Biosensors and Drug Delivery in Oncotheranostics Using Inorganic Synthetic and Biogenic Magnetic Nanoparticles
Abstract
:1. Introduction
2. Synthetic Magnetic Nanoparticles
2.1. Characterization of Synthetic Magnetic Nanoparticles
2.2. Applications of Biosensors for Cancer Diagnostics
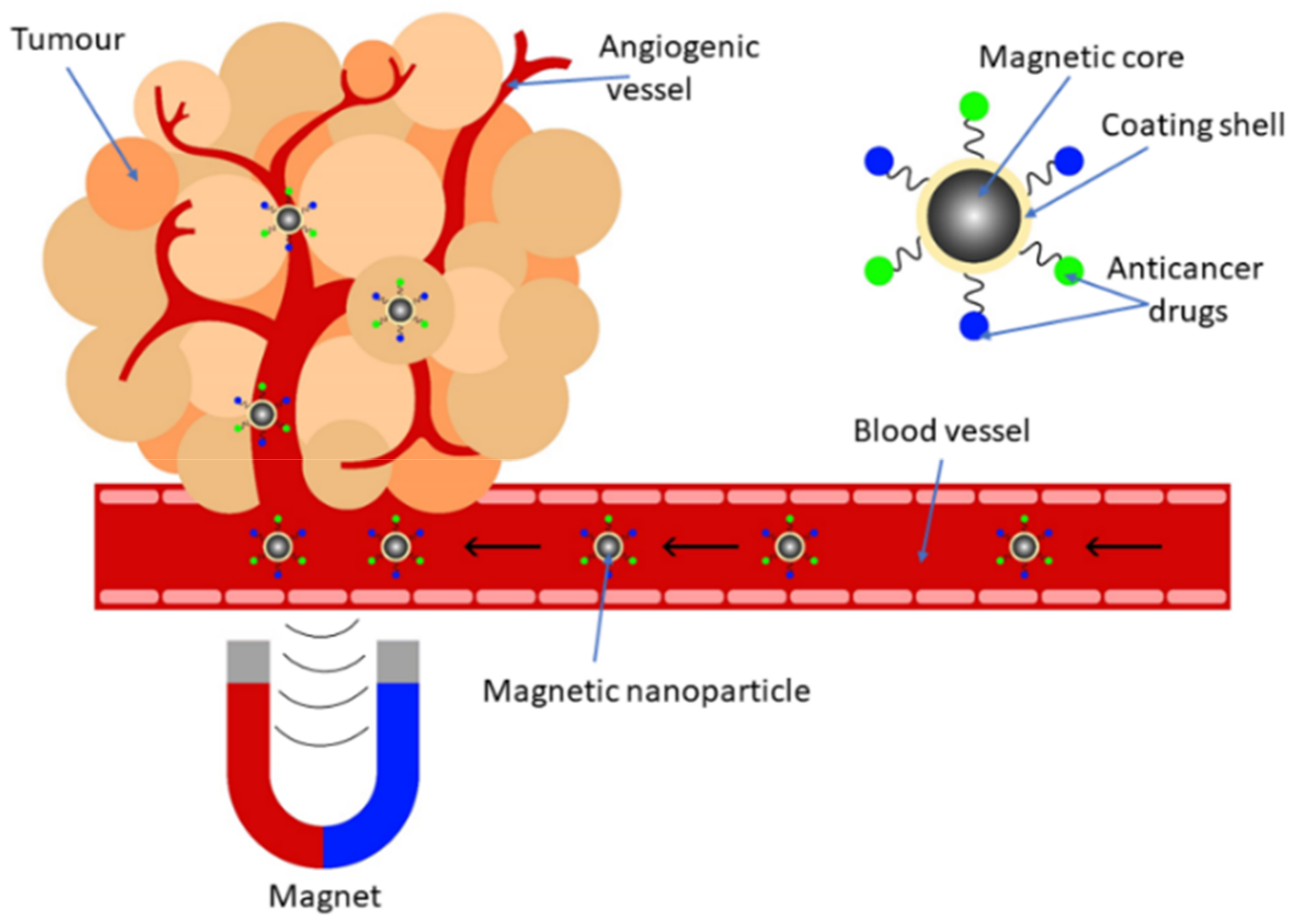
2.3. Synthetic Magnetic Nanoparticles for Drug Delivery
3. Biogenic Nanoparticles
3.1. Biogenic Synthesis and Diversity of Magnetic Nanoparticles
3.2. Applications of Magnetosomes in Cancer Theranostics
3.2.1. Biosensors on the Basis of Magnetosomes
3.2.2. Drug Delivery in Cancer Theranostics Using Magnetosomes
4. Comparative Analysis of the Relevance of Synthesized and Biogenic Particles in Biosensors and Drug Delivery for Cancer Theranostics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schiffman, J.D.; Fisher, P.G.; Gibbs, P. Early Detection of Cancer: Past, Present, and Future. Am. Soc. Clin. Oncol. Educ. Book 2015, 35, 57–65. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Solaimuthu, A.; Vijayan, A.N.; Murali, P.; Korrapati, P.S. Nano-biosensors and their relevance in tissue engineering. Curr. Opin. Biomed. Eng. 2020, 13, 84–93. [Google Scholar] [CrossRef]
- Singh, A.; Sahoo, S.K. Magnetic nanoparticles: A novel platform for cancer theranostics. Drug Discov. Today 2013, 19, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Veiseh, O.; Gunn, J.W.; Zhang, M. Design and fabrication of magnetic nanoparticles for targeted drug delivery and imaging. Adv. Drug Deliv. Rev. 2010, 62, 284–304. [Google Scholar] [CrossRef]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic nanoparticles in MR imaging and drug delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef]
- Shubayev, V.I.; Pisanic, T.R., II; Jin, S. Magnetic nanoparticles for theragnostics. Adv. Drug Deliv. Rev. 2009, 61, 467–477. [Google Scholar] [CrossRef]
- Al-Karagoly, H.; Rhyaf, A.; Naji, H.; Albukhaty, S.; AlMalki, F.A.; Alyamani, A.A.; Albaqami, J.; Aloufi, S. Green synthesis, characterization, cytotoxicity, and antimicrobial activity of iron oxide nanoparticles using Nigella sativa seed extract. Green Process. Synth. 2022, 11, 254–265. [Google Scholar] [CrossRef]
- Kuchma, E.; Kubrin, S.; Soldatov, A. The Local Atomic Structure of Colloidal Superparamagnetic Iron Oxide Nanoparticles for Theranostics in Oncology. Biomedicines 2018, 6, 78. [Google Scholar] [CrossRef]
- Mughal, B.; Zaidi, S.Z.J.; Zhang, X.; Hassan, S.U. Biogenic Nanoparticles: Synthesis, Characterisation and Applications. Appl. Sci. 2021, 11, 2598. [Google Scholar] [CrossRef]
- Šafařík, I.; Šafaříková, M. Magnetic Nanoparticles and Biosciences. In Nanostructured Materials; Hofmann, H., Rahman, Z., Schubert, U., Eds.; Springer: Vienna, Austria, 2002. [Google Scholar] [CrossRef]
- Blakemore, R. Magnetotactic Bacteria. Science 1975, 190, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Markande, A.R.; Mistry, K.; Undaviya, S.; Jha, A. Magnetic Nanoparticles from Bacteria. In Biobased Nanotechnology for Green Applications. Nanotechnology in the Life Sciences; Sarma, H., Joshi, S.J., Prasad, R., Jampilek, J., Eds.; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Tay, A.; McCausland, H.; Komeili, A.; Di Carlo, D. Nano and Microtechnologies for the Study of Magnetotactic Bacteria. Adv. Funct. Mater. 2019, 29, 1904178. [Google Scholar] [CrossRef]
- Rajalakshmi, A.; Anjukam, E.; Ramesh, M.; Kavitha, K.; Puvanakrishnan, R.; Ramesh, B. A novel colorimetric technique for estimating iron in magnetosomes of magnetotactic bacteria based on linear regression. Arch. Microbiol. 2022, 204, 282. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.; Pai, S.; Panda, M.; Aadrika; Anjali, K.; Ram, H.N.A.; Pai, A.; Venkatesh, K.B. Isolation of microbes possessing magnetosomes and their potential role in drug delivery. Res. J. Pharm. Technol. 2020, 13, 5042. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Zhao, J.; Yao, H.; Chu, S.; Song, Z.; He, Z.; Zhang, W. Magnetotactic bacteria: Characteristics and environmental applications. Front. Environ. Sci. Eng. 2020, 14, 56. [Google Scholar] [CrossRef]
- Ying, G.; Zhang, G.; Yang, J.; Hao, Z.; Xing, W.; Lu, D.; Zhang, S.; Yan, L. Biomineralization and biotechnological applications of bacterial magnetosomes. Colloids Surfaces. B Biointerfaces 2022, 216, 112556. [Google Scholar] [CrossRef]
- Yan, L.; Xing, W. Methods to Study Magnetotactic Bacteria and Magnetosomes. Methods Microbiol. 2018, 45, 357–386. [Google Scholar] [CrossRef]
- Lin, W.; Pan, Y.; Bazylinski, D.A. Diversity and ecology of and biomineralization by magnetotactic bacteria. Environ. Microbiol. Rep. 2017, 9, 345–356. [Google Scholar] [CrossRef]
- Lins, U.; Farina, M. Magnetosome chain arrangement and stability in magnetotactic cocci. Antonie van Leeuwenhoek. Int. J. Gen. Mol. Microbiol. 2004, 85, 335–341. [Google Scholar] [CrossRef]
- Alphandery, E.; Guyot, F.; Chebbi, I. Preparation of chains of magnetosomes, isolated from Magnetospirillum magneticum strain AMB-1 magnetotactic bacteria, yielding efficient treatment of tumors using magnetic hyperthermia. Int. J. Pharm. 2012, 434, 444–452. [Google Scholar] [CrossRef]
- Benoit, M.R.; Mayer, D.; Barak, Y.; Chen, I.Y.; Hu, W.; Cheng, Z.; Wang, S.X.; Spielman, D.M.; Gambhir, S.S.; Matin, A. Visualizing Implanted Tumors in Mice with Magnetic Resonance Imaging Using Magnetotactic Bacteria. Clin. Cancer Res. 2009, 15, 5170–5177. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.-J.; Lee, H.; Shao, H.; Hilderbrand, S.A.; Weissleder, R. Multicore Assemblies Potentiate Magnetic Properties of Biomagnetic Nanoparticles. Adv. Mater. 2011, 23, 4793–4797. [Google Scholar] [CrossRef] [PubMed]
- Nuñez-Magos, L.; Lira-Escobedo, J.; Rodríguez-López, R.; Muñoz-Navia, M.; Castillo-Rivera, F.; Viveros-Méndez, P.X.; Araujo, E.; Encinas, A.; Saucedo-Anaya, S.A.; Aranda-Espinoza, S. Effects of DC Magnetic Fields on Magnetoliposomes. Front. Mol. Biosci. 2021, 8, 703417. [Google Scholar] [CrossRef] [PubMed]
- Dadfar, S.M.; Roemhild, K.; Drude, N.I.; von Stillfried, S.; Knüchel, R.; Kiessling, F.; Lammers, T. Iron oxide nanoparticles: Diagnostic, therapeutic and theranostic applications. Adv. Drug Deliv. Rev. 2019, 138, 302–325. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Su, D.; Liu, J.; Saha, R.; Wang, J.-P. Magnetic nanoparticles in nanomedicine: A review of recent advances. Nanotechnology 2019, 30, 502003. [Google Scholar] [CrossRef] [PubMed]
- Ryvolova, M.; Chomoucka, J.; Drbohlavova, J.; Kopel, P.; Babula, P.; Hynek, D.; Adam, V.; Eckschlager, T.; Hubalek, J.; Stiborova, M.; et al. Modern Micro and Nanoparticle-Based Imaging Techniques. Sensors 2012, 12, 14792–14820. [Google Scholar] [CrossRef] [PubMed]
- Kianfar, E. Magnetic Nanoparticles in Targeted Drug Delivery: A Review. J. Supercond. Nov. Magn. 2021, 34, 1709–1735. [Google Scholar] [CrossRef]
- Khot, V.; Pawar, S. Magnetic Hyperthermia with Magnetic Nanoparticles: A Status Review. Curr. Top. Med. Chem. 2014, 14, 572–594. [Google Scholar] [CrossRef]
- Elingarami, S.; Zeng, X. A Short Review on Current Use of Magnetic Nanoparticles for Bio-Separation, Sequencing, Diagnosis and Drug Delivery. Adv. Sci. Lett. 2011, 4, 3295–3300. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Xu, C. Using magnetic nanoparticles to manipulate biological objects. Chin. Phys. B 2013, 22, 097503. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.; Grüttner, C.; Bodnar, W.; Burkel, E. Magnetic Nanoparticles for Biomedical Applications. In Encyclopedia of Nanotechnology; Bhushan, B., Ed.; Springer: Dordrecht, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Li, K.; Xu, J.; Li, P.; Fan, Y. A review of magnetic ordered materials in biomedical field: Constructions, applications and prospects. Compos. Part B Eng. 2022, 228, 109401. [Google Scholar] [CrossRef]
- Khizar, S.; Ahmad, N.M.; Zine, N.; Jaffrezic-Renault, N.; Errachid-El-Salhi, A.; Elaissari, A. Magnetic Nanoparticles: From Synthesis to Theranostic Applications. ACS Appl. Nano Mater. 2021, 4, 4284–4306. [Google Scholar] [CrossRef]
- Kargol, A.; Malkinski, L.; Caruntu, G. Biomedical Applications of Multiferroic Nanoparticles. In Advanced Magnetic Materials; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Kopyl, S.; Surmenev, R.; Surmeneva, M.; Fetisov, Y.; Kholkin, A. Magnetoelectric effect: Principles and applications in biology and medicine—A review. Mater. Today Bio 2021, 12, 100149. [Google Scholar] [CrossRef] [PubMed]
- Bedoya-Hincapié, C.M.; Restrepo-Parra, E.; López-Carreño, L.D. Applications of magnetic and multiferroic core/shell nanostructures and their physical properties. DYNA 2018, 85, 29–35. [Google Scholar] [CrossRef]
