Electroanalytical Overview: Electrochemical Sensing Platforms for Food and Drink Safety
Abstract
:1. Introduction
2. Electrochemical Sensors towards Food Safety
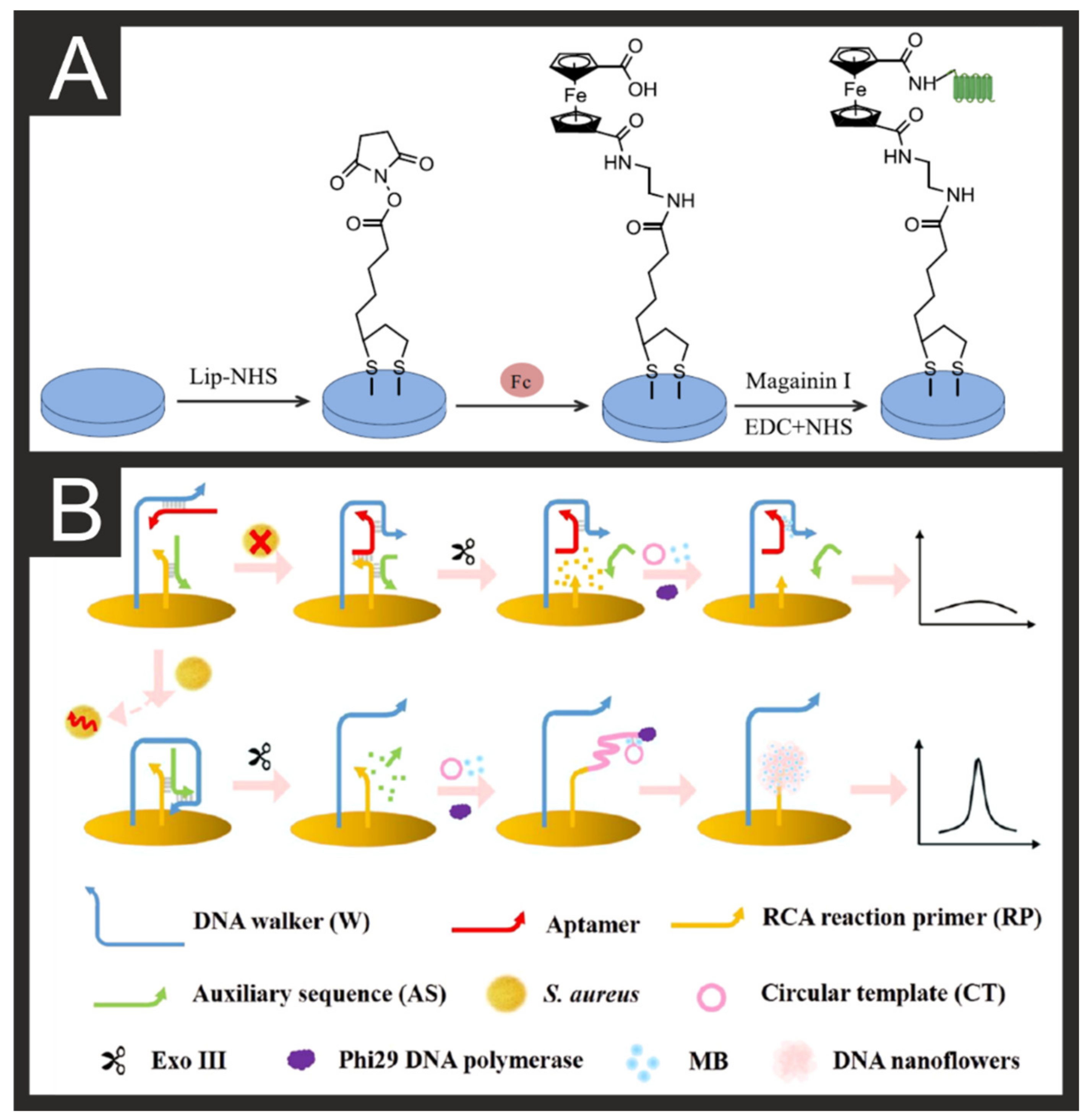
2.1. Pathogen Detection
2.2. Toxins/Mycotoxins
2.3. Allergen Examples
2.4. Veterinary Drugs
2.5. Pesticides
3. Future Trends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- (WHO), W.H.O. Food Safety. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 15 July 2021).
- FAO/WHO. Codex Alimentarius. Available online: http://www.fao.org/fao-who-codexalimentarius/en (accessed on 15 July 2021).
- FAO-WHO. Codex Alimentarius Text. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/en (accessed on 15 July 2021).
- Yáñez, L.; Ortiz, D.; Calderón, J.; Batres, L.; Carrizales, L.; Mejía, J.; Martínez, L.; García-Nieto, E.; Díaz-Barriga, F. Overview of human health and chemical mixtures: Problems facing developing countries. Environ. Health Perspect. 2002, 110, 901–909. [Google Scholar] [CrossRef] [Green Version]
- (CDC), C.f.D.C.a.P. Available online: http://www.cdc.gov/foodborneburden/2011-foodborne-estimates.html (accessed on 15 July 2021).
