Detection of Hypertension-Induced Changes in Erythrocytes by SERS Nanosensors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanostructure Synthesis
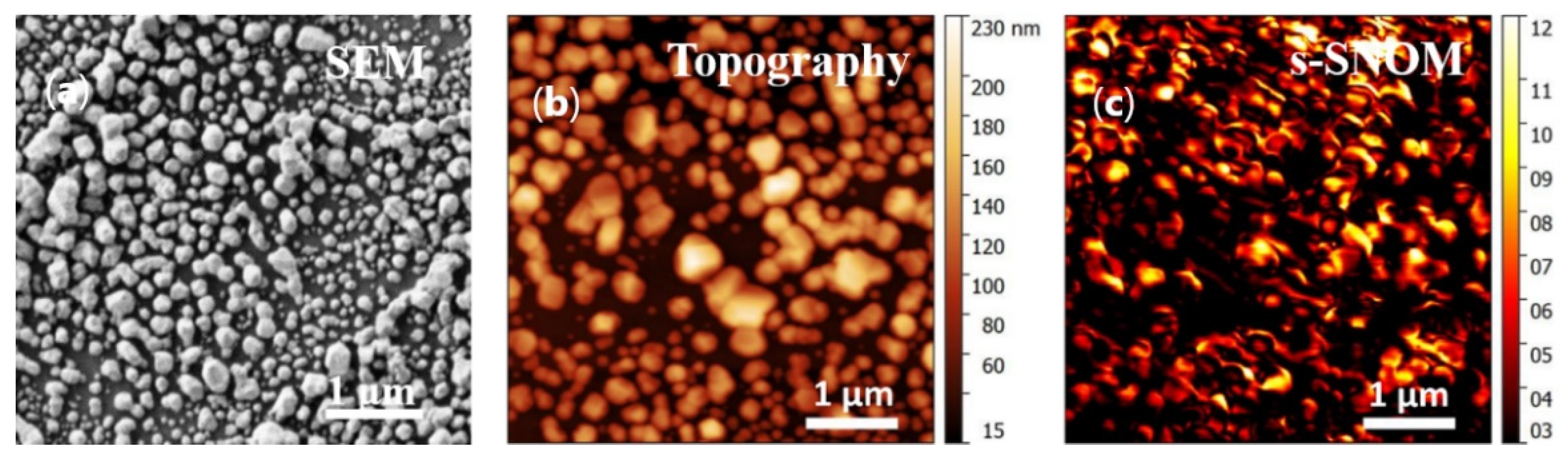
2.2. Characterization of Nanostructures
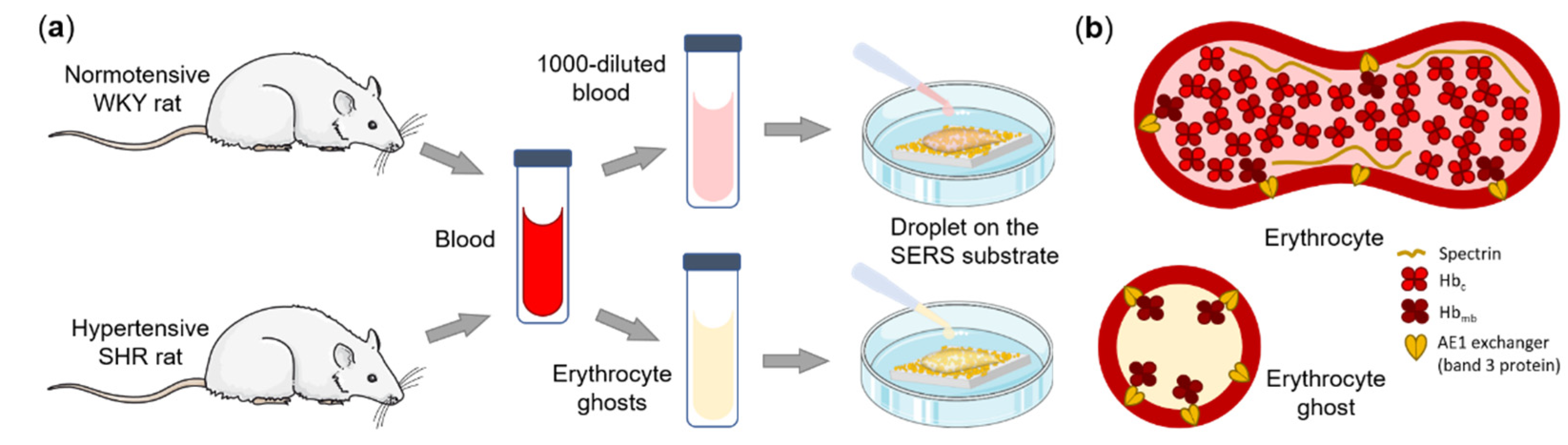
2.3. Animals
2.4. Preparation of Erythrocyte Ghosts
2.5. SERS Measurements
2.6. SERS Spectra Analysis and Statistics
3. Results
3.1. Experimental Design and AgNSS Characterization
3.2. Spontaneous Hypertension Affects Conformation of Heme in Hb Bound to AE1-Exchanger
3.3. Decreased Fluidity of Plasma Membrane of Erythrocytes under Spontaneous Hypertension
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ganau, L.; Prisco, L.; Ligarotti, G.; Ambu, R.; Ganau, M. Understanding the Pathological Basis of Neurological Diseases Through Diagnostic Platforms Based on Innovations in Biomedical Engineering: New Concepts and Theranostics Perspectives. Medicines 2018, 5, 22. [Google Scholar] [CrossRef] [Green Version]
- Beverung, S.; Wu, J.; Steward, R. Lab-on-a-chip for cardiovascular physiology and pathology. Micromachines 2020, 11, 898. [Google Scholar] [CrossRef]
- Valpapuram, I.; Candeloro, P.; Coluccio, M.L.; Parrotta, E.I.; Giugni, A.; Das, G.; Cuda, G.; Di Fabrizio, E.; Perozziello, G. Waveguiding and SERS simplified Raman spectroscopy on biological samples. Biosensors 2019, 9, 37. [Google Scholar] [CrossRef] [Green Version]
- Pires, P.W.; Dams Ramos, C.M.; Matin, N.; Dorrance, A.M. The effects of hypertension on the cerebral circulation. Am. J. Physiol. Circ. Physiol. 2013, 304, H1598–H1614. [Google Scholar] [CrossRef]
- Zhou, W.; Brown, J.M.; Bajaj, N.S.; Chandra, A.; Divakaran, S.; Weber, B.; Bibbo, C.F.; Hainer, J.; Taqueti, V.R.; Dorbala, S.; et al. Hypertensive coronary microvascular dysfunction: A subclinical marker of end organ damage and heart failure. Eur. Heart, J. 2020, 41, 2366–2375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L. Oxygen sensing, hypoxia-inducible factors, and disease pathophysiology. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 47–71. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Guazzi, M.; Testani, J.M.; Borlaug, B.A. Altered Hemodynamics and End-Organ Damage in Heart Failure. Circulation 2020, 142, 998–1012. [Google Scholar] [CrossRef]