- Rao, B.N.; Kaviraj, P.; Vaibavi, S.R.; Kumar, A.; Bajpai, S.K.; Arockiarajan, A. Investigation of magnetoelectric properties and biocompatibility of CoFe2O4-BaTiO3 core-shell nanoparticles for biomedical applications. J. Appl. Phys. 2017, 122, 164102. [Google Scholar] [CrossRef]
- Praveena, M.G.; Thoufeeq, S.; Manikanta, B.; Rahul, M.T.; Bhowmik, R.N.; Nair, S.S.; Kalarikkal, N.; Mohammed, E.M.; Kala, M.S.; Anantharaman, M.R. A magnetoelectric nanocomposite based on two dimensional Cr2O3 and CoFe2O4. Solid State Commun. 2022, 354, 114865. [Google Scholar] [CrossRef]
- Adamiano, A.; Iafisco, M.; Tampieri, A. Magnetic core-shell nanoparticles: Remote driving, hyperthermia, and controlled drug release. In Core-Shell Nanostructures for Drug Delivery and Theranostics; Woodhead Publishing: Sawston, UK, 2018; pp. 259–296. [Google Scholar] [CrossRef]
- Jenjob, R.; Phakkeeree, T.; Crespy, D. Core–shell particles for drug-delivery, bioimaging, sensing, and tissue engineering. Biomater. Sci. 2020, 8, 2756–2770. [Google Scholar] [CrossRef]
- Mosayebi, J.; Kiyasatfar, M.; Laurent, S. Synthesis, Functionalization, and Design of Magnetic Nanoparticles for Theranostic Applications. Adv. Heal. Mater. 2017, 6, 23–29. [Google Scholar] [CrossRef]
- Chen, Y.; Xianyu, Y.; Dong, M.; Zhang, J.; Zheng, W.; Qian, Z.; Jiang, X. Cascade Reaction-Mediated Assembly of Magnetic/Silver Nanoparticles for Amplified Magnetic Biosensing. Anal. Chem. 2018, 90, 6906–6912. [Google Scholar] [CrossRef]
- Chen, D.; Wu, Y. Rapid and Ultrasensitive Electrochemical Detection of TP53 Gene Mutation in Blood: Hybridization with a DNA/Gold-Coated Magnetic Nanoparticle Network. Anal. Sens. 2022, 2, e202200032. [Google Scholar] [CrossRef]
- Yu, H.; Yu, J.; Li, L.; Zhang, Y.; Xin, S.; Ni, X.; Sun, Y.; Song, K. Recent Progress of the Practical Applications of the Platinum Nanoparticle-Based Electrochemistry Biosensors. Front. Chem. 2021, 9, 677876. [Google Scholar] [CrossRef] [PubMed]
- Kwizera, E.A.; Chaffin, E.; Wang, Y.; Huang, X. Synthesis and properties of magnetic-optical core–shell nanoparticles. RSC Adv. 2017, 7, 17137–17153. [Google Scholar] [CrossRef]
- Anik, M.I.; Hossain, M.K.; Hossain, I.; Mahfuz, A.M.U.B.; Rahman, M.T.; Ahmed, I. Recent progress of magnetic nanoparticles in biomedical applications: A review. Nano Sel. 2021, 2, 1146–1186. [Google Scholar] [CrossRef]
- Materón, E.M.; Miyazaki, C.M.; Carr, O.; Joshi, N.; Picciani, P.H.; Dalmaschio, C.J.; Davis, F.; Shimizu, F.M. Magnetic nanoparticles in biomedical applications: A review. Appl. Surf. Sci. Adv. 2021, 6, 100163. [Google Scholar] [CrossRef]
- Chen, Z.; Song, S.; Ma, J.; Da Ling, S.; Wang, Y.D.; Kong, T.T.; Xu, J.H. Fabrication of magnetic core/shell hydrogels via microfluidics for controlled drug delivery. Chem. Eng. Sci. 2022, 248, 117216. [Google Scholar] [CrossRef]
- Zou, L.; Huang, B.; Zheng, X.; Pan, H.; Zhang, Q.; Xie, W.; Zhao, Z.; Li, X. Microfluidic synthesis of magnetic nanoparticles in droplet-based microreactors. Mater. Chem. Phys. 2022, 276, 125384. [Google Scholar] [CrossRef]
- Abedini-Nassab, R.; Pouryosef Miandoab, M.P.; Şaşmaz, M. Microfluidic Synthesis, Control, and Sensing of Magnetic Nanoparticles: A Review. Micromachines 2021, 12, 768. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, Z.; Hasanzadeh, M. Nanotechnology-assisted microfluidic systems for chemical sensing, biosensing, and bioanalysis. TrAC Trends Anal. Chem. 2022, 152, 116637. [Google Scholar] [CrossRef]
- Haun, J.B.; Yoon, T.-J.; Lee, H.; Weissleder, R. Molecular Detection of Biomarkers and Cells Using Magnetic Nanoparticles and Diagnostic Magnetic Resonance. Methods Mol. Biol. 2011, 726, 33–49. [Google Scholar] [CrossRef]
- Spitzberg, J.D.; Zrehen, A.; Van Kooten, X.F.; Meller, A. Plasmonic-Nanopore Biosensors for Superior Single-Molecule Detection. Adv. Mater. 2019, 31, e1900422. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, A.; Gurbuz, Y.; Niazi, J.H. Biosensors for cardiac biomarkers detection: A review. Sens. Actuators B Chem. 2012, 171–172, 62–76. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef] [PubMed]
- Holzinger, M.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2, 63. [Google Scholar] [CrossRef]
- Chen, D.; Wu, Y.; Tilley, R.D.; Gooding, J.J. Rapid and ultrasensitive electrochemical detection of DNA methylation for ovarian cancer diagnosis. Biosens. Bioelectron. 2022, 206, 114126. [Google Scholar] [CrossRef]
- Chen, D.; Wu, Y.; Hoque, S.; Tilley, R.D.; Gooding, J.J. Rapid and ultrasensitive electrochemical detection of circulating tumor DNA by hybridization on the network of gold-coated magnetic nanoparticles. Chem. Sci. 2021, 12, 5196–5201. [Google Scholar] [CrossRef]
- Shamsazar, A.; Asadi, A.; Seifzadeh, D.; Mahdavi, M. A novel and highly sensitive sandwich-type immunosensor for prostate-specific antigen detection based on MWCNTs-Fe3O4 nanocomposite. Sens. Actuators B Chem. 2021, 346, 130459. [Google Scholar] [CrossRef]
- Khoshfetrat, S.M.; Mehrgardi, M.A. Amplified detection of leukemia cancer cells using an aptamer-conjugated gold-coated magnetic nanoparticles on a nitrogen-doped graphene modified electrode. Bioelectrochemistry 2017, 114, 24–32. [Google Scholar] [CrossRef]
- Sharafeldin, M.; Bishop, G.W.; Bhakta, S.; El-Sawy, A.; Suib, S.L.; Rusling, J.F. Fe3O4 nanoparticles on graphene oxide sheets for isolation and ultrasensitive amperometric detection of cancer biomarker proteins. Biosens. Bioelectron. 2016, 91, 359–366. [Google Scholar] [CrossRef]
- Yang, Q.; Li, N.; Li, Q.; Chen, S.; Wang, H.-L.; Yang, H. Amperometric sarcosine biosensor based on hollow magnetic Pt–Fe3O4@C nanospheres. Anal. Chim. Acta 2019, 1078, 161–167. [Google Scholar] [CrossRef]
- Jahanbani, S.; Benvidi, A. A novel electrochemical DNA biosensor based on a modified magnetic bar carbon paste electrode with Fe3O4NPs-reduced graphene oxide/PANHS nanocomposite. Mater. Sci. Eng. C 2016, 68, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Nuñez, L.F.E.; Gutierrez-Iglesias, G.; Martínez-Cuazitl, A.; Mata-Miranda, M.M.; Alvarez-Jiménez, V.D.; Sánchez-Monroy, V.; Golberg, A.; González-Díaz, C.A. A biosensor capable of identifying low quantities of breast cancer cells by electrical impedance spectroscopy. Sci. Rep. 2019, 9, 6419. [Google Scholar] [CrossRef] [PubMed]
- Bonaiuto, E.; Magro, M.; Baratella, D.; Jakubec, P.; Sconcerle, E.; Terzo, M.; Miotto, G.; Macone, A.; Agostinelli, E.; Fasolato, S.; et al. Ternary Hybrid γ-Fe2O3/CrVI/Amine Oxidase Nanostructure for Electrochemical Sensing: Application for Polyamine Detection in Tumor Tissue. Chem.–A Eur. J. 2016, 22, 6846–6852. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Gan, X. Antibody-functionalized magnetic nanoparticles for electrochemical immunoassay of α-1-fetoprotein in human serum. Mikrochim. Acta 2009, 164, 231–237. [Google Scholar] [CrossRef]
- Zou, K.; Gao, Z.; Deng, Q.; Luo, Y.; Zou, L.; Lu, Y.; Zhao, W.; Lin, B. Picomolar detection of carcinoembryonic antigen in whole blood using microfluidics and surface-enhanced Raman spectroscopy. Electrophoresis 2016, 37, 786–789. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Wang, C.; Wang, J.; Sun, Z.; Xiao, R.; Wang, S. Fe3O4@Ag magnetic nanoparticles for microRNA capture and duplex-specific nuclease signal amplification based SERS detection in cancer cells. Biosens. Bioelectron. 2016, 79, 574–580. [Google Scholar] [CrossRef]
- Turan, E.; Zengin, A.; Suludere, Z.; Kalkan, N.; Tamer, U. Construction of a sensitive and selective plasmonic biosensor for prostate specific antigen by combining magnetic molecularly-imprinted polymer and surface-enhanced Raman spectroscopy. Talanta 2022, 237, 122926. [Google Scholar] [CrossRef]
- Shipunova, V.O.; Nikitin, M.P.; Belova, M.M.; Deyev, S.M. Label-free methods of multiparametric surface plasmon resonance and MPQ-cytometry for quantitative real-time measurements of targeted magnetic nanoparticles complexation with living cancer cells. Mater. Today Commun. 2021, 29, 102978. [Google Scholar] [CrossRef]
- Mohammadzadeh-Asl, S.; Aghanejad, A.; de la Guardia, M.; Dolatabadi, J.E.N.; Keshtkar, A. Surface plasmon resonance signal enhancement based on erlotinib loaded magnetic nanoparticles for evaluation of its interaction with human lung cancer cells. Opt. Laser Technol. 2021, 133, 106521. [Google Scholar] [CrossRef]
- Hu, Y.; Li, L.; Guo, L. The sandwich-type aptasensor based on gold nanoparticles/DNA/magnetic beads for detection of cancer biomarker protein AGR2. Sens. Actuators B Chem. 2015, 209, 846–852. [Google Scholar] [CrossRef]
- Xu, Q.; Liang, K.; Liu, R.-Y.; Deng, L.; Zhang, M.; Shen, L.; Liu, Y.-N. Highly sensitive fluorescent detection of p53 protein based on DNA functionalized Fe3O4 nanoparticles. Talanta 2018, 187, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, J.-H.; Mondal, J.; Hwang, J.; Kim, H.S.; Kumar, V.; Raj, A.; Hwang, S.R.; Lee, Y.-K. Magnetofluoro-Immunosensing Platform Based on Binary Nanoparticle-Decorated Graphene for Detection of Cancer Cell-Derived Exosomes. Int. J. Mol. Sci. 2022, 23, 9619. [Google Scholar] [CrossRef] [PubMed]