- Nolvachai, Y.; Kulsing, C.; Marriott, P.J. Multidimensional gas chromatography in food analysis. TrAC Trends Anal. Chem. 2017, 96, 124–137. [Google Scholar] [CrossRef]
- Armutcu, C.; Uzun, L.; Denizli, A. Determination of Ochratoxin A traces in foodstuffs: Comparison of an automated on-line two-dimensional high-performance liquid chromatography and off-line immunoaffinity-high-performance liquid chromatography system. J. Chromatogr. A 2018, 1569, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Nilghaz, A.; Choi, J.R.; Liu, X.; Lu, X. Rapid detection of clenbuterol in milk using microfluidic paper-based ELISA. Food Chem. 2018, 246, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Raeisossadati, M.J.; Danesh, N.M.; Borna, F.; Gholamzad, M.; Ramezani, M.; Abnous, K.; Taghdisi, S.M. Lateral flow based immunobiosensors for detection of food contaminants. Biosens. Bioelectron. 2016, 86, 235–246. [Google Scholar] [CrossRef]
- Zeng, L.; Peng, L.; Wu, D.; Yang, B. Electrochemical sensors for food safety. In Nutrition in Health and Disease: Our Challenges Now and Forthcoming Time; IntechOpen: London, UK, 2018. [Google Scholar]
- Yadav, N.; Mishra, A.; Narang, J. 31—Electrochemical sensor method for food quality evaluation. In Evaluation Technologies for Food Quality; Zhong, J., Wang, X., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 793–815. [Google Scholar] [CrossRef]
- Petersen, M.; Yu, Z.; Lu, X. Application of Raman Spectroscopic Methods in Food Safety: A Review. Biosensors 2021, 11, 187. [Google Scholar] [CrossRef]
- Tang, H.; Zhu, C.; Meng, G.; Wu, N. Review—Surface-Enhanced Raman Scattering Sensors for Food Safety and Environmental Monitoring. J. Electrochem. Soc. 2018, 165, B3098–B3118. [Google Scholar] [CrossRef]
- Nelis, J.L.D.; Tsagkaris, A.S.; Dillon, M.J.; Hajslova, J.; Elliott, C.T. Smartphone-based optical assays in the food safety field. TrAC Trends Anal. Chem. 2020, 129, 115934. [Google Scholar] [CrossRef]
- Kadara, R.O.; Jenkinson, N.; Banks, C.E. Disposable Bismuth Oxide Screen Printed Electrodes for the High Throughput Screening of Heavy Metals. Electroanalysis 2009, 21, 2410–2414. [Google Scholar] [CrossRef]
- Foster, C.W.; de Souza, A.P.; Metters, J.P.; Bertotti, M.; Banks, C.E. Metallic modified (bismuth, antimony, tin and combinations thereof) film carbon electrodes. Analyst 2015, 140, 7598–7612. [Google Scholar] [CrossRef] [Green Version]
- García-Miranda Ferrari, A.; Carrington, P.; Rowley-Neale, S.J.; Banks, C.E. Recent advances in portable heavy metal electrochemical sensing platforms. Environ. Sci. Water Res. Technol. 2020. [Google Scholar] [CrossRef]
- Elbardisy, H.M.; Ferrari, A.G.M.; Foster, C.W.; Sutcliffe, O.B.; Brownson, D.A.C.; Belal, T.S.; Talaat, W.; Daabees, H.G.; Banks, C.E. Forensic Electrochemistry: The Electroanalytical Sensing of Mephedrone Metabolites. ACS Omega 2019, 4, 1947–1954. [Google Scholar] [CrossRef]
- Smith, J.P.; Randviir, E.P.; Banks, C.E. An Introduction to Forensic Electrochemistry. In Forensic Science; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 89–102. [Google Scholar]
- Zuway, K.Y.; Smith, J.P.; Foster, C.W.; Kapur, N.; Banks, C.E.; Sutcliffe, O.B. Detection and quantification of new psychoactive substances (NPSs) within the evolved “legal high” product, NRG-2, using high performance liquid chromatography-amperometric detection (HPLC-AD). Analyst 2015, 140, 6283–6294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Ibáñez, N.; Sanjuán, I.; Montiel, M.Á.; Foster, C.W.; Banks, C.E.; Iniesta, J. l-Cysteine determination in embryo cell culture media using Co (II)-phthalocyanine modified disposable screen-printed electrodes. J. Electroanal. Chem. 2016, 780, 303–310. [Google Scholar] [CrossRef]
- Randviir, E.P.; Metters, J.P.; Stainton, J.; Banks, C.E. Electrochemical impedance spectroscopy versus cyclic voltammetry for the electroanalytical sensing of capsaicin utilising screen printed carbon nanotube electrodes. Analyst 2013, 138, 2970–2981. [Google Scholar] [CrossRef] [PubMed]
- Ngamchuea, K.; Hurst, P.; Batchelor-McAuley, C.; Compton, R.G. Handheld electrochemical device for the determination of the strength of garlic. Sens. Actuators B Chem. 2016, 232, 138–142. [Google Scholar] [CrossRef]
- García-Miranda Ferrari, A.; Rowley-Neale, S.J.; Banks, C.E. Screen-printed electrodes: Transitioning the laboratory in-to-the field. Talanta Open 2021, 3, 100032. [Google Scholar] [CrossRef]
- Adkins, J.; Boehle, K.; Henry, C. Electrochemical paper-based microfluidic devices. ELECTROPHORESIS 2015, 36, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; McCord, C.P.; Clark, K.M.; Jang, I.; Henry, C.S. Electrochemical paper-based devices: Sensing approaches and progress toward practical applications. Lab Chip 2020, 20, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Naresh, V.; Lee, N. A Review on Biosensors and Recent Development of Nanostructured Materials-Enabled Biosensors. Sensors 2021, 21, 1109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Geng, P.; Liu, H.; Teng, Y.; Liu, Y.; Wang, Q.; Zhang, W.; Jin, L.; Jiang, L. Development of an electrochemical immunoassay for rapid detection of E. coli using anodic stripping voltammetry based on Cu@Au nanoparticles as antibody labels. Biosens. Bioelectron. 2009, 24, 2155–2159. [Google Scholar] [CrossRef]
- Mittelmann, A.S.; Ron, E.Z.; Rishpon, J. Amperometric Quantification of Total Coliforms and Specific Detection of Escherichia coli. Anal. Chem. 2002, 74, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Serra, B.; Morales, M.D.; Zhang, J.; Reviejo, A.J.; Hall, E.H.; Pingarron, J.M. In-a-Day Electrochemical Detection of Coliforms in Drinking Water Using a Tyrosinase Composite Biosensor. Anal. Chem. 2005, 77, 8115–8121. [Google Scholar] [CrossRef]
