Recent Progress of SERS Nanoprobe for pH Detecting and Its Application in Biological Imaging
Abstract
1. Introduction
2. The Significance of pH SERS Probe in the Study of Physiological Activities of Cells
2.1. Surface Enhanced Raman Scattering (SERS)
2.2. Significance of Cell pH Value in the Study of Cell Physiological Activities
3. pH SERS Probe Molecules
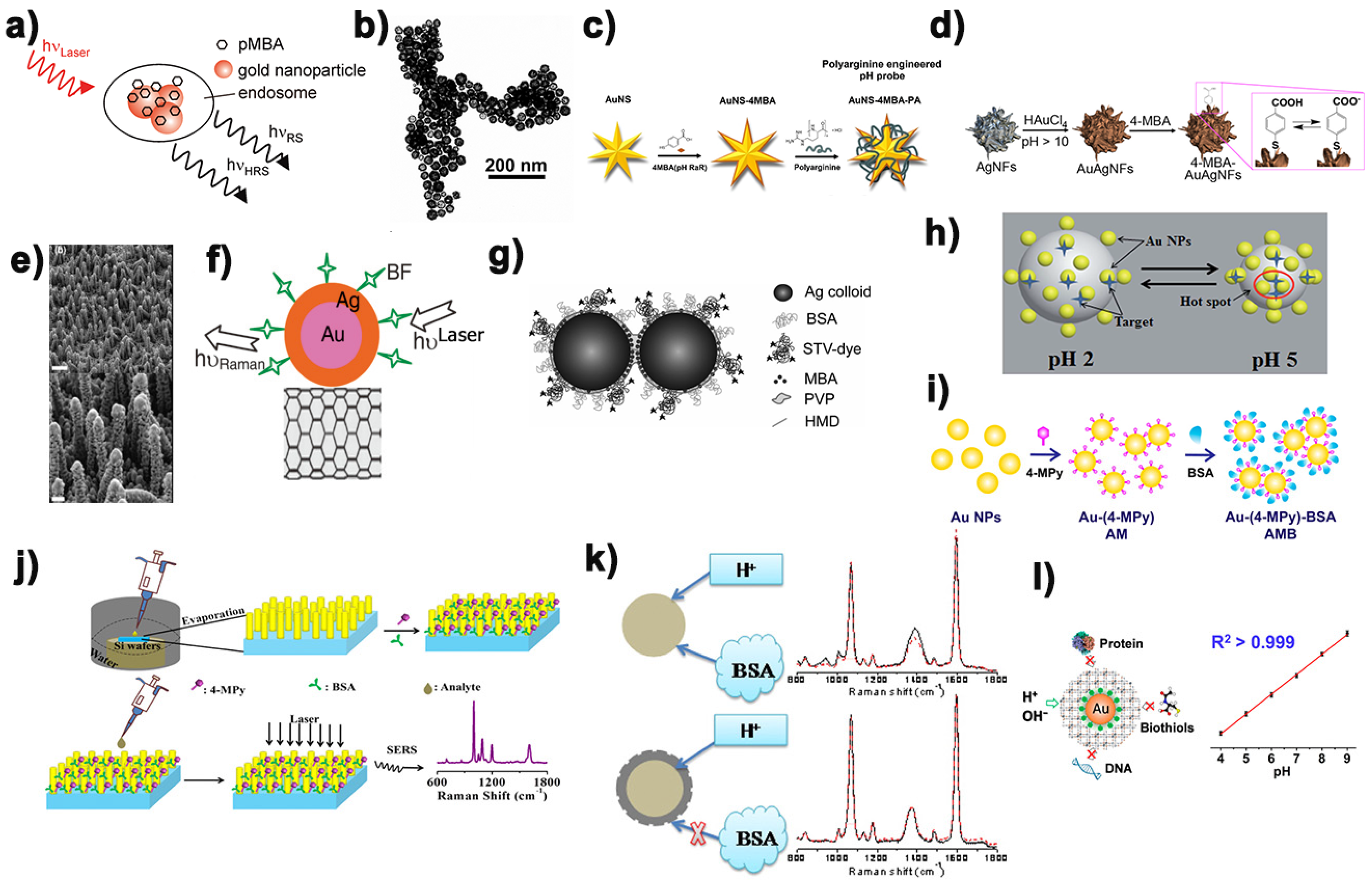
3.1. 4-Mercaptobenzoic Acid
3.2. 4-Mercaptopyridine
3.3. 4-Aminobenzene Mercaptan
3.4. Other Novel pH Probe Molecules
4. pH SERS Probe
5. pH SERS Probe Was Used for Cell pH Value Monitoring and pH Imaging
6. pH SERS Probe Combined with Other Technologies
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swietach, P. What is pH regulation, and why do cancer cells need it? Cancer Metastasis Rev. 2019, 38, 5–15. [Google Scholar] [CrossRef]
- Malek, K.; Jaworska, A.; Krala, P.; Kachamakova-Trojanowska, N.; Baranska, M. Imaging of macrophages by surface enhanced Raman spectroscopy (SERS). Bio. Spectrosc. Imaging 2013, 2, 349–357. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, L.; Piper, J.A.; Wang, Y. SERS nanotags and their applications in biosensing and bioimaging. J. Anal. Test. 2018, 2, 26–44. [Google Scholar] [CrossRef]
- Ma, D.; Zheng, J.; Tang, P.; Xu, W.; Qing, Z.; Yang, S.; Li, J.; Yang, R. Quantitative monitoring of hypoxia-Induced intracellular acidification in lung tumor cells and tissues using activatable surface-enhanced Raman scattering nanoprobes. Anal. Chem. 2016, 88, 11852–11859. [Google Scholar] [CrossRef] [PubMed]
- Talley, C.E.; Jusinski, L.; Hollars, C.W.; Lane, S.M.; Huser, T. Intracellular pH sensors based on surface-enhanced Raman scattering. Anal. Chem. 2004, 76, 7064–7068. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Anomalously intense raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Moskovits, M. Surface roughness and the enhanced intensity of Raman scattering by molecules adsorbed on metals. J. Chem. Phys. 1978, 69, 4159–4161. [Google Scholar] [CrossRef]
- Qiu, C.; Cheng, Z.; Lv, C.; Wang, R.; Yu, F. Development of bioorthogonal SERS imaging probe in biological and biomedical applications. Chin. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Jensen, L.; Aikens, C.M.; Schatz, G.C. Electronic structure methods for studying surface-enhanced Raman scattering. Chem. Soc. Rev. 2008, 37, 1061–1073. [Google Scholar] [CrossRef] [PubMed]
- Langer, J.; De Aberasturi, D.J.; Aizpurua, J.; Alvarez-Puebla, R.A.; Auguié, B.; Baumberg, J.J.; Bazan, G.C.; Bell, S.E.J.; Boisen, A.; Brolo, A.G.; et al. Present and future of surface-enhanced Raman scattering. ACS Nano 2020, 14, 28–117. [Google Scholar] [CrossRef] [PubMed]
- Coman, C.; Leopold, L.F. Raman mapping: Emerging applications. In Raman Spectroscopy and Applications; Khan, M., Ed.; IntechOpen: London, UK, 2017; pp. 59–77. [Google Scholar] [CrossRef]
