Novel SH-SAW Biosensors for Ultra-Fast Recognition of Growth Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
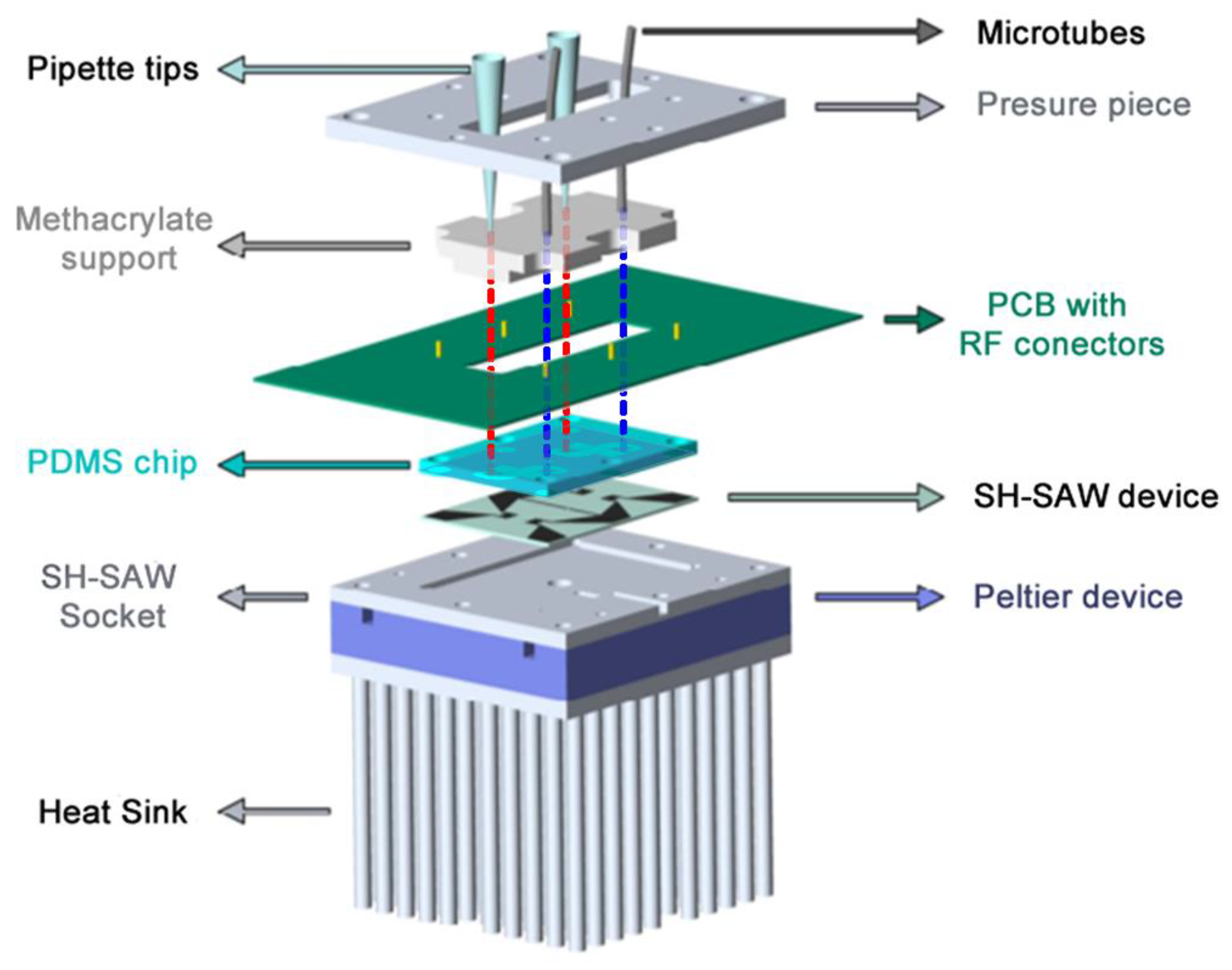
2.2. Assembly of the Biosensor (SH-SAW)
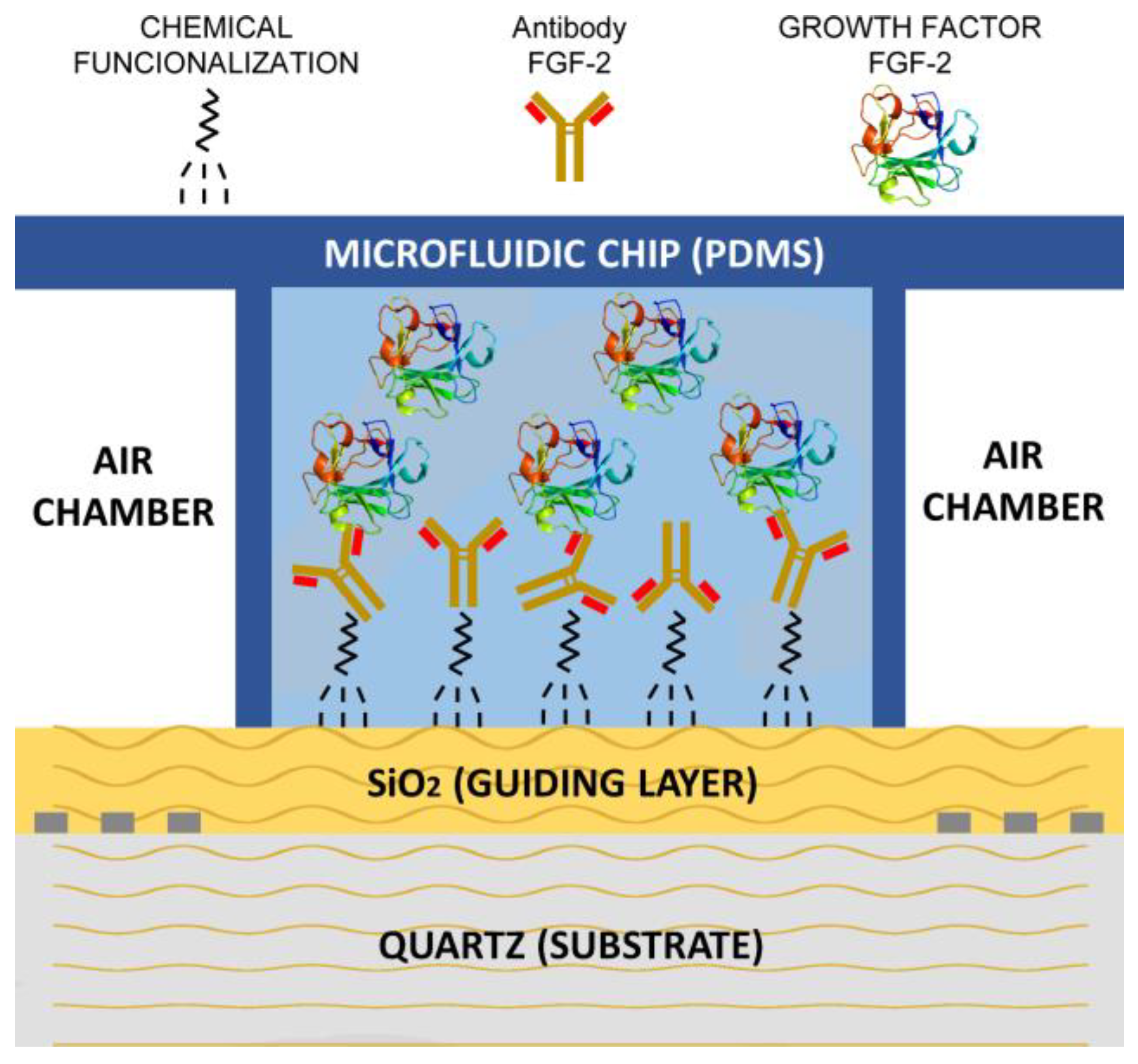
2.3. PDMS Chip and Liquid Cell
2.4. System Temperature Control
- A Peltier device that allows thermostatization of the socket where the Love device is located. Said plinth made of aluminum, due to its great thermal conductivity, allows the temperature of the device to be homogeneous, and also, due to its low heat capacity, the Peltier device is able to act on its temperature in a short time;
- A Platinum resistor (Pt100) that is located in a cavity between the socket and the SH-SAW device, and is capable of measuring temperature with an uncertainty of ±0.1 °C;
- A PID controller, which every two seconds measures the resistance of the Pt100, processing and storing the temperature data to finally act on the Peltier device through a power source;
- An aluminum heat sink that is used to cool and heats in short times, and besides promotes heat exchange with the environment;
- A fan is necessary so that the heat exchange between the heat sink and the environment is faster.