- Ramanujam, A.; Neyhouse, B.; Keogh, R.A.; Muthuvel, M.; Carroll, R.K.; Botte, G.G. Rapid electrochemical detection of Escherichia coli using nickel oxidation reaction on a rotating disk electrode. Chem. Eng. J. 2021, 411, 128453. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Kang, H.Y.; Yang, H. Permeabilization-free β-galactosidase-induction-based electrochemical detection of Escherichia coli. Sens. Actuators B Chem. 2021, 337, 129768. [Google Scholar] [CrossRef]
- Qaanei, M.; Taheri, R.A.; Eskandari, K. Electrochemical aptasensor for Escherichia coli O157: H7 bacteria detection using a nanocomposite of reduced graphene oxide, gold nanoparticles and polyvinyl alcohol. Anal. Methods 2021. [Google Scholar] [CrossRef] [PubMed]
- Vu, Q.K.; Tran, Q.H.; Vu, N.P.; Anh, T.-L.; Le Dang, T.T.; Matteo, T.; Nguyen, T.H.H. A label-free electrochemical biosensor based on screen-printed electrodes modified with gold nanoparticles for quick detection of bacterial pathogens. Mater. Today Commun. 2021, 26, 101726. [Google Scholar] [CrossRef]
- Li, Y.; Afrasiabi, R.; Fathi, F.; Wang, N.; Xiang, C.; Love, R.; She, Z.; Kraatz, H.-B. Impedance based detection of pathogenic E. coli O157:H7 using a ferrocene-antimicrobial peptide modified biosensor. Biosens. Bioelectron. 2014, 58, 193–199. [Google Scholar] [CrossRef]
- Qian, X.; Qu, Q.; Li, L.; Ran, X.; Zuo, L.; Huang, R.; Wang, Q. Ultrasensitive Electrochemical Detection of Clostridium perfringens DNA Based Morphology-Dependent DNA Adsorption Properties of CeO₂ Nanorods in Dairy Products. Sensors 2018, 18, 1878. [Google Scholar] [CrossRef] [Green Version]
- Rochelet, M.; Solanas, S.; Betelli, L.; Chantemesse, B.; Vienney, F.; Hartmann, A. Rapid amperometric detection of Escherichia coli in wastewater by measuring β-D glucuronidase activity with disposable carbon sensors. Anal. Chim. Acta 2015, 892, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Leong, M.C.; Leong, E.Z.W.; Kuan, W.S.; Leong, D.T. Clinically Relevant Detection of Streptococcus pneumoniae with DNA-Antibody Nanostructures. Anal. Chem. 2017, 89, 6900–6906. [Google Scholar] [CrossRef]
- Low, K.-F.; Chuenrangsikul, K.; Rijiravanich, P.; Surareungchai, W.; Chan, Y.-Y. Electrochemical genosensor for specific detection of the food-borne pathogen, Vibrio cholerae. World J. Microbiol. Biotechnol. 2012, 28, 1699–1706. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yang, Y.; Wang, L.; Xu, T.; Wang, R. Electrochemical immunosensor based on mussel inspired coating for simultaneous detection and elimination of Staphylococcus aureus in drinks. RSC Adv. 2021, 11, 18252–18258. [Google Scholar] [CrossRef]
- Cai, R.; Zhang, S.; Chen, L.; Li, M.; Zhang, Y.; Zhou, N. Self-Assembled DNA Nanoflowers Triggered by a DNA Walker for Highly Sensitive Electrochemical Detection of Staphylococcus aureus. ACS Appl. Mater. Interfaces 2021, 13, 4905–4914. [Google Scholar] [CrossRef] [PubMed]
- Ozoemena, O.C.; Maphumulo, T.; Shai, J.L.; Ozoemena, K.I. Electrospun Carbon Nanofibers as an Electrochemical Immunosensing Platform for Vibrio cholerae Toxin: Aging Effect of the Redox Probe. ACS Omega 2020, 5, 5762–5771. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, J.; Sharma, M.K.; Ponmariappan, S.; Shaik, M.; Upadhyay, S. Electrochemical immunosensor for botulinum neurotoxin type-E using covalently ordered graphene nanosheets modified electrodes and gold nanoparticles-enzyme conjugate. Biosens. Bioelectron. 2015, 69, 249–256. [Google Scholar] [CrossRef]
- Caratelli, V.; Fillo, S.; D’Amore, N.; Rossetto, O.; Pirazzini, M.; Moccia, M.; Avitabile, C.; Moscone, D.; Lista, F.; Arduini, F. Based electrochemical peptide sensor for on-site detection of botulinum neurotoxin serotype A and C. Biosens. Bioelectron. 2021, 183, 113210. [Google Scholar] [CrossRef]
- Radi, A.-E.; Eissa, A.; Wahdan, T. Voltammetric behavior of mycotoxin zearalenone at a single walled carbon nanotube screen-printed electrode. Anal. Methods 2019, 11, 4494–4500. [Google Scholar] [CrossRef]
- Zhao, H.; Tian, J.; Quan, X. A graphene and multienzyme functionalized carbon nanosphere-based electrochemical immunosensor for microcystin-LR detection. Colloids Surf. B Biointerfaces 2013, 103, 38–44. [Google Scholar] [CrossRef]
- Nelis, J.L.; Migliorelli, D.; Mühlebach, L.; Generelli, S.; Stewart, L.; Elliott, C.T.; Campbell, K. Highly sensitive electrochemical detection of the marine toxins okadaic acid and domoic acid with carbon black modified screen printed electrodes. Talanta 2021, 228, 122215. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.L.; Barros, P.; Santos, M.; Delerue-Matos, C.; Gomes, A.C.; Barroso, M.F. Electrochemical genosensor for the detection of Alexandrium minutum dinoflagellates. Talanta 2021, 222, 121416. [Google Scholar] [CrossRef] [PubMed]
- Marín-Barroso, E.; Messina, G.A.; Bertolino, F.A.; Raba, J.; Pereira, S.V. Electrochemical immunosensor modified with carbon nanofibers coupled to a paper platform for the determination of gliadins in food samples. Anal. Methods 2019, 11, 2170–2178. [Google Scholar] [CrossRef]
- Benedé, S.; Ruiz-Valdepeñas Montiel, V.; Povedano, E.; Villalba, M.; Mata, L.; Galán-Malo, P.; Torrente-Rodríguez, R.M.; Vargas, E.; Reviejo, A.J.; Campuzano, S.; et al. Fast amperometric immunoplatform for ovomucoid traces determination in fresh and baked foods. Sens. Actuators B Chem. 2018, 265, 421–428. [Google Scholar] [CrossRef]
- Baldo, T.A.; dos Anjos Proença, C.; da Silva Felix, F.; Freitas, T.A.; Sakata, S.K.; Angnes, L.; Faria, R.C. Disposable electrochemical microfluidic device for ultrasensitive detection of egg allergen in wine samples. Talanta 2021, 232, 122447. [Google Scholar] [CrossRef]