- Benz, F.; Chikkaraddy, R.; Salmon, A.; Ohadi, H.; de Nijs, B.; Mertens, J.; Carnegie, C.; Bowman, R.W.; Baumberg, J.J. SERS of individual nanoparticles on a mirror: Size does matter, but so does shape. J. Phys. Chem. Lett. 2016, 7, 2264–2269. [Google Scholar] [CrossRef] [PubMed]
- Seney, C.S.; Gutzman, B.M.; Goddard, R.H. Correlation of size and surface-enhanced Raman scattering activity of optical and spectroscopic properties for silver nanoparticles. J. Phys. Chem. C 2009, 113, 74–80. [Google Scholar] [CrossRef]
- Sumitra, T.F.A.; Barber, D.; Nystul, T. Cell fate decisions: Emerging roles for metabolic signals and cell morphology. EMBO Rep. 2017, 18, 2105–2118. [Google Scholar] [CrossRef]
- Schotthofer, S.K.; Bohrmann, J. Bioelectrical and cytoskeletal patterns correlate with altered axial polarity in the follicular epithelium of the Drosophila mutant gurken. BMC Dev. Biol. 2020, 20, 5. [Google Scholar] [CrossRef]
- Miller, S.; Ptok, M.; Jungheim, M. Influence of acid swallows on the dynamics of the upper esophageal sphincter. Dysphagia 2021, 36, 443–455. [Google Scholar] [CrossRef]
- Park, J.; Tabet, A.; Moon, J.; Chiang, P.H.; Koehler, F.; Sahasrabudhe, A.; Anikeeva, P. Remotely controlled proton generation for neuromodulation. Nano Lett. 2020, 20, 6535–6541. [Google Scholar] [CrossRef]
- Zong, S.; Wang, Z.; Yang, J.; Cui, Y. Intracellular pH sensing using p-aminothiophenol functionalized gold nanorods with low cytotoxicity. Anal. Chem. 2011, 83, 4178–4183. [Google Scholar] [CrossRef]
- Schulz, E.; Munzel, T. Intracellular pH: A fundamental modulator of vascular function. Circulation 2011, 124, 1806–1807. [Google Scholar] [CrossRef][Green Version]
- Fang, B.; Wang, D.; Huang, M.; Yu, G.; Li, H. Hypothesis on the relationship between the change in intracellular pH and incidence of sporadic Alzheimer’s disease or vascular dementia. Int. J. Neurosci. 2010, 120, 591–595. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, B.; Chen, L. SERS tags: Novel optical nanoprobes for bioanalysis. Chem. Rev. 2012, 113, 1391–1428. [Google Scholar] [CrossRef]
- Xie, M.; Li, F.; Gu, P.; Wang, F.; Qu, Z.; Li, J.; Wang, L.; Zuo, X.; Zhang, X.; Shen, J. Gold nanoflower-based surface enhanced-Raman probes for pH mapping of tumor cell microenviroment. Cell Prolif. 2019, 52, e12618. [Google Scholar] [CrossRef]
- Potara, M.; Nagy-Simon, T.; Craciun, A.M.; Suarasan, S.; Licarete, E.; Imre-Lucaci, F.; Astilean, S. Carboplatin-loaded, Raman-encoded, chitosan-coated Silver nanotriangles as multimodal traceable nanotherapeutic delivery systems and pH reporters inside human ovarian cancer cells. ACS Appl. Mater. Interfaces 2017, 9, 32565–32576. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Yonet-Tanyeri, N.; Ende, E.V.; Henry, A.I.; Perez White, B.E.; Mrksich, M.; Van Duyne, R.P. Plasmonic microneedle arrays for in situ sensing with surface enhanced Raman spectroscopy (SERS). Nano Lett. 2019, 19, 6862–6868. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.L.; Carron, K.T. Dynamic Raman scattering studies of coated gold nanoparticles: 4-mercaptopyridine, 4-mercaptophenol, and benzenethiol. J. Phys. Chem. C 2016, 120, 20905–20913. [Google Scholar] [CrossRef]