2.5. Activation of the SH-SAW Devices
2.6. Experimental Setup
2.7. Detection of the Biomolecules (GFs)
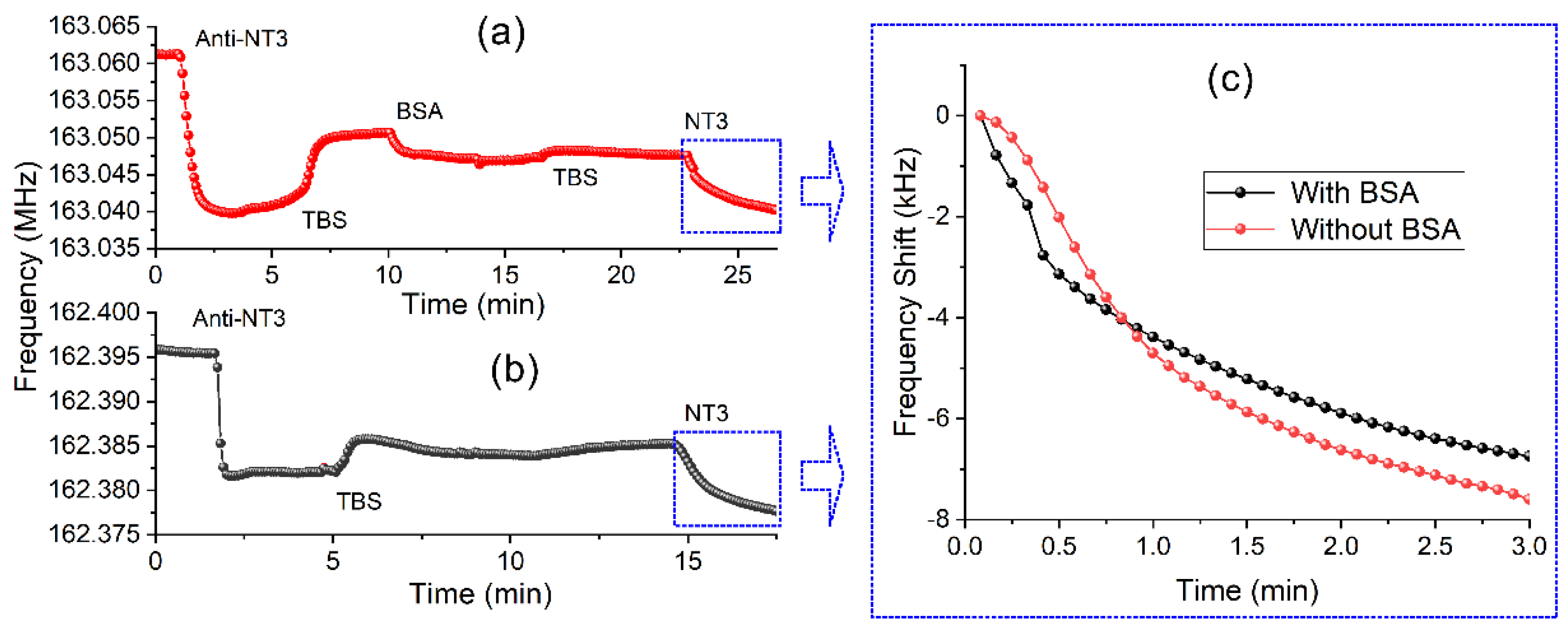
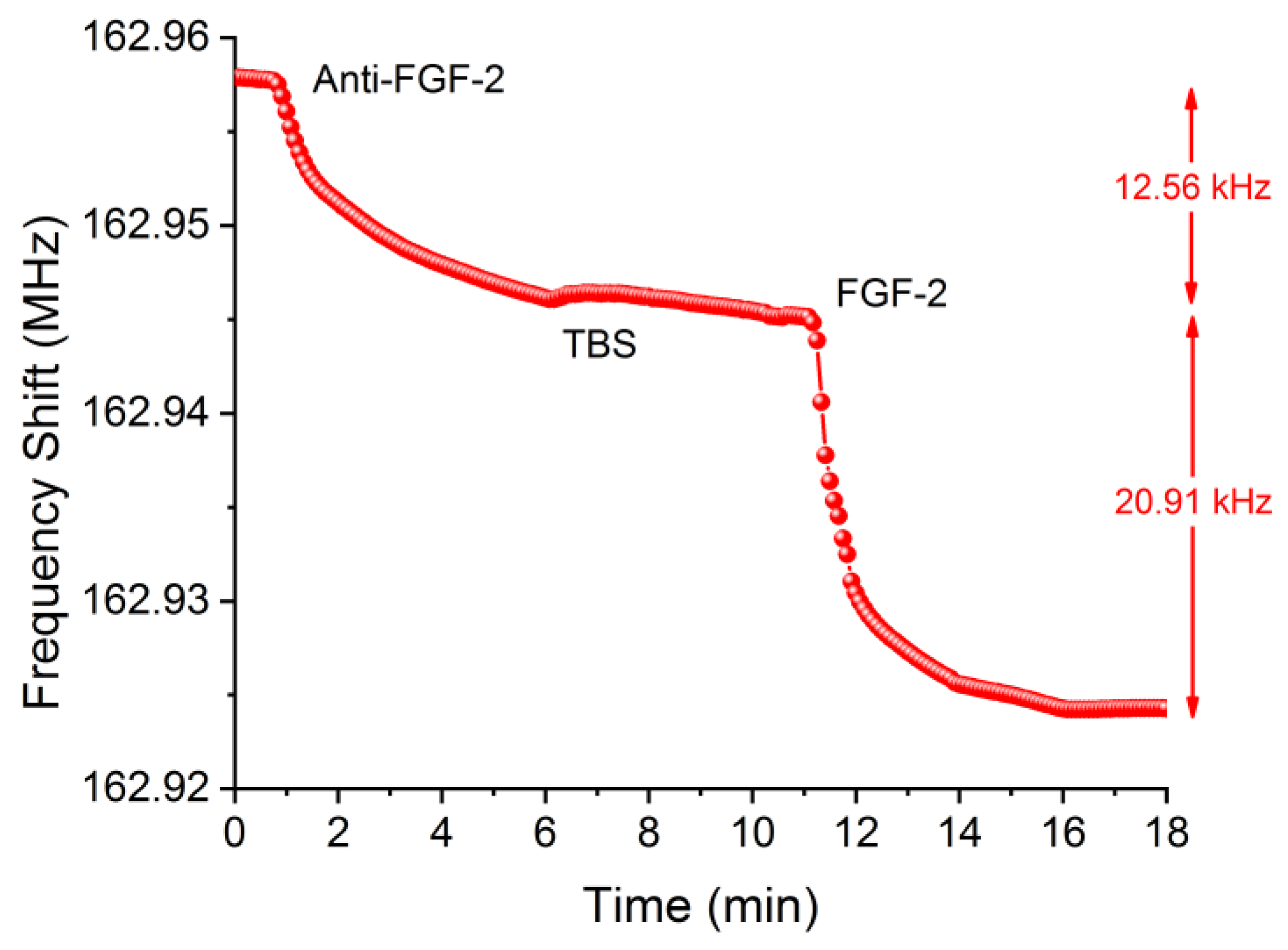
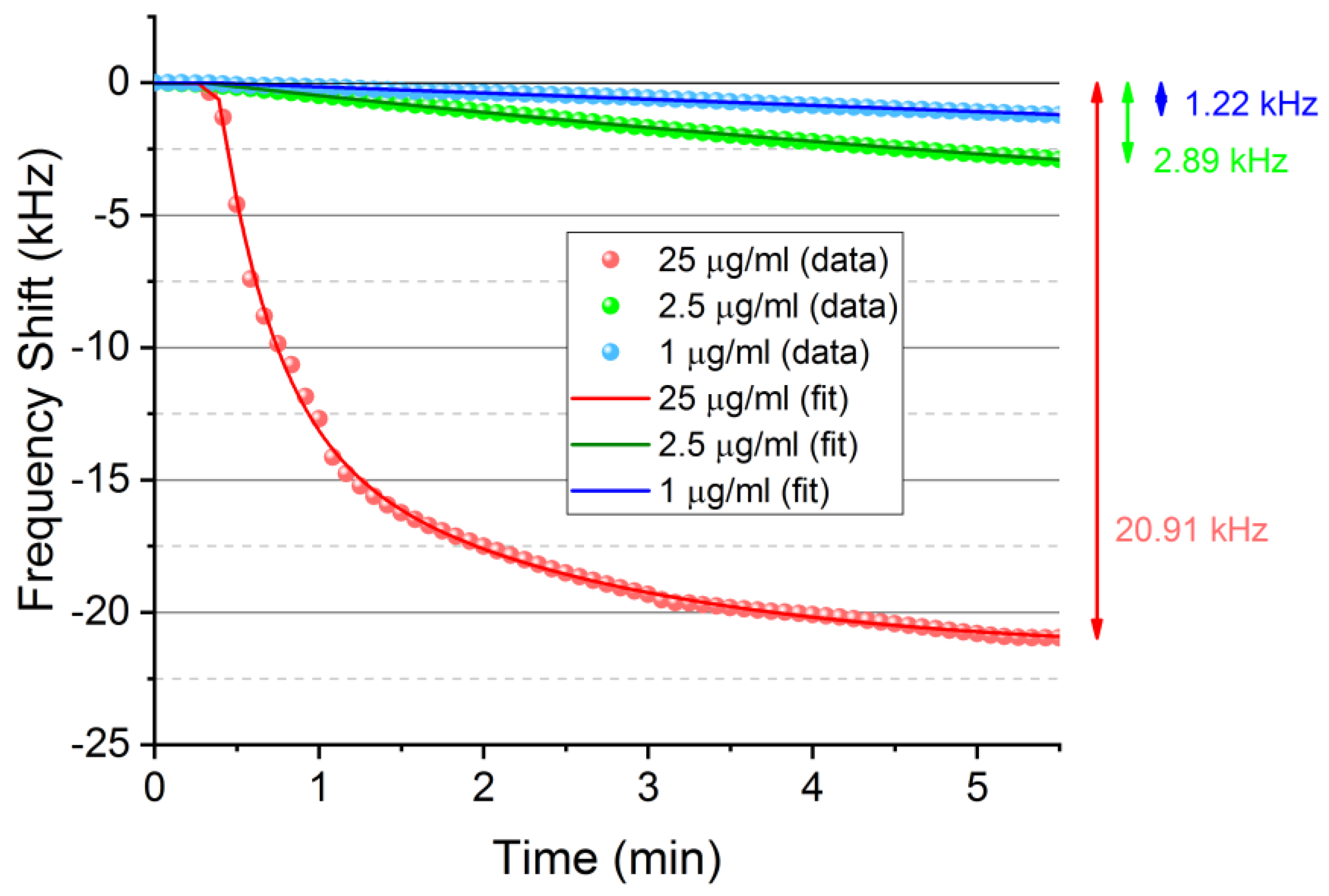
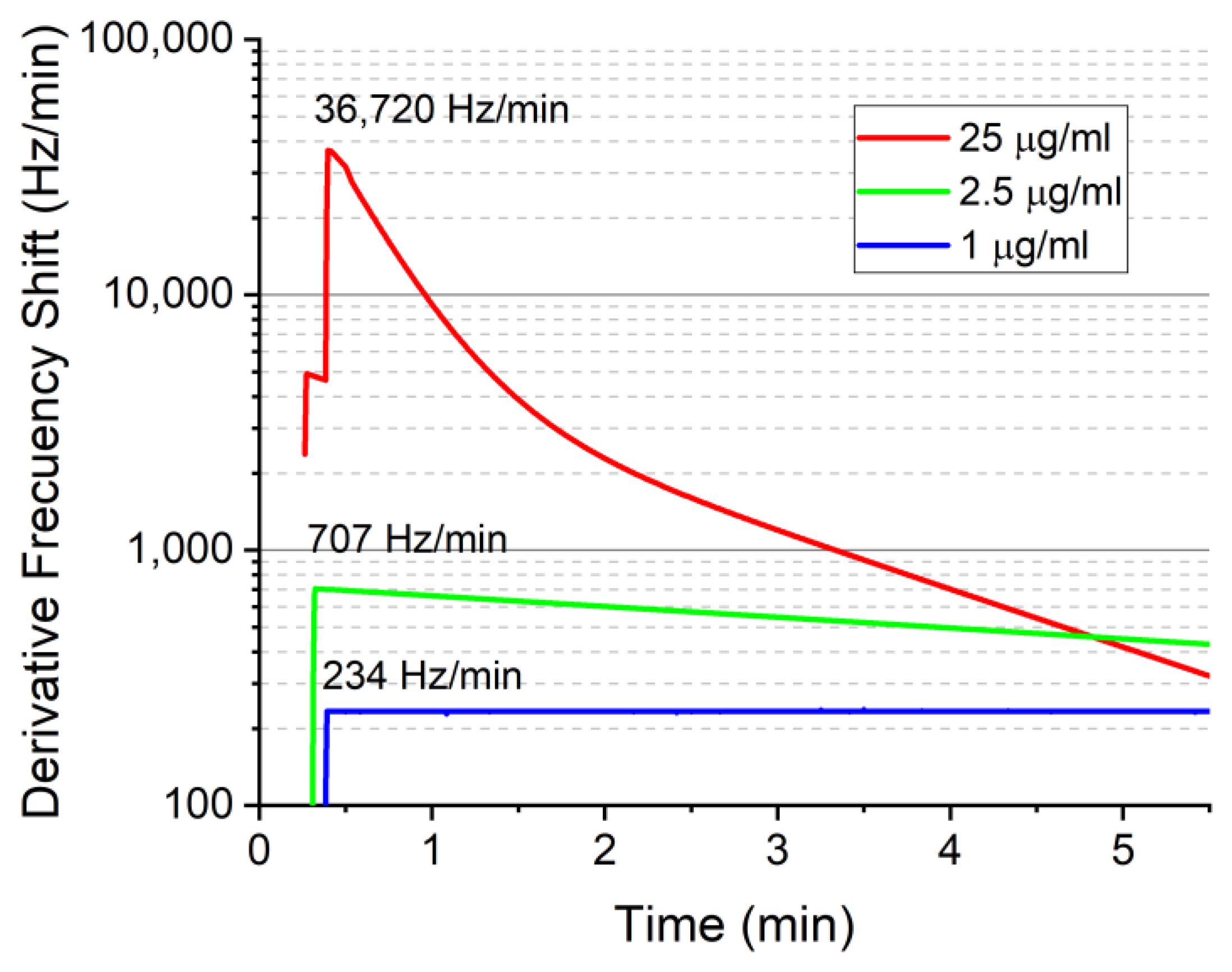
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, Y.S.; Lee, H.-J.; Han, M.-H.; Yoon, N.-K.; Kim, Y.-C.; Ahn, J. Effective production of human growth factors in Escherichia coli by fusing with small protein 6HFh8. Microb. Cell Fact. 2021, 20, 9. [Google Scholar] [CrossRef] [PubMed]
- Katoh, M. Therapeutics targeting fgf signaling network in human diseases. Trends Pharmacol. Sci. 2016, 37, 1081–1096. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [Green Version]
- Butte, M.; Hwang, P.K.; Mobley, W.C.; Fletterick, R.J. Crystal structure of neurotrophin-3 homodimer shows distinct regions are used to bind its receptors. Biochemistry 1998, 37, 16846–16852. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Nerve growth factor. Nature 1973, 244, 471. [Google Scholar] [CrossRef] [Green Version]
- Ernfors, P.; Wetmore, C.; Olson, L.; Persson, H. Identification of cells in rat brain and peripheral tissues expressing mRNA for members of the nerve growth factor family. Neuron 1990, 5, 511–526. [Google Scholar] [CrossRef]
- DeChant, G.; Tsoulfas, P.; Parada, L.F.; Barde, Y.-A. The neurotrophin receptor p75 binds neurotrophin-3 on sympathetic neurons with high affinity and specificity. J. Neurosci. 1997, 17, 5281–5287. [Google Scholar] [CrossRef]
