Detection of Alpha-Fetoprotein Using Aptamer-Based Sensors
Abstract
:1. Introduction
2. AFP Detection Based on Optical Aptamer Biosensor
2.1. AFP Detection Based on Raman Spectroscopy
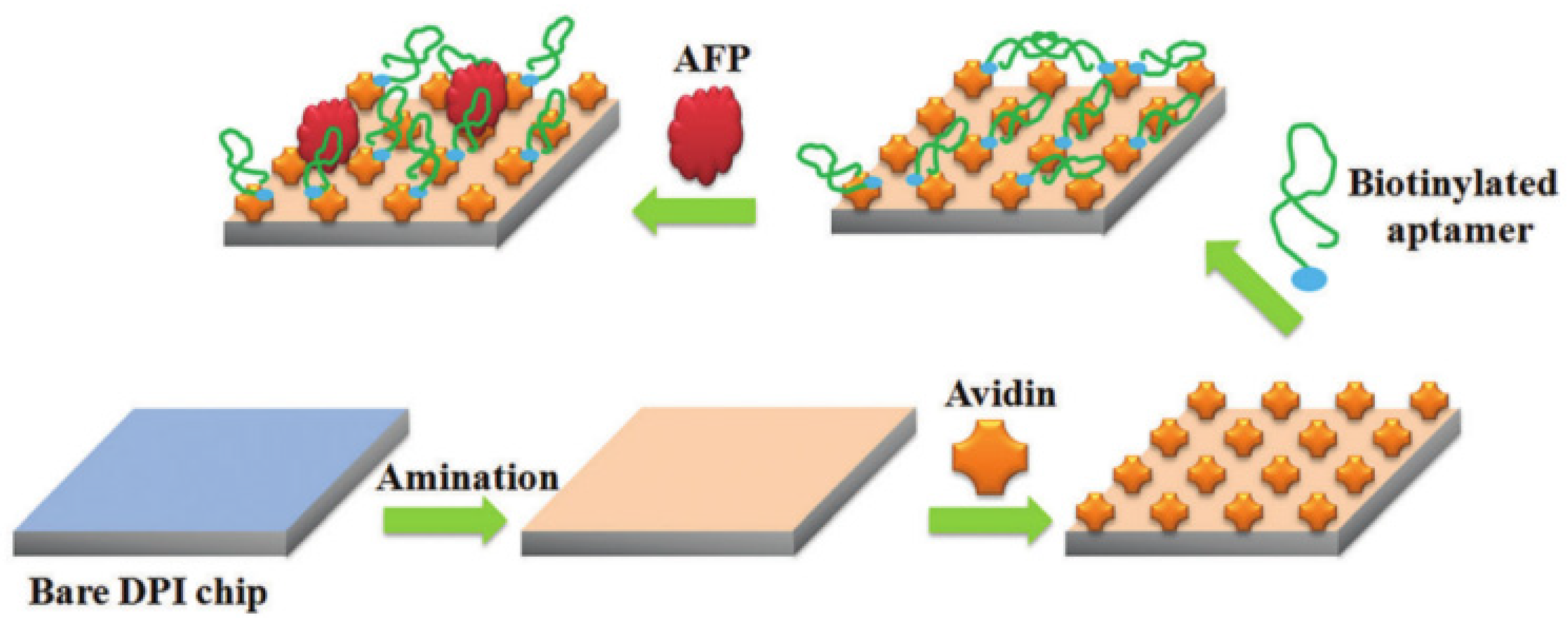
2.2. AFP Detection Based on Dual-Polarization Interferometry
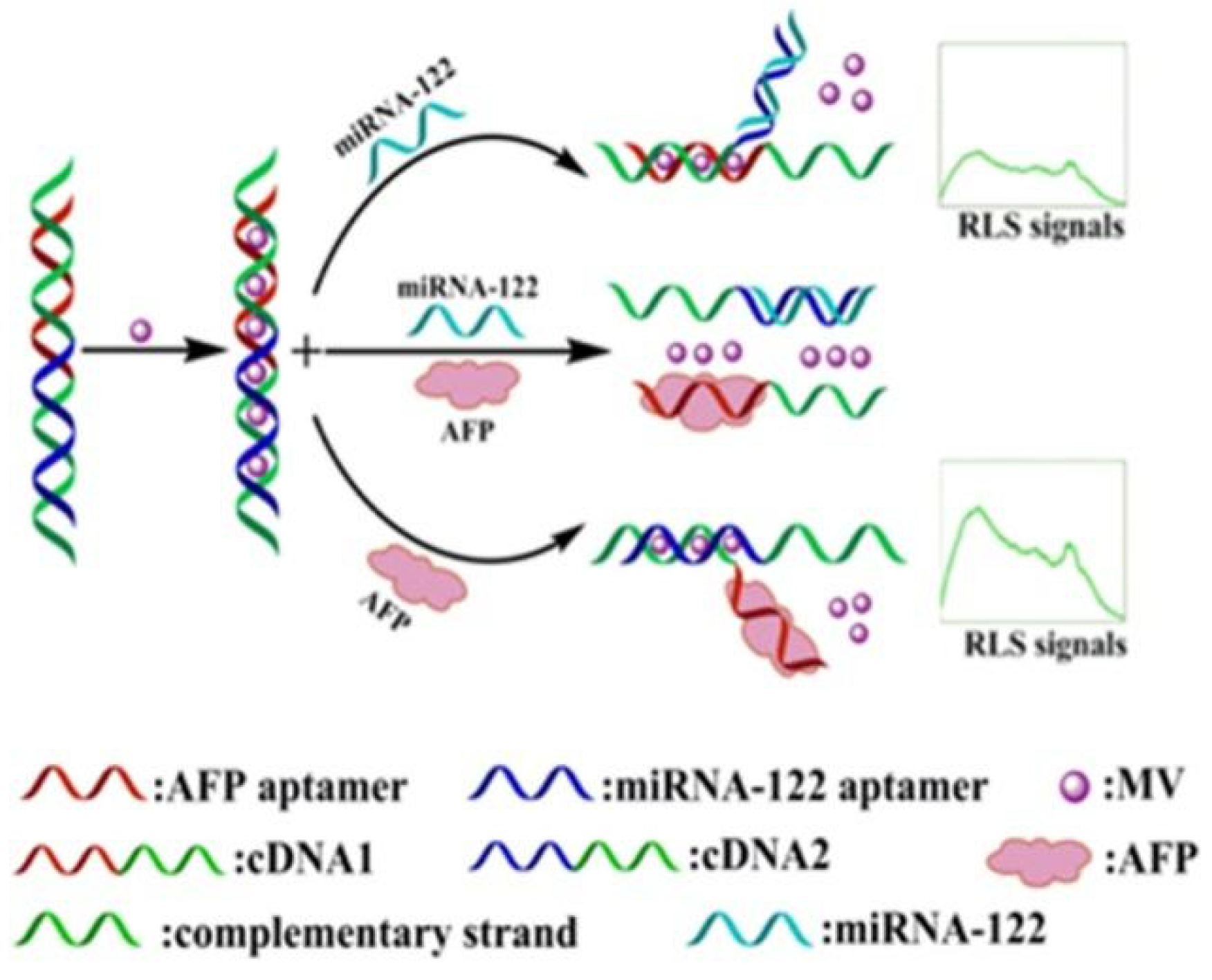
2.3. AFP Detection Based on Resonance Light-Scattering
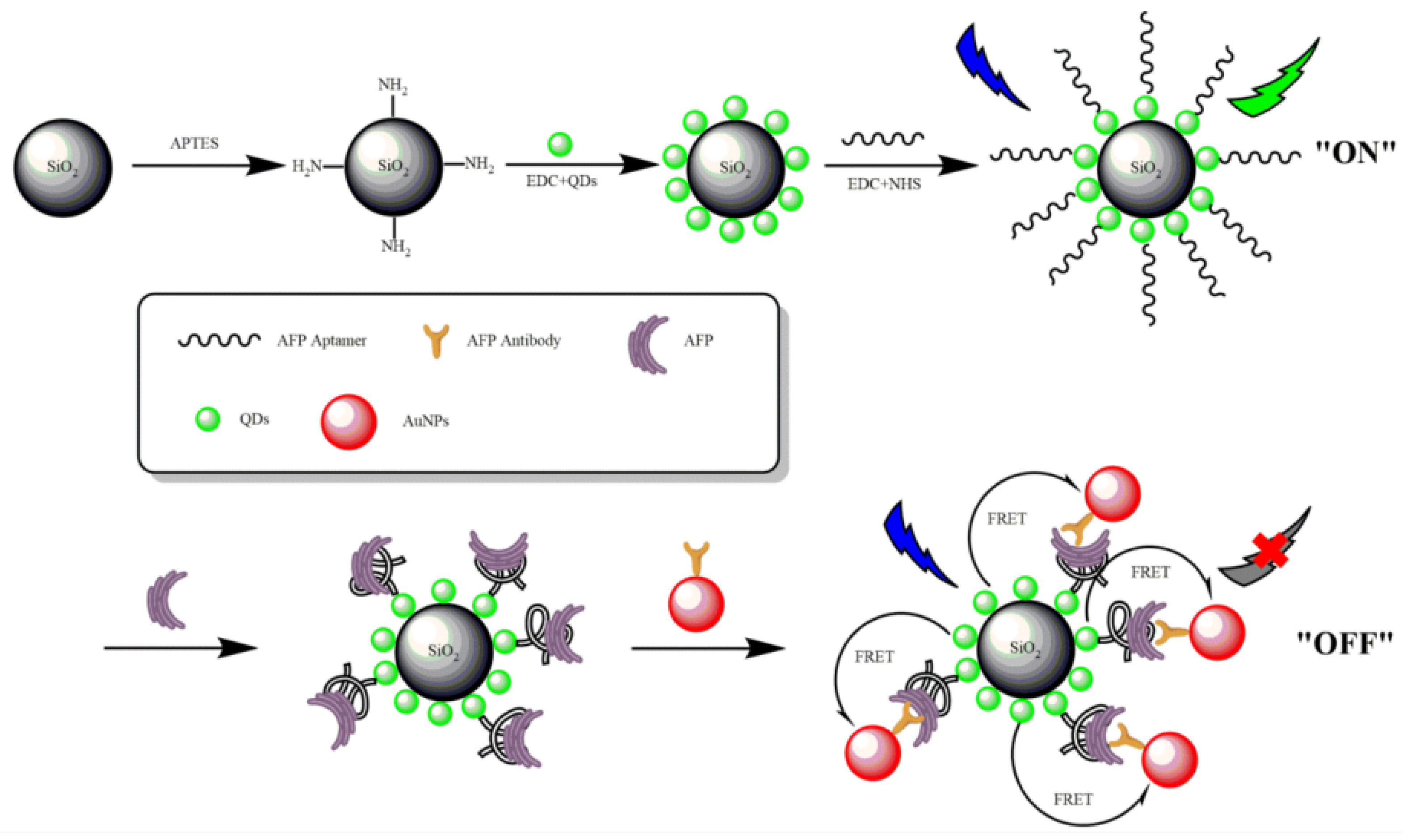
2.4. AFP Detection Based on Fluorescence
2.5. AFP Detection Based on Chemiluminescence
3. AFP Detection Based on Electrochemical Aptamer Biosensor
3.1. AFP Detection Based on Cyclic Voltammetry
3.2. AFP Detection Based on Electrochemical Impedance Spectroscopy
3.3. AFP Detection Based on Giant Magnetic Impedance
4. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, N.; Perera, M.T.; Silva, M.A. Current treatment approaches to HCC with a special consideration to transplantation. J. Transplant. 2016, 2016, 7926264. [Google Scholar] [CrossRef]
- Benson, A.B.; D’Angelica, M.I.; Abrams, T.A.; Are, C.; Bloomston, P.M.; Chang, D.T.; Clary, B.M.; Covey, A.M.; Ensminger, W.D.; Iyer, R.; et al. Hepatobiliary Cancers, Version 2.2014. J. Natl. Compr. Cancer Netw. 2014, 12, 1152. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Gao, T.; Gu, S.; Zhi, J.; Yang, J.; Li, G. An electrochemical biosensor for the assay of alpha-fetoprotein-L3 with practical applications. Biosens. Bioelectron. 2017, 87, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Li, S.; Xue, Y.; Liang, J.; Cui, L.; Li, Q.; Zhou, S.; Huang, Y.; Li, G.; Zhao, Y. A Fe3O4@Au-basedpseudo-homogeneous electrochemical immunosensor for AFP measurement using AFP antibody-GNPs-HRP as detection probe. Anal. Biochem. 2017, 534, 56–63. [Google Scholar] [CrossRef]
- Sauzay, C.; Petit, A.; Bourgeois, A.M.; Barbare, J.C.; Chauffert, B.; Galmiche, A.; Houessinon, A. Alpha-foetoprotein (AFP): A multi-purpose marker in hepatocellular carcinoma. Clin. Chim. Acta 2016, 463, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, Y.; Du, R.; Li, J.; Yu, X. An ultrasensitive electrochemical immunosensor based on the synergistic effect of quaternary Cu2SnZnS4 NCs and cyclodextrin functionalized-graphene. Analyst 2017, 142, 780–786. [Google Scholar] [CrossRef]
- Guo, J.; Han, X.; Wang, J.; Zhao, J.; Guo, Z.; Zhang, Y. Horseradish peroxidase functionalized gold nanorods as a label for sensitive electrochemical detection of alpha-fetoprotein antigen. Anal. Biochem. 2015, 491, 58–64. [Google Scholar] [CrossRef]
- Vivekanandarajah, A.; Atallah, J.P.; Gupta, S. Alpha-fetoprotein-producing nonmetastatic gastric adenocarcinoma: A rare entity. J. Gastrointest. Canc. 2014, 45, 225–227. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.J.; Wu, Y.S.; Lin, G.F.; Hou, J.Y.; Li, M.; Liu, T.C. Quantum-dot-based homogeneous time-resolved fluoroimmunoassay of alpha-fetoprotein. Anal. Chim. Acta 2012, 741, 100–105. [Google Scholar] [CrossRef]
- Schoental, R. Diagnosis of primary cancer of the liver. Lancet 1945, 245, 226–227. [Google Scholar] [CrossRef]
- Kew, M.C.; Dos Santos, H.A.; Sherlock, S. Diagnosis of primary cancer of the liver. Brit. Med. J. 1971, 4, 408–411. [Google Scholar] [CrossRef]
