Label-Free Detection and Spectrometrically Quantitative Analysis of the Cancer Biomarker CA125 Based on Lyotropic Chromonic Liquid Crystal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PI-Coated Glass Substrates
2.3. Construction of the LCLC-Based Protein Detection Platform
2.4. LCLC-Based ca125 Immunoassay
2.5. Transmission Spectrometric Measurements
2.6. Optical Texture Observation
3. Results and Discussion
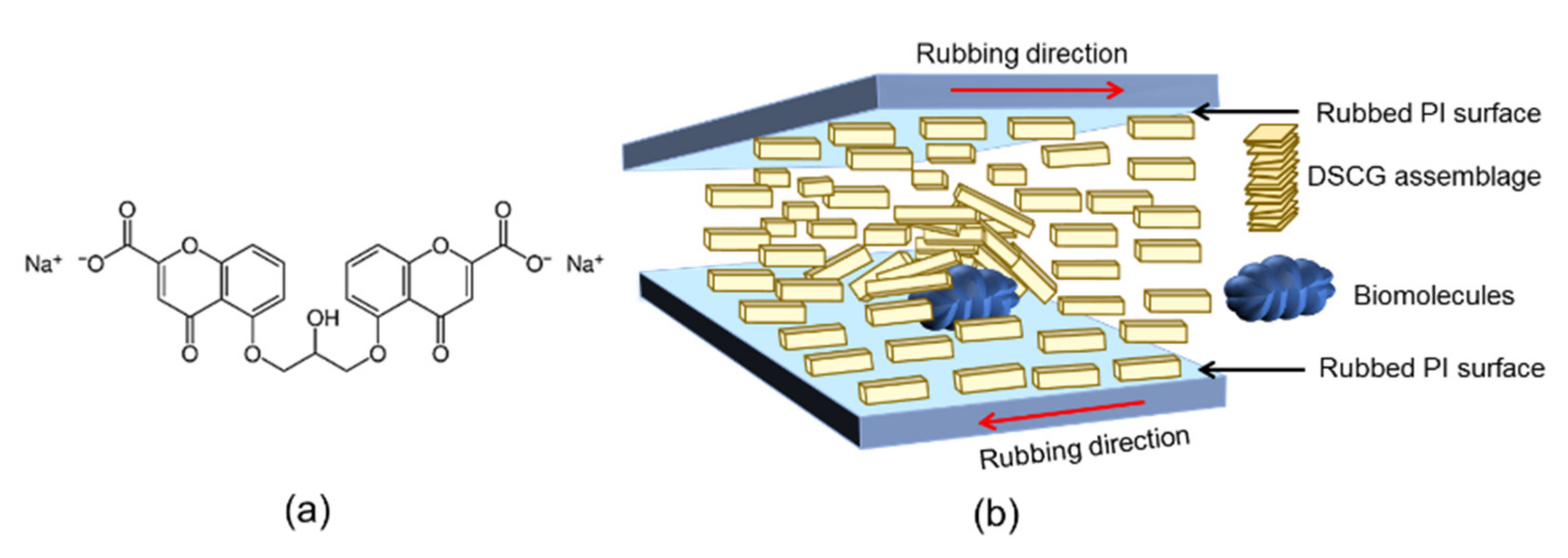
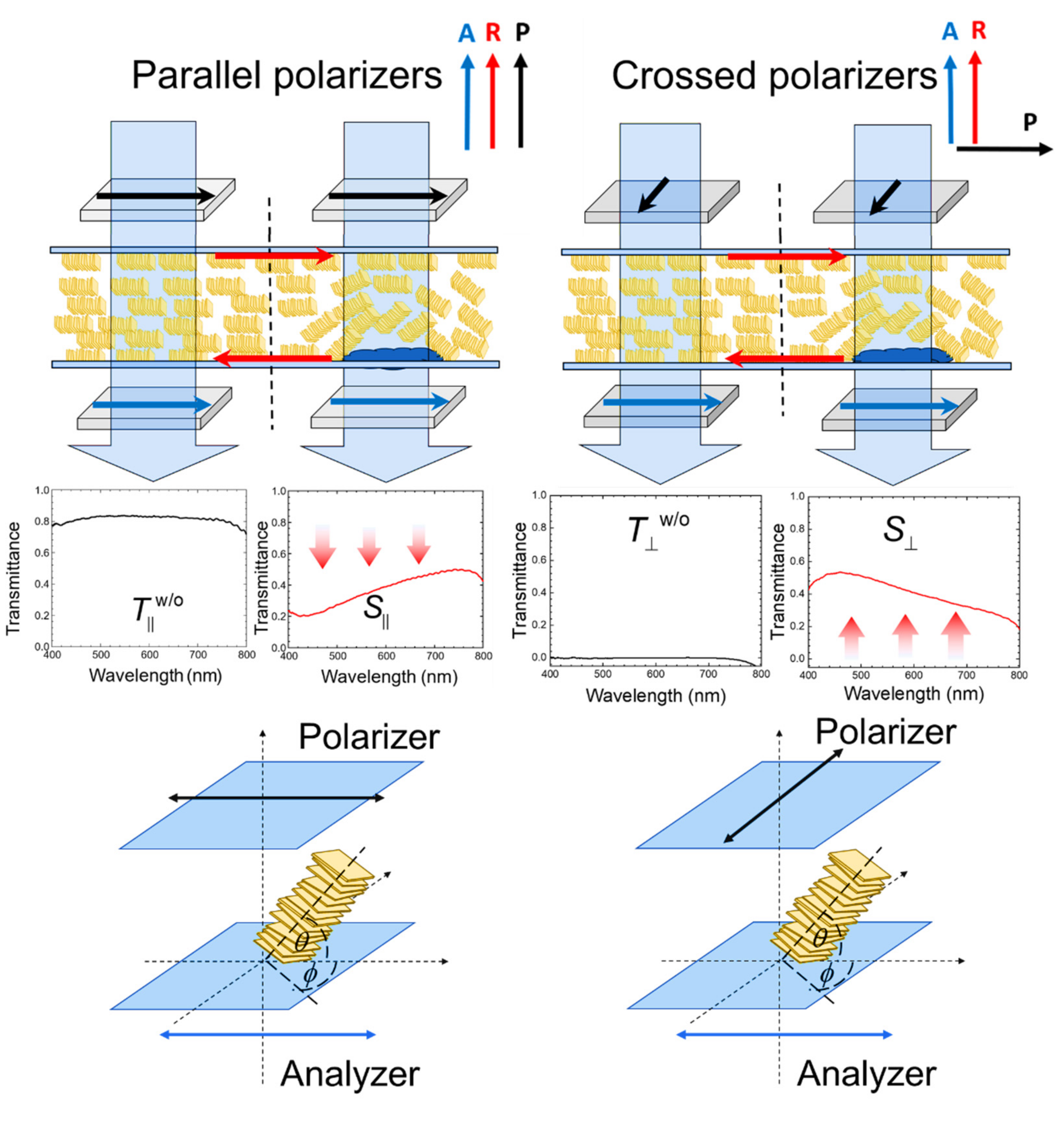
3.1. Principle of Detection by Transmission Spectrometry in the LCLC-Based Biosensor
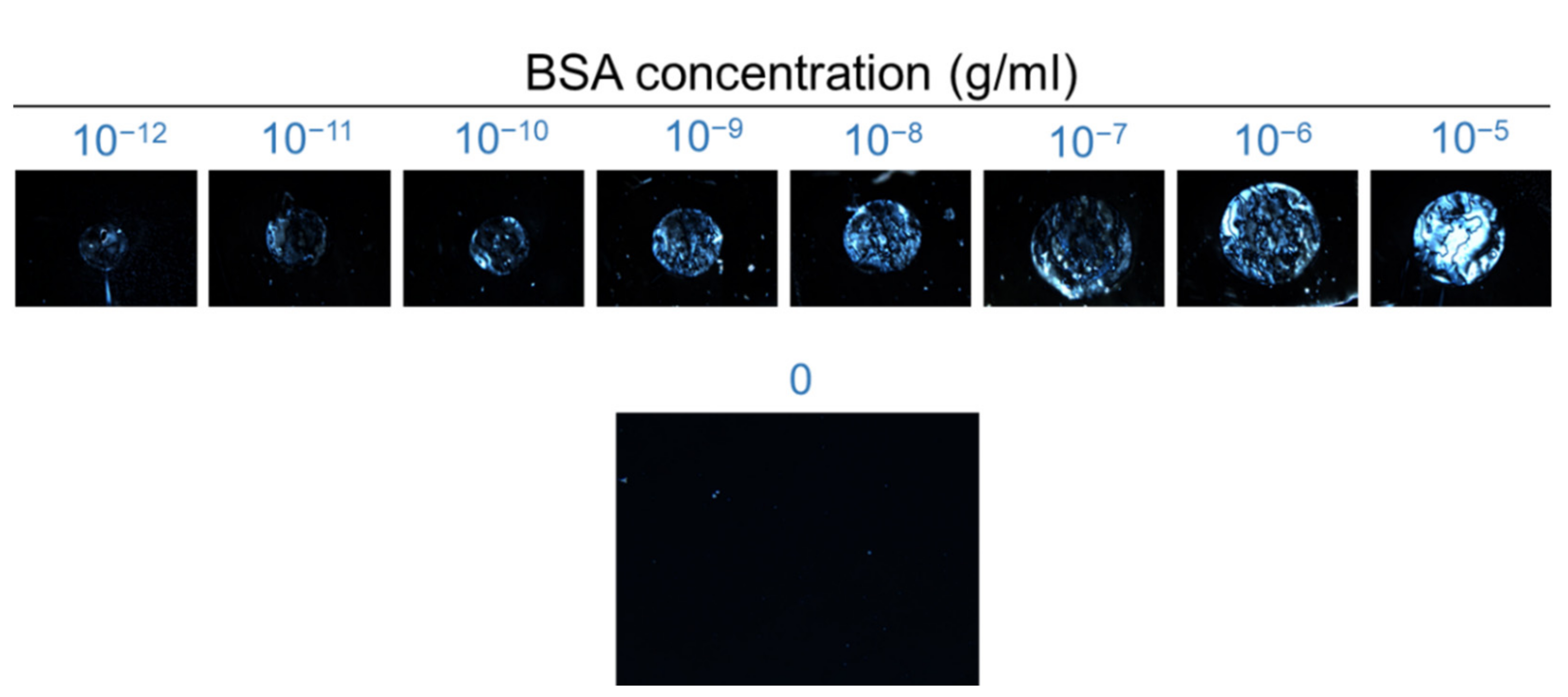
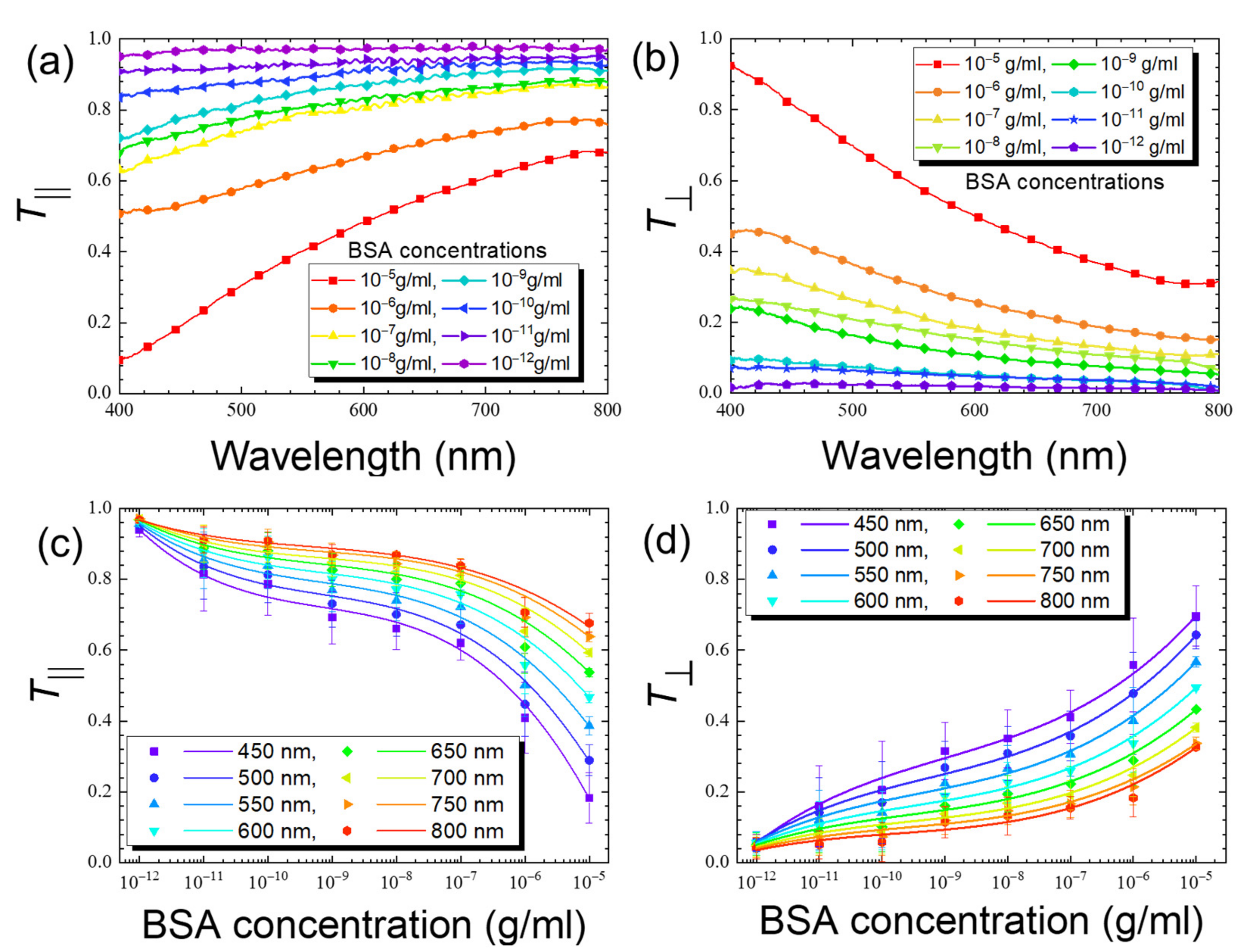
3.2. LCLC-Based Spectrometric Quantitation of BSA
3.3. LCLC-Based Spectrometric Quantitation of the Anti-CA125 Antibody
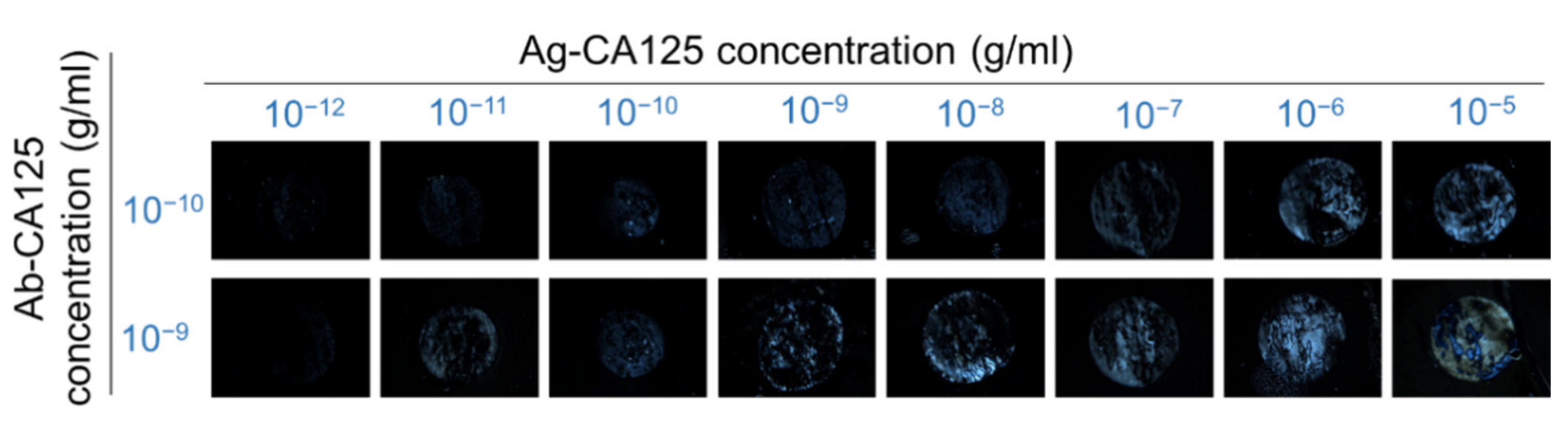
3.4. LCLC-Based Quantitative Immunoassay of the Cancer Biomarker CA125
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luan, C.; Luan, H.; Luo, D. Application and technique of liquid crystal-based biosensors. Micromachines 2020, 11, 176. [Google Scholar] [CrossRef] [Green Version]
- Prakash, J.; Parveen, A.; Mishra, Y.K.; Kaushik, A. Nanotechnology-assisted liquid crystals-based biosensors: Towards fundamental to advanced applications. Biosens. Bioelectron. 2020, 168, 112562. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, T.; Noel, A.; Chen, Y.-C.; Liu, T. Applications of liquid crystals in biosensing. Soft Matter 2021, 17, 4675–4702. [Google Scholar] [CrossRef]