- Weerathunge, P.; Pooja, D.; Singh, M.; Kulhari, H.; Mayes, E.L.; Bansal, V.; Ramanathan, R. Transferrin-conjugated quasi-cubic SPIONs for cellular receptor profiling and detection of brain cancer. Sens. Actuators B Chem. 2019, 297, 126737. [Google Scholar] [CrossRef]
- Kim, M.I.; Ye, Y.; Woo, M.-A.; Lee, J.; Park, H.G. A Highly Efficient Colorimetric Immunoassay Using a Nanocomposite Entrapping Magnetic and Platinum Nanoparticles in Ordered Mesoporous Carbon. Adv. Healthc. Mater. 2014, 3, 36–41. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Cai, Y.; Zhang, S.; Ma, K.; Hua, K.; Cui, Y. A novel DNA methylation biosensor by combination of isothermal amplification and lateral flow device. Sens. Actuators B Chem. 2021, 333, 129624. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Zhang, S.; Cai, Y.; Ma, K.; Hua, K.; Cui, Y. A site-specific DNA methylation biosensor for both visual and magnetic determination based on lateral flow assay. Analyst 2021, 146, 2248–2254. [Google Scholar] [CrossRef]
- Huang, C.-C.; Ray, P.; Chan, M.; Zhou, X.; Hall, D.A. An aptamer-based magnetic flow cytometer using matched filtering. Biosens. Bioelectron. 2020, 169, 112362. [Google Scholar] [CrossRef]
- Kim, S.J.; Lee, S.-W.; Song, J.D.; Kwon, Y.-W.; Lee, K.-J.; Koo, H.C. An InSb-based magnetoresistive biosensor using Fe3O4 nanoparticles. Sens. Actuators B Chem. 2018, 255, 2894–2899. [Google Scholar] [CrossRef]
- Nagesetti, A.; Rodzinski, A.; Stimphil, E.; Stewart, T.; Khanal, C.; Wang, P.; Guduru, R.; Liang, P.; Agoulnik, I.; Horstmyer, J.; et al. Multiferroic coreshell magnetoelectric nanoparticles as NMR sensitive nanoprobes for cancer cell detection. Sci. Rep. 2017, 7, 1610. [Google Scholar] [CrossRef]
- Zhu, F.; Li, D.; Ding, Q.; Lei, C.; Ren, L.; Ding, X.; Sun, X. 2D magnetic MoS2–Fe3O4 hybrid nanostructures for ultrasensitive exosome detection in GMR sensor. Biosens. Bioelectron. 2020, 147, 111787. [Google Scholar] [CrossRef]
- Javed, K.R.; Ahmad, M.; Ali, S.; Butt, M.Z.; Nafees, M.; Butt, A.; Nadeem, M.; Shahid, A. Comparison of Doxorubicin Anticancer Drug Loading on Different Metal Oxide Nanoparticles. Medicine 2015, 94, e617. [Google Scholar] [CrossRef]
- Li, H.; Xu, W.; Li, F.; Zeng, R.; Zhang, X.; Wang, X.; Zhao, S.; Weng, J.; Li, Z.; Sun, L. Amplification of anticancer efficacy by co-delivery of doxorubicin and lonidamine with extracellular vesicles. Drug Deliv. 2022, 29, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Gui, G.; Fan, Z.; Ning, Y.; Yuan, C.; Zhang, B.; Xu, Q. Optimization, Characterization and in vivo Evaluation of Paclitaxel-Loaded Folate-Conjugated Superparamagnetic Iron Oxide Nanoparticles. Int. J. Nanomed. 2021, 16, 2283–2295. [Google Scholar] [CrossRef]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef] [PubMed]
- Nosrati, H.; Sefidi, N.; Sharafi, A.; Danafar, H.; Manjili, H.K. Bovine Serum Albumin (BSA) coated iron oxide magnetic nanoparticles as biocompatible carriers for curcumin-anticancer drug. Bioorg. Chem. 2018, 76, 501–509. [Google Scholar] [CrossRef]
- Attari, E.; Nosrati, H.; Danafar, H.; Manjili, H.K. Methotrexate anticancer drug delivery to breast cancer cell lines by iron oxide magnetic based nanocarrier. J. Biomed. Mater. Res. Part A 2019, 107, 2492–2500. [Google Scholar] [CrossRef] [PubMed]
- Ayyanaar, S.; Bhaskar, R.; Esthar, S.; Vadivel, M.; Rajesh, J.; Rajagopal, G. Design and development of 5-fluorouracil loaded biodegradable magnetic microspheres as site-specific drug delivery vehicle for cancer therapy. J. Magn. Magn. Mater. 2022, 546, 168853. [Google Scholar] [CrossRef]
- Zhu, Y.; Liao, L. Applications of Nanoparticles for Anticancer Drug Delivery: A Review. J. Nanosci. Nanotechnol. 2015, 15, 4753–4773. [Google Scholar] [CrossRef] [PubMed]
- Mandal, A.; Bisht, R.; Pal, D.; Mitra, A.K. Diagnosis and Drug Delivery to the Brain: Novel strategies. In Emerging Nanotechnologies for Diagnostics, Drug Delivery and Medical Devices; Elsevier: Amsterdam, The Netherlands, 2017; pp. 59–83. [Google Scholar] [CrossRef]
- McBain, S.C.; Yiu, H.H.; Dobson, J. Magnetic nanoparticles for gene and drug delivery. Int. J. Nanomed. 2008, 3, 169–180. [Google Scholar] [CrossRef]
- Chen, M.; Li, J.; Shu, G.; Shen, L.; Qiao, E.; Zhang, N.; Fang, S.; Chen, X.; Zhao, Z.; Tu, J.; et al. Homogenous multifunctional microspheres induce ferroptosis to promote the anti-hepatocarcinoma effect of chemoembolization. J. Nanobiotechnol. 2022, 20, 179. [Google Scholar] [CrossRef]
- Hu, W.; Qi, Q.; Hu, H.; Wang, C.; Zhang, Q.; Zhang, Z.; Zhao, Y.; Yu, X.; Guo, M.; Du, S.; et al. Fe3O4 liposome for photothermal/chemo-synergistic inhibition of metastatic breast tumor. Colloids Surfaces A Physicochem. Eng. Asp. 2022, 634, 127921. [Google Scholar] [CrossRef]
- Al-Musawi, S.; Albukhaty, S.; Al-Karagoly, H.; Sulaiman, G.M.; Jabir, M.S.; Naderi-Manesh, H. Dextran-coated superparamagnetic nanoparticles modified with folate for targeted drug delivery of camptothecin. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 045009. [Google Scholar] [CrossRef]
- Satpathy, M.; Wang, L.; Zielinski, R.J.; Qian, W.; Wang, Y.A.; Mohs, A.; Kairdolf, B.A.; Ji, X.; Capala, J.; Lipowska, M.; et al. Targeted Drug Delivery and Image-Guided Therapy of Heterogeneous Ovarian Cancer Using HER2-Targeted Theranostic Nanoparticles. Theranostics 2019, 9, 778–795. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Badiei, A.; Sorouri, A.M.; Fasihi-Ramandi, M.; Ganjali, M.R.; Rahimi-Nasrabadi, M.; Ahmadi, F. Cur-loaded magnetic ZnFe2O4@L-cysteine—Ox, N-rich mesoporous -gC3N4 nanocarriers as a targeted sonodynamic chemotherapeutic agent for enhanced tumor eradication. Surf. Interfaces 2022, 30, 101900. [Google Scholar] [CrossRef]
- Ramezani Farani, M.; Azarian, M.; Heydari Sheikh Hossein, H.; Abdolvahabi, Z.; Mohammadi Abgarmi, Z.; Moradi, A.; Mousavi, S.M.; Ashrafizadeh, M.; Makvandi, P.; Saeb, M.R.; et al. Folic Acid-Adorned Curcumin-Loaded Iron Oxide Nanoparticles for Cervical Cancer. ACS Appl. Bio Mater. 2022, 5, 1305–1318. [Google Scholar] [CrossRef]
- Patitsa, M.; Karathanou, K.; Kanaki, Z.; Tzioga, L.; Pippa, N.; Demetzos, C.; Verganelakis, D.A.; Cournia, Z.; Klinakis, A. Magnetic nanoparticles coated with polyarabic acid demonstrate enhanced drug delivery and imaging properties for cancer theranostic applications. Sci. Rep. 2017, 7, 775. [Google Scholar] [CrossRef]
- Noh, K.; Uthaman, S.; Lee, C.-S.; Kim, Y.; Pillarisetti, S.; Hwang, H.S.; Park, I.-K.; Huh, K.M. Tumor intracellular microenvironment-responsive nanoparticles for magnetically targeted chemotherapy. J. Ind. Eng. Chem. 2022, 111, 121–128. [Google Scholar] [CrossRef]
- Abolhasani Zadeh, F.; Abdalkareem Jasim, S.; Atakhanova, N.E.; Majdi, H.S.; Abed Jawad, M.; Khudair Hasan, M.; Borhani, F.; Khatami, M. Drug delivery and anticancer activity of biosynthesised mesoporous Fe 2 O 3 nanoparticles. IET Nanobiotechnol. 2022, 16, 85–91. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zeng, Y.; Wang, G.; Han, S.; Yang, Z.; Li, B.; Wang, X.; Gao, J.; Zheng, L.; et al. Leucine-coated cobalt ferrite nanoparticles: Synthesis, characterization and potential biomedical applications for drug delivery. Phys. Lett. A 2020, 384, 126600. [Google Scholar] [CrossRef]
- Li, J.; Yang, N.; Yang, M.; Lu, C.; Xie, M. Development of a magnetic MoS2 system camouflaged by lipid for chemo/phototherapy of cancer. Colloids Surf. B Biointerfaces 2022, 213, 112389. [Google Scholar] [CrossRef]
- Liu, E.; Zhang, M.; Cui, H.; Gong, J.; Huang, Y.; Wang, J.; Cui, Y.; Dong, W.; Sun, L.; He, H.; et al. Tat-functionalized Ag-Fe3O4 nano-composites as tissue-penetrating vehicles for tumor magnetic targeting and drug delivery. Acta Pharm. Sin. B 2018, 8, 956–968. [Google Scholar] [CrossRef] [PubMed]
- Shahzada, K.; Mushtaqbc, S.; Rizwana, M.; Khalida, W.; Atifa, M.; Din, F.U.; Ahmadc, N.; Abbasic, R.; Alia, Z. Field-controlled magnetoelectric core-shell CoFe2O4@BaTiO3 nanoparticles as effective drug carriers and drug release in vitro. Mater. Sci. Eng. C 2021, 119, 111444. [Google Scholar] [CrossRef] [PubMed]
- Foroughi-Nia, B.; Aghanejad, A.; Kadkhoda, J.; Barar, J.; Nosrati, H.; Davaran, S. AS1411 conjugated magnetic-based poly N -isopropyl acrylamide nanoparticles for delivery of erlotinib to prostate cancer cells. Appl. Organomet. Chem. 2022, 36, e6691. [Google Scholar] [CrossRef]
- Stewart, T.S.; Nagesetti, A.; Guduru, R.; Liang, P.; Stimphil, E.; Hadjikhani, A.; Salgueiro, L.; Horstmyer, J.; Cai, R.; Schally, A.; et al. Magnetoelectric nanoparticles for delivery of antitumor peptides into glioblastoma cells by magnetic fields. Nanomedicine 2018, 13, 423–438. [Google Scholar] [CrossRef]