- Youns, M.M.; Abdel Wahab, A.H.; Hassan, Z.A.; Attia, M.S. Serum talin-1 is a potential novel biomarker for diagnosis of hepatocellular carcinoma in Egyptian patients. Asian Pac. J. Cancer Prev. 2013, 14, 3819–3823. [Google Scholar] [CrossRef] [PubMed]
- Shafik, H.M.; Ayoub, S.M.; Ebeid, N.H.; Someda, H.H. New adjuvant design using layered double hydroxide for production of polyclonal antibodies in radioimmunoassay techniques. J. Radioanal. Nucl. Chem. 2014, 301, 81–89. [Google Scholar] [CrossRef]
- Li, Y.; Dong, L.; Wang, X.; Liu, Y.; Liu, H.; Xie, M. Development of graphite carbon nitride based fluorescent immune sensor for detection of alpha fetoprotein. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 196, 103–109. [Google Scholar] [CrossRef]
- Li, X.; Guo, Q.; Cao, W.; Li, Y.; Du, B.; Wei, Q. Enhanced electrochemiluminescence from luminol at carboxyl graphene for detection of alpha-fetoprotein. Anal. Biochem. 2014, 457, 59–64. [Google Scholar] [CrossRef]
- Wang, A.; Ruan, W.; Song, W.; Chen, L.; Zhao, B.; Jung, Y.M.; Wang, X. Detection of the potential tumor marker of AFP using surface-enhanced Raman scattering-based immunoassay. J. Raman Spectrosc. 2013, 44, 1649–1653. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Mao, K.; Li, Y.; Du, B.; Zhang, Y.; Wei, Q. Electrochemical immunosensor for alpha-fetoprotein detection using ferroferric oxide and horseradish peroxidase as signal amplification labels. Anal. Biochem. 2014, 465, 121–126. [Google Scholar] [CrossRef]
- Cox, W.G.; Singer, V.L. Fluorescent DNA hybridization probe preparation using amine modification and reactive dye coupling. Biotechniques 2004, 36, 114–122. [Google Scholar] [CrossRef]
- Xu, T.; Chi, B.; Wu, F.; Ma, S.; Zhan, S.; Yi, M.; Xu, H.; Mao, C. A sensitive label-free immunosensor for detection alpha-Fetoprotein in whole blood based on anticoagulating magnetic nanoparticles. Biosens. Bioelectron. 2017, 95, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.J.; Lin, H.I.; Shiesh, S.C.; Lee, G.B. An integrated microflfluidic system for rapid screening of alpha-fetoprotein-specifific aptamers. Biosens. Bioelectron. 2012, 35, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Tan, Q.; Ye, W.; Liu, D.; Chen, H.; Hu, H.; Wen, D.; Liu, Y.; Cao, Y.; Kang, J.; et al. Screening and identifying a novel ssDNA aptamer against alpha-fetoprotein using CE-SELEX. Sci. Rep. 2015, 5, 15552. [Google Scholar] [CrossRef]
- Myszka, D.G. Survey of the 1998 optical biosensor literature. J. Mol. Recognit. 1999, 12, 390–408. [Google Scholar] [CrossRef]
- Damborsky, P.; Svitel, J.; Katrlik, J. Optical biosensors. Essays Biochem. 2016, 60, 91–100. [Google Scholar]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Popp, J.; Mayerhofer, T. Surface-enhanced raman spectroscopy. Anal. Bioanal. Chem. 2009, 394, 1717–1718. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Paidi, S.K.; Sakowski, E.; Preheim, S.; Barman, I. Ultrasensitive detection of hepatotoxic microcystin production from cyanobacteria using surface-enhanced raman scattering immunosensor. ACS Sens. 2019, 4, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Choi, N.; Wang, R.; Lee, S.; Moon, K.C.; Yoon, S.Y.; Chen, L.; Choo, J. Simultaneous detection of dual prostate specific antigens using surface-enhanced raman scattering-based immunoassay for accurate diagnosis of prostate cancer. ACS Nano 2017, 11, 4926–4933. [Google Scholar] [CrossRef]