- Nayani, K.; Yang, Y.; Yu, H.; Jani, P.; Mavrikakis, M.; Abbott, N. Areas of opportunity related to design of chemical and biological sensors based on liquid crystals. Liq. Cryst. Today 2020, 29, 24–35. [Google Scholar] [CrossRef]
- Popov, P.; Mann, E.K.; Jákli, A. Thermotropic liquid crystal films for biosensors and beyond. J. Mater. Chem. B 2017, 5, 5061–5078. [Google Scholar] [CrossRef]
- Munir, S.; Kang, I.-K.; Park, S.-Y. Polyelectrolytes functionalized nematic liquid crystal-based biosensors: An overview. TrAC Trends Anal. Chem. 2016, 83, 80–94. [Google Scholar] [CrossRef]
- Lee, M.-J.; Lee, W. Liquid crystal-based capacitive, electro-optical and dielectric biosensors for protein quantitation. Liq. Cryst. 2020, 47, 1145–1153. [Google Scholar] [CrossRef]
- Concellón, A.; Fong, D.; Swager, T.M. Complex liquid crystal emulsions for biosensing. J. Am. Chem. Soc. 2021, 143, 9177–9182. [Google Scholar] [CrossRef]
- Wu, P.-C.; Karn, A.; Lee, M.-J.; Lee, W.; Chen, C.-Y. Dye-liquid-crystal-based biosensing for quantitative protein assay. Dyes Pigment. 2018, 150, 73–78. [Google Scholar] [CrossRef]
- Lin, C.-M.; Wu, P.-C.; Lee, M.-J.; Lee, W. Label-free protein quantitation by dielectric spectroscopy of dual-frequency liquid crystal. Sens. Actuators B Chem. 2019, 282, 158–163. [Google Scholar] [CrossRef]
- Lee, M.-J.; Lee, W. Unconventional Liquid Crystals and Their Applications; Lee, W., Kumar, S., Eds.; Chapter 5; De Gruyter: Berlin, Germany, 2021; pp. 239–264. [Google Scholar] [CrossRef]