- Tomeh, M.A.; Hadianamrei, R.; Xu, D.; Brown, S.; Zhao, X. Peptide-functionalised magnetic silk nanoparticles produced by a swirl mixer for enhanced anticancer activity of ASC-J9. Colloids Surf. B Biointerfaces 2022, 216, 112549. [Google Scholar] [CrossRef]
- Farmanbar, N.; Mohseni, S.; Darroudi, M. Green synthesis of chitosan-coated magnetic nanoparticles for drug delivery of oxaliplatin and irinotecan against colorectal cancer cells. Polym. Bull. 2022, 1–19. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Q.; Chen, J.; Wang, Z.; Xin, H.; Zhang, D. Efficient Delivery of Therapeutic siRNA by Fe3O4 Magnetic Nanoparticles into Oral Cancer Cells. Pharmaceutics 2019, 11, 615. [Google Scholar] [CrossRef]
- Chi, H.; Zhu, G.; Yin, Y.; Diao, H.; Liu, Z.; Sun, S.; Guo, Z.; Xu, W.; Xu, J.; Cui, C.; et al. Dual-Responsive multifunctional “core-shell” magnetic nanoparticles promoting Fenton reaction for tumor ferroptosis therapy. Int. J. Pharm. 2022, 622, 121898. [Google Scholar] [CrossRef]
- Pandit, P.; Bhagat, S.; Rananaware, P.; Mohanta, Z.; Kumar, M.; Tiwari, V.; Singh, S.; Brahmkhatri, V.P. Iron oxide nanoparticle encapsulated; folic acid tethered dual metal organic framework-based nanocomposite for MRI and selective targeting of folate receptor expressing breast cancer cells. Microporous Mesoporous Mater. 2022, 340, 112008. [Google Scholar] [CrossRef]
- Arabzadeh, A.; Akhlaghi, N.; Najafpour-Darzi, G. Quercetin loading on mesoporous magnetic MnFe2O4@ hydroxyapatite core-shell nanoparticles for treating cancer cells. Adv. Powder Technol. 2022, 33, 103609. [Google Scholar] [CrossRef]
- Ghasemzadeh, F.; Mohammadi, M.; Najafpour, G.D.; Moghadamnia, A.A. Ursolic acid loaded β-cyclodextrin/folic acid/Fe3O4 nanocomplex for drug delivery to tumor cells. J. Drug Deliv. Sci. Technol. 2022, 72, 103412. [Google Scholar] [CrossRef]
- Kanelli, M.; Saleh, B.; Webster, T.J.; Vouyiouka, S.; Topakas, E. Co-Encapsulation of Violacein and Iron Oxide in Poly(lactic acid) Nanoparticles for Simultaneous Antibacterial and Anticancer Applications. J. Biomed. Nanotechnol. 2022, 18, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Joshy, K.S.; Augustine, R.; Mayeen, A.; Alex, S.M.; Hasan, A.; Thomas, S.; Chi, H. NiFe2O4/poly(ethylene glycol)/lipid–polymer hybrid nanoparticles for anti-cancer drug delivery. New J. Chem. 2020, 44, 18162–18172. [Google Scholar] [CrossRef]
- Tokmedash, M.A.; Zadeh, E.S.; Balouchi, E.N.; Salehi, Z.; Ardestani, M.S. Synthesis of smart carriers based on tryptophan-functionalized magnetic nanoparticles and its application in 5-fluorouracil delivery. Biomed. Mater. 2022, 17, 045026. [Google Scholar] [CrossRef]
- Nie, Z.; Vahdani, Y.; Cho, W.C.; Bloukh, S.H.; Edis, Z.; Haghighat, S.; Falahati, M.; Kheradmandi, R.; Jaragh-Alhadad, L.A.; Sharifi, M. 5-Fluorouracil-containing inorganic iron oxide/platinum nanozymes with dual drug delivery and enzyme-like activity for the treatment of breast cancer. Arab. J. Chem. 2022, 15, 103966. [Google Scholar] [CrossRef]
- Ali, A.; Shah, T.; Ullah, R.; Zhou, P.; Guo, M.; Ovais, M.; Tan, Z.; Rui, Y. Review on Recent Progress in Magnetic Nanoparticles: Synthesis, Characterization, and Diverse Applications. Front. Chem. 2021, 9, 629054. [Google Scholar] [CrossRef]
- Husseiny, M.I.; El-Aziz, M.A.; Badr, Y.; Mahmoud, M.A. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1003–1006. [Google Scholar] [CrossRef]
- Khan, A.A.; Khan, S.; Khan, S.; Rentschler, S.; Laufer, S.; Deigner, H.-P. Biosynthesis of iron oxide magnetic nanoparticles using clinically isolated Pseudomonas aeruginosa. Sci. Rep. 2021, 11, 20503. [Google Scholar] [CrossRef]
- Rai, M.; Gade, A.; Yadav, A. Biogenic Nanoparticles: An Introduction to What They Are, How They Are Synthesized and Their Applications. In Metal Nanoparticles in Microbiology; Rai, M., Duran, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–14. ISBN 978-3-642-18311-9. [Google Scholar]
- Golinska, P.; Wypij, M.; Ingle, A.P.; Gupta, I.; Dahm, H.; Rai, M. Biogenic synthesis of metal nanoparticles from actinomycetes: Biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 2014, 98, 8083–8097. [Google Scholar] [CrossRef]
- Govender, Y.; Riddin, T.; Gericke, M.; Whiteley, C.G. Bioreduction of platinum salts into nanoparticles: A mechanistic perspective. Biotechnol. Lett. 2009, 31, 95–100. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rashid, M.; Rahman, A.; Tajuddin; Husen, A.; Rehman, S. Biogenic fabrication and characterization of silver nanoparticles using aqueous-ethanolic extract of lichen (Usnea longissima) and their antimicrobial activity. Biomater. Res. 2018, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, N.; Banerjee, A.; Lahiri, S.; Panda, A.; Ghosh, A.N.; Pal, R. Biorecovery of gold using cyanobacteria and an eukaryotic alga with special reference to nanogold formation—A novel phenomenon. J. Appl. Phycol. 2009, 21, 145–152. [Google Scholar] [CrossRef]
- Gade, A.K.; Bonde, P.; Ingle, A.P.; Marcato, P.D.; Durán, N.; Rai, M.K. Exploitation of Aspergillus niger for Synthesis of Silver Nanoparticles. J. Biobased Mater. Bioenergy 2008, 2, 243–247. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological synthesis of metallic nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Natural Metallic Nanoparticles for Application in Nano-Oncology. Int. J. Mol. Sci. 2020, 21, 4412. [Google Scholar] [CrossRef] [PubMed]
- Assa, F.; Jafarizadeh-Malmiri, H.; Ajamein, H.; Anarjan, N.; Vaghari, H.; Sayyar, Z.; Berenjian, A. A biotechnological perspective on the application of iron oxide nanoparticles. Nano Res. 2016, 9, 2203–2225. [Google Scholar] [CrossRef]
- Arakaki, A.; Nakazawa, H.; Nemoto, M.; Mori, T.; Matsunaga, T. Formation of magnetite by bacteria and its application. J. R. Soc. Interface 2008, 5, 977–999. [Google Scholar] [CrossRef]
- Uebe, R.; Schüler, D. Magnetosome biogenesis in magnetotactic bacteria. Nat. Rev. Microbiol. 2016, 14, 621–637. [Google Scholar] [CrossRef]
- Barber-Zucker, S.; Keren-Khadmy, N.; Zarivach, R. From invagination to navigation: The story of magnetosome-associated proteins in magnetotactic bacteria. Protein Sci. 2016, 25, 338–351. [Google Scholar] [CrossRef]
- Ben-Shimon, S.; Stein, D.; Zarivach, R. Current view of iron biomineralization in magnetotactic bacteria. J. Struct. Biol. X 2021, 5, 100052. [Google Scholar] [CrossRef]
- Komeili, A. Molecular mechanisms of compartmentalization and biomineralization in magnetotactic bacteria. FEMS Microbiol. Rev. 2012, 36, 232–255. [Google Scholar] [CrossRef]
- Komeili, A.; Li, Z.; Newman, D.K.; Jensen, G.J. Magnetosomes Are Cell Membrane Invaginations Organized by the Actin-Like Protein MamK. Science 2006, 311, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Okamura, Y.; Arakaki, A.; Tanaka, T.; Takeyama, H.; Matsunaga, T. Origin of magnetosome membrane: Proteomic analysis of magnetosome membrane and comparison with cytoplasmic membrane. Proteomics 2006, 6, 5234–5247. [Google Scholar] [CrossRef]
- Zeytuni, N.; Ozyamak, E.; Ben-Harush, K.; Davidov, G.; Levin, M.; Gat, Y.; Moyal, T.; Brik, A.; Komeili, A.; Zarivach, R. Self-recognition mechanism of MamA, a magnetosome-associated TPR-containing protein, promotes complex assembly. Proc. Natl. Acad. Sci. USA 2011, 108, E480–E487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raschdorf, O.; Bonn, F.; Zeytuni, N.; Zarivach, R.; Becher, D.; Schüler, D. A quantitative assessment of the membrane-integral sub-proteome of a bacterial magnetic organelle. J. Proteom. 2018, 172, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G.; Cypriano, J.; Correa, T.; Leão, P.; Bazylinski, D.A.; Abreu, F. Applications of Magnetotactic Bacteria, Magnetosomes and Magnetosome Crystals in Biotechnology and Nanotechnology: Mini-Review. Molecules 2018, 23, 2438. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Taoka, A.; Uchihashi, T.; Sasaki, H.; Watanabe, H.; Ando, T.; Fukumori, Y. Visualization and structural analysis of the bacterial magnetic organelle magnetosome using atomic force microscopy. Proc. Natl. Acad. Sci. USA 2010, 107, 9382–9387. [Google Scholar] [CrossRef] [PubMed]
- Lohße, A.; Borg, S.; Raschdorf, O.; Kolinko, I.; Tompa, E.; Pósfai, M.; Faivre, D.; Baumgartner, J.; Schüler, D. Genetic Dissection of the mamAB and mms6 Operons Reveals a Gene Set Essential for Magnetosome Biogenesis in Magnetospirillum gryphiswaldense. J. Bacteriol. 2014, 196, 2658–2669. [Google Scholar] [CrossRef] [PubMed]
- Schüler, D. Genetics and cell biology of magnetosome formation in magnetotactic bacteria. FEMS Microbiol. Rev. 2008, 32, 654–672. [Google Scholar] [CrossRef]
- Uebe, R.; Junge, K.; Henn, V.; Poxleitner, G.; Katzmann, E.; Plitzko, J.M.; Zarivach, R.; Kasama, T.; Wanner, G.; Pósfai, M.; et al. The cation diffusion facilitator proteins MamB and MamM of Magnetospirillum gryphiswaldense have distinct and complex functions, and are involved in magnetite biomineralization and magnetosome membrane assembly. Mol. Microbiol. 2011, 82, 818–835. [Google Scholar] [CrossRef]
