1. Introduction
The rapid growth of research interest in magnetogenetics in the past decade has resulted in a broad range of bioelectromagnetic stimulation applications [
1], creating a demand for sophisticated stimulus delivery systems. Many biological systems can be magnetically stimulated to regulate gene expression or neural activity, and stimulation parameters can vary significantly depending on the mechanisms employed to elicit responses [
1]. In contrast to visible light, low frequency and DC magnetic fields easily penetrate soft tissue and bone, potentially allowing for minimally invasive and wireless stimulation. High costs of bioelectromagnetic stimulation devices and a lack of systematic analysis of electromagnetic stimulus fields serve as a barrier to designing quantitative studies and replicating results in magnetogenetics experiments. Development of magnetic sensitive pathways, like those using nanoparticles [
2] and proteins like the electromagnetic perceptive gene (EPG) [
3,
4,
5,
6,
7], have contributed to making magnetic stimulus delivery for wide ranging applications increasingly important. Furthermore, recent studies which show that humans may also have magnetoperception [
8] serve to increase the demand for easy to implement and versatile electromagnetic stimulation devices.
One solution, which is well suited for stimulating multi-well plates, includes the coil array of [
9], which consists of an array of coils positioned to fit underneath each well of a 24-well plate. The coils are then used to deliver a pulsed time varying electromagnetic field stimulus having magnetic flux densities in the range of 1.0 to 1.2 mT.
Another solution for magnetic stimulus delivery includes the use of an induction heater [
2]; however, these are limited in their ability to be integrated into a wide variety of experimental protocols. Therefore, it is beneficial to design and build electromagnets which can be more easily incorporated into a wide variety of applications. Custom stimulation coils demonstrate improved integration into microscopy applications [
10,
11], and we aim to build on this flexibility and emphasize detailed stimulation validation. Furthermore, repeatability, uniformity, a negative control condition, and ease of use are critical properties of interest in magnetic stimulus systems.
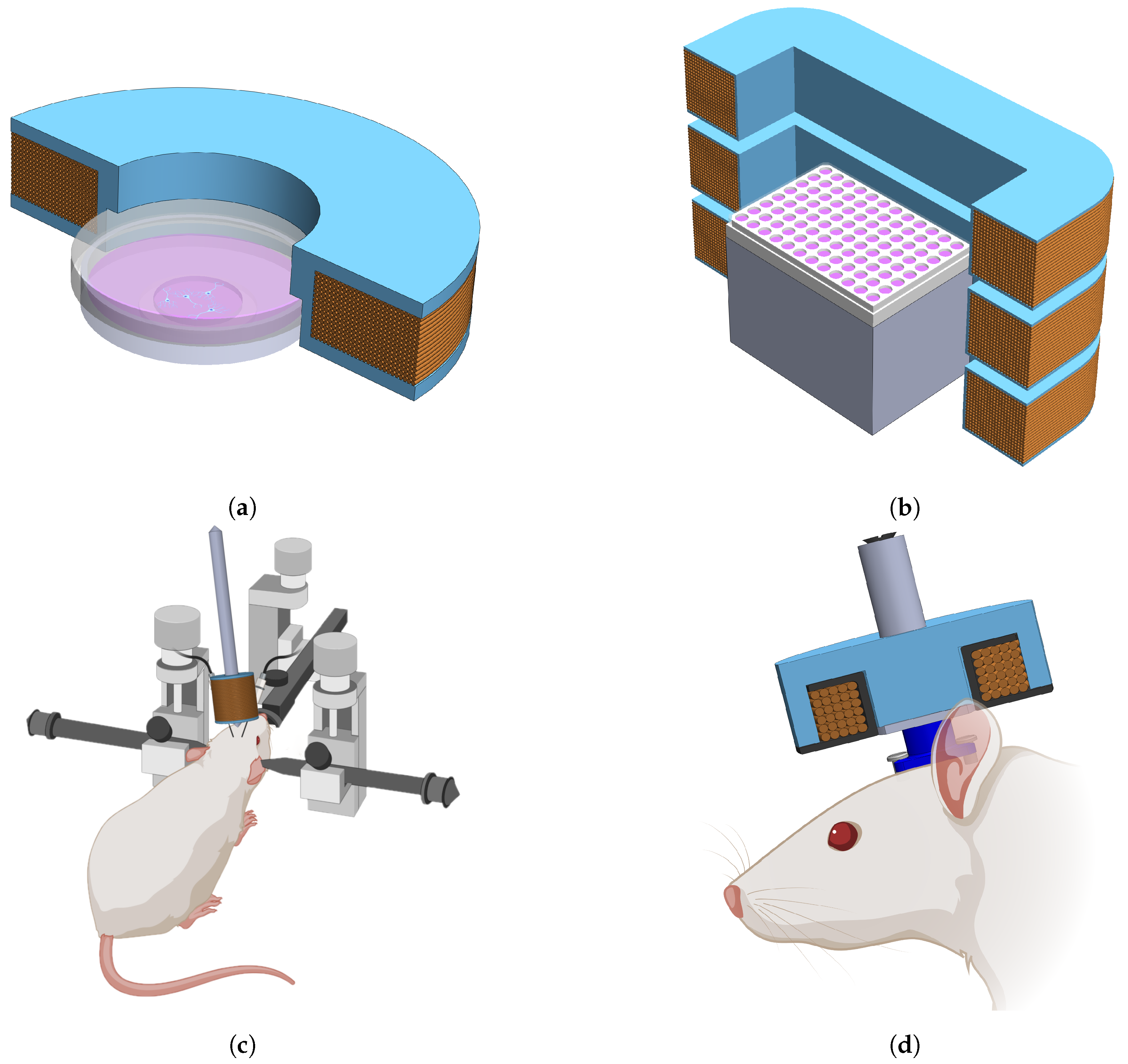
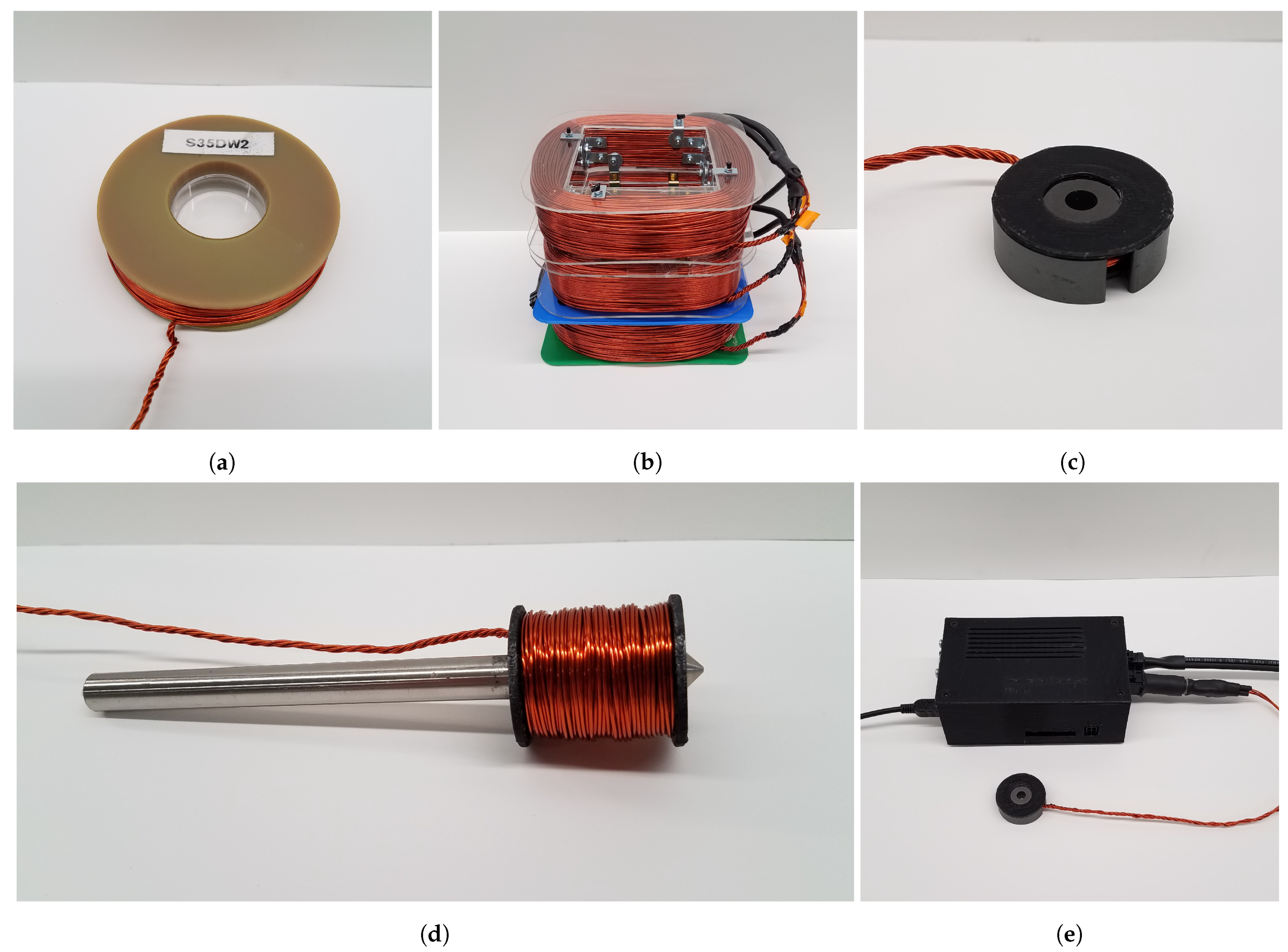
Here, we present work conducted toward developing a magnetogenetics bioelectromagnet stimulation platform which is low cost, versatile, easy to use, and affords a high degree of control over stimulation parameters. The electromagnet designs presented in this paper are applicable for electrophysiology, microscopy, fluorescence and luminescence imaging, and also to stimulate in freely behaving animals.