- Demir, E.; Silah, H. Development of a new analytical method for determination of veterinary drug oxyclozanide by electrochemical sensor and its application to pharmaceutical formulation. Chemosensors 2020, 8, 25. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.; Hu, X.; Zhang, Y.; Teng, M.; Deng, R.; Xing, G.; Tao, J.; Xu, G.; Chen, J.; Zhang, Y. Label-free electrochemical immunosensor based on AuNPs/Zn/Ni-ZIF-8-800@ graphene composites for sensitive detection of monensin in milk. Sens. Actuators B Chem. 2019, 288, 571–578. [Google Scholar] [CrossRef]
- El Hassani, N.E.A.; Baraket, A.; Boudjaoui, S.; Neto, E.T.T.; Bausells, J.; El Bari, N.; Bouchikhi, B.; Elaissari, A.; Errachid, A.; Zine, N. Development and application of a novel electrochemical immunosensor for tetracycline screening in honey using a fully integrated electrochemical Bio-MEMS. Biosens. Bioelectron. 2019, 130, 330–337. [Google Scholar] [CrossRef]
- Mendes, L.F.; e Silva, Â.R.S.; Bacil, R.P.; Serrano, S.H.P.; Angnes, L.; Paixão, T.R.L.C.; de Araujo, W.R. Forensic electrochemistry: Electrochemical study and quantification of xylazine in pharmaceutical and urine samples. Electrochim. Acta 2019, 295, 726–734. [Google Scholar] [CrossRef]
- Saisahas, K.; Soleh, A.; Promsuwan, K.; Phonchai, A.; Mohamed Sadiq, N.S.; Teoh, W.K.; Chang, K.H.; Lim Abdullah, A.F.; Limbut, W. A portable electrochemical sensor for detection of the veterinary drug xylazine in beverage samples. J. Pharm. Biomed. Anal. 2021, 198, 113958. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, X.; Zhu, N.; Li, R.; Kang, H.; Zhang, Q. An aptasensor for the detection of ampicillin in milk using a personal glucose meter. Anal. Methods 2020, 12, 3376–3381. [Google Scholar] [CrossRef]
- Umesh, N.; Jesila, J.A.; Wang, S.-F.; Devi, K.S.; Govindasamy, M.; Alothman, A.A.; Alshgari, R.A. An enhanced electrochemical performance of in milk, pigeon meat and eggs samples using se nanorods capped with Co3O4 nanoflowers decorated on graphene oxide. Colloids Surf. B Biointerfaces 2021, 200, 111577. [Google Scholar] [CrossRef]
- Ali, M.; Bacchu, M.; Daizy, M.; Tarafder, C.; Hossain, M.; Rahman, M.; Khan, M. A highly sensitive poly-arginine based MIP as an electrochemical sensor for selective detection of dimetridazole. Anal. Chim. Acta 2020, 1121, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Soulis, D.; Trigazi, M.; Tsekenis, G.; Chandrinou, C.; Klinakis, A.; Zergioti, I. Facile and Low-Cost SPE Modification Towards Ultra-Sensitive Organophosphorus and Carbamate Pesticide Detection in Olive Oil. Molecules 2020, 25, 4988. [Google Scholar] [CrossRef] [PubMed]
- Della Pelle, F.; Angelini, C.; Sergi, M.; Del Carlo, M.; Pepe, A.; Compagnone, D. Nano carbon black-based screen printed sensor for carbofuran, isoprocarb, carbaryl and fenobucarb detection: Application to grain samples. Talanta 2018, 186, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Govindasamy, M.; Umamaheswari, R.; Chen, S.-M.; Mani, V.; Su, C. Graphene Oxide Nanoribbons Film Modified Screen-Printed Carbon Electrode for Real-Time Detection of Methyl Parathion in Food Samples. J. Electrochem. Soc. 2017, 164, B403–B408. [Google Scholar] [CrossRef]
- Arduini, F.; Cinti, S.; Caratelli, V.; Amendola, L.; Palleschi, G.; Moscone, D. Origami multiple paper-based electrochemical biosensors for pesticide detection. Biosens. Bioelectron. 2019, 126, 346–354. [Google Scholar] [CrossRef]
- Cioffi, A.; Mancini, M.; Gioia, V.; Cinti, S. Office Paper-Based Electrochemical Strips for Organophosphorus Pesticide Monitoring in Agricultural Soil. Environ. Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Dong, H.; Zhao, Q.; Li, J.; Xiang, Y.; Liu, H.; Guo, Y.; Yang, Q.; Sun, X. Broad-spectrum electrochemical immunosensor based on one-step electrodeposition of AuNP–Abs and Prussian blue nanocomposite for organophosphorus pesticide detection. Bioprocess Biosyst. Eng. 2021, 44, 585–594. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Q.; Li, Q.; Li, H.; Li, F. Two-Dimensional MnO2 Nanozyme-Mediated Homogeneous Electrochemical Detection of Organophosphate Pesticides without the Interference of H2O2 and Color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef]
- Li, X.; Gao, X.; Gai, P.; Liu, X.; Li, F. Degradable metal-organic framework/methylene blue composites-based homogeneous electrochemical strategy for pesticide assay. Sens. Actuators B Chem. 2020, 323, 128701. [Google Scholar] [CrossRef]
- Fu, J.; An, X.; Yao, Y.; Guo, Y.; Sun, X. Electrochemical aptasensor based on one step co-electrodeposition of aptamer and GO-CuNPs nanocomposite for organophosphorus pesticide detection. Sens. Actuators B Chem. 2019, 287, 503–509. [Google Scholar] [CrossRef]
- Renganathan, V.; Balaji, R.; Chen, S.-M.; Kokulnathan, T. Coherent design of palladium nanostructures adorned on the boron nitride heterojunctions for the unparalleled electrochemical determination of fatal organophosphorus pesticides. Sens. Actuators B Chem. 2020, 307, 127586. [Google Scholar] [CrossRef]
- Wang, W.; Xu, Y.; Cheng, N.; Xie, Y.; Huang, K.; Xu, W. Dual-recognition aptazyme-driven DNA nanomachine for two-in-one electrochemical detection of pesticides and heavy metal ions. Sens. Actuators B Chem. 2020, 321, 128598. [Google Scholar] [CrossRef]
- Alves, R.C.; Barroso, M.F.; González-García, M.B.; Oliveira, M.B.P.P.; Delerue-Matos, C. New Trends in Food Allergens Detection: Toward Biosensing Strategies. Crit. Rev. Food Sci. Nutr. 2016, 56, 2304–2319. [Google Scholar] [CrossRef] [PubMed]