- Arakaki, A.; Yamagishi, A.; Fukuyo, A.; Tanaka, M.; Matsunaga, T. Co-ordinated functions of Mms proteins define the surface structure of cubo-octahedral magnetite crystals in magnetotactic bacteria. Mol. Microbiol. 2014, 93, 554–567. [Google Scholar] [CrossRef] [PubMed]
- Toro-Nahuelpan, M.; Giacomelli, G.; Raschdorf, O.; Borg, S.; Plitzko, J.M.; Bramkamp, M.; Schüler, D.; Müller, F.-D. MamY is a membrane-bound protein that aligns magnetosomes and the motility axis of helical magnetotactic bacteria. Nat. Microbiol. 2019, 4, 1978–1989. [Google Scholar] [CrossRef]
- Gareev, K.G.; Grouzdev, D.S.; Kharitonskii, P.V.; Kosterov, A.; Koziaeva, V.V.; Sergienko, E.S.; Shevtsov, M.A. Magnetotactic Bacteria and Magnetosomes: Basic Properties and Applications. Magnetochemistry 2021, 7, 86. [Google Scholar] [CrossRef]
- Uzun, M.; Koziaeva, V.; Dziuba, M.; Alekseeva, L.; Krutkina, M.; Sukhacheva, M.; Baslerov, R.; Grouzdev, D. Looking for a Needle in a Haystack: Magnetotactic Bacteria Help in “Rare Biosphere” Investigations. bioRxiv 2022. [Google Scholar] [CrossRef]
- Uzun, M.; Alekseeva, L.; Krutkina, M.; Koziaeva, V.; Grouzdev, D. Unravelling the diversity of magnetotactic bacteria through analysis of open genomic databases. Sci. Data 2020, 7, 252. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, W.; Paterson, G.A.; Zhu, Q.; Zhao, X.; Knight, R.; Bazylinski, D.A.; Roberts, A.P.; Pan, Y. Expanding magnetic organelle biogenesis in the domain Bacteria. Microbiome 2020, 8, 152. [Google Scholar] [CrossRef]
- Spring, S.; Amann, R.; Ludwig, W.; Schleifer, K.-H.; van Gemerden, H.; Petersen, N. Dominating Role of an Unusual Magnetotactic Bacterium in the Microaerobic Zone of a Freshwater Sediment. Appl. Environ. Microbiol. 1993, 59, 2397–2403. [Google Scholar] [CrossRef]
- Descamps, E.C.T.; Monteil, C.L.; Menguy, N.; Ginet, N.; Pignol, D.; Bazylinski, D.A.; Lefèvre, C.T. Desulfamplus magnetovallimortis gen. nov., sp. nov., a magnetotactic bacterium from a brackish desert spring able to biomineralize greigite and magnetite, that represents a novel lineage in the Desulfobacteraceae. Syst. Appl. Microbiol. 2017, 40, 280–289. [Google Scholar] [CrossRef]
- Epósfai, M.; Lefèvre, C.T.; Etrubitsyn, D.; Bazylinski, D.A.; Frankel, R.B. Phylogenetic significance of composition and crystal morphology of magnetosome minerals. Front. Microbiol. 2013, 4, 344. [Google Scholar] [CrossRef]
- Dziuba, M.; Koziaeva, V.; Grouzdev, D.; Burganskaya, E.; Baslerov, R.; Kolganova, T.; Chernyadyev, A.; Osipov, G.; Andrianova, E.; Gorlenko, V.; et al. Magnetospirillum caucaseum sp. nov., Magnetospirillum marisnigri sp. nov. and Magnetospirillum moscoviense sp. nov., freshwater magnetotactic bacteria isolated from three distinct geographical locations in European Russia. Int. J. Syst. Evol. Microbiol. 2016, 66, 2069–2077. [Google Scholar] [CrossRef]
- Gareev, K.G.; Grouzdev, D.S.; Kharitonskii, P.V.; Kirilenko, D.A.; Kosterov, A.; Koziaeva, V.V.; Levitskii, V.S.; Multhoff, G.; Nepomnyashchaya, E.K.; Nikitin, A.V.; et al. Magnetic Properties of Bacterial Magnetosomes Produced by Magnetospirillum caucaseum SO-1. Microorganisms 2021, 9, 1854. [Google Scholar] [CrossRef] [PubMed]
- Grouzdev, D.S.; Dziuba, M.V.; Sukhacheva, M.S.; Mardanov, A.V.; Beletskiy, A.V.; Kuznetsov, B.B.; Skryabin, K.G. Draft Genome Sequence of Magnetospirillum sp. Strain SO-1, a Freshwater Magnetotactic Bacterium Isolated from the Ol’khovka River, Russia. Genome Announc. 2014, 2, e00235-14. [Google Scholar] [CrossRef] [PubMed]
- Grouzdev, D.S.; Dziuba, M.V.; Kurek, D.V.; Ovchinnikov, A.I.; Zhigalova, N.A.; Kuznetsov, B.B.; Skryabin, K.G. Optimized Method for Preparation of IgG-Binding Bacterial Magnetic Nanoparticles. PLoS ONE 2014, 9, e109914. [Google Scholar] [CrossRef]
- Faivre, D.; Menguy, N.; Pósfai, M.; Schüler, D. Environmental parameters affect the physical properties of fast-growing magnetosomes. Am. Miner. 2008, 93, 463–469. [Google Scholar] [CrossRef]
- Schleifer, K.H.; Schüler, D.; Spring, S.; Weizenegger, M.; Amann, R.; Ludwig, W.; Köhler, M. The Genus Magnetospirillum gen. nov. Description of Magnetospirillum gryphiswaldense sp. nov. and Transfer of Aquaspirillum magnetotacticum to Magnetospirillum magnetotacticum comb. nov. Syst. Appl. Microbiol. 1991, 14, 379–385. [Google Scholar] [CrossRef]
- Fdez-Gubieda, M.L.; Muela, A.; Alonso, J.; García-Prieto, A.; Olivi, L.; Fernández-Pacheco, R.; Barandiarán, J.M. Magnetite Biomineralization in Magnetospirillum gryphiswaldense: Time-Resolved Magnetic and Structural Studies. ACS Nano 2013, 7, 3297–3305. [Google Scholar] [CrossRef]
- Koziaeva, V.V.; Rusakova, S.A.; Slobodova, N.V.; Uzun, M.; Kolganova, T.V.; Skryabin, K.G.; Grouzdev, D.S. Magnetospirillum kuznetsovii sp. nov., a novel magnetotactic bacterium isolated from a lake in the Moscow region. Int. J. Syst. Evol. Microbiol. 2019, 69, 1953–1959. [Google Scholar] [CrossRef]
- Li, J.; Pan, Y.; Chen, G.; Liu, Q.; Tian, L.; Lin, W. Magnetite magnetosome and fragmental chain formation of Magnetospirillum magneticum AMB-1: Transmission electron microscopy and magnetic observations. Geophys. J. Int. 2009, 177, 33–42. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, S.-D.; Chen, L.; Cai, Y.; Zhang, W.-J.; Song, T.; Wu, L.-F. Efficient Genome Editing of Magnetospirillum magneticum AMB-1 by CRISPR-Cas9 System for Analyzing Magnetotactic Behavior. Front. Microbiol. 2018, 9, 1569. [Google Scholar] [CrossRef]
- Matsunaga, T.; Okamura, Y.; Fukuda, Y.; Wahyudi, A.T.; Murase, Y.; Takeyama, H. Complete Genome Sequence of the Facultative Anaerobic Magnetotactic Bacterium Magnetospirillum sp. strain AMB-1. DNA Res. 2005, 12, 157–166. [Google Scholar] [CrossRef]
- Devouard, B.; Posfai, M.; Hua, X.; Bazylinski, D.A.; Frankel, R.B.; Buseck, P.R. Magnetite from magnetotactic bacteria; size distributions and twinning. Am. Miner. 1998, 83, 1387–1398. [Google Scholar] [CrossRef]
- Maratea, D.; Blakemore, R.P. Aquaspirillum magnetotacticum sp. nov., a Magnetic Spirillum. Int. J. Syst. Bacteriol. 1981, 31, 452–455. [Google Scholar] [CrossRef]
- Kozyaeva, V.V.; Grouzdev, D.S.; Dziuba, M.V.; Kolganova, T.V.; Kuznetsov, B.B. Diversity of magnetotactic bacteria of the Moskva River. Microbiology 2017, 86, 106–112. [Google Scholar] [CrossRef]
- Koziaeva, V.V.; Dziuba, M.V.; Ivanov, T.M.; Kuznetsov, B.B.; Skryabin, K.G.; Grouzdev, D.S. Draft Genome Sequences of Two Magnetotactic Bacteria, Magnetospirillum moscoviense BB-1 and Magnetospirillum marisnigri SP-1. Genome Announc. 2016, 4, e00814-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monteil, C.; Grouzdev, D.S.; Perrière, G.; Alonso, B.; Rouy, Z.; Cruveiller, S.; Ginet, N.; Pignol, D.; Lefevre, C.T. Repeated horizontal gene transfers triggered parallel evolution of magnetotaxis in two evolutionary divergent lineages of magnetotactic bacteria. ISME J. 2020, 14, 1783–1794. [Google Scholar] [CrossRef] [PubMed]
- Lefèvre, C.T.; Schmidt, M.L.; Viloria, N.; Trubitsyn, D.; Schüler, D.; Bazylinski, D.A. Insight into the Evolution of Magnetotaxis in Magnetospirillum spp., Based on mam Gene Phylogeny. Appl. Environ. Microbiol. 2012, 78, 7238–7248. [Google Scholar] [CrossRef] [PubMed]
- Bazylinski, D.A.; Williams, T.J.; Lefèvre, C.T.; Trubitsyn, D.; Fang, J.; Beveridge, T.J.; Moskowitz, B.M.; Ward, B.; Schübbe, S.; Dubbels, B.L.; et al. Magnetovibrio blakemorei gen. nov., sp. nov., a magnetotactic bacterium (Alphaproteobacteria: Rhodospirillaceae) isolated from a salt marsh. Int. J. Syst. Evol. Microbiol. 2013, 63, 1824–1833. [Google Scholar] [CrossRef]
- Bazylinski, D.A.; Frankel, R.B.; Jannasch, H.W. Anaerobic magnetite production by a marine, magnetotactic bacterium. Nature 1988, 334, 518–519. [Google Scholar] [CrossRef]
- Clemett, S.J.; Thomas-Keprta, K.L.; Shimmin, J.; Morphew, M.; McIntosh, J.R.; Bazylinski, D.A.; Kirschvink, J.L.; Wentworth, S.J.; McKay, D.S.; Vali, H.; et al. Crystal morphology of MV-1 magnetite. Am. Miner. 2002, 87, 1727–1730. [Google Scholar] [CrossRef]
- Monteil, C.L.; Perrière, G.; Menguy, N.; Ginet, N.; Alonso, B.; Waisbord, N.; Cruveiller, S.; Pignol, D.; Lefèvre, C.T. Genomic study of a novel magnetotactic Alphaproteobacteria uncovers the multiple ancestry of magnetotaxis. Environ. Microbiol. 2018, 20, 4415–4430. [Google Scholar] [CrossRef]
- Zhu, K.; Pan, H.; Li, J.; Yu-Zhang, K.; Zhang, S.-D.; Zhang, W.-Y.; Zhou, K.; Yue, H.; Pan, Y.; Xiao, T.; et al. Isolation and characterization of a marine magnetotactic spirillum axenic culture QH-2 from an intertidal zone of the China Sea. Res. Microbiol. 2010, 161, 276–283. [Google Scholar] [CrossRef]