- Böning, D.; Kuebler, W.M.; Bloch, W. The oxygen dissociation curve of blood in COVID-19. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2021, 321, L349–L357. [Google Scholar] [CrossRef] [PubMed]
- Yudin, J.; Verhovsek, M. How we diagnose and manage altered oxygen affinity hemoglobin variants. Am. J. Hematol. 2019, 94, 597–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smolsky, J.; Kaur, S.; Hayashi, C.; Batra, S.K.; Krasnoslobodtsev, A.V. Surface-enhanced raman scattering-based immunoassay technologies for detection of disease biomarkers. Biosensors 2017, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Pilot, R.; Signorini, R.; Durante, C.; Orian, L.; Bhamidipati, M.; Fabris, L. A review on surface-enhanced Raman scattering. Biosensors 2019, 9, 57. [Google Scholar] [CrossRef] [Green Version]
- Brazhe, N.A.; Abdali, S.; Brazhe, A.R.; Luneva, O.G.; Bryzgalova, N.Y.; Parshina, E.Y.; Sosnovtseva, O.V.; Maksimov, G.V. New Insight into Erythrocyte through In Vivo Surface-Enhanced Raman Spectroscopy. Biophys. J. 2009, 97, 3206–3214. [Google Scholar] [CrossRef] [Green Version]
- Semenova, A.A.; Goodilin, E.A.; Brazhe, N.A.; Ivanov, V.K.; Baranchikov, A.E.; Lebedev, V.A.; Goldt, A.E.; Sosnovtseva, O.V.; Savilov, S.V.; Egorov, A.V.; et al. Planar SERS nanostructures with stochastic silver ring morphology for biosensor chips. J. Mater. Chem. 2012, 22, 24530–24544. [Google Scholar] [CrossRef]
- Sarycheva, A.S.; Brazhe, N.A.; Baizhumanov, A.A.; Nikelshparg, E.I.; Semenova, A.A.; Garshev, A.V.; Baranchikov, A.E.; Ivanov, V.K.; Maksimov, G.V.; Sosnovtseva, O.; et al. New nanocomposites for SERS studies of living cells and mitochondria. J. Mater. Chem. B 2016, 4, 539–546. [Google Scholar] [CrossRef]
- Nikelshparg, E.I.; Grivennikova, V.G.; Baizhumanov, A.A.; Semenova, A.A.; Sosnovtseva, V.; Goodilin, E.A.; Maksimov, G.V.; Brazhe, N.A. Probing lipids in biological membranes using SERS. Mendeleev Commun. 2019, 29, 635–637. [Google Scholar] [CrossRef]