- Vasilescu, A.; Nunes, G.; Hayat, A.; Latif, U.; Marty, J.-L. Electrochemical Affinity Biosensors Based on Disposable Screen-Printed Electrodes for Detection of Food Allergens. Sensors 2016, 16, 1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campuzano, S.; Ruiz-Valdepeñas Montiel, V.; Serafín, V.; Yáñez-Sedeño, P.; Pingarrón, J.M. Cutting-Edge Advances in Electrochemical Affinity Biosensing at Different Molecular Level of Emerging Food Allergens and Adulterants. Biosensors 2020, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, O.; Soares, T.C.; de Faria, B.A.; Hudson, A.; Mecozzi, F.; Rowley-Neale, S.J.; Banks, C.E.; Gruber, J.; Novakovic, K.; Peeters, M. Screen printed electrode based detection systems for the antibiotic amoxicillin in aqueous samples utilising molecularly imprinted polymers as synthetic receptors. Chemosensors 2020, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Crapnell, R.D.; Canfarotta, F.; Czulak, J.; Johnson, R.; Betlem, K.; Mecozzi, F.; Down, M.P.; Eersels, K.; van Grinsven, B.; Cleij, T.J. Thermal detection of cardiac biomarkers heart-fatty acid binding protein and ST2 using a molecularly imprinted nanoparticle-based multiplex sensor platform. ACS Sens. 2019, 4, 2838–2845. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Dempsey-Hibbert, N.C.; Peeters, M.; Tridente, A.; Banks, C.E. Molecularly imprinted polymer based electrochemical biosensors: Overcoming the challenges of detecting vital biomarkers and speeding up diagnosis. Talanta Open 2020, 100018. [Google Scholar] [CrossRef]
- Saylan, Y.; Akgönüllü, S.; Yavuz, H.; Ünal, S.; Denizli, A. Molecularly Imprinted Polymer Based Sensors for Medical Applications. Sensors 2019, 19, 1279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wackerlig, J.; Schirhagl, R. Applications of Molecularly Imprinted Polymer Nanoparticles and Their Advances toward Industrial Use: A Review. Anal. Chem. 2016, 88, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, O.; Betlem, K.; Mansouri, N.; Crapnell, R.; Vieira, F.; Hudson, A.; Banks, C.; Liauw, C.; Gruber, J.; Zubko, M. Electropolymerised molecularly imprinted polymers for the heat-transfer based detection of microorganisms: A proof-of-concept study using yeast. Therm. Sci. Eng. Prog. 2021, 24, 100956. [Google Scholar] [CrossRef]
- Dye, C. After 2015: Infectious diseases in a new era of health and development. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesewski, E.; Johnson, B.N. Electrochemical biosensors for pathogen detection. Biosens. Bioelectron. 2020, 159, 112214. [Google Scholar] [CrossRef]
- Furst, A.L.; Francis, M.B. Impedance-Based Detection of Bacteria. Chem. Rev. 2019, 119, 700–726. [Google Scholar] [CrossRef]
- Monzó, J.; Insua, I.; Fernandez-Trillo, F.; Rodriguez, P. Fundamentals, achievements and challenges in the electrochemical sensing of pathogens. Analyst 2015, 140, 7116–7128. [Google Scholar] [CrossRef]
- Amiri, M.; Bezaatpour, A.; Jafari, H.; Boukherroub, R.; Szunerits, S. Electrochemical Methodologies for the Detection of Pathogens. ACS Sens. 2018, 3, 1069–1086. [Google Scholar] [CrossRef]
- Rainbow, J.; Sedlackova, E.; Jiang, S.; Maxted, G.; Moschou, D.; Richtera, L.; Estrela, P. Integrated Electrochemical Biosensors for Detection of Waterborne Pathogens in Low-Resource Settings. Biosensors 2020, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Simoska, O.; Stevenson, K.J. Electrochemical sensors for rapid diagnosis of pathogens in real time. Analyst 2019, 144, 6461–6478. [Google Scholar] [CrossRef]
- Min, J.; Baeumner, A.J. Highly Sensitive and Specific Detection of Viable Escherichia coli in Drinking Water. Anal. Biochem. 2002, 303, 186–193. [Google Scholar] [CrossRef]
- Brynestad, S.; Granum, P.E. Clostridium perfringens and foodborne infections. Int. J. Food Microbiol. 2002, 74, 195–202. [Google Scholar] [CrossRef]
- Sharma, H.; Mutharasan, R. Review of biosensors for foodborne pathogens and toxins. Sens. Actuators B Chem. 2013, 183, 535–549. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors - A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Musher, D.M.; Thorner, A.R. Community-acquired pneumonia. N. Engl. J. Med. 2014, 371, 1619–1628. [Google Scholar] [CrossRef] [PubMed]
- Ghose, A.C. Lessons from cholera & Vibrio cholerae. Indian J. Med. Res. 2011, 133, 164–170. [Google Scholar] [PubMed]
- Charles, R.C.; Ryan, E.T. Cholera in the 21st century. Curr. Opin. Infect. Dis. 2011, 24, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Sack, D.A.; Sack, R.B.; Nair, G.B.; Siddique, A.K. Cholera. Lancet 2004, 363, 223–233. [Google Scholar] [CrossRef]
- Navaneethan, U.; Giannella, R.A. Mechanisms of infectious diarrhea. Nat. Clin. Pract. Gastroenterol. Hepatol. 2008, 5, 637–647. [Google Scholar] [CrossRef]
- Wang, C.-H.; Lien, K.-Y.; Wu, J.-J.; Lee, G.-B. A magnetic bead-based assay for the rapid detection of methicillin-resistant Staphylococcus aureus by using a microfluidic system with integrated loop-mediated isothermal amplification. Lab Chip 2011, 11, 1521–1531. [Google Scholar] [CrossRef]
- Suaifan, G.A.R.Y.; Alhogail, S.; Zourob, M. Rapid and low-cost biosensor for the detection of Staphylococcus aureus. Biosens. Bioelectron. 2017, 90, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Staphylococcus aureus toxins. Curr. Opin. Microbiol. 2014, 17, 32–37. [Google Scholar] [CrossRef] [Green Version]
- Mason, S.D.; Tang, Y.; Li, Y.; Xie, X.; Li, F. Emerging bioanalytical applications of DNA walkers. TrAC Trends Anal. Chem. 2018, 107, 212–221. [Google Scholar] [CrossRef]
- Jin, Y.; Li, Z.; Liu, H.; Chen, S.; Wang, F.; Wang, L.; Li, N.; Ge, K.; Yang, X.; Liang, X.-J. Biodegradable, multifunctional DNAzyme nanoflowers for enhanced cancer therapy. NPG Asia Mater. 2017, 9, e365. [Google Scholar] [CrossRef]
- (CDC), C.f.D.C.a.P. Listeria (Listeriosis). Available online: https://www.cdc.gov/listeria/index.html (accessed on 19 July 2021).