- Bai, L.; Wang, X.; Zhang, K.; Tan, X.; Zhang, Y.; Xie, W. Etchable SERS nanosensor for accurate pH and hydrogen peroxide sensing in living cells. Chem. Commun. 2019, 55, 12996–12999. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liang, L.; Zhang, S.; Huang, D.; Zhang, J.; Xu, S.; Liang, C.; Xu, W. Organelle-targeting surface-enhanced Raman scattering (SERS) nanosensors for subcellular pH sensing. Nanoscale 2018, 10, 1622–1630. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, W.; Wang, D.; Gong, Z.; Fan, M. Evaluation of the intrinsic pH sensing performance of surface-enhanced Raman scattering pH probes. Microchem. J. 2020, 154. [Google Scholar] [CrossRef]
- Ma, C.; Harris, J.M. Surface-enhanced Raman spectroscopy investigation of the potential-dependent acid-base chemistry of silver-immobilized 2-mercaptobenzoic acid. Langmuir 2011, 27, 3527–3533. [Google Scholar] [CrossRef]
- Zhao, X.C.S.; Wallace, G.Q.; Claing, A.; Bazuin, G.; Masson, J. Branched Au nanoparticles on nanofibers for surface-enhanced Raman scattering sensing of intracellular pH and extracellular pH gradients. ACS Sens. 2020, 5, 2155–2167. [Google Scholar] [CrossRef]
- Scarpitti, B.T.; Morrison, A.M.; Buyanova, M.; Schultz, Z.D. Comparison of 4-mercaptobenzoic acid surface-enhanced Raman spectroscopy-based methods for pH determination in cells. Appl. Spectrosc. 2020, 74, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Zhang, P.; Bai, T.; Galvan, D.D.; Hung, H.C.; Zhou, N.; Jiang, S.; Yu, Q. Functionalized plasmonic nanostructure arrays for direct and accurate mapping extracellular pH of living cells in complex media using SERS. Biosens. Bioelectron. 2015, 73, 202–207. [Google Scholar] [CrossRef]
- Xu, M.; Ma, X.; Wei, T.; Lu, Z.X.; Ren, B. In situ imaging of live-cell extracellular pH during cell apoptosis with surface-enhanced Raman spectroscopy. Anal. Chem. 2018, 90, 13922–13928. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Spegazzini, N.; Kitahama, Y.; Chen, Y.; Zhao, B.; Ozaki, Y. pH-response mechanism of p-aminobenzenethiol on Ag nanoparticles revealed by two-dimensional correlation surface enhanced Raman scattering spectroscopy. J. Phys. Chem. Lett. 2012, 3, 3204–3209. [Google Scholar] [CrossRef]
- Yang, T.; Ma, J.; Zhen, S.J.; Huang, C.Z. Electrostatic assemblies of well-dispersed AgNPs on the surface of electrospun nanofibers as highly active SERS substrates for wide-range pH sensing. ACS Appl. Mater. Interfaces 2016, 8, 14802–14811. [Google Scholar] [CrossRef]