- Ma, L.; Reis, G.; Parada, L.F.; Schuman, E.M. Neuronal NT-3 is not required for synaptic transmission or long-term potentiation in area CA1 of the adult rat hippocampus. Learn. Mem. 1999, 6, 267–275. [Google Scholar] [CrossRef]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef]
- Hur, Y.; Han, J.; Seon, J.; Pak, Y.E.; Roh, Y. Development of an SH-SAW sensor for the detection of DNA hybridization. Sens. Actuators A Phys. 2005, 120, 462–467. [Google Scholar] [CrossRef]
- Son, K.J.; Gheibi, P.; Stybayeva, G.; Rahimian, A.; Revzin, A. Detecting cell-secreted growth factors in microfluidic devices using bead-based biosensors. Microsyst. Nanoeng. 2017, 3, 17025. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, S.; Nemade, H.B. Coupled resonance in SH-SAW resonator with S1813 micro-ridges for high mass sensitivity biosensing applications. Sens. Actuators B Chem. 2018, 273, 288–297. [Google Scholar] [CrossRef]
- Freeman, R.; Girsh, J.; Jou, A.F.-J.; Ho, J.-A.A.; Hug, T.; Dernedde, J.; Willner, I. Optical Aptasensors for the Analysis of the Vascular Endothelial Growth Factor (VEGF). Anal. Chem. 2012, 84, 6192–6198. [Google Scholar] [CrossRef]
- Zandieh, M.; Hosseini, S.N.; Vossoughi, M.; Khatami, M.; Abbasian, S.; Moshaii, A. Label-free and simple detection of endotoxins using a sensitive LSPR biosensor based on silver nanocolumns. Anal. Biochem. 2018, 548, 96–101. [Google Scholar] [CrossRef]