- Ruan, C.; Wang, H.; Yang, L.; Li, Y. Detection of viable listeria monocytogenes in milk using an electrochemical method. J. Rapid Methods Autom. Microbiol. 2003, 11, 11–22. [Google Scholar] [CrossRef]
- Janik, E.; Ceremuga, M.; Saluk-Bijak, J.; Bijak, M. Biological Toxins as the Potential Tools for Bioterrorism. Int. J. Mol. Sci. 2019, 20, 1181. [Google Scholar] [CrossRef] [Green Version]
- Shrivastava, A.; Sharma, R.K. Biosensors for the detection of mycotoxins. Toxin Rev. 2021, 1–21. [Google Scholar] [CrossRef]
- Hodgson, E. Chapter Fourteen—Toxins and Venoms. In Progress in Molecular Biology and Translational Science; Hodgson, E., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 112, pp. 373–415. [Google Scholar]
- Arnon, S.S.; Schechter, R.; Inglesby, T.V.; Henderson, D.A.; Bartlett, J.G.; Ascher, M.S.; Eitzen, E.; Fine, A.D.; Hauer, J.; Layton, M.; et al. Botulinum toxin as a biological weapon: Medical and public health management. J. Am. Med. Assoc. 2001, 285, 1059–1070. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.; Mendoza, M.C.; Rodicio, M.R. Food poisoning and Staphylococcus aureus enterotoxins. Toxins 2010, 2, 1751–1773. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Raza, N.; Bhardwaj, S.K.; Vikrant, K.; Kim, K.-H.; Bhardwaj, N. Advances in nanomaterial-based electrochemical biosensors for the detection of microbial toxins, pathogenic bacteria in food matrices. J. Hazard. Mater. 2021, 401, 123379. [Google Scholar] [CrossRef]
- Zhang, W.; Dixon, M.B.; Saint, C.; Teng, K.S.; Furumai, H. Electrochemical Biosensing of Algal Toxins in Water: The Current State-of-the-Art. ACS Sens. 2018, 3, 1233–1245. [Google Scholar] [CrossRef]
- Zhang, W. Visible-light sensitive TiO2 synthesis via wet chemical N-doping for the degradation of dissolved organic compounds in wastewater treatment: A review. J. Nanopart. Res. 2015, 17, 221. [Google Scholar] [CrossRef]
- Zhang, W. Visible-light assisted methylene blue (MB) removal by novel TiO2/adsorbent nanocomposites. Water Sci. Technol. 2010, 61, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.; Mastan, S.A. Algal Toxins and their Impact on Human Health. Biomed. Pharmacol. J. 2011, 4, 129–134. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, Y.; Fan, R.; Lewis, R. A facile TiO2/PVDF composite membrane synthesis and their application in water purification. J. Nanoparticle Res. 2016, 18, 31. [Google Scholar] [CrossRef]
- Anderson, D.M. Harmful Algal Blooms (HABs) and Desalination: A Guide to Impacts, Monitoring and Management; Intergovernmental Oceanographic Commission of UNESCO: Paris, France, 2017. [Google Scholar]
- Carmichael, W.W. Cyanobacteria secondary metabolites—the cyanotoxins. J. Appl. Bacteriol. 1992, 72, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.M.; Anderson, E.J.; Beletsky, D.; Boland, S.; Bosch, N.S.; Bridgeman, T.B.; Chaffin, J.D.; Cho, K.; Confesor, R.; Daloğlu, I.; et al. Record-setting algal bloom in Lake Erie caused by agricultural and meteorological trends consistent with expected future conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6448–6452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, J.C.; Michalak, A.M. Phytoplankton blooms in Lake Erie impacted by both long-term and springtime phosphorus loading. J. Great Lakes Res. 2017, 43, 221–228. [Google Scholar] [CrossRef]
- Vikesland, P.J. Nanomaterial enabled biosensors for pathogen monitoring-a review. Environ. Sci. Technol. 2010, 44, 3656–3669. [Google Scholar] [CrossRef]
- Clark, S.L.; Remcho, V.T. Aptamers as analytical reagents. ELECTROPHORESIS 2002, 23, 1335–1340. [Google Scholar] [CrossRef]
- Lin, Z.; Huang, H.; Xu, Y.; Gao, X.; Qiu, B.; Chen, X.; Chen, G. Determination of microcystin-LR in water by a label-free aptamer based electrochemical impedance biosensor. Talanta 2013, 103, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Jiang, L.; Song, Y.; Ding, Y.; Zhang, J.; Wu, X.; Tang, D. Amperometric aptasensor for saxitoxin using a gold electrode modified with carbon nanotubes on a self-assembled monolayer, and methylene blue as an electrochemical indicator probe. Microchim. Acta 2016, 183, 1971–1980. [Google Scholar] [CrossRef]
- Elshafey, R.; Siaj, M.; Zourob, M. In Vitro Selection, Characterization, and Biosensing Application of High-Affinity Cylindrospermopsin-Targeting Aptamers. Anal. Chem. 2014, 86, 9196–9203. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Watson, C.J.; Kennedy, R.T. Aptamer affinity chromatography for rapid assay of adenosine in microdialysis samples collected in vivo. J. Chromatogr. A 2003, 1005, 123–130. [Google Scholar] [CrossRef]
- Eissa, S.; Ng, A.; Siaj, M.; Zourob, M. Label-Free Voltammetric Aptasensor for the Sensitive Detection of Microcystin-LR Using Graphene-Modified Electrodes. Anal. Chem. 2014, 86, 7551–7557. [Google Scholar] [CrossRef]
- Penna, A.; Magnani, M. Identification of Alexandrium (Dinophyceae) species using PCR and rDNA-targeted probes. J. Phycol. 1999, 35, 615–621. [Google Scholar] [CrossRef]
- Walker, M.J.; Burns, D.T.; Elliott, C.T.; Gowland, M.H.; Mills, E.N.C. Is food allergen analysis flawed? Health and supply chain risks and a proposed framework to address urgent analytical needs. Analyst 2016, 141, 24–35. [Google Scholar] [CrossRef] [Green Version]
- Montalto, M.; Santoro, L.; D’Onofrio, F.; Curigliano, V.; Gallo, A.; Visca, D.; Cammarota, G.; Gasbarrini, A.; Gasbarrini, G. Adverse Reactions to Food: Allergies and Intolerances. Dig. Dis. 2008, 26, 96–103. [Google Scholar] [CrossRef]
- Ortolani, C.; Pastorello, E.A. Food allergies and food intolerances. Best Pract. Res. Clin. Gastroenterol. 2006, 20, 467–483. [Google Scholar] [CrossRef]
- European Commission. EU law on food information to consumers Regulation (EU) No 1169/2011. In (EU) No 1169/2011; European Commission, Ed.; European Commission: Brussels, Belgium, 2014; Volume (EU) No 1169/2011, Available online: https://ec.europa.eu/food/food/labelling-and-nutrition/food-information-consumers-legislation_en (accessed on 17 August 2021).