- Verdin, A.; Malherbe, C.; Eppe, G. Spatially resolved determination of the abundance of the HER2 marker in microscopic breast tumors using targeted SERS imaging. Microchim. Acta 2021, 188, 228. [Google Scholar] [CrossRef]
- Fu, X.; Chen, L.; Choo, J. Optical nanoprobes for ultrasensitive immunoassay. Anal. Chem. 2017, 89, 124–137. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, Y.; Jiang, N.; Wang, J.; Yu, M.; Zhuang, X. Preparation of aptamer responsive DNA functionalized hydrogels for the sensitive detection of alpha-fetoprotein using SERS method. Bioconjug. Chem. 2020, 31, 813–820. [Google Scholar] [CrossRef]
- Qu, A.; Wu, X.L.; Xu, L.; Liu, L.G.; Ma, W.; Kuang, H.; Xu, C.L. SERS- and luminescence-active Au-Au-UCNP trimers for attomolar detection of two cancer biomarkers. Nanoscale 2017, 9, 3865–3872. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, I.R.; Xiao, Y.; Cho, M.; Csordas, A.T.; Sheehan, J.H.; Meiler, J.; Soh, H.T.; Bornhop, D.J. Measurement of aptamer-protein interactions with back-scattering interferometry. Anal. Chem. 2011, 83, 8867–8870. [Google Scholar] [CrossRef] [PubMed]
- Biehle, S.J.; Carrozzella, J.; Shukla, R.; Popplewell, J.; Swann, M.; Freeman, N.; Clark, J.F. Apolipoprotein Eisoprotein-specific interactions with tissue plasminogen activator. Biochim. Biophys. Acta 2004, 1689, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Swann, M.J.; Peel, L.L.; Carrington, S.; Freeman, N.J. Dual-polarization interferometry: An analytical technique to measure changes in protein structure in real time, to determine the stoichiometry of binding events, and to differentiate between specific and nonspecific interactions. Anal. Biochem. 2004, 329, 190–198. [Google Scholar] [CrossRef]
- Escorihuela, J.; González-Martínez, M.Á.; López-Paz, J.L.; Puchades, R.; Maquieira, Á.; Gimenez-Romero, D. Dual-polarization interferometry: A novel technique to light up the nanomolecular world. Chem. Rev. 2014, 115, 265–294. [Google Scholar] [CrossRef] [PubMed]
- Shao, C.G.; Liu, Y.X.; Qi, J.X.; Su, Y.; Chen, Y.H.; Xu, H.G.; Lin, Z.K.; Guan, H.Q. Real-time detection of the interaction between alpha-fetoprotein and its ssDNA aptamer by dual polarization interferometry. New J. Chem. 2018, 42, 19564–19570. [Google Scholar] [CrossRef]
- Collings, P.J.; Gibbs, E.J.; Starr, T.E.; Vafek, O.; Yee, C.; Pomerance, L.A.; Pasternack, R.F. Resonance light scattering and its application in determining the size, shape, and aggregation number for supramolecular assemblies of chromophores. J. Phys. Chem. B 1999, 103, 8474–8481. [Google Scholar] [CrossRef]
- Ling, J.; Huang, C.Z.; Li, Y.F.; Long, Y.F.; Liao, Q.G. Recent developments of the resonance light scattering technique: Technical evolution, new probes and applications. Appl. Spectrosc. Rev. 2007, 42, 177–201. [Google Scholar] [CrossRef]
- Bao, P.; Frutos, A.G.; Greef, C.; Lahiri, J.; Muller, U.; Peterson, T.C.; Warden, L.; Xie, X. High-sensitivity detection of DNA hybridization on microarrays using resonance light scattering. Anal. Chem. 2002, 74, 1792–1797. [Google Scholar] [CrossRef]