- Sharma, R.; Premachandra, B.R. Membrane-bound hemoglobin as a marker of oxidative injury in adult and neonatal red blood cells. Biochem. Med. Metab. Biol. 1991, 46, 33–44. [Google Scholar] [CrossRef]
- Chu, H.; McKenna, M.M.; Krump, N.A.; Zheng, S.; Mendelsohn, L.; Thein, S.L.; Garrett, L.J.; Bodine, D.M.; Low, P.S. Reversible binding of hemoglobin to band 3 constitutes the molecular switch that mediates O2 regulation of erythrocyte properties. Blood 2016, 128, 2708–2716. [Google Scholar] [CrossRef] [PubMed]
- Brazhe, N.A.; Evlyukhin, A.B.; Goodilin, E.A.; Semenova, A.A.; Novikov, S.M.; Bozhevolnyi, S.I.; Chichkov, B.N.; Sarycheva, A.S.; Baizhumanov, A.A.; Nikelshparg, E.I.; et al. Probing cytochrome c in living mitochondria with surface-enhanced Raman spectroscopy. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Yamakoshi, K.I.; Kamiya, A. Noninvasive measurement of arterial blood pressure and elastic properties using photoelectric plethysmography technique. Med. Prog. Technol. 1987, 12, 123–143. [Google Scholar] [CrossRef]
- Brazhe, N.A.; Parshina, E.Y.; Khabatova, V.V.; Semenova, A.A.; Brazhe, A.R.; Yusipovich, A.I.; Sarycheva, A.S.; Churin, A.A.; Goodilin, E.A.; Maksimov, G.V.; et al. Tuning SERS for living erythrocytes: Focus on nanoparticle size and plasmon resonance position. J. Raman Spectrosc. 2013, 44, 686–694. [Google Scholar] [CrossRef]
- Bukara, K.; Jovanić, S.Z.; Drvenica, I.T.; Stančić, A.; Ilić, V.; Rabasović, M.D.; Pantelić, D.V.; Jelenković, B.M.; Bugarski, B.; Krmpot, A.J. Mapping of hemoglobin in erythrocytes and erythrocyte ghosts using two photon excitation fluorescence microscopy. J. Biomed. Opt. 2017, 22, 026003. [Google Scholar] [CrossRef] [Green Version]
- Shiohara, A.; Novikov, S.M.; Solís, D.M.; Taboada, J.M.; Obelleiro, F.; Liz-Marzán, L.M. Plasmon Modes and Hot Spots in Gold Nanostar-Satellite Clusters. J. Phys. Chem. C 2015, 119, 10836–10843. [Google Scholar] [CrossRef] [Green Version]