- Li, H.; Hitchins, V.M.; Wickramasekara, S. Rapid detection of bacterial endotoxins in ophthalmic viscosurgical device materials by direct analysis in real time mass spectrometry. Anal. Chim. Acta 2016, 943, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zawistowski, A.; Ehrmann, M.; Yi, T.; Schmuck, C. Peptide functionalized polydiacetylene liposomes act as a fluorescent turn-on sensor for bacterial lipopolysaccharide. J. Am. Chem. Soc. 2011, 133, 9720–9723. [Google Scholar] [CrossRef] [PubMed]
- Heras, J.Y.; Pallarola, D.; Battaglini, F. Electronic tongue for simultaneous detection of endotoxins and other contaminants of microbiological origin. Biosens. Bioelectron. 2010, 25, 2470–2476. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Meng, F.; Cheng, W.; Sun, H.; Luo, Y.; Tang, Y.; Miao, P. Preparation of a peptide-modified electrode for capture and voltammetric determination of endotoxin. ACS Omega 2017, 2, 2469–2473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fetter, L.; Richards, J.; Daniel, J.; Roon, L.; Rowland, T.J.; Bonham, A.J. Electrochemical aptamer scaffold biosensors for detection of botulism and ricin toxins. Chem. Commun. 2015, 51, 15137–15140. [Google Scholar] [CrossRef]
- Länge, K.; Rapp, B.E.; Rapp, M. Surface acoustic wave biosensors: A review. Anal. Bioanal. Chem. 2008, 391, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fang, J.; Zou, L.; Zou, Y.; Lang, L.; Gao, F.; Hu, N.; Wang, P. A novel sensitive cell-based Love Wave biosensor for marine toxin detection. Biosens. Bioelectron. 2015, 77, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Pang, Y.; Li, D.; Huang, Z.; Zhang, Z.; Xue, N.; Xu, Y.; Mu, X. An aptamer-based shear horizontal surface acoustic wave biosensor with a CVD-grown single-layered graphene film for high-sensitivity detection of a label-free endotoxin. Microsyst. Nanoeng. 