- Chen, P.; Wang, Z.; Zong, S.; Chen, H.; Zhu, D.; Zhong, Y.; Cui, Y. A wide range optical pH sensor for living cells using Au@Ag nanoparticles functionalized carbon nanotubes based on SERS signals. Anal. Bioanal. Chem. 2014, 406, 6337–6346. [Google Scholar] [CrossRef] [PubMed]
- Capocefalo, A.; Mammucari, D.; Brasili, F.; Fasolato, C.; Bordi, F.; Postorino, P.; Domenici, F. Exploring the potentiality of a SERS-active pH nano-biosensor. Front. Chem. 2019, 7, 413. [Google Scholar] [CrossRef]
- Lawson, L.; Huser, T. Synthesis and characterization of a disulfide reporter molecule for enhancing pH measurements based on surface-enhanced Raman scattering. Anal. Chem. 2012, 84, 3574–3580. [Google Scholar] [CrossRef] [PubMed]
- Paulo, T.F.; Ando, R.A.; Diógenes, I.C.N.; Temperini, M.L.A. Understanding the equilibria of thio compounds adsorbed on gold by surface-enhanced Raman scattering and density functional theory calculations. J. Phys. Chem. C 2013, 117, 6275–6283. [Google Scholar] [CrossRef]
- Kong, K.V.; Dinish, U.S.; On Lau, W.K.; Olivo, M. Sensitive SERS-pH sensing in biological media using metal carbonyl functionalized planar substrates. Biosens. Bioelectron. 2014, 54, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Hu, Y.; Wang, M.; Chi, M.; Yin, Y. Fully alloyed Ag/Au nanospheres: Combining the plasmonic property of Ag with the stability of Au. J. Am. Chem. Soc. 2014, 136, 7474–7479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Bando, K.; Mochizuki, K.; Taguchi, A.; Fujita, K.; Kawata, S. Quantitative evaluation of surface-enhanced Raman scattering nanoparticles for intracellular pH sensing at a single particle level. Anal. Chem. 2019, 91, 3254–3262. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Khanadeev, V.A.; Burov, A.M.; Le Ru, E.C.; Khlebtsov, N.G. Reexamination of surface-enhanced Raman scattering from gold nanorods as a function of aspect ratio and shape. J. Phys. Chem. C 2020, 124, 10647–10658. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, Q.; Lee, J.Y.; Wang, D.L.C. The synthesis of SERS-active gold nanoflower tags for in vivo applications. ACS Nano 2008, 2, 2473–2480. [Google Scholar] [CrossRef]
- Jimenez de Aberasturi, D.; Serrano-Montes, A.B.; Langer, J.; Henriksen-Lacey, M.; Parak, W.J.; Liz-Marzán, L.M. Surface enhanced Raman scattering encoded gold nanostars for multiplexed cell discrimination. Chem. Mater. 2016, 28, 6779–6790. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, K.; Li, Y.; Ji, J.; Liu, B. High-resolution and universal visualization of latent fingerprints based on aptamer-functionalized core-shell nanoparticles with embedded SERS reporters. ACS Appl. Mater. Interfaces 2016, 8, 14389–14395. [Google Scholar] [CrossRef]