- Moskovits, M. Spot the hotspot. Nature 2011, 469, 307–308. [Google Scholar] [CrossRef] [PubMed]
- Zenin, V.A.; Andryieuski, A.; Malureanu, R.; Radko, I.P.; Volkov, V.S.; Gramotnev, D.K.; Lavrinenko, A.V.; Bozhevolnyi, S.I. Boosting Local Field Enhancement by on-Chip Nanofocusing and Impedance-Matched Plasmonic Antennas. Nano Lett. 2015, 15, 8148–8154. [Google Scholar] [CrossRef] [Green Version]
- Novikov, S.M.; Frydendahl, C.; Beermann, J.; Zenin, V.A.; Stenger, N.; Coello, V.; Mortensen, N.A.; Bozhevolnyi, S.I. White Light Generation and Anisotropic Damage in Gold Films near Percolation Threshold. ACS Photonics 2017, 4, 1207–1215. [Google Scholar] [CrossRef] [Green Version]
- Bozhevolnyi, S.I.; Volkov, V.S.; Leosson, K.; Boltasseva, A. Bend loss in surface plasmon polariton band-gap structures. Appl. Phys. Lett. 2001, 79, 1076–1078. [Google Scholar] [CrossRef] [Green Version]
- Torres Filho, I.P.; Terner, J.; Pittman, R.N.; Proffitt, E.; Ward, K.R. Measurement of hemoglobin oxygen saturation using Raman microspectroscopy and 532-nm excitation. J. Appl. Physiol. 2008, 104, 1809–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkins, C.G.; Buckley, K.; Blades, M.W.; Turner, R.F.B. Raman Spectroscopy of Blood and Blood Components. Appl. Spectrosc. 2017, 71, 767–793. [Google Scholar] [CrossRef]
- Gautam, R.; Oh, J.Y.; Marques, M.B.; Dluhy, R.A.; Patel, R.P. Characterization of storage-induced red blood cell hemolysis using Raman spectroscopy. Lab. Med. 2018, 49, 298–310. [Google Scholar] [CrossRef]
- Senge, M.O.; MacGowan, S.A.; O’Brien, J.M. Conformational control of cofactors in nature-the influence of protein-induced macrocycle distortion on the biological function of tetrapyrroles. Chem. Commun. 2015, 51, 17031–17063. [Google Scholar] [CrossRef] [Green Version]