- Nehra, M.; Lettieri, M.; Dilbaghi, N.; Kumar, S.; Marrazza, G. Nano-biosensing platforms for detection of cow’s milk allergens: An overview. Sensors 2020, 20, 32. [Google Scholar] [CrossRef] [Green Version]
- Neethirajan, S.; Weng, X.; Tah, A.; Cordero, J.O.; Ragavan, K.V. Nano-biosensor platforms for detecting food allergens—New trends. Sens. Bio-Sens. Res. 2018, 18, 13–30. [Google Scholar] [CrossRef]
- Wu, D.; Du, D.; Lin, Y. Recent progress on nanomaterial-based biosensors for veterinary drug residues in animal-derived food. TrAC Trends Anal. Chem. 2016, 83, 95–101. [Google Scholar] [CrossRef]
- Lan, L.; Yao, Y.; Ping, J.; Ying, Y. Recent advances in nanomaterial-based biosensors for antibiotics detection. Biosens. Bioelectron. 2017, 91, 504–514. [Google Scholar] [CrossRef]
- Majdinasab, M.; Yaqub, M.; Rahim, A.; Catanante, G.; Hayat, A.; Marty, J.L. An Overview on Recent Progress in Electrochemical Biosensors for Antimicrobial Drug Residues in Animal-Derived Food. Sensors 2017, 17, 1947. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, I.; Casewell, M.; Cox, T.; De Groot, B.; Friis, C.; Jones, R.; Nightingale, C.; Preston, R.; Waddell, J. Does the use of antibiotics in food animals pose a risk to human health? A critical review of published data. J. Antimicrob. Chemother. 2004, 53, 28–52. [Google Scholar] [CrossRef]
- Hao, H.; Cheng, G.; Iqbal, Z.; Ai, X.; Hussain, H.I.; Huang, L.; Dai, M.; Wang, Y.; Liu, Z.; Yuan, Z. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014, 5, 288. [Google Scholar] [CrossRef] [Green Version]
- Rico, A.; Burgat-Sacaze, V. Veterinary drugs and food safety: A toxilogical approach. Rev. Sci. Tech. (Int. Off. Epizoot.) 1985, 4, 111–130. [Google Scholar] [CrossRef] [Green Version]
- Du, L.; Liu, W. Occurrence, fate, and ecotoxicity of antibiotics in agro-ecosystems. A review. Agron. Sustain. Dev. 2012, 32, 309–327. [Google Scholar] [CrossRef] [Green Version]
- Leibovici, L.; Paul, M.; Garner, P.; Sinclair, D.J.; Afshari, A.; Pace, N.L.; Cullum, N.; Williams, H.C.; Smyth, A.; Skoetz, N.; et al. Addressing resistance to antibiotics in systematic reviews of antibiotic interventions. J. Antimicrob. Chemother. 2016, 71, 2367–2369. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Colón, K.; Chavez-Arias, C.; Díaz-Alcalá, J.E.; Martínez, M.A. Xylazine intoxication in humans and its importance as an emerging adulterant in abused drugs: A comprehensive review of the literature. Forensic Sci. Int. 2014, 240, 1–8. [Google Scholar] [CrossRef]
- Samanta, A.; Roffe, C.; Woods, K. Accidental self administration of xylazine in a veterinary nurse. Postgrad. Med. J. 1990, 66, 244–245. [Google Scholar] [CrossRef] [PubMed]
- De Meo, M.; Vanelle, P.; Bernadini, E.; Laget, M.; Maldonado, J.; Jentzer, O.; Crozet, M.; Dumenil, G. Evaluation of the mutagenic and genotoxic activities of 48 nitroimidazoles and related imidazole derivatives by the Ames test and the SOS chromotest. Environ. Mol. Mutagenesis 1992, 19, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Nsibande, S.A.; Forbes, P.B. Fluorescence detection of pesticides using quantum dot materials—A review. Anal. Chim. Acta 2016, 945, 9–22. [Google Scholar] [CrossRef] [PubMed]
- London, L.; Nell, V.; Thompson, M.L.; Myers, J.E. Effects of long-term organophosphate exposures on neurological symptoms, vibration sense and tremor among South African farm workers. Scand. J. Work Environ. Health 1998, 24, 18–29. [Google Scholar] [CrossRef] [Green Version]
- Papazoglou, S.; Zergioti, I. Laser Induced Forward Transfer (LIFT) of nano-micro patterns for sensor applications. Microelectron. Eng. 2017, 182, 25–34. [Google Scholar] [CrossRef]
- Chatzipetrou, M.; Massaouti, M.; Tsekenis, G.; Trilling, A.K.; van Andel, E.; Scheres, L.; Smulders, M.M.; Zuilhof, H.; Zergioti, I. Direct Creation of Biopatterns via a Combination of Laser-Based Techniques and Click Chemistry. Langmuir: ACS J. Surf. Colloids 2017, 33, 848–853. [Google Scholar] [CrossRef]
- Crapnell, R.D.; Banks, C.E. Electroanalytical overview: Utilising micro- and nano-dimensional sized materials in electrochemical-based biosensing platforms. Microchim. Acta 2021, 188, 268. [Google Scholar] [CrossRef]
- Khoshmanesh, S.M.; Hamishehkar, H.; Razmi, H. Trace analysis of organophosphorus pesticide residues in fruit juices and vegetables by an electrochemically fabricated solid-phase microextraction fiber coated with a layer-by-layer graphenized graphite/graphene oxide/polyaniline nanocomposite. Anal. Methods 2020, 12, 3268–3276. [Google Scholar] [CrossRef]
- Tan, X.; Liu, Y.; Zhang, T.; Luo, S.; Liu, X.; Tian, H.; Yang, Y.; Chen, C. Ultrasensitive electrochemical detection of methyl parathion pesticide based on cationic water-soluble pillar [5] arene and reduced graphene nanocomposite. RSC Adv. 2019, 9, 345–353. [Google Scholar] [CrossRef] [Green Version]
- Kumar, T.V.; Sundramoorthy, A.K. Electrochemical biosensor for methyl parathion based on single-walled carbon nanotube/glutaraldehyde crosslinked acetylcholinesterase-wrapped bovine serum albumin nanocomposites. Anal. Chim. Acta 2019, 1074, 131–141. [Google Scholar] [CrossRef]