- Tam-Chang, S.-W.; Huang, L. Chromonic liquid crystals: Properties and applications as functional materials. Chem. Commun. 2008, 17, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Nastishin, Y.A.; Liu, H.; Schneider, T.; Nazarenko, V.; Vasyuta, R.; Shiyanovskii, S.V.; Lavrentovich, O.D. Optical characterization of the nematic lyotropic chromonic liquid crystals: Light absorption, birefringence, and scalar order parameter. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2005, 72, 41711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agra-Kooijman, D.M.; Singh, G.; Lorenz, A.; Collings, P.J.; Kitzerow, H.S.; Kumar, S. Columnar molecular aggregation in the aqueous solutions of disodium cromoglycate. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2014, 89, 062504. [Google Scholar] [CrossRef] [Green Version]
- Luk, Y.Y.; Jang, C.H.; Cheng, L.L.; Israel, B.A.; Abbott, N.L. Influence of lyotropic liquid crystals on the ability of antibodies to bind to surface-immobilized antigens. Chem. Mater. 2005, 17, 4774–4782. [Google Scholar] [CrossRef]
- Lydon, J. Chromonic review. J. Mater. Chem. 2010, 20, 10071–10099. [Google Scholar] [CrossRef]
- Dhakal, N.P.; Jiang, J.; Guo, Y.; Peng, C. Self-assembly of aqueous soft matter patterned by liquid-crystal polymer networks for controlling the dynamics of bacteria. ACS Appl. Mater. Inter. 2020, 12, 13680–13685. [Google Scholar] [CrossRef]
- Kumar, A.; Galstian, T.; Pattanayek, S.K.; Rainville, S. The motility of bacteria in an anisotropic liquid environment. Mol. Cryst. Liq. Cryst. 2013, 574, 33–39. [Google Scholar] [CrossRef]