- Kinschel, D.; Bacellar, C.; Cannelli, O.; Sorokin, B.; Katayama, T.; Mancini, G.F.; Rouxel, J.R.; Obara, Y.; Nishitani, J.; Ito, H.; et al. Femtosecond X-ray emission study of the spin cross-over dynamics in haem proteins. Nat. Commun. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Perutz, M.F.; Wilkinson, A.J.; Paoli, M.; Dodson, G.G. The stereochemical mechanism of the cooperative effects in hemoglobin revisited. Annu. Rev. Biophys. Biomol. Struct. 1998, 27, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Brazhe, N.A.; Nikelshparg, E.I.; Baizhumanov, A.A.; Grivennikova, V.G.; Semenova, A.A.; Novikov, S.M.; Volkov, V.S.; Arsenin, A.V.; Yakubovsky, D.I.; Evlyukhin, A.B.; et al. SERS uncovers the link between conformation of cytochrome c heme and mitochondrial membrane potential. bioRxiv 2021, 1–33. [Google Scholar] [CrossRef]
- Chertkova, R.V.; Brazhe, N.A.; Bryantseva, T.V.; Nekrasov, A.N.; Dolgikh, D.A.; Yusipovich, A.I.; Sosnovtseva, O.; Maksimov, G.V.; Rubin, A.B.; Kirpichnikov, M.P. New insight into the mechanism of mitochondrial cytochrome c function. PLoS ONE 2017, 12, e0178280. [Google Scholar] [CrossRef] [PubMed]
- Czamara, K.; Majzner, K.; Pacia, M.Z.; Kochan, K.; Kaczor, A.; Baranska, M. Raman spectroscopy of lipids: A review. J. Raman Spectrosc. 2015, 46, 4–20. [Google Scholar] [CrossRef]
- Kutuzov, N.P.; Brazhe, A.R.; Yusipovich, A.I.; Maksimov, G.V.; Dracheva, O.E.; Lyaskovskiy, V.L.; Bulygin, F.V.; Rubin, A.B. ATP-induced lipid membrane reordering in the myelinated nerve fiber identified using Raman spectroscopy. Laser Phys. Lett. 2013, 10, 075606. [Google Scholar] [CrossRef]
- Shvalya, V.; Filipič, G.; Zavašnik, J.; Abdulhalim, I.; Cvelbar, U. Surface-enhanced Raman spectroscopy for chemical and biological sensing using nanoplasmonics: The relevance of interparticle spacing and surface morphology. Appl. Phys. Rev. 2020, 7, 031307. [Google Scholar] [CrossRef]
- Luneva, O.G.; Brazhe, N.A.; Maksimova, N.V.; Rodnenkov, O.V.; Parshina, E.Y.; Bryzgalova, N.Y.; Maksimov, G.V.; Rubin, A.B.; Orlov, S.N.; Chazov, E.I. Ion transport, membrane fluidity and haemoglobin conformation in erythrocyte from patients with cardiovascular diseases: Role of augmented plasma cholesterol. Pathophysiology 2007, 14, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Yusipovich, A.I.; Braze, N.A.; Luneva, O.G.; Parshina, E.Y.; Churin, A.A.; Rodnenkov, O.V.; Maksimov, G.V. Changes in the state of hemoglobin in patients with coronary heart disease and patients with circulatory failure. Bull. Exp. Biol. Med. 2013, 155, 233–235. [Google Scholar] [CrossRef] [PubMed]