- Sanles-Sobrido, M.; Exner, W.; Rodríguez-Lorenzo, L.R.; Rodríguez-González, B.; Correa-Duarte, M.A.; Álvarez-Puebla, R.A.; Liz-Marzán, L.M. Design of SERS-encoded, submicron, hollow particles through confined growth of encapsulated metal nanoparticles. J. Am. Chem. Soc. 2009, 131, 2699–2705. [Google Scholar] [CrossRef]
- Chang, C.C.; Wu, H.L.; Kuo, C.H.; Huang, M.H. Hydrothermal synthesis of monodispersed octahedral gold nanocrystals with five different size ranges and their self-assembled structures. Chem. Mater. 2008, 20, 7570–7574. [Google Scholar] [CrossRef]
- Rong, Z.; Xiao, R.; Wang, C.; Wang, D.; Wang, S. Plasmonic Ag core-satellite nanostructures with a tunable silica-spaced nanogap for surface-enhanced Raman scattering. Langmuir 2015, 31, 8129–8137. [Google Scholar] [CrossRef]
- Kneipp, J.; Kneipp, H.; Wittig, B.; Kneipp, K. Following the dynamics of pH in endosomes of live cells with SERS nanosensors. J. Phys. Chem. C 2010, 114, 7421–7426. [Google Scholar] [CrossRef]
- Schwartzberg, A.M.; Oshiro, T.Y.; Zhang, J.Z.; Huser, T.; Talley, C.E. Improving nanoprobes using surface-enhanced Raman scattering from 30-nm hollow gold particles. Anal. Chem. 2006, 78, 4732–4736. [Google Scholar] [CrossRef]
- Zhang, Y.; De Aberasturi, D.J.; Henriksen-Lacey, M.; Langer, J.; Liz-Marzán, L.M. Live-cell surface-enhanced Raman spectroscopy imaging of intracellular pH: From two dimensions to three dimensions. ACS Sens. 2020, 5, 3194–3206. [Google Scholar] [CrossRef]
- Zhang, Q.; Wen, H.; Watanabe, K.; Kotani, I.; Ricci, M.; Fortuni, B.; Dao, A.T.N.; Masuhara, A.; Hirai, K.; Kasai, H.; et al. Low-cytotoxic gold-coated silver nanoflowers for intracellular pH sensing. ACS Appl. Nano Mater. 2020, 3, 7643–7650. [Google Scholar] [CrossRef]
- Dzięcielewski, I.; Krajczewski, J.; Dzwolak, W. pH-responsive mixed-thiol-modified surface of roughened GaN: A wettability and SERS study. Appl. Surf. Sci. 2020, 502. [Google Scholar] [CrossRef]
- Zhao, L.; Shingaya, Y.; Tomimoto, H.; Huang, Q.; Nakayama, T. Functionalized carbon nanotubes for pH sensors based on SERS. J. Mater. Chem. C 2008, 18, 4759–4761. [Google Scholar] [CrossRef]
- Pallaoro, A.; Braun, G.; Reich, N.O.; Moskovits, M. Mapping local pH in live cells using encapsulated fluorescent SERS nanotags. Small 2010, 5, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; You, T.; Jiang, L.; Gao, Y.; Yin, P. Creating dynamic SERS hotspots on the surface of pH-responsive microgels for direct detection of crystal violet in solution. RSC Adv. 2017, 7, 32743–32748. [Google Scholar] [CrossRef]
- Zheng, X.S.; Hu, P.; Cui, Y.; Zong, C.; Feng, J.M.; Wang, X.; Ren, B. BSA-coated nanoparticles for improved SERS-based intracellular pH sensing. Anal. Chem. 2014, 86, 12250–12257. [Google Scholar] [CrossRef] [PubMed]