- Mushenheim, P.C.; Trivedi, R.R.; Weibel, D.B.; Abbott, N.L. Using liquid crystals to reveal how mechanical anisotropy changes interfacial behaviors of motile bacteria. Biophys. J. 2014, 107, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Tovkach, O.; Golovaty, D.; Sokolov, A.; Aranson, I.S.; Lavrentovich, O.D. Dynamic states of swimming bacteria in a nematic liquid crystal cell with homeotropic alignment. New J. Phys. 2017, 19, 055006. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.L.; Luk, Y.Y.; Murphy, C.J.; Israel, B.A.; Abbott, N.L. Compatibility of lyotropic liquid crystals with viruses and mammalian cells that support the replication of viruses. Biomaterials 2005, 26, 7173–7182. [Google Scholar] [CrossRef]
- Shiyanovskii, S.V.; Lavrentovich, O.D.; Schneider, T.; Ishikawa, T.; Smalyukh, I.I.; Woolverton, C.J.; Niehaus, G.D.; Doane, K.J. Lyotropic chromonic liquid crystals for biological sensing applications. Mol. Cryst. Liq. Cryst. 2005, 434, 259–270. [Google Scholar] [CrossRef]
- Skates, S.J.; Xu, F.-J.; Yu, Y.-H.; Sjövall, K.; Einhorn, N.; Chang, Y.; Bast, R.C.; Knapp, R.C. Toward an optimal algorithm for ovarian cancer screening with longitudinal tumor markers. Cancer 1995, 76, 2004–2010. [Google Scholar] [CrossRef]
- Cotchim, S.; Thavarungkul, P.; Kanatharana, P.; Limbut, W. Multiplexed label-free electrochemical immunosensor for breast cancer precision medicine. Anal. Chim. Acta 2020, 1130, 60–71. [Google Scholar] [CrossRef]