- Zou, Q.C.; Yan, Q.J.; Song, G.W.; Zhang, S.L.; Wu, L.M. Detection of DNA using cationic polyhedral oligomeric silsesquioxane nanoparticles as the probe by resonance light scattering technique. Biosens. Bioelectron. 2007, 22, 1461–1465. [Google Scholar] [CrossRef]
- Zhong, H.; Li, N.; Zhao, F.; Li, K.A. Determination of proteins with Alizarin Red S by Rayleigh light scattering technique. Talanta 2004, 62, 37–42. [Google Scholar] [CrossRef]
- Liu, S.P.; Liu, Z.F.; Luo, H.Q. Resonance Rayleigh scattering method for the determination of trace amounts of cadmium with iodide–rhodamine dye systems. Anal. Chim. Acta 2000, 407, 255–260. [Google Scholar] [CrossRef]
- Feng, P.; Shu, W.Q.; Huang, C.Z.; Li, Y.F. Total internal reflected resonance light scattering determination of chlortetracycline in body fluid with the complex cation of chlortetracycline-europium-trioctyl phosphine oxide at the water/tetrachloromethane interface. Anal. Chem. 2001, 73, 4307–4312. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, F.; Liu, Y.; Cai, C. Simply and sensitively simultaneous detection hepatocellular carcinoma markers AFP and miRNA-122 by a label-free resonance light scattering sensor. Talanta 2018, 186, 473–480. [Google Scholar] [CrossRef]
- Li, W.; Yang, X.; Wang, K.; Tan, W.; Li, H.; Ma, C. FRET-based aptamer probe for rapid angiogenin detection. Talanta 2008, 75, 770–774. [Google Scholar] [CrossRef]
- Jares-Erijman, E.A.; Jovin, T.M. FRET imaging. Nat. Biotechnol. 2003, 21, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Wegner, K.D.; Linden, S.; Jin, Z.; Jennings, T.L.; Khoulati, R.E.; van Bergen en Henegouwen, P.M.; Hildebrandt, N. Nanobodies and nanocrystals: Highly sensitive quantum dot-based homogeneous FRET immunoassay for serum-based EGFR detection. Small 2014, 10, 734–740. [Google Scholar] [CrossRef]
- Lao, Y.H.; Chi, C.W.; Friedrich, S.M.; Peck, K.; Wang, T.H.; Leong, K.W.; Chen, L.C. Signal-on protein detection via dye translocation between aptamer and quantum dot. ACS Appl. Mater. Interfaces 2016, 8, 12048–12055. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, K.; Zhu, J.; Zhang, L.; Lin, H. A FRET-based carbon dots-MnO2 nanosheets architecture for glutathione sensing in human whole blood samples. Chem. Commun. 2015, 51, 12748–12751. [Google Scholar] [CrossRef]
- Arola, H.O.; Tullila, A.; Kiljunen, H.; Campbell, K.; Siitari, H.; Nevanen, T.K. Specific noncompetitive immunoassay for HT-2 mycotoxin detection. Anal. Chem. 2016, 88, 2446–2452. [Google Scholar] [CrossRef]
- Chen, G.; Jin, Y.; Wang, L.; Deng, J.; Zhang, C. Gold nanorods-based FRET assay for ultrasensitive detection of Hg2+. Chem. Commun. 2011, 47, 12500–12502. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Karthigeyan, D.; Kundu, T.K.; Govindaraju, T. FRET-based rational strategy for ratiometric detection of Cu2+ and live cell imaging. Sensor. Actuat. B Chem. 2013, 176, 831–837. [Google Scholar] [CrossRef]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, biology, and medicine of fluorescent nanomaterials and related systems: New insights into biosensing, bioimaging, genomics, diagnostics, and therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, Y.; Xiao, F.; Xia, Z.; Rao, J. Quantum dot/bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angew. Chem. Int. Ed. 2007, 46, 4346–4349. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ren, J. Nanomaterial-based chemiluminescence resonance energy transfer: A strategy to develop new analytical methods. TrAC Trend. Anal. Chem. 2012, 40, 77–89. [Google Scholar] [CrossRef]