- Bi, L.; Wang, Y.; Yang, Y.; Li, Y.; Mo, S.; Zheng, Q.; Chen, L. Highly sensitive and reproducible SERS sensor for biological pH detection based on a uniform gold nanorod array platform. ACS Appl. Mater. Interfaces 2018, 10, 15381–15387. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Widejko, R.G.; Yang, Z.; Nguyen, K.T.; Chen, H.; Fernando, L.P.; Christensen, K.A.; Anker, J.N. Surface-enhanced raman scattering detection of pH with silica-encapsulated 4-mercaptobenzoic acid-functionalized silver nanoparticles. Anal. Chem. 2012, 84, 8013–8019. [Google Scholar] [CrossRef]
- Bi, Y.; Di, H.; Zeng, E.; Li, Q.; Li, W.; Yang, J.; Liu, D. Reliable quantification of pH variation in live cells using prussian blue-caged surface-enhanced Raman scattering probes. Anal. Chem. 2020, 92, 9574–9582. [Google Scholar] [CrossRef]
- Jaworska, A.; Malek, K.; Kudelski, A. Intracellular pH–Advantages and pitfalls of surface-enhanced Raman scattering and fluorescence microscopy–A review. Spectrochim. Acta Part A 2021, 251, 119410. [Google Scholar] [CrossRef]
- Lardner, A. The effects of extracellular pH on immune function. J. Leukocyte Biol. 2001, 69, 522–530. [Google Scholar]
- Liu, L.; Dou, C.X.; Liu, J.W.; Wang, X.N.; Ying, Z.M.; Jiang, J.H. Cell surface-anchored DNA nanomachine for dynamically tunable sensing and imaging of extracellular pH. Anal. Chem. 2018, 90, 11198–11202. [Google Scholar] [CrossRef]
- Edamaghi, M.; Wojtkowiak, J.W.; Gillies, R.J. pH sensing and regulation in cancer. Front Physiol. 2013, 4, 1–10. [Google Scholar] [CrossRef]
- Hashim, A.I.; Zhang, X.; Wojtkowiak, J.W.; Martinez, G.V.; Gillies, R.J. Imaging pH and metastasis. NMR Biomed. 2011, 24, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, R.E.; Stanica, L.; Gheorghiu, M.; Gaspar, S. Measurement of the extracellular pH of adherently growing mammalian cells with high spatial resolution using a voltammetric pH microsensor. Anal. Chem. 2018, 90, 6899–6905. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Kang, H.C.; Hu, J.; Nichols, J.W.; Jeon, Y.S.; Bae, Y.H. pH-sensitive polymeric micelle-based pH probe for detecting and imaging acidic biological environments. Biomacromolecules 2012, 13, 2945–2951. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef]
- Dey, S.; Trau, M.; Koo, K.M. Surface-enhanced Raman spectroscopy for cancer immunotherapy applications: Opportunities, challenges, and current progress in nanomaterial strategies. Nanomaterials 2020, 10, 1145. [Google Scholar] [CrossRef]
- Zhang, Y.; Mi, X.; Tan, X.; Xiang, R. Recent progress on liquid biopsy analysis using surface-enhanced Raman spectroscopy. Theranostics 2019, 9, 491–525. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Kneipp, H.; McLaughlin, M.; Brown, D.; Kneipp, K. In vivo molecular probing of cellular compartments with gold nanoparticles and nanoaggregates. Nano Lett. 2006, 6, 2225–2231. [Google Scholar] [CrossRef]