2020, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voiculescu, I.; Nordin, A.N. Acoustic wave based MEMS devices for biosensing applications. Biosens. Bioelectron. 2012, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ricco, A.; Martin, S.; Zipperian, T. Surface acoustic wave gas sensor based on film conductivity changes. Sens. Actuators 1985, 8, 319–333. [Google Scholar] [CrossRef]
- Tom-Moy, M.; Baer, R.L.; Spira-Solomon, D.; Doherty, T.P. Atrazine measurements using surface transverse wave devices. Anal. Chem. 1995, 67, 1510–1516. [Google Scholar] [CrossRef]
- Borodina, I.; Zaitsev, B.; Burygin, G.; Guliy, O. Sensor based on the slot acoustic wave for the non-contact analysis of the bacterial cells—Antibody binding in the conducting suspensions. Sens. Actuators B Chem. 2018, 268, 217–222. [Google Scholar] [CrossRef]
- Vasudev, A.; Kaushik, A.; Bhansali, S. Electrochemical immunosensor for label free epidermal growth factor receptor (EGFR) detection. Biosens. Bioelectron. 2013, 39, 300–305. [Google Scholar] [CrossRef]
- Shin, D.-M.; Shin, Y.-C.; Lee, J.-H.; Kim, T.-H.; Han, D.-W.; Kim, J.-M.; Kim, H.K.; Kim, K.; Hwang, Y.-H. Highly sensitive detection of epidermal growth factor receptor expression levels using a capacitance sensor. Sens. Actuators B Chem. 2015, 209, 438–443. [Google Scholar] [CrossRef]