- Tennico, Y.H.; Hutanu, D.; Koesdjojo, M.T.; Bartel, C.M.; Remcho, V.T. On-Chip Aptamer-Based Sandwich Assay for Thrombin Detection Employing Magnetic Beads and Quantum Dots. Anal. Chem. 2010, 82, 5591–5597. [Google Scholar] [CrossRef]
- Zhou, L.; Ji, F.; Zhang, T.; Wang, F.; Li, Y.; Yu, Z.; Jin, X.; Ruan, B. An fluorescent aptasensor for sensitive detection of tumor marker based on the FRET of a sandwich structured QDs-AFP-AuNPs. Talanta 2019, 197, 444–450. [Google Scholar] [CrossRef]
- Cheng, R.; Ge, C.; Qi, L.; Zhang, Z.; Ma, J.; Huang, H.; Pan, T.; Dai, Q.; Dai, L. Label-Free Graphene oxide förster resonance energy transfer sensors for selective detection of dopamine in human serums and cells. J. Phys. Chem. C 2017, 122, 13314–13321. [Google Scholar] [CrossRef]
- Raj, M.; Moon, J.M.; Goyal, R.N.; Park, D.S.; Shim, Y.B. Simultaneous detection of ATP metabolites in human plasma and urine based on palladium nanoparticle and poly(bromocresol green) composite sensor. Biosens. Bioelectron. 2019, 126, 758–766. [Google Scholar] [CrossRef]
- Gao, R.; Hao, C.; Xu, L.; Xu, C.; Kuang, H. Spiny nanorod and upconversion nanoparticle satellite assemblies for ultrasensitive detection of messenger RNA in living cells. Anal. Chem. 2018, 90, 5414–5421. [Google Scholar] [CrossRef]
- Tan, H.; Wu, X.; Weng, Y.; Lu, Y.; Huang, Z.Z. Self-assembled FRET nanoprobe with metal-organic framework as a scaffold for ratiometric detection of hypochlorous acid. Anal. Chem. 2020, 92, 3447–3454. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Sun, D.E.; Liu, Z. Ultrasensitive biosensing platform based on the luminescence quenching ability of plasmonic palladium nanoparticles. Chem. Eur. J. 2015, 21, 4944–4948. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, D.; Li, P.; Zhang, Q.; Zhang, W.; Ding, X.; Mao, J.; Wu, J. Palladium nanoparticles-based fluorescence resonance energy transfer aptasensor for highly sensitive detection of Aflatoxin M(1) in milk. Toxins 2017, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, L.; Sun, D.E.; Li, P.; Liu, Z. Fluorescence resonance energy transfer biosensor between upconverting nanoparticles and palladium nanoparticles for ultrasensitive CEA detection. Biosens. Bioelectron. 2016, 86, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zeng, J.; Liu, H.; Ding, P.; Liang, J.; Nie, X.; Zhou, Z. A fluorometric aptamer nanoprobe for alpha-fetoprotein by exploiting the FRET between 5-carboxyfluorescein and palladium nanoparticles. Mikrochim. Acta 2019, 186, 314. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, J.; Zhang, Z.; Zhang, C.; Zhang, X. Development of a gas sensor utilizing chemiluminescence on nanosized titanium dioxide. Anal. Chem. 2002, 74, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Lan, D.; Li, B.; Zhang, Z. Chemiluminescence flow biosensor for glucose based on gold nanoparticle-enhanced activities of glucose oxidase and horseradish peroxidase. Biosens. Bioelectron. 2008, 24, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Li, B.; Xu, C.; Wang, L. Chemiluminescence flow biosensor for hydrogen peroxide using DNAzyme immobilized on eggshell membrane as a thermally stable biocatalyst. Biosens. Bioelectron. 2009, 24, 2534–2540. [Google Scholar] [CrossRef]