- Guo, J.; Sesena Rubfiaro, A.; Lai, Y.; Moscoso, J.; Chen, F.; Liu, Y.; Wang, X.; He, J. Dynamic single-cell intracellular pH sensing using a SERS-active nanopipette. Analyst 2020, 145, 4852–4859. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S.; Zhang, M.; Wang, J.H.; Yang, F.; Kang, B.; Xu, J.J.; Chen, H.Y. Monitoring the changes of pH in lysosomes during autophagy and apoptosis by plasmon enhanced Raman imaging. Anal. Chem. 2019, 91, 8398–8405. [Google Scholar] [CrossRef]
- Dong, B.; Song, X.; Wang, C.; Kong, X.; Tang, Y.; Lin, W. Dual site-controlled and lysosome-targeted intramolecular charge transfer-photoinduced electron transfer-fluorescence resonance energy transfer fluorescent probe for monitoring pH changes in living cells. Anal. Chem. 2016, 88, 4085–4091. [Google Scholar] [CrossRef]
- Yu, F.; Jing, X.; Lin, W. Single-/dual-responsive pH fluorescent probes based on the hybridization of unconventional fluorescence and fluorophore for imaging lysosomal pH changes in HeLa cells. Anal. Chem. 2019, 91, 15213–15219. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, Q.; Liang, Y.; Liu, H.; Qu, L.; Li, H. Fluorescence-SERS dual-signal probes for pH sensing in live cells. Colloids Surf. A 2019, 562, 289–295. [Google Scholar] [CrossRef]
- Yue, S.; Sun, X.; Wang, Y.; Wang, Y.; Xu, Z.; Chen, M.; Wang, J. SERS-fluorescence dual-mode pH-sensing method based on Janus microparticles. ACS Appl. Mater. Interfaces 2017, 9, 39699–39707. [Google Scholar] [CrossRef]
- Yue, J.; Shen, Y.; Liang, L.; Cong, L.; Xu, W.; Shi, W.; Liang, C.; Xu, S. Revealing mitochondrial microenvironmental evolution triggered by photodynamic therapy. Anal. Chem. 2020, 92, 6081–6087. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, X.; Wang, S.; Ma, H.; Shen, Y.; Wang, X. A multifunctional electrochemical sensor for the simultaneous detection of ascorbic acid, dopamine, uric acid, and nitrite. J. AOAC Int. 2021, 104, 860–866. [Google Scholar] [CrossRef]
- Pan, C.; Li, X.; Sun, J.; Li, Z.; Zhang, L.; Qian, W.; Wang, P.; Dong, J. A multiplexed SERS-active microneedle for simultaneous redox potential and pH measurements in rat joints. ACS Appl. Bio. Mater. 2019, 2, 2102–2108. [Google Scholar] [CrossRef]
- Jung, S.; Nam, J.; Hwang, S.; Park, J.; Hur, J.; Im, K.; Park, N.; Kim, S. Theragnostic pH-sensitive gold nanoparticles for the selective surface enhanced Raman scattering and photothermal cancer therapy. Anal. Chem. 2013, 85, 7674–7681. [Google Scholar] [CrossRef] [PubMed]






| SERS Substrate | Reporter of pH | Range of pH | Target | Ref. |
|---|---|---|---|---|
| Gold nanospheres | 4-mercaptobenzoic acid | 5.0–6.9 | NIH/3T3 cells. | [51] |
| Hollow gold nanospheres | 4-mercaptobenzoic acid | 3.5–9.0 | —— | [52] |
| Gold nanostars | 4-mercaptobenzoic acid | 3.2–9.4 | MCF7 cells | [53] |