- Purohit, B.; Vernekar, P.R.; Shetti, N.P.; Chandra, P. Biosensor nanoengineering: Design, operation, and implementation for biomolecular analysis. Sens. Int. 2020, 1, 100040. [Google Scholar] [CrossRef]
- Cecchini, M.; Girardo, S.; Pisignano, D.; Cingolani, R.; Beltram, F. Acoustic-counterflow microfluidics by surface acoustic waves. Appl. Phys. Lett. 2008, 92, 104103. [Google Scholar] [CrossRef]
- Greco, G.; Agostini, M.; Tonazzini, I.; Sallemi, D.; Barone, S.; Cecchini, M. Surface-acoustic-wave (SAW)-driven device for dynamic cell cultures. Anal. Chem. 2018, 90, 7450–7457. [Google Scholar] [CrossRef]
- Greco, G.; Agostini, M.; Shilton, R.; Travagliati, M.; Signore, G.; Cecchini, M. Surface acoustic wave (SAW)-enhanced chemical functionalization of gold films. Sensors 2017, 17, 2452. [Google Scholar] [CrossRef] [Green Version]
- Agostini, M.; Greco, G.; Cecchini, M. Full-SAW microfluidics-based lab-on-a-chip for biosensing. IEEE Access 2019, 7, 70901–70909. [Google Scholar] [CrossRef]
- Lurz, F.; Ostertag, T.; Scheiner, B.; Weigel, R.; Koelpin, A. Reader architectures for wireless surface acoustic wave sensors. Sensors 2018, 18, 1734. [Google Scholar] [CrossRef] [Green Version]
- Matatagui, D.; Fontecha, J.; Fernández, M.; Oliver, M.; Hernando-García, J.; Sánchez-Rojas, J.; Gràcia, I.; Cané, C.; Santos, J.; Horrillo, M. Comparison of two types of acoustic biosensors to detect immunoreactions: Love-wave sensor working in dynamic mode and QCM working in static mode. Sens. Actuators B Chem. 2013, 189, 123–129. [Google Scholar] [CrossRef]