- Han, R.; Sun, Y.; Lin, Y.; Liu, H.; Dai, Y.; Zhu, X.; Gao, D.; Wang, X.; Luo, C. A simple chemiluminescent aptasensor for the detection of α-fetoprotein based on iron-based metal organic frameworks. New J. Chem. 2020, 44, 4099–4107. [Google Scholar] [CrossRef]
- Wang, J.D.; Hou, Y.N.; Sun, Y.L.; Fang, F.; Luo, C.N.; Wang, X.Y. A chemiluminescence aptasensor for sensitive detection of alpha-fetoprotein based on hemin@ZIF-67. Anal. Bioanal. Chem. 2022, 414, 4757–4765. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. Analytical Electrochemistry, 3rd ed.; John and Wiley and Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Ronkainen-Matsuno, N.J.; Thomas, J.H.; Halsall, H.B.; Heineman, W.R. Electrochemical immunoassay moving into the fast lane. Trend. Anal. Chem. 2002, 21, 213–225. [Google Scholar] [CrossRef]
- Brajter-Toth, A.; Chambers, J.Q. Electroanalytical Methods of Biological Materials; CRC Press: Boca Raton, FL, USA, 2002; pp. 1–538. [Google Scholar]
- Lang, X.Y.; Fu, H.Y.; Hou, C.; Han, G.F.; Yang, P.; Liu, Y.B.; Jiang, Q. Nanoporous gold supported cobalt oxide microelectrodes as high-performance electrochemical biosensors. Nat. Commun. 2013, 4, 2169. [Google Scholar] [CrossRef] [PubMed]
- Taşdemir, İ.H. Electrochemistry and determination of cefdinir by voltammetric and computational approaches. J. Food Drug Anal. 2014, 22, 527–536. [Google Scholar] [CrossRef]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical immunosensors for detection of cancer protein biomarkers. ACS Nano 2012, 6, 4546–4561. [Google Scholar] [CrossRef] [PubMed]
- Pourali, A.; Rashidi, M.R.; Barar, J.; Pavon-Djavid, G.; Omidi, Y. Voltammetric biosensors for analytical detection of cardiac troponin biomarkers in acute myocardial infarction. TrAC Trend. Anal. Chem. 2021, 134, 116123. [Google Scholar] [CrossRef]
- Durai, L.; Badhulika, S. Highly selective trace level detection of DNA damage biomarker using iron-based MAX compound modified screen-printed carbon electrode using differential pulse voltammetry. Sens. Actuators Rep. 2021, 3, 100057. [Google Scholar] [CrossRef]
- Jiang, L.; Li, F.; Feng, J.; Wang, P.; Liu, Q.; Li, Y.; Dong, Y.; Wei, Q. An optionality further amplification of an sandwich-type electrochemical immunosensor based on biotin–streptavidin–biotin strategy for detection of alpha fetoprotein. RSC Adv. 2016, 6, 24373–24380. [Google Scholar] [CrossRef]
- Suchacz, B.; Wesolowski, M. Voltammetric quantitation of acetaminophen in tablets using solid graphite electrodes. Anal. Methods 2016, 8, 3307–3315. [Google Scholar] [CrossRef]
- Fadr, M.; Amro, A.N.; Aoun, S.B. Voltammetric determination of vildagliptin in a pharmaceutical formulation. Trop. J. Pharm. Res. 2018, 17, 1847–1852. [Google Scholar] [CrossRef]
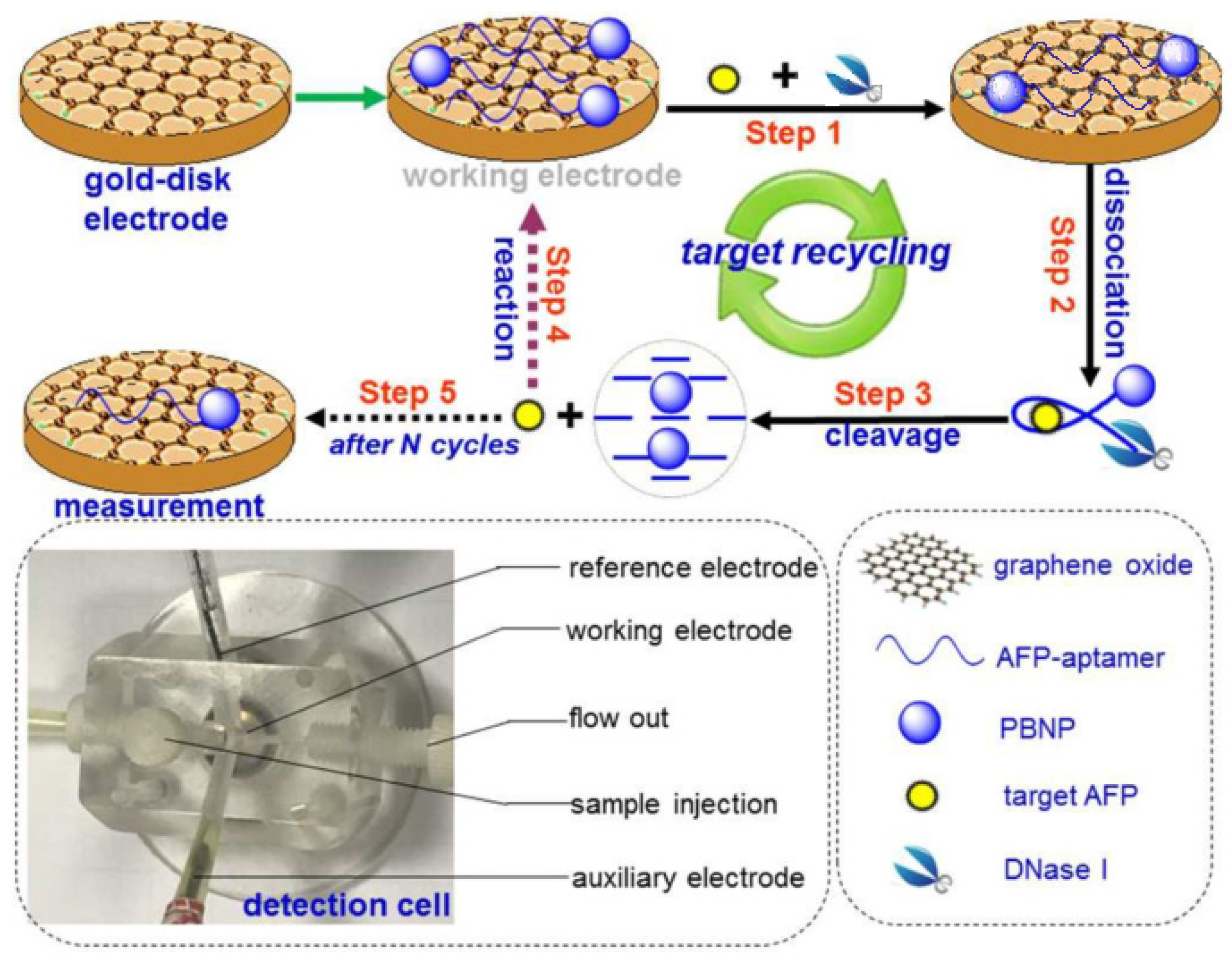