- Geurink, C.; Lefevre, C.T.; Monteil, C.L.; Morillo-Lopez, V.; Abreu, F.; Bazylinski, D.A.; Trubitsyn, D. Complete Genome Sequence of Strain BW-2, a Magnetotactic Gammaproteobacterium in the Family Ectothiorhodospiraceae, Isolated from a Brackish Spring in Death Valley, California. Microbiol. Resour. Announc. 2020, 9, e01144-19. [Google Scholar] [CrossRef]
- Lefèvre, C.T.; Viloria, N.; Schmidt, M.L.; Pósfai, M.; Frankel, R.B.; Bazylinski, D.A. Novel magnetite-producing magnetotactic bacteria belonging to the Gammaproteobacteria. ISME J. 2012, 6, 440–450. [Google Scholar] [CrossRef] [PubMed]
- Taoka, A.; Kondo, J.; Oestreicher, Z.; Fukumori, Y. Characterization of uncultured giant rod-shaped magnetotactic Gammaproteobacteria from a freshwater pond in Kanazawa, Japan. Microbiology 2014, 160, 2226–2234. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.; Tamaxia, A.; Liu, Y.; Qiu, H.; Pan, J.; Jin, Z.; Zhao, X.; Roberts, A.P.; Pan, Y.; Li, J. Identification and characterization of magnetotactic Gammaproteobacteria from a salt evaporation pool, Bohai Bay, China. Environ. Microbiol. 2021, 24, 938–950. [Google Scholar] [CrossRef]
- Leão, P.; Teixeira, L.C.R.S.; Cypriano, J.; Farina, M.; Abreu, F.; Bazylinski, D.A.; Lins, U. North-Seeking Magnetotactic Gammaproteobacteria in the Southern Hemisphere. Appl. Environ. Microbiol. 2016, 82, 5595–5602. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, H.; Menguy, N.; Benzerara, K.; Wang, F.; Lin, X.; Chen, Z.; Pan, Y. Single-Cell Resolution of Uncultured Magnetotactic Bacteria via Fluorescence-Coupled Electron Microscopy. Appl. Environ. Microbiol. 2017, 83, e00409-17. [Google Scholar] [CrossRef] [PubMed]
- Trubitsyn, D.; Monteil, C.L.; Geurink, C.; Morillo-Lopez, V.; de Almeida, L.G.P.; de Vasconcelos, A.T.R.; Abreu, F.; Bazylinski, D.A.; Lefevre, C.T. Complete Genome Sequence of Strain SS-5, a Magnetotactic Gammaproteobacterium Isolated from the Salton Sea, a Shallow, Saline, Endorheic Rift Lake Located on the San Andreas Fault in California. Microbiol. Resour. Announc. 2021, 10, e00928-20. [Google Scholar] [CrossRef]
- Meldrum, F.C.; Mann, S.; Heywood, B.R.; Frankel, R.B.; Bazylinski, D.A. Electron microscopy study of magnetosomes in a cultured coccoid magnetotactic bacterium. Proc. R. Soc. B Boil. Sci. 1993, 251, 231–236. [Google Scholar] [CrossRef]
- Schübbe, S.; Williams, T.J.; Xie, G.; Kiss, H.E.; Brettin, T.S.; Martinez, D.; Ross, C.A.; Schüler, D.; Cox, B.L.; Nealson, K.H.; et al. Complete Genome Sequence of the Chemolithoautotrophic Marine Magnetotactic Coccus Strain MC-1. Appl. Environ. Microbiol. 2009, 75, 4835–4852. [Google Scholar] [CrossRef]
- Bazylinski, D.A.; Williams, T.J.; Lefevre, C.T.; Berg, R.J.; Zhang, C.L.; Bowser, S.S.; Dean, A.J.; Beveridge, T.J. Magnetococcus marinus gen. nov., sp. nov., a marine, magnetotactic bacterium that represents a novel lineage (Magnetococcaceae fam. nov., Magnetococcales ord. nov.) at the base of the Alphaproteobacteria. Int. J. Syst. Evol. Microbiol. 2013, 63, 801–808. [Google Scholar] [CrossRef]
- Koziaeva, V.; Dziuba, M.; Leão, P.; Uzun, M.; Krutkina, M.; Grouzdev, D. Genome-Based Metabolic Reconstruction of a Novel Uncultivated Freshwater Magnetotactic coccus “Ca. Magnetaquicoccus inordinatus” UR-1, and Proposal of a Candidate Family “Ca. Magnetaquicoccaceae”. Front. Microbiol. 2019, 10, 2290. [Google Scholar] [CrossRef]
- Lefevre, C.T.; Bernadac, A.; Yu-Zhang, K.; Pradel, N.; Wu, L.-F. Isolation and characterization of a magnetotactic bacterial culture from the Mediterranean Sea. Environ. Microbiol. 2009, 11, 1646–1657. [Google Scholar] [CrossRef]
- Ji, B.; Zhang, S.-D.; Zhang, W.-J.; Rouy, Z.; Alberto, F.; Santini, C.-L.; Mangenot, S.; Gagnot, S.; Philippe, N.; Pradel, N.; et al. The chimeric nature of the genomes of marine magnetotactic coccoid-ovoid bacteria defines a novel group of Proteobacteria. Environ. Microbiol. 2017, 19, 1103–1119. [Google Scholar] [CrossRef]
- Werckmann, J.; Cypriano, J.; Lefèvre, C.T.; Dembelé, K.; Ersen, O.; Bazylinski, D.A.; Lins, U.; Farina, M. Localized iron accumulation precedes nucleation and growth of magnetite crystals in magnetotactic bacteria. Sci. Rep. 2017, 7, 8291. [Google Scholar] [CrossRef]
- Morillo, V.; Abreu, F.; Araujo, A.C.; De Almeida, L.G.P.; Enrich-Prast, A.; Farina, M.; De Vasconcelos, A.T.R.; Bazylinski, D.A.; Lins, U. Isolation, cultivation and genomic analysis of magnetosome biomineralization genes of a new genus of South-seeking magnetotactic cocci within the Alphaproteobacteria. Front. Microbiol. 2014, 5, 72. [Google Scholar] [CrossRef] [PubMed]
- Uzun, M.; Koziaeva, V.; Dziuba, M.; Leão, P.; Krutkina, M.; Grouzdev, D. Detection of interphylum transfers of the magnetosome gene cluster in magnetotactic bacteria. Front. Microbiol. 2022, 13, 945734. [Google Scholar] [CrossRef]
- Koziaeva, V.V.; Alekseeva, L.M.; Uzun, M.M.; Leão, P.; Sukhacheva, M.V.; Patutina, E.O.; Kolganova, T.V.; Grouzdev, D.S. Biodiversity of Magnetotactic Bacteria in the Freshwater Lake Beloe Bordukovskoe, Russia. Microbiology 2020, 89, 348–358. [Google Scholar] [CrossRef]
- Lefèvre, C.T.; Menguy, N.; Abreu, F.; Lins, U.; Pósfai, M.; Prozorov, T.; Pignol, D.; Frankel, R.B.; Bazylinski, D.A. A Cultured Greigite-Producing Magnetotactic Bacterium in a Novel Group of Sulfate-Reducing Bacteria. Science 2011, 334, 1720–1723. [Google Scholar] [CrossRef] [PubMed]
- Nakazawa, H.; Arakaki, A.; Narita-Yamada, S.; Yashiro, I.; Jinno, K.; Aoki, N.; Tsuruyama, A.; Okamura, Y.; Tanikawa, S.; Fujita, N.; et al. Whole genome sequence of Desulfovibrio magneticus strain RS-1 revealed common gene clusters in magnetotactic bacteria. Genome Res. 2009, 19, 1801–1808. [Google Scholar] [CrossRef] [PubMed]
- Pósfai, M.; Moskowitz, B.M.; Arató, B.; Schüler, D.; Flies, C.; Bazylinski, D.A.; Frankel, R.B. Properties of intracellular magnetite crystals produced by Desulfovibrio magneticus strain RS-1. Earth Planet. Sci. Lett. 2006, 249, 444–455. [Google Scholar] [CrossRef]
- Sakaguchi, T.; Burgess, J.G.; Matsunaga, T. Magnetite formation by a sulphate-reducing bacterium. Nature 1993, 365, 47–49. [Google Scholar] [CrossRef]
- Chen, Y.-R.; Zhang, R.; Du, H.-J.; Pan, H.-M.; Zhang, W.-Y.; Zhou, K.; Li, J.-H.; Xiao, T.; Wu, L.-F. A novel species of ellipsoidal multicellular magnetotactic prokaryotes from Lake Yuehu in China: The Ellipsoidal MMPs from the Lake Yuehu. Environ. Microbiol. 2014, 17, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Leão, P.; Chen, Y.-R.; Abreu, F.; Wang, M.; Zhang, W.-J.; Zhou, K.; Xiao, T.; Wu, L.-F.; Lins, U. Ultrastructure of ellipsoidal magnetotactic multicellular prokaryotes depicts their complex assemblage and cellular polarity in the context of magnetotaxis: Ultrastructure and Magnetotaxis in Ellipsoidal MMP. Environ. Microbiol. 2017, 19, 2151–2163. [Google Scholar] [CrossRef]
- Abreu, F.; Cantão, M.E.; Nicolás, M.F.; Barcellos, F.G.; Morillo, V.; Almeida, L.G.; do Nascimento, F.F.; Lefèvre, C.T.; Bazylinski, D.A.; de Vasconcelos, A.T.R.; et al. Common ancestry of iron oxide- and iron-sulfide-based biomineralization in magnetotactic bacteria. ISME J. 2011, 5, 1634–1640. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.; Martins, J.L.; Silveira, T.S.; Keim, C.N.; De Barros, H.G.P.L.; Filho, F.J.G.; Lins, U. ‘Candidatus Magnetoglobus multicellularis’, a multicellular, magnetotactic prokaryote from a hypersaline environment. Int. J. Syst. Evol. Microbiol. 2007, 57, 1318–1322. [Google Scholar] [CrossRef]
- Abreu, F.; Silva, K. Greigite magnetosome membrane ultrastructure in ‘Candidatus Magnetoglobus multicellularis’. Int. Microbiol. 2008, 1, 75–80. [Google Scholar] [CrossRef]
- Kolinko, S.; Richter, M.; Glöckner, F.-O.; Brachmann, A.; Schüler, D. Single-cell genomics of uncultivated deep-branching magnetotactic bacteria reveals a conserved set of magnetosome genes. Environ. Microbiol. 2016, 18, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Deng, A.; Wang, Z.; Li, Y.; Wen, T.; Wu, L.-F.; Wu, M.; Pan, Y. Genomic insights into the uncultured genus ‘Candidatus Magnetobacterium’ in the phylum Nitrospirae. ISME J. 2014, 8, 2463–2477. [Google Scholar] [CrossRef]
- Li, J.; Menguy, N.; Gatel, C.; Boureau, V.; Snoeck, E.; Patriarche, G.; Leroy, E.; Pan, Y. Crystal growth of bullet-shaped magnetite in magnetotactic bacteria of the Nitrospirae phylum. J. R. Soc. Interface 2015, 12, 20141288. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Liu, L.; Pan, Y.; Lin, W. Identification and Genomic Characterization of Two Previously Unknown Magnetotactic Nitrospirae. Front. Microbiol. 2021, 12, 690052. [Google Scholar] [CrossRef]
- Kolinko, S.; Jogler, C.; Katzmann, E.; Wanner, G.; Peplies, J.; Schüler, D. Single-cell analysis reveals a novel uncultivated magnetotactic bacterium within the candidate division OP3. Environ. Microbiol. 2012, 14, 1709–1721. [Google Scholar] [CrossRef]