- Matatagui, D.; Moynet, D.; Fernández, M.; Fontecha, J.; Esquivel, J.; Gràcia, I.; Cané, C.; Déjous, C.; Rebière, D.; Santos, J.; et al. Detection of bacteriophages in dynamic mode using a Love-wave immunosensor with microfluidics technology. Sens. Actuators B Chem. 2013, 185, 218–224. [Google Scholar] [CrossRef]
- Lo, X.-C.; Li, J.-Y.; Lee, M.-T.; Yao, D.-J. Frequency shift of a SH-SAW biosensor with glutaraldehyde and 3-aminopropyltriethoxysilane functionalized films for detection of epidermal growth factor. Biosensors 2020, 10, 92. [Google Scholar] [CrossRef] [PubMed]
- Matatagui, D.; Fontecha, J.L.; Fernández, M.J.; Gracia, I.; Cane, C.; Santos, J.P.; Horrillo, M.C. Love-wave sensors combined with microfluidics for fast detection of biological warfare agents. Sensors 2014, 14, 12658–12669. [Google Scholar] [CrossRef] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matatagui, D.; Bastida, Á.; Horrillo, M.C. Novel SH-SAW Biosensors for Ultra-Fast Recognition of Growth Factors. Biosensors 2022, 12, 17. https://doi.org/10.3390/bios12010017
Matatagui D, Bastida Á, Horrillo MC. Novel SH-SAW Biosensors for Ultra-Fast Recognition of Growth Factors. Biosensors. 2022; 12(1):17. https://doi.org/10.3390/bios12010017
Chicago/Turabian StyleMatatagui, Daniel, Ágatha Bastida, and M. Carmen Horrillo. 2022. "Novel SH-SAW Biosensors for Ultra-Fast Recognition of Growth Factors" Biosensors 12, no. 1: 17. https://doi.org/10.3390/bios12010017
APA StyleMatatagui, D., Bastida, Á., & Horrillo, M. C. (2022). Novel SH-SAW Biosensors for Ultra-Fast Recognition of Growth Factors. Biosensors, 12(1), 17. https://doi.org/10.3390/bios12010017