- de Castro, A.C.H.; Alves, L.M.; Siquieroli, A.C.S.; Madurro, J.M.; Brito-Madurro, A.G. Label-free electrochemical immunosensor for detection of oncomarker CA125 in serum. Microchem. J. 2020, 155, 104746. [Google Scholar] [CrossRef]
- Mansouri Majd, S.; Salimi, A. Ultrasensitive flexible FET-type aptasensor for CA 125 cancer marker detection based on carboxylated multiwalled carbon nanotubes immobilized onto reduced graphene oxide film. Anal. Chim. Acta 2018, 1000, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Ravalli, A.; Pilon Dos Santos, G.; Ferroni, M.; Faglia, G.; Yamanaka, H.; Marrazza, G. New label free CA125 detection based on gold nanostructured screen-printed electrode. Sens. Actuat. B Chem. 2013, 179, 194–200. [Google Scholar] [CrossRef]
- Su, H.-W.; Lee, Y.-H.; Lee, M.-J.; Hsu, Y.-C.; Lee, W. Label-free immunodetection of the cancer biomarker CA125 using high-Δn liquid crystals. J. Biomed. Opt. 2014, 19, 077006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, H.-W.; Lee, M.-J.; Lee, W. Surface modification of alignment layer by ultraviolet irradiation to dramatically improve the detection limit of liquid-crystal-based immunoassay for the cancer biomarker CA125. J. Biomed. Opt. 2015, 20, 057004. [Google Scholar] [CrossRef]
- Lee, M.-J.; Duan, F.-F.; Wu, P.-C.; Lee, W. Liquid crystal–photopolymer composite films for label-free single-substrate protein quantitation and immunoassay. Biomed. Opt. Express 2020, 11, 4915. [Google Scholar] [CrossRef]