- Tziakas, D.N.; Kaski, J.C.; Chalikias, G.K.; Romero, C.; Fredericks, S.; Tentes, I.K.; Kortsaris, A.X.; Hatseras, D.I.; Holt, D.W. Total Cholesterol Content of Erythrocyte Membranes Is Increased in Patients With Acute Coronary Syndrome. A New Marker of Clinical Instability? J. Am. Coll. Cardiol. 2007, 49, 2081–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Tang, H.; Zeng, Q.; Wang, X.; Yi, G.; Meng, K.; Mao, Y.; Mao, X. Total cholesterol content of erythrocyte membranes is associated with the severity of coronary artery disease and the therapeutic effect of rosuvastatin. Ups. J. Med. Sci. 2012, 117, 390–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khatibzadeh, N.; Gupta, S.; Farrell, B.; Brownell, W.E.; Anvari, B. Effects of cholesterol on nano-mechanical properties of the living cell plasma membrane. Soft Matter 2012, 8, 8350–8360. [Google Scholar] [CrossRef] [PubMed]
- Ayee, M.A.; Levitan, I. Paradoxical impact of cholesterol on lipid packing and cell stiffness. Front. Biosci. - Landmark 2016, 21, 1245–1259. [Google Scholar] [CrossRef] [Green Version]
- Forsyth, A.M.; Braunmüller, S.; Wan, J.; Franke, T.; Stone, H.A. The effects of membrane cholesterol and simvastatin on red blood cell deformability and ATP release. Microvasc. Res. 2012, 83, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Faried, M.; Suga, K.; Okamoto, Y.; Shameli, K.; Miyake, M.; Umakoshi, H. Membrane Surface-Enhanced Raman Spectroscopy for Cholesterol-Modified Lipid Systems: Effect of Gold Nanoparticle Size. ACS Omega 2019, 4, 13687–13695. [Google Scholar] [CrossRef]
- Yao, J.; Wang, L.; Yang, J.M.; Maslov, K.I.; Wong, T.T.W.; Li, L.; Huang, C.H.; Zou, J.; Wang, L.V. High-speed label-free functional photoacoustic microscopy of mouse brain in action. Nat. Methods 2015, 12, 407–410. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- De Vecchis, D.; Reithmeier, R.A.F.; Kalli, A.C. Molecular Simulations of Intact Anion Exchanger 1 Reveal Specific Domain and Lipid Interactions. Biophys. J. 2019, 117, 1364–1379. [Google Scholar] [CrossRef] [Green Version]