We developed four designs, where each design is unique to accommodate application specific physical constraints as well as maintain uniformity in the target area. Double wrapping coils as described in Kirschvink et al. [
12] allows experiments to be tested with a negative control. Additionally, a low-cost stimulation controller along with an accompanying graphical interface provides a user-friendly way to switch between active and sham stimulation conditions and reproduce specific stimulus parameters.
3. Results and Discussion
It is crucial to validate simulated results with experimental measurements to fully characterize the core and coil parameters in an electromagnet stimulation system. For a quantitative comparison between simulation and experiment, we have utilized a TLE493D-A2B6 three-axis Gaussmeter along with an Aerotech AGS1000 programmable XYZ scanner to measure the distribution of
B in the regions of interest as shown in
Figure 2 produced by the different configurations of stimulation devices. All experimental scans were performed with step sizes of 0.5 mm.
All experimental measurements were performed with a 1 Ampere excitation current. At each position, five sensor measurements were averaged to generate the resulting |B|. To compare the experimental measurements with corresponding simulation results, magnetic flux density images were first aligned based on the maximum of their cross-correlation.
A qualitative comparison of the experimental and simulated field data are shown in
Figure 4 for the air core, three-coil, ferromagnetic core, and pot core systems. The images represent the spatial distribution of |
B|. Results for the air core coil, shown in
Figure 4a, indicate that a maximum |
B| of 5.20 mT is recorded just above the surface of the coil. For the three-coil system, the first region of interest consists of the central XZ plane passing through the coils. A region of 60 mm by 120 mm centered at the center of the coil was scanned in the XZ plane, as shown in
Figure 4b. In addition, measurements along the central XY plane were performed along a 40 mm by 120 mm region approximately centered on the coil axis, as shown in
Figure 4c. In
Figure 4d, we see the measured distribution of |
B| for the ferromagnetic core coil, reaching a maximum of 24.55 mT. It is not surprising that, of the four geometries, this design produces the highest |
B| at the target location due to the effect of the ferromagnetic core. The pot core measurements are presented in
Figure 4e, where the |
B| achieves a maximum of 3.29 mT.
In addition to measuring the |
B| during the active condition, similar measurements were taken in the sham configuration as well as with no stimulation current. The air core sham results are seen in
Figure 4f, while the results for the air core no stimulation current control are shown in
Figure 4g. Low amplitude fringing fields are observed near the coil at the bottom of the image in the case of the sham condition. Otherwise, the sham condition performed similarly to the no current case. Similar results were observed in the case of other geometries.
A quantitative comparison of the predicted and measured fields for the no current, sham, experimental, and simulated stimulations for each coil design is shown in
Figure 5 and summarized quantitatively in
Figure 5f. The values listed in
Figure 5f for the cases of air, ferromagnetic, and pot core coils are measured at a distance of 10 mm from the coil along the coil axis, while measurements for the three-coil system are taken at the center of the XZ and XY planes.
With regard to the uniformity of the stimulus delivered by the three-coil system,
Figure 5b shows that the flux density along the three-coil system’s XZ axis drops on average only 2.99% in strength at the extrema of the line scan compared to the center. In the XY plane, the |
B| increases on average 5.98% at ±40 mm from the center, whereas, at the extrema of the line scan, it increases on average by 14.33% compared to the center.
It is worth noting that the ferromagnetic core does retain a low level of magnetization. However, the rapid decay in |B| means the magnetization has little effect at a distance of 10 mm from the tip.
Performance of the pot core coil showed that it did not reach the stimulation strength of the simulated target value. It is possible that the wide tolerance of the magnetic permeability of the pot core, ±30%, played some role in contributing to the reduced strength. Regardless, achieving a stimulation strength of 9.45 mT at the target distance is thought be a successful implementation of such a compact stimulation device. Future work will aim to increase the strength of the device by reducing the target distance to less than 10 mm and using a smaller diameter magnet wire to increase the number of turns. In the case of the latter method for increasing stimulation strength, careful consideration must be given to the trade-off between added performance in strength and the decreased thermal performance, which is a consequence of using smaller wire.
Despite the use of DC stimulation in our study, induction of a transient electric field is inevitable during the powering on and powering off of the stimulation. To characterize the induced electric field at the target locations during these periods, electrical characteristics of the coils were measured and the induced electric field was estimated.