| Electrode Materials | Electrode Modification | Target Analyte | Detection Method | Limit of Detection | Linear Range | Sample Composition | Reference |
|---|---|---|---|---|---|---|---|
| ITO/GCE | Cu@Au labelled Abs/Nafion and Hg | E. coli | ASV | 30 CFU/mL | 50–50,000 CFU/mL | Surface water | [29] |
| SPE | Abs | E. coli | CA | 103 CFU/mL | 103–107 CFU/mL | Water | [30] |
| Graphite | Teflon/tyrosinase | E. coli | CA | 10 CFU/mL | 10–107 CFU/mL | Drinking water | [31] |
| Ni disk | NiOOH/Ni(OH)2 | E. coli | CA | 104 CFU/mL | 6.4 × 104–3.3 × 109 CFU/mL | Water | [32] |
| ITO | CNT | E. coli | CC | 2 × 103 CFU/mL | 105–107 CFU/mL | Drinking/Tap water | [33] |
| GCE | rGO-PVA/AuNP/Aptamer | E. coli | DPV | 9.34 CFU/mL | 9.2–9.2 × 108 CFU/mL | Tap water, milk, meat | [34] |
| SPE | AuNP/Abs | E. coli | EIS | 15 CFU/mL | 101–106 CFU/mL | Water | [35] |
| Au | SAM/FcD/Peptide | E. coli | EIS | 103 CFU/mL | 103–107 CFU/mL | Water | [36] |
| GCE | dsDNA/CeO2/ CHIT | C. perfringens | EIS | 1.95 fM | 10 fM–100 nM | Dairy products | [37] |
| SPE | N/A | E. coli | CV | 10 ng/mL | 10–1000 ng/mL | Wastewater | [38] |
| Au | DNA-TH/Abs | S. pneumoniae | SWV | 0.093 CFU/mL | 5–100 CFU/mL | Nasal, mouth and axilla samples | [39] |
| SPE | ExtrAvidin®/ VHMR | V. cholerae | CA | 0.95 ng/µL | 0.49–15.6 nM | Water | [40] |
| GCE | PDA/EPD/Abs | S. aureus | DPV | 28.55 CFU/mL | 104–1010 CFU/mL | Milk | [41] |
| Au | DNA walker/RP | S. aureus | DPV | 9 CFU/mL | 60–6 × 107 CFU/mL | Water, honey | [42] |
| GCE | CNF/Abs | V. cholerae | EIS | 1.2 × 10−13 g/mL | 10−13–10−5 g/mL | Water samples | [43] |
| GCE | Ph-PhNH2/GNS/Abs | BoNT/E | LSV | 5 pg/mL | 0.01–10 ng/mL | Orange juice, milk | [44] |
| SPE | AuNPs/Peptide | BoNT/A&C | SWV | 10 pM | 0.01–1 nM | Orange juice | [45] |
| SPE | SWCNT | ZEA | DPASV | 5 nM | 0.0025–1 µM | Cornflakes | [46] |
| GCE | GS/CHIT | Microcystin-LR | DPV | 0.016 µg/L | 0.05–15 µg/L | Water | [47] |
| SPE | CB/ovalbumin | DA/OA | DPV | 1.9/0.18 ng/mL | 4–34/0.35–3.9 ng/mL | Mussel extract | [48] |
| Au-SPE | DNA-capture probe | A. minutum | CA | 25 pM | 0.12–1 nM | Ocean sample | [49] |
| SPE | CNF/Abs | gliadin | CA | 0.005 mg/kg | 0–80 µg/kg | Flour samples | [50] |
| SPE | MBs/Abs | ovomucoid | CA | 0.1 ng/mL | 0.3–25 ng/mL | Eggs, flour, bread | [51] |
| SPE | GO/MBs/Abs/ HRP | ovalbumin | CA | 0.2 fg/mL | 0.01–10 pg/mL | Wine | [52] |
| CPE | - | oxyclozanide | SWASV | 17.42 µg/L | 0.058–4 mg/L | Pharmaceutical formulation | [53] |
| GCE | Zn/Ni-ZIF-8 800/G/AuNp/Abs | monensin | DPV | 0.25–100 ng/mL | 0.11 ng/mL | Milk | [54] |
| Au | MBs | tetracycline | EIS | 1.2 pg/mL | 0.1–1000 pg/mL | Honey | [55] |
| GCE | - | xylazine | DPV | 120 nM | 0.5–256 µM | Pharmaceutical formulation/urine | [56] |
| GCE | GNP | xylazine | ASV | 0.1 mg/L | 0.4–6 mg/L | Beverages | [57] |
| PGM | MBs/Aptamer | ampicillin | - | 0.25 nM | 0.25–100 nM | Milk | [58] |
| GCE | Se-Co3O4/GO | dimetridazole | DPV | 3.4 nM | 0.02–83.72 µM | Pigeon meat, eggs | [59] |
| GCE | P-Arg-MIP | dimetridazole | DPV | 0.1 nM | 0.1 nM–10 µM | Egg, milk, honey | [60] |
| SPE | CB/acetylcholinesterase | Carbofuran chlorpyrifos | CA | 0.6 nM 0.4 nM | 1.1–23 nM 0.7–14 nM | Olive oil | [61] |
| SPE | CB | Carbofuran Isoprocarb Carbaryl fenobucarb | DPV | 0.048 µM 0.049 µM 0.079 µM 0.80 µM | 0.1–100 µM | Wheat and maize | [62] |
| SPE | GONRs | Metyl parathion | CA | 0.5 nM | 100 nM–100 µM 100–2500 µM | Tomato, beetroot, broccoli | [63] |
| SPE | CB/PB/Enzyme | Paraoxen 2,4-dichlorophenoxyacetic acid atrazine | CA | 2–20 ppb 100–600 ppb 10-100 ppb | 2 ppb 50 ppb - | River water | [64] |
| SPE | CB/PB/BChE | paraoxon | CA | 1.3 ng/mL | 0.0013–3 µg/mL | Soil, fruit, vegetables | [65] |
| SPE | AuNP/PB/Abs | OPs | DPV | 0.003 ng/mL | 1.82 × 10−3–3.29 × 104 ng/mL | Cabbage | [66] |
| ITO | MnNS | OPs | DPV | 0.025 ng/mL | 0.1–20 ng/mL | Pakchoi | [67] |
| ITO | MB/ZIF-8/AChE | paraoxon | DPV | 1.7 ng/mL | 20–4000 ng/mL | Apple, aubergine | [68] |
| SPE | rGO-CuNPs/Aptamer | Profenofos Phorate Isocarbophos omethoate | DPV | 0.003 nM 0.3 nM 0.03 nM 0.3 nM | 0.01–100 nM 1–1000 nM 0.1–1000 nM 1–500 nM | Spinach, rapeseed | [69] |
| GCE | PdNPs/BN | Paraoxon ethyl | LSV | 3 nM | 0.01–610.5 µM | River water | [70] |
| Au | DRAB | Chlorpyrifos Pb | DPV | 0.178 nM 0.034 nM | 0.5–500 nM 0.1–500 nM | Apple, orange, cabbage | [71] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, A.G.-M.; Crapnell, R.D.; Banks, C.E. Electroanalytical Overview: Electrochemical Sensing Platforms for Food and Drink Safety. Biosensors 2021, 11, 291. https://doi.org/10.3390/bios11080291
Ferrari AG-M, Crapnell RD, Banks CE. Electroanalytical Overview: Electrochemical Sensing Platforms for Food and Drink Safety. Biosensors. 2021; 11(8):291. https://doi.org/10.3390/bios11080291
Chicago/Turabian StyleFerrari, Alejandro Garcia-Miranda, Robert D. Crapnell, and Craig E. Banks. 2021. "Electroanalytical Overview: Electrochemical Sensing Platforms for Food and Drink Safety" Biosensors 11, no. 8: 291. https://doi.org/10.3390/bios11080291
APA StyleFerrari, A. G.-M., Crapnell, R. D., & Banks, C. E. (2021). Electroanalytical Overview: Electrochemical Sensing Platforms for Food and Drink Safety. Biosensors, 11(8), 291. https://doi.org/10.3390/bios11080291