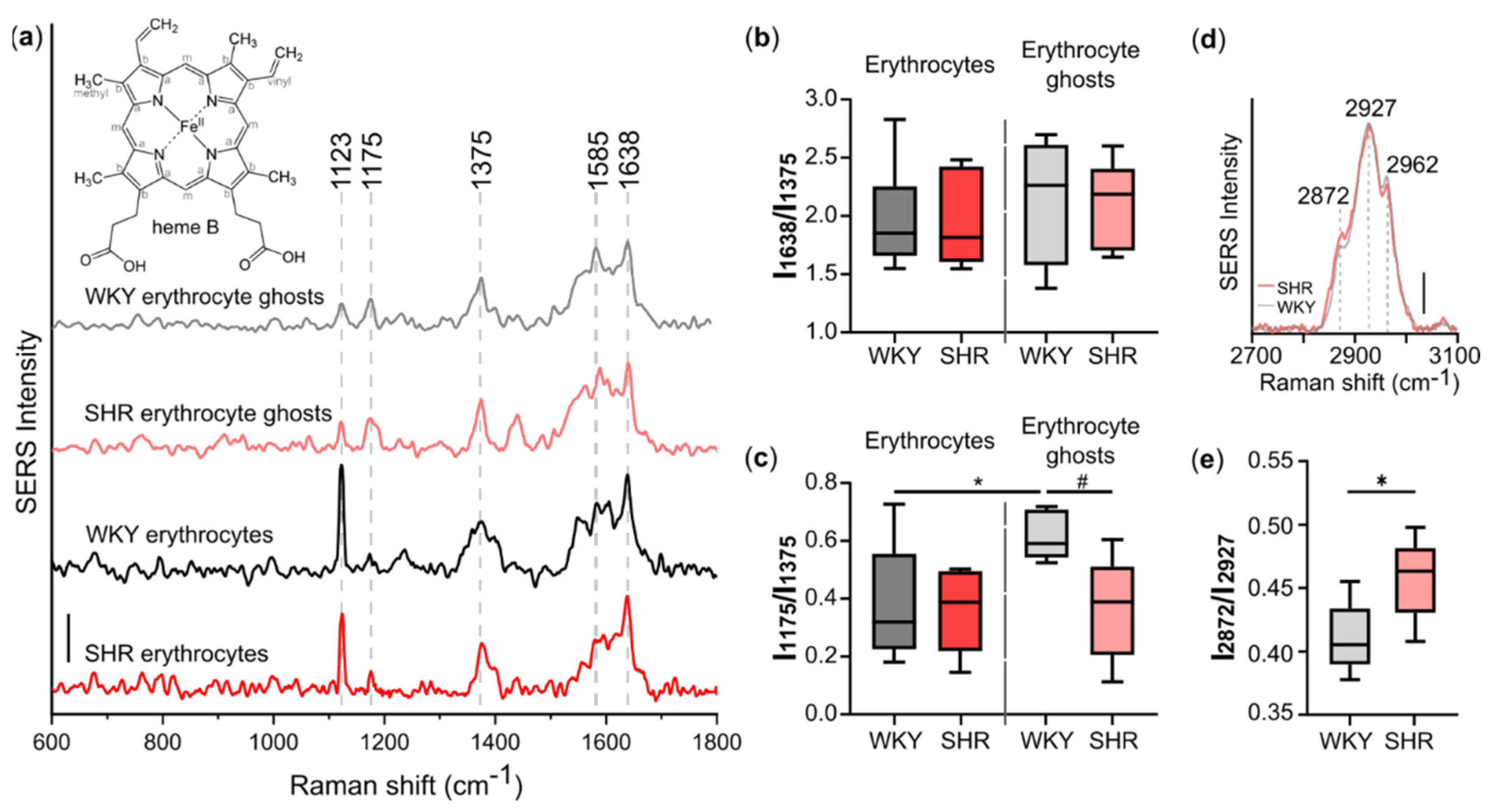

| Peak Position (cm−1) | Assignment | Main Contribution from Molecules: |
|---|---|---|
| 1121 | Cb–CH3 | HbO2 |
| 1175 | Pyrrole half-ring, asymmetric | HbO2 |
| 1375 | Pyrrole half-ring, symmetric | HbO2 |
| 1638 | CaCm, CaCmH, CaCb | HbO2 in planar conformation |
| 2872 | νas ( =CH2) | lipids in trans conformation and cholesterol |
| 2927 | νs (–CH3) | lipids and proteins |
| 2962 | νas (–CH3) | lipids and proteins |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikelshparg, E.I.; Baizhumanov, A.A.; Bochkova, Z.V.; Novikov, S.M.; Yakubovsky, D.I.; Arsenin, A.V.; Volkov, V.S.; Goodilin, E.A.; Semenova, A.A.; Sosnovtseva, O.; et al. Detection of Hypertension-Induced Changes in Erythrocytes by SERS Nanosensors. Biosensors 2022, 12, 32. https://doi.org/10.3390/bios12010032
Nikelshparg EI, Baizhumanov AA, Bochkova ZV, Novikov SM, Yakubovsky DI, Arsenin AV, Volkov VS, Goodilin EA, Semenova AA, Sosnovtseva O, et al. Detection of Hypertension-Induced Changes in Erythrocytes by SERS Nanosensors. Biosensors. 2022; 12(1):32. https://doi.org/10.3390/bios12010032
Chicago/Turabian StyleNikelshparg, Evelina I., Adil A. Baizhumanov, Zhanna V. Bochkova, Sergey M. Novikov, Dmitry I. Yakubovsky, Aleksey V. Arsenin, Valentyn S. Volkov, Eugene A. Goodilin, Anna A. Semenova, Olga Sosnovtseva, and et al. 2022. "Detection of Hypertension-Induced Changes in Erythrocytes by SERS Nanosensors" Biosensors 12, no. 1: 32. https://doi.org/10.3390/bios12010032
APA StyleNikelshparg, E. I., Baizhumanov, A. A., Bochkova, Z. V., Novikov, S. M., Yakubovsky, D. I., Arsenin, A. V., Volkov, V. S., Goodilin, E. A., Semenova, A. A., Sosnovtseva, O., Maksimov, G. V., & Brazhe, N. A. (2022). Detection of Hypertension-Induced Changes in Erythrocytes by SERS Nanosensors. Biosensors, 12(1), 32. https://doi.org/10.3390/bios12010032







