhou, X.; Jiang, H. Amperometric detection of dopamine in human serum by electrochemical sensor based on gold nanoparticles doped molecularly imprinted polymers. Biosens. Bioelectron. 2013, 49, 199–203. [Google Scholar] [CrossRef]
- Wu, D.; Li, H.; Xue, X.; Fan, H.; Xin, Q.; Wei, Q. Sensitive and selective determination of dopamine by electrochemical sensor based on molecularly imprinted electropolymerization of o-phenylenediamine. Anal. Methods 2013, 5, 1469–1473. [Google Scholar] [CrossRef]
- Prasad, B.B.; Kumar, D.; Madhuri, R.; Tiwari, M.P. Sol–gel derived multiwalled carbon nanotubes ceramic electrode modified with molecularly imprinted polymer for ultra-trace sensing of dopamine in real samples. Electrochim. Acta 2011, 56, 7202–7211. [Google Scholar] [CrossRef]
- Qian, T.; Yu, C.; Zhou, X.; Ma, P.; Wu, S.; Xu, L.; Shen, J. Ultrasensitive dopamine sensor based on novel molecularly imprinted polypyrrole coated carbon nanotubes. Biosens. Bioelectron. 2014, 58, 237–241. [Google Scholar] [CrossRef] [PubMed]


| Active Layer | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| FePt–Fe3O4 | 0.1–90 µM | 1 nM | [71] |
| NiO | 0.5–5 μM | 85 nM | [72] |
| MoS2 | 0.006–181 μM | 2 nM | [73] |
| MoS2 | 10 pM–10 µM | 2.3 pM | [74] |
| ZnO | 0.1–800 µM | 60 nM | [75] |
| Fe3O4 | 2–600 nM | 0.8 nM | [76] |
| ZnFe2O4 | 2–600 nM | 0.4 nM | [77] |
| Active Layer | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| SWCNT | 1.2–900 µM | 0.57 µM | [86] |
| MWCNT | 1.2–800 µM | 0.16 µM | [87] |
| Carbonyl–SWCNT | 10–200 nM | 15 nM | [88] |
| SDS–SWCNT | 5–100 µM | 20 nM | [89] |
| Nafion+poly(3-methylthiophene)-SWCNT | 5–177 μM | 2 µM | [90] |
| Ionic liquid–SWCNT | 0.5–30 µM | 0.16 µM | [91] |
| Polypyrrole–SWCNT | 0.1–100 µM | 136 pM | [92] |
| AgAu–MWCNT | 3 nM–2.3 µM | 0.23 nM | [93] |
| Graphene–MWCNT | 5 nM–100 µM | 0.87 nM | [94] |
| MoS2–MWCNT | 0.03–1950 µM | 13 nM | [95] |
| Active Layer | Linear Range | Detection Limit | Reference |
|---|---|---|---|
| Polypyrrole–SiO2 | 2 µM–0.23 mM | 0.9 µM | [159] |
| Phenylenediamine–Ni | 0.05–50 pM | 0.017 pM | [160] |
| Polypyrrole/phenylenediamine | 50 nM–100 µM | 33 nM | [161] |
| Polypyrrole–ZnO | 0.02–800 µM | 1 nM | [162] |
| Poly(thioaniline)–Au NPs | 1 nM–5 µM | 33 pM | [163] |
| Poly(Aminobenzenethiol)–Au NPs | 0.02–0.74 µM | 7.8 nM | [164] |
| Phenylenediamine–Graphene–SO3- | 3–50 µM | 0.7 µM | [165] |
| Poly(nitrophenyl acrylate)–ceramic MWCNT | 6.5–550 µM | 1 mM | [166] |
| Polypyrrole–CNT | 50 pM–5 µM | 10 pM | [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakard, S.; Pavel, I.-A.; Lakard, B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors 2021, 11, 179. https://doi.org/10.3390/bios11060179
Lakard S, Pavel I-A, Lakard B. Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors. 2021; 11(6):179. https://doi.org/10.3390/bios11060179
Chicago/Turabian StyleLakard, Sophie, Ileana-Alexandra Pavel, and Boris Lakard. 2021. "Electrochemical Biosensing of Dopamine Neurotransmitter: A Review" Biosensors 11, no. 6: 179. https://doi.org/10.3390/bios11060179
APA StyleLakard, S., Pavel, I.-A., & Lakard, B. (2021). Electrochemical Biosensing of Dopamine Neurotransmitter: A Review. Biosensors, 11(6), 179. https://doi.org/10.3390/bios11060179