- Jacob, J.J.; Suthindhiran, K. Magnetotactic bacteria and magnetosomes—Scope and challenges. Mater. Sci. Eng. C 2016, 68, 919–928. [Google Scholar] [CrossRef]
- Han, L.; Li, S.; Yang, Y.; Zhao, F.; Huang, J.; Chang, J. Comparison of magnetite nanocrystal formed by biomineralization and chemosynthesis. J. Magn. Magn. Mater. 2007, 313, 236–242. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Liang, X.-J.; Wang, P.C. Bacterial Magnetosome: A Novel Biogenetic Magnetic Targeted Drug Carrier with Potential Multifunctions. J. Nanomater. 2011, 2011, 469031. [Google Scholar] [CrossRef]
- Alphandéry, E. Applications of Magnetosomes Synthesized by Magnetotactic Bacteria in Medicine. Front. Bioeng. Biotechnol. 2014, 2, 5. [Google Scholar] [CrossRef]
- Lower, B.H.; Bazylinski, D.A. The Bacterial Magnetosome: A Unique Prokaryotic Organelle. Microb. Physiol. 2013, 23, 63–80. [Google Scholar] [CrossRef]
- Basit, A.; Wang, J.; Guo, F.; Niu, W.; Jiang, W. Improved methods for mass production of magnetosomes and applications: A review. Microb. Cell Fact. 2020, 19, 197. [Google Scholar] [CrossRef] [PubMed]
- Alphandéry, E. Applications of magnetotactic bacteria and magnetosome for cancer treatment: A review emphasizing on practical and mechanistic aspects. Drug Discov. Today 2020, 25, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, G.R.; Guo, F.F.; Jiang, W.; Li, Y.; Li, L.J. Large-scale production of magnetosomes by chemostat culture of Magnetospirillum gryphiswaldense at high cell density. Microb. Cell Fact. 2010, 9, 99. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Jiang, W.; Li, Y.; Li, J. Semicontinuous Culture of Magnetospirillum gryphiswaldense MSR-1 Cells in an Autofermentor by Nutrient-Balanced and Isosmotic Feeding Strategies. Appl. Environ. Microbiol. 2011, 77, 5851–5856. [Google Scholar] [CrossRef]
- Kolinko, I.; Lohße, A.; Borg, S.; Raschdorf, O.; Jogler, C.; Tu, Q.; Pósfai, M.; Tompa, É.; Plitzko, J.M.; Brachmann, A.; et al. Biosynthesis of magnetic nanostructures in a foreign organism by transfer of bacterial magnetosome gene clusters. Nat. Nanotechnol. 2014, 9, 193–197. [Google Scholar] [CrossRef]
- Tay, A.; Pfeiffer, D.; Rowe, K.; Tannenbaum, A.; Popp, F.; Strangeway, R.; Schüler, D.; Di Carlo, D. High-Throughput Microfluidic Sorting of Live Magnetotactic Bacteria. Appl. Environ. Microbiol. 2018, 84, e01308-18. [Google Scholar] [CrossRef]
- Gorby, Y.A.; Beveridge, T.J.; Blakemore, R.P. Characterization of the bacterial magnetosome membrane. J. Bacteriol. 1988, 170, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Grünberg, K.; Wawer, C.; Tebo, B.M.; Schüler, D. A Large Gene Cluster Encoding Several Magnetosome Proteins Is Conserved in Different Species of Magnetotactic Bacteria. Appl. Environ. Microbiol. 2001, 67, 4573–4582. [Google Scholar] [CrossRef] [PubMed]
- Salam, M.A. Isolation of magnetotactic bacteria from environmental samples and optimization and characterization of extracted magnetosomes. Appl. Ecol. Environ. Res. 2019, 17, 5355–5367. [Google Scholar] [CrossRef]
- Baki, A.; Wiekhorst, F.; Bleul, R. Advances in Magnetic Nanoparticles Engineering for Biomedical Applications—A Review. Bioengineering 2021, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C.; Chambel, L.; Rodrigues, M.S.; Ferreira, L.P.; Cruz, M.M. Magnetotactic Bacteria: Magnetism Beyond Magnetosomes. IEEE Trans. NanoBiosci. 2018, 17, 555–559. [Google Scholar] [CrossRef]
- Mathuriya, A.S. Magnetotactic bacteria: Nanodrivers of the future. Crit. Rev. Biotechnol. 2016, 36, 788–802. [Google Scholar] [CrossRef]
- Roda, A.; Cevenini, L.; Borg, S.; Michelini, E.; Calabretta, M.M.; Schüler, D. Bioengineered bioluminescent magnetotactic bacteria as a powerful tool for chip-based whole-cell biosensors. Lab Chip 2013, 13, 4881–4889. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, S.; Arumugasamy, S.K.; Mathiyarasu, J.; Sudhakaran, R.; Suthindhiran, K. Detection of white spot syndrome virus in seafood samples using a magnetosome-based impedimetric biosensor. Arch. Virol. 2021, 166, 2763–2778. [Google Scholar] [CrossRef] [PubMed]
- Sannigrahi, S.; Arumugasamy, S.K.; Mathiyarasu, J.; Suthindhiran, K. Magnetosome-anti-Salmonella antibody complex based biosensor for the detection of Salmonella typhimurium. Mater. Sci. Eng. C 2020, 114, 111071. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, L.; He, J.; Ma, S.; Li, S.; Wang, Z.; Xu, T.; Jiang, W.; Wen, Y.; Li, Y.; et al. Engineered magnetosomes fused to functional molecule (protein A) provide a highly effective alternative to commercial immunomagnetic beads. J. Nanobiotechnol. 2019, 17, 37. [Google Scholar] [CrossRef] [PubMed]
- Orlando, T.; Mannucci, S.; Fantechi, E.; Conti, G.; Tambalo, S.; Busato, A.; Innocenti, C.; Ghin, L.; Bassi, R.; Arosio, P.; et al. Characterization of magnetic nanoparticles from Magnetospirillum Gryphiswaldense as potential theranostics tools. Contrast Media Mol. Imaging 2016, 11, 139–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ni, Q.; Xu, C.; Wan, B.; Geng, Y.; Zheng, G.; Yang, Z.; Tao, J.; Zhao, Y.; Wen, J.; et al. Smart Bacterial Magnetic Nanoparticles for Tumor-Targeting Magnetic Resonance Imaging of HER2-Positive Breast Cancers. ACS Appl. Mater. Interfaces 2019, 11, 3654–3665. [Google Scholar] [CrossRef] [PubMed]
- Mériaux, S.; Boucher, M.; Marty, B.; Lalatonne, Y.; Prévéral, S.; Motte, L.; Lefèvre, C.T.; Geffroy, F.; Lethimonnier, F.; Péan, M.; et al. Magnetosomes, Biogenic Magnetic Nanomaterials for Brain Molecular Imaging with 17.2 T MRI Scanner. Adv. Healthc. Mater. 2015, 4, 1076–1083. [Google Scholar] [CrossRef]
- Xiang, Z.; Yang, X.; Xu, J.; Lai, W.; Wang, Z.; Hu, Z.; Tian, J.; Geng, L.; Fang, Q. Tumor detection using magnetosome nanoparticles functionalized with a newly screened EGFR/HER2 targeting peptide. Biomaterials 2017, 115, 53–64. [Google Scholar] [CrossRef]
- Ertas, Y.N.; Abedi Dorcheh, K.; Akbari, A.; Jabbari, E. Nanoparticles for Targeted Drug Delivery to Cancer Stem Cells: A Review of Recent Advances. Nanomaterials 2021, 11, 1755. [Google Scholar] [CrossRef]
- Zhao, N.; Honert, J.; Schmid, B.; Klas, M.; Isoya, J.; Markham, M.; Twitchen, D.; Jelezko, F.; Liu, R.-B.; Fedder, H.; et al. Sensing single remote nuclear spins. Nat. Nanotechnol. 2012, 7, 657–662. [Google Scholar] [CrossRef]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef]
- Tanaka, M.; Mazuyama, E.; Arakaki, A.; Matsunaga, T. MMS6 Protein Regulates Crystal Morphology during Nano-sized Magnetite Biomineralization in Vivo. J. Biol. Chem. 2011, 286, 6386–6392. [Google Scholar] [CrossRef]
- Taher, Z.; Legge, C.; Winder, N.; Lysyganicz, P.; Rawlings, A.; Bryant, H.; Muthana, M.; Staniland, S. Magnetosomes and Magnetosome Mimics: Preparation, Cancer Cell Uptake and Functionalization for Future Cancer Therapies. Pharmaceutics 2021, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Xavierselvan, M.; Divecha, H.R.; Hajra, M.; Silwal, S.; Macwan, I. Towards Tumor Targeting via Invasive Assay Using Magnetospirillum magneticum. Front. Microbiol. 2021, 12, 697132. [Google Scholar] [CrossRef] [PubMed]
- Martel, S.; Mohammadi, M.; Felfoul, O.; Lu, Z.; Pouponneau, P. Flagellated Magnetotactic Bacteria as Controlled MRI-trackable Propulsion and Steering Systems for Medical Nanorobots Operating in the Human Microvasculature. Int. J. Robot. Res. 2009, 28, 571–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuzajewska, D.; Wszołek, A.; Żwierełło, W.; Kirczuk, L.; Maruszewska, A. Magnetotactic Bacteria and Magnetosomes as Smart Drug Delivery Systems: A New Weapon on the Battlefield with Cancer? Biology 2020, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Majedi, Y.; Loghin, D.; Mohammadi, M.; Martel, S. Characterizations of magnetotactic bacteria conjugated versus unconjugated with carboxylate-Functionalized superparamagnetic iron oxide nanoparticles for tumor targeting purposes. In Proceedings of the 2017 International Conference on Manipulation, Automation and Robotics at Small Scales (MARSS), Montreal, QC, Canada, 17–21 July 2017; pp. 1–6. [Google Scholar] [CrossRef]
- De Lanauze, D.; Felfoul, O.; Turcot, J.-P.; Mohammadi, M.; Martel, S. Three-dimensional remote aggregation and steering of magnetotactic bacteria microrobots for drug delivery applications. Int. J. Robot. Res. 2013, 33, 359–374. [Google Scholar] [CrossRef]
- Wang, J.; Geng, Y.; Zhang, Y.; Wang, X.; Liu, J.; Basit, A.; Miao, T.; Liu, W.; Jiang, W. Bacterial magnetosomes loaded with doxorubicin and transferrin improve targeted therapy of hepatocellular carcinoma. Nanotheranostics 2019, 3, 284–298. [Google Scholar] [CrossRef]
- Piacenza, E.; Presentato, A.; Turner, R.J. Stability of biogenic metal(loid) nanomaterials related to the colloidal stabilization theory of chemical nanostructures. Crit. Rev. Biotechnol. 2018, 38, 1137–1156. [Google Scholar] [CrossRef]