Table 2 shows the resistance and inductance used to determine the rise and fall times according to the relationship
where
is rise time,
is fall time,
L is inductance, and
R is resistance. Equation (
2) can be derived from the equations governing a step response in an RL circuit. The rise or fall times indicate the time required for the stimulation to ramp up from 10% strength to 90% strength or vice versa. An estimation of the induced transient electric field at the target locations is also shown in
Table 2, calculated using Faraday’s Law of induction over the transition from 10% to 90% stimulation strength.
Thermal imaging was also performed with a FLIR One thermal infrared camera. Safe operation of stimulation coils requires identification of maximum operating times for each coil geometry to stay below 75 °C. Such analysis is important to carefully design experiments that will allow the coils to stay within the defined temperature limits. Currents ranging from 1 to 15 Amperes at 1 Ampere intervals were applied to stimulate each coil while sampling coil temperature at one sample per second until the coil temperature reached 75 °C.
Results plotted in
Figure 6 can be used to determine both the maximum excitation current and stimulation time based on the desired stimulus strength. For each of the four geometries, composite plots of maximum flux density magnitude and maximum operating time are shown for increasing stimulation current values ranging from 1 to 15 Amperes. The blue
y-axis on the left shows the flux density magnitude in mT and the orange
y-axis on the right shows the maximum operating times for the given stimulation current. Stimulation times are cutoff after one hour for the three-coil system and three minutes for the remaining geometries. An exponential best fit line for the maximum stimulation times is also shown in each graph of
Figure 6.
Temperatures were also measured at sample target locations for each coil configuration with a 15 Ampere stimulation current. Target locations for temperature measurements were the same locations as the measurements from
Figure 5f. Additionally, temperature measurements were taken at the corner wells of a 96-well plate placed in the three-coil system. For the three-coil system, temperature was observed after application of a five minute stimulation. The air core, ferromagnetic core, and pot core coils were stimulated for the duration of their respective maximum operating times indicated in
Figure 6.
With the air core coil, sample temperature was seen to rise by 0.5 °C, whereas the ferromagnetic core sample temperature increased by 1.1 °C and the pot core sample temperature increased by 0.7 °C throughout the stimulations. The temperature of the corner well samples in the three-coil system showed an increase of 1.3 °C after five minutes, while the temperature at the center remained essentially unchanged, decreasing by 0.1 °C.
4. Conclusions
This study presented a magnetogenetics stimulation platform that supports four electromagnet stimulation coil designs and a controller for selecting stimulation conditions. The various coil geometries were chosen so that at least one of the designs satisfied the needs of magnetogenetics experiments including microscopy, in vivo electrophysiology, freely moving behavioral experiments, and some fluorescence and luminescence imaging setups.
Regarding the use of ferromagnetic materials in stimulation coils, the added benefit of increased stimulation strength for an otherwise similar coil without a ferromagnetic core must be weighed against the necessity to account for the residual magnetization of the material. Negative effects can be mitigated by either demagnetizing the core between stimulations or placing the coil at a distance such that the residual field of the core does not interfere with the experiment design.
While sham conditions are required to ensure proper experimental controls, it is important to understand their limitations as they apply to a given experimental protocol and stimulation conditions. The sham conditions all demonstrated at least an order of magnitude reduction in |B|; however, some residual magnetic flux is unavoidable. To properly incorporate sham conditions into an experiment, it would be best to know the minimum stimulation threshold necessary to produce a meaningful target response. With this knowledge, stimulations can be performed such that active conditions provide suprathreshold stimulation while sham conditions provide only subthreshold stimulation.
Accounting for the effects of temperature change is important when studying pathways with thermal sensitivity. Our designs showed minimal temperature increases (0.5–1.3 °C) at sample locations under maximum field strength conditions in all cases studied.
The versatility of various magnet designs presented allows for multiple choices of electromagnets based on the size constraints of the application. The analysis of the |B| distributions is important for selecting an appropriate electromagnet system to achieve the proper strength of the applied stimulus at the target location to successfully elicit a response. Additionally, our analysis of the sham stimulus strength is important in designing experiments with a negative control which can help eliminate the role of confounding variables on observed effects.
Studies presented here also provide a useful tool for selecting experimental design parameters for magnetogenetics experiments. For example, knowing that the three-coil system has a field distribution that varies less than 6% over a range of 40 mm from the central axis of the coils means that the sample placement should be restricted to this range in order to maintain a high degree of stimulation uniformity. In addition to limiting the effects of temperature on experimental observations, thermal analysis also allowed for determination of safe operating limits for the coils.
Figure 6 can be used to find the operating limits, in terms of time and current, for a desired |
B| with each coil geometry. Lastly, the use of a custom stimulation controller allows for easily configurable stimulation patterns, in either the sham or experimental modes, which improves repeatability.