| Gold-coated silver nanoparticles and nanaflowers | 4-mercaptobenzoic acid | 5.0–8.0 | A549 cells | [54] |
| Au-sputtered GaN nanowhiskers | 4-mercaptopyridine | 2.23–12.35 | —— | [55] |
| Bimetallic-nanoparticle-decorated carbon nanotubes | Biotin-fluorescein moleules | 5.6–8.2 | Bimetallic-nanoparticle-decorated carbon nanotubes | [56] |
| Silver nanospheres dimer | 4-mercaptobenzoic acid | 3.2–9.0 | HeLa cells | [57] |
| Gold nanoparticles/poly(2-vinylpyridine) microgels | 4-mercaptobenzoic acid | 2.0–5.0 | —— | [58] |
| Gold nanoparticles coated with bovine serum protein | 4-mercaptopyridine | 4.0–9.0 | CaSki cells | [59] |
| Gold nanorods array platform | 4-mercaptopyridine | 3.0–8.0 | Mouse boold | [60] |
| Silver nanoparticles coated with silica layer | 4-aminobenzene mercaptan | 2.0–10.0 | Macrophage cells | [61] |
| gold nanoparticles coated with Prussian blue cage | 4-mercaptopyridine | 1.69–11.2 | Adenocarcinoma cells | [62] |
| Abbreviations | Full Name | Abbreviations | Full Name |
|---|---|---|---|
| AuNPs | Gold nanoparticles | AuNRs | Gold nanorods |
| SERS | Surface enhanced Raman scattering | Hpyt | 5-(4-pyridyl)-1,3,4-oxadiazole-2-thiol |
| ER | Endoplasmic reticulum | LSPR | Local surface plasmonic resonance |
| EM | Electromagnetic enhancement mechanism | HMD | Hexanediamine |
| CM | Chemical enhancement mechanism | PVP | Polyvinylpyrrolidone |
| 4-MBA | 4-mercaptobenzoic acid | PB | Prussian blue |
| 4-Mpy | 4-mercaptopyridine | pHe | Extracellular pH |
| 4-ATP | 4-aminobenzene mercaptan | pHi | Intracellular pH |
| 2-ABT | 2-mercaptobenzoic acid | Q3D-PNA | Au Quasi-3D plasma nanostructure array |
| TNA | Thionicotinamide | PDT | Photodynamic therapy |
| iTNA | Thioisonicotinamide | BSA | Bovine serum protein |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Zhao, Q.; Jiang, Z.; Shen, J.; Wu, W.; Liu, X.; Fan, Q.; Huang, W. Recent Progress of SERS Nanoprobe for pH Detecting and Its Application in Biological Imaging. Biosensors 2021, 11, 282. https://doi.org/10.3390/bios11080282
Zhang L, Zhao Q, Jiang Z, Shen J, Wu W, Liu X, Fan Q, Huang W. Recent Progress of SERS Nanoprobe for pH Detecting and Its Application in Biological Imaging. Biosensors. 2021; 11(8):282. https://doi.org/10.3390/bios11080282
Chicago/Turabian StyleZhang, Lei, Qianqian Zhao, Zhitao Jiang, Jingjing Shen, Weibing Wu, Xingfen Liu, Quli Fan, and Wei Huang. 2021. "Recent Progress of SERS Nanoprobe for pH Detecting and Its Application in Biological Imaging" Biosensors 11, no. 8: 282. https://doi.org/10.3390/bios11080282
APA StyleZhang, L., Zhao, Q., Jiang, Z., Shen, J., Wu, W., Liu, X., Fan, Q., & Huang, W. (2021). Recent Progress of SERS Nanoprobe for pH Detecting and Its Application in Biological Imaging. Biosensors, 11(8), 282. https://doi.org/10.3390/bios11080282