- Chiang, Y.L.; Lee, M.J.; Lee, W. Enhancing detection sensitivity in quantitative protein detection based on dye-doped liquid crystals. Dyes Pigment. 2018, 157, 117–122. [Google Scholar] [CrossRef]
- Yang, K.H. Measurements of empty cell gap for liquid-crystal displays using interferometric methods. J. Appl. Phys. 1988, 64, 4780–4781. [Google Scholar] [CrossRef]
- Zhou, S.; Neupane, K.; Nastishin, Y.A.; Baldwin, A.R.; Shiyanovskii, S.V.; Lavrentovich, O.D.; Sprunt, S. Elasticity, viscosity, and orientational fluctuations of a lyotropic chromonic nematic liquid crystal disodium cromoglycate. Soft Matter 2014, 10, 6571–6581. [Google Scholar] [CrossRef]
- Kumar, A.; Pattanayek, S.K. Exploitation of orientation of liquid crystals 5CB and DSCG near surfaces to detect low protein concentration. Liq. Cryst. 2015, 42, 1506–1514. [Google Scholar] [CrossRef]
- Nazarenko, V.G.; Boiko, O.P.; Park, H.S.; Brodyn, O.M.; Omelchenko, M.M.; Tortora, L.; Nastishin, Y.A.; Lavrentovich, O.D. Surface alignment and anchoring transitions in nematic lyotropic chromonic liquid crystal. Phys. Rev. Lett. 2010, 105, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Shahsavan, H.; Davidson, Z.S.; Sitti, M. Precise Control of Lyotropic Chromonic Liquid Crystal Alignment through Surface Topography. ACS Appl. Mater. Interfaces 2019, 11, 36110–36117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.; Han, G.; Johnson, A.T.C.; Collings, P.J.; Lubensky, T.C.; Yodh, A.G. Homeotropic alignment of lyotropic chromonic liquid crystals using noncovalent interactions. Langmuir 2014, 30, 2914–2920. [Google Scholar] [CrossRef]