- Zhang, B.; Ding, H.; Chen, Q.; Wang, T.; Zhang, K. Prussian blue nanoparticle-labeled aptasensing platform on graphene oxide for voltammetric detection of α-fetoprotein in hepatocellular carcinoma with target recycling. Analyst 2019, 144, 4858–4864. [Google Scholar] [CrossRef] [PubMed]
- Koehne, J.E.; Chen, H.; Cassell, A.M.; Ye, Q.; Han, J.; Meyyappan, M.; Li, J. Miniaturized multiplex label-free electronic chip for rapid nucleic acid analysis based on carbon nanotube nanoelectrode arrays. Clin. Chem. 2004, 50, 1886–1893. [Google Scholar] [CrossRef] [PubMed]
- Endo, T.; Kerman, K.; Nagatani, N.; Takamura, Y.; Tamiya, E. Label-free detection of peptide nucleic acid-DNA hybridization using localized surface plasmon resonance based optical biosensor. Anal. Chem. 2005, 77, 6976–6984. [Google Scholar] [CrossRef]
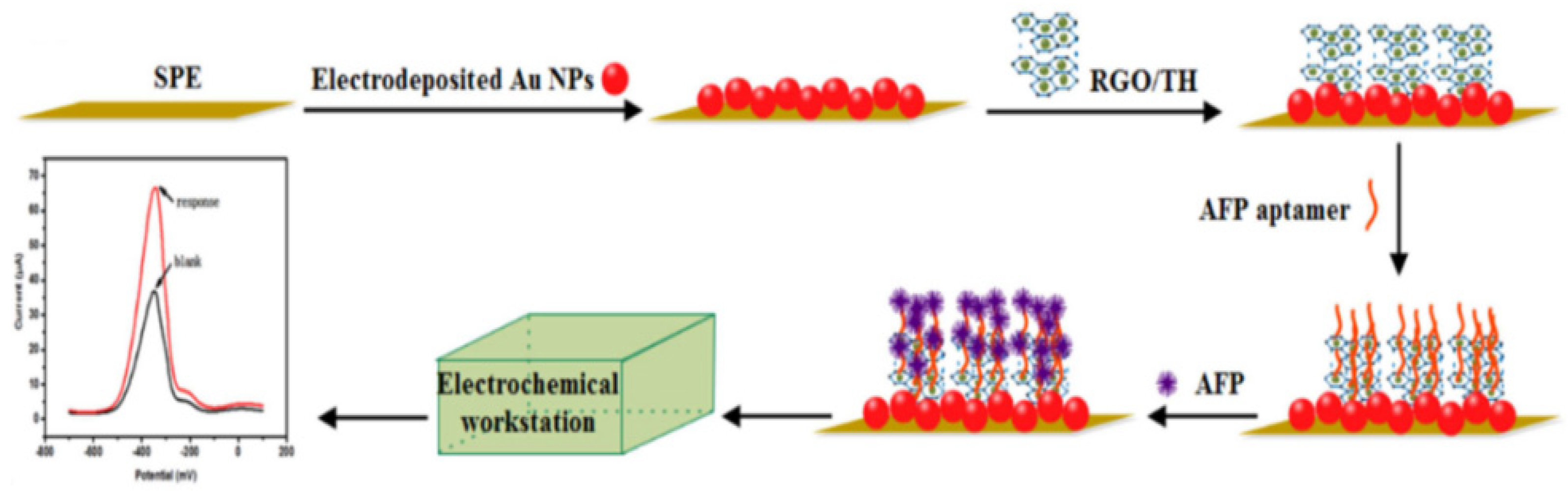
- Li, G.; Li, S.; Wang, Z.; Xue, Y.; Dong, C.; Zeng, J.; Huang, Y.; Liang, J.; Zhou, Z. Label-free electrochemical aptasensor for detection of alpha-fetoprotein based on AFP-aptamer and thionin/reduced graphene oxide/gold nanoparticles. Anal. Biochem. 2018, 547, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pui, T.S.; Kongsuphol, P.; Arya, S.K.; Bansal, T. Detection of tumor necrosis factor (TNF-α) in cell culture medium with label free electrochemical impedance spectroscopy. Sens. Actuat. B Chem. 2013, 181, 494–500. [Google Scholar] [CrossRef]
- Lisdat, F.; Schafer, D. The use of electrochemical impedance spectroscopy for biosensing. Anal. Bioanal. Chem. 2008, 391, 1555–1567. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.; Cheng, Z.; Wang, E.; Yang, X. Amplification of antigen–antibody interactions based on biotin labeled protein–streptavidin network complex using impedance spectroscopy. Biosens. Bioelectron. 2001, 16, 355–361. [Google Scholar] [CrossRef]
- Taheri, N.; Khoshsafar, H.; Ghanei, M.; Ghazvini, A.; Bagheri, H. Dual-template rectangular nanotube molecularly imprinted polypyrrole for label-free impedimetric sensing of AFP and CEA as lung cancer biomarkers. Talanta 2022, 239, 123146. [Google Scholar] [CrossRef]
- Qi, H.; Wang, C.; Cheng, N. Label-free electrochemical impedance spectroscopy biosensor for the determination of human immunoglobulin G. Microchim. Acta 2010, 170, 33–38. [Google Scholar] [CrossRef]
- Li, B.; Wang, Y.; Wei, H.; Dong, S. Amplified electrochemical aptasensor taking AuNPs based sandwich sensing platform as a model. Biosens. Bioelectron. 2008, 23, 965–970. [Google Scholar] [CrossRef]
- Li, X.; Shen, L.; Zhang, D.; Qi, H.; Gao, Q.; Ma, F.; Zhang, C. Electrochemical impedance spectroscopy for study of aptamer–thrombin interfacial interactions. Biosens. Bioelectron. 2008, 23, 1624–1630. [Google Scholar] [CrossRef] [PubMed]
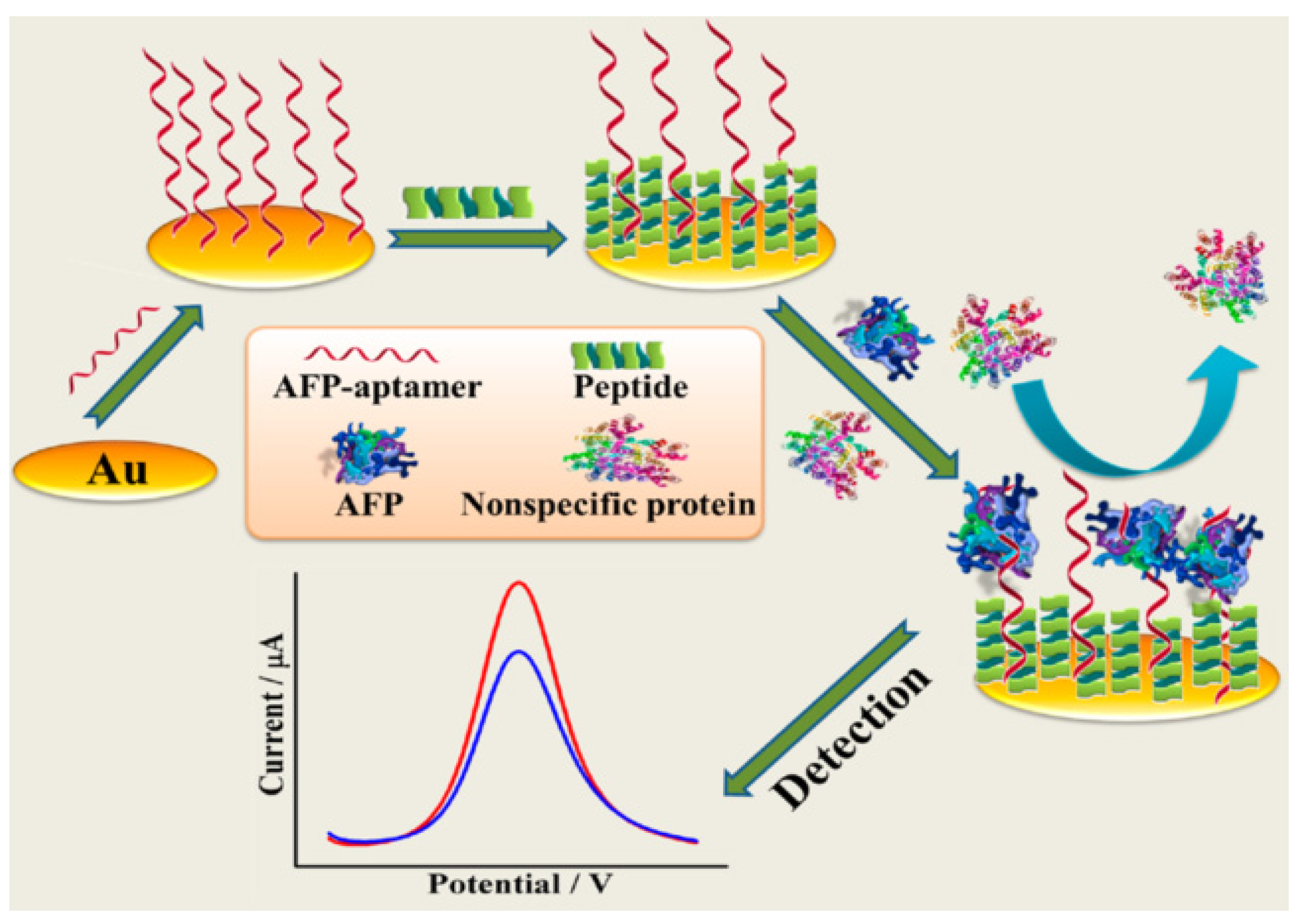
- Yang, H.; Li, Z.; Wei, X.; Huang, R.; Qi, H.; Gao, Q.; Li, C.; Zhang, C. Detection and discrimination of alpha-fetoprotein with a label-free electrochemical impedance spectroscopy biosensor array based on lectin functionalized carbon nanotubes. Talanta 2013, 111, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zhang, F.; Wang, Z.; Liang, Q. A graphene oxide-based label-free electrochemical aptasensor for the detection of alpha-fetoprotein. Biosens. Bioelectron. 2018, 112, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Wang, Y.; Jiao, M.; Jayachandran, S.; Wu, Y.; Fan, X.; Luo, X. Mixed self-assembled aptamer and newly designed zwitterionic peptide as antifouling biosensing interface for electrochemical detection of alpha-fetoprotein. ACS Sens. 2017, 2, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhang, Q.; Li, X.; Pan, H.; Wang, J.; Zhao, Z. Detection of AFP with an ultra-sensitive giant magnetoimpedance biosensor. Sensor. Actuat. B Chem. 2019, 293, 53–58. [Google Scholar] [CrossRef]