- Collings, P.J.; van der Asdonk, P.; Martinez, A.; Tortora, L.; Kouwer, P.H.J. Anchoring strength measurements of a lyotropic chromonic liquid crystal on rubbed polyimide surfaces. Liq. Cryst. 2017, 44, 1165–1172. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Hu, Y.J.; Liu, Y.; Sun, T.Q.; Bai, A.M.; Lü, J.Q.; Pi, Z.B. Binding of anti-inflammatory drug cromolyn sodium to bovine serum albumin. Int. J. Biol. Macromol. 2006, 39, 280–285. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaban, H.; Lee, M.-J.; Lee, W. Label-Free Detection and Spectrometrically Quantitative Analysis of the Cancer Biomarker CA125 Based on Lyotropic Chromonic Liquid Crystal. Biosensors 2021, 11, 271. https://doi.org/10.3390/bios11080271
Shaban H, Lee M-J, Lee W. Label-Free Detection and Spectrometrically Quantitative Analysis of the Cancer Biomarker CA125 Based on Lyotropic Chromonic Liquid Crystal. Biosensors. 2021; 11(8):271. https://doi.org/10.3390/bios11080271
Chicago/Turabian StyleShaban, Hassanein, Mon-Juan Lee, and Wei Lee. 2021. "Label-Free Detection and Spectrometrically Quantitative Analysis of the Cancer Biomarker CA125 Based on Lyotropic Chromonic Liquid Crystal" Biosensors 11, no. 8: 271. https://doi.org/10.3390/bios11080271
APA StyleShaban, H., Lee, M.-J., & Lee, W. (2021). Label-Free Detection and Spectrometrically Quantitative Analysis of the Cancer Biomarker CA125 Based on Lyotropic Chromonic Liquid Crystal. Biosensors, 11(8), 271. https://doi.org/10.3390/bios11080271