- Sutomo, A.D.; Nuryani, N.; Purnama, B. A short review of magneto-impedance effect for biosensor. J. Phys. Conf. Ser. 2019, 1153, 012045. [Google Scholar] [CrossRef]
- Chen, L.; Bao, C.C.; Yang, H.; Li, D.; Lei, C.; Wang, T.; Hu, H.Y.; He, M.; Zhou, Y.; Cui, D.X. A prototype of giant magnetoimpedance-based biosensing system for targeted detection of gastric cancer cells. Biosens. Bioelectron. 2011, 26, 3246–3253. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Chen, L.; Lei, C.; Zhang, J.; Li, D.; Zhou, Z.-M.; Bao, C.-C.; Hu, H.-Y.; Chen, X.; Cui, F.; et al. Giant magnetoimpedance-based microchannel system for quick and parallel genotyping of human papilloma virus type 16/18. Appl. Phys. Lett. 2010, 97, 043702. [Google Scholar] [CrossRef]
- Lorenzo-Gomez, R.; Gonzalez-Robles, D.; Miranda-Castro, R.; de-Los-Santos-Alvarez, N.; Lobo-Castanon, M.J. On the electrochemical detection of alpha-fetoprotein using aptamers: DNA isothermal amplification strategies to improve the performance of weak aptamers. Biosensors 2020, 10, 46. [Google Scholar] [CrossRef]

| Method | Analyst | Linear Range | LOD | Reference | |
|---|---|---|---|---|---|
| SERS | Aptamer/nanoparticles | Aptamer/Au–Au–UCNP | 1–100 aM | 0.059 aM | [31] |
| FRET | Aptamer/QDs–AuNPs | 0.5~45 ng/mL | 400 pg/mL | [57] | |
| FRET | Aptamer/PdNP | 5~150 ng/mL | 1.38 ng/mL | [65] | |
| CV | Aptamer/PBNPs | 0.01~300 ng/mL | 6.3 pg/mL | [83] | |
| CV | Aptamer/TH/RGO/AuNPs | 0.1~100.0 μg/mL | 0.050 μg/mL | [86] | |
| CL | Aptamer/other nanomaterials | Aptamer/Fe-MOF | 100 fg/mL~30 ng/mL | 77 fg/mL | [69] |
| CL | Aptamer/hemin@ZIF-67 composites | 4 × 10−10 to 200 × 10−10 mg/mL | 1.3 × 10−10 mg/mL | [70] | |
| RLS | Aptamer/methyl violet | - | 0.94 μg/mL | [44] | |
| SERS | Aptamer/hydrogel | 50~100 ng/mL | 50 pg/mL | [30] | |
| EIS | Aptamer/graphene oxide | 0.01~100 ng/mL | 3 pg/mL | [95] | |
| EIS | Aptamer/peptide/Au | 10 fg/mL~100 pg/mL | 3.1 fg/mL | [96] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wang, H.; Xie, B.; Zhang, B.; Lin, Y.; Gao, L. Detection of Alpha-Fetoprotein Using Aptamer-Based Sensors. Biosensors 2022, 12, 780. https://doi.org/10.3390/bios12100780
Liu L, Wang H, Xie B, Zhang B, Lin Y, Gao L. Detection of Alpha-Fetoprotein Using Aptamer-Based Sensors. Biosensors. 2022; 12(10):780. https://doi.org/10.3390/bios12100780
Chicago/Turabian StyleLiu, Lei, Huixing Wang, Bing Xie, Bianjiang Zhang, Yuanwei Lin, and Li Gao. 2022. "Detection of Alpha-Fetoprotein Using Aptamer-Based Sensors" Biosensors 12, no. 10: 780. https://doi.org/10.3390/bios12100780
APA StyleLiu, L., Wang, H., Xie, B., Zhang, B., Lin, Y., & Gao, L. (2022). Detection of Alpha-Fetoprotein Using Aptamer-Based Sensors. Biosensors, 12(10), 780. https://doi.org/10.3390/bios12100780