- Lang, C.; Schüler, D.; Faivre, D. Synthesis of Magnetite Nanoparticles for Bio- and Nanotechnology: Genetic Engineering and Biomimetics of Bacterial Magnetosomes. Macromol. Biosci. 2007, 7, 144–151. [Google Scholar] [CrossRef]
- Rong, G.; Corrie, S.R.; Clark, H.A. In Vivo Biosensing: Progress and Perspectives. ACS Sens. 2017, 2, 327–338. [Google Scholar] [CrossRef]
- Ozbakir, H.F.; Miller, A.D.C.; Fishman, K.B.; Martins, A.F.; Kippin, T.E.; Mukherjee, A. A Protein-Based Biosensor for Detecting Calcium by Magnetic Resonance Imaging. ACS Sens. 2021, 6, 3163–3169. [Google Scholar] [CrossRef] [PubMed]
- Finsen, N.R. Nobel Lectures, Physiology or Medicine 1901–1921; Elsevier Publishing Company: Amsterdam, The Netherlands, 1967. [Google Scholar]
- Erdal, E.; Demirbilek, M.; Yeh, Y.; Akbal, Ö.; Ruff, L.; Bozkurt, D.; Cabuk, A.; Senel, Y.; Gumuskaya, B.; Algın, O.; et al. A Comparative Study of Receptor-Targeted Magnetosome and HSA-Coated Iron Oxide Nanoparticles as MRI Contrast-Enhancing Agent in Animal Cancer Model. Appl. Biochem. Biotechnol. 2018, 185, 91–113. [Google Scholar] [CrossRef] [PubMed]






















| Detection Principle | Biorecognition Interface | Target | Detection Limit | Refs. | |
|---|---|---|---|---|---|
| Electrochemical | Square wave voltammetry (SWV) | DNA-modified gold-coated magnetic nanoparticles (DNA-Au@MNPs) | DNA methylation for ovarian cancer diagnosis | 2 aM | [60] |
| SWV | DNA-Au@MNPs | Circulating tumor DNA (ctDNA) | 5 fM | [61] | |
| Differential pulse voltammetry (DPV) | MWCNT/Fe3O4 modified with anti-PSA antibodies | Prostate-specific antigen (PSA) | 0.39 pg·mL−1 | [62] | |
| DPV | Apt-GMNPs | Human T-cell acute lymphocytic leukemia cells (CCRF-CEM) | 10 cells·mL−1 | [63] | |
| Amperometry | Fe3O4@GO modified with anti-PSA antibodies | PSA and prostate-specific membrane antigen (PSMA) | 15 fg·mL−1 and 4.8 fg·mL−1, respectively | [64] | |
| Amperometry | Sox/Pt–Fe3O4@C/GCE | Sarcosine (prostate cancer biomarker) | 0.43 μM | [65] | |
| Electrochemical impedance spectroscopy (EIS) | MBCPE/Fe3O4-RGO/PANHS/ssDNA | Breast cancer mutation BRCA1 5382 insC | 2.8 × 10−19 mol·L−1 | [66] | |
| EIS | MNPs + antibodies | EpCAM, MUC-1, and HER-2 | 0.5 μg, 1.0 μg and 0.125 μg per 106 cells, respectively | [67] | |
| Chronoamperometry | γ-Fe2O3 /CrVI/Amine Oxidase | Polyamine in tumor tissue | 2.47 µM | [68] | |
| Potentiometry | Anti-AFP with the nanogold/MPS–CoFe2O4 particles | AFP (α-1-fetoprotein) | 0.3 ng·mL−1 | [69] | |
| Optical | Surface-enhanced Raman spectroscopy (SERS) | Magnetic nanoparticle–antibody–CEA–antibody–gold nanoparticle–Raman reporter | Carcinoembryonic antigen (CEA) | 10−12 M | [70] |
| SERS | Raman tags-DNA probes modified Fe3O4@Ag NPs | MicroRNA in cancer cells (HeLa, MCF-7, A549) | 0.3 fM | [71] | |
| SERS | Magnetic molecularly imprinted polymers (MMIPs) with anti-PSA@DTNB@Au nanoparticles | Prostate-specific antigen (PSA) | 0.9 pg·mL−1 | [72] | |
| Surface plasmon resonance and MPQ cytometry | Magnetite nanoparticles modified by phytolectins (SBA, WGA, ConA) | Epidermoid carcinoma cells | up to 4.2 ± 0.1 pg·cell−1 2.2 ± 0.5 pg·cell−1 and 0.45 ± 0.07 pg·cell−1, respectively | [73] | |
| Surface plasmon resonance | Erlotinib conjugated MNP (erlotinib-MNP) | Human lung cancer cells (A549 cells) | 5 µg·mL−1 | [74] | |
| UV–vis spectrometry | Au nanoparticles/DNA/magnetic beads | Anterior gradient homolog 2 (AGR2) | 6.6 pM | [75] | |
| Fluorescent detection | DNA/dextran/PAA/Fe3O4 NPs | p53 protein | 8 pM | [76] | |
| Magnetofluoro-immunosensing (MFI) system | Ag/iron oxide NP-decorated graphene | Prostate-cancer-cell-derived exosome | 134.32 NPs·mL−1 | [77] | |
| Colorimetry | superparamagnetic iron oxide nanoparticles (SPIONs)/NanoZyme/Transferrine | Transferrin receptors in human U87MG glioblastoma cells | 50 cells | [78] | |
| Colorimetry | Nanocomposite MNP and Pt NP in ordered mesoporous carbon | Human epidermal growth factor receptor 2 (HER2) | 1.5 ng·mL−1 | [79] | |
| Other principles | Loop-mediated isothermal amplification (LAMP) and lateral flow device (LFD) with magnetometric detection | Biotin-labeled inner primer and digoxigenin-labeled dUTP and gold magnetic nanoparticle (GMNP) as a signal generator | DNA methylation pattern of miR-34a | - | [80] |
| Methylation-specific lateral flow assay (MS-LFA) with magnetometric detection | Amplicon recognizing and capture by gold magnetic nanoparticles (GMNPs) | DNA methylation pattern of miR-34a | 0.01 pg | [81] | |
| Magnetic flow cytometry | Magnetic nanoparticles with aptamers | Pancreatic cancer cells | - | [82] | |
| Magnetoresistance | Fe3O4 NPs/Ab in InSb-based semiconductor channel | Liver cancer antigen | 0.14 pg·mL−1 | [83] | |
| Nanoprobe-based nuclear magnetic resonance (NMR) spectroscopy | Core-shell CoFe2O4@BaTiO3 magnetoelectric (ME) nanoparticles (MENs) | Ovarian carcinoma cells Skov3, glioblastoma cells U87-MG, and breast adenocarcinoma cells MCF-7 | - | [84] | |
| Giant magnetoresistance detection | MoS2–Fe3O4-Aptamer | Exosomes derived from human A431 epidermoid carcinoma cells | 100 exosomes | [85] | |
| Anticancer Drug | Type of MNPs | Coating Agents | Target Cell | Refs. |
|---|---|---|---|---|
| Adriamycin | Fe3O4 | Homogenous gelatin microspheres | Hepatocellular carcinoma (HCC) | [96] |
| Bufalin | Fe3O4 | Liposomes | 4T1 breast cancer cells | [97] |
| Camptothecin (CPT) | Fe3O4 | Dextran + folate | Prostate cancer cells | [98] |
| Сisplatin | Fe3O4 | Amphiphilic polymer + near-infrared dye-labeled HER2 affibody | HER2-expressing tumor cells | [99] |
| Curcumin (Cur) | Fe3O4 | Bovine serum albumin | MCF7 cells | [90] |
| Cur | ZnFe2O4 | L-cysteine (L-Cys) + oxygen-containing functional groups and nitrogen-rich mesoporous graphite-phase carbon nitride (Ox, N-rich mpg-C3N4) | Human lung adenocarcinoma A549 cells | [100] |
| Cur | Fe3O4 | Hyperbranched polyglycerol (HPG) and folic acid (FA) | HeLa cells | [101] |
| Doxorubicin (DOX) | Fe3O4 | Polyethylene Glycol (PEG) + polyarabic acid | Human breast cancer cell line MDA-MB-231 | [102] |
| DOX | Superparamagnetic iron oxide nanoparticles (SPIONs) | Poly(ethylene glycol)-poly(aspartic acid) [PEG-P(Asp)] copolymer | Colon carcinoma and fibroblast cell lines | [103] |
| DOX | mesoporous haematite Fe2O3 | - | Human breast cancer, MCF-7 | [104] |
| DOX | CoFe2O4 | Leucine (Leu) | HeLa cells | [105] |
| DOX | Fe3O4 | Magnetic molybdenum disulfide (mMoS2) + Liposomes | Human breast cancer, MCF-7 | [106] |
| DOX | Ag-Fe3O4 | Dextrin + cell-penetrating peptide (Tat) | MCF-7 cells | [107] |
| DOX and methotrexate | CoFe2O4@BaTiO3 | - | Human hepatocellular carcinoma (HepG2) and human malignant melanoma (HT144) | [108] |
| Erlotinib (ERL) | SPIONs | Poly N-isopropyl acrylamide (PNIPAM) with aptamer AS1411 | Prostate cancer cells | [109] |
| Growth hormone-releasing hormone antagonist of the MIA class (MIA690) | CoFe2O4@BaTiO3 | - | Human glioblastoma cells (U-87MG) | [110] |
| Hydrophobic anticancer agent ASC-J9 | Fe3O4 | Silk fibroin + cationic amphiphilic anticancer peptide, G(IIKK)3I-NH2 (G3) | Colorectal cancer cells HCT 116 | [111] |
| Methotrexate | Fe3O4 | Arginine | MCF-7, 4T1, and HFF-2 cell lines | [90] |
| Oxaliplatin (OXA), and irinotecan (IRI) | Fe3O4 | Chitosan (CS) | CT-26 cancer cells | [112] |
| Paclitaxel (PTX) | SPIONs | FA-conjugated Polyethylene glycol (PEG)/ polyethyleneimine (PEI)-SPIONs SPTX-loaded nanoparticles (SPTX@FA@PEG/PEI-SPIONs) | Nasopharyngeal carcinoma | [88] |
| siRNA | Fe3O4 | Polyethyleneimine (PEI) | B-cell lymphoma-2 (BCL2), Ca9-22 oral cancer cells | [113] |
| Sorafenib | Fe3O4 | Mesoporous organosilica + MnO2 + hyaluronic acid | Human lung adenocarcinoma A549 cells | [114] |
| Quercetin 5-fluorouracil | SPIONs | Zeolitic imidazolate frameworks (ZIF) + FA | Breast cancer MDA-MB-231 cells | [115] |
| Quercetin | MnFe2O4 | Mesoporous hydroxyapatite (HA) | Human breast cancer MCF-7 cells | [116] |
| Ursolic acid (UA) | Fe3O4 | β-cyclodextrin, folate | Human breast cancer MCF-7 cells | [117] |
| Violacein | Fe3O4 | Polylactic acid | Glioblastoma and melanoma cancer cell lines | [118] |
| Zidovudine | NiFe2O4 | Poly(vinyl alcohol)/stearic acid with poly(ethylene glycol) PEG | Human SK-BR-3 breast cancer cell lines | [119] |
| 5-fluorouracil (FLU) | Fe3O4 | (3-aminopropyl) triethoxysilane + tryptophan (TRP) | Human breast cancer MCF-7 cells | [120] |
| FLU | Fe3O4-Pt | FLU@PEG nanospheres | 4T1 cells | [121] |
| Name of Organism | Crystal Composition | Crystal Shape | Magnetosome | Ref. | ||
|---|---|---|---|---|---|---|
| Number | Length (nm) | Width (nm) | ||||
| Alphaproteobacteria | ||||||
| Magnetospirillum caucaseum SO-1. | Fe3O4 | cuboctahedral | ~25 | 40–50 | 40–50 | [157,158,159,160] |
| Magnetospirillum gryphiswaldense MSR-1 | Fe3O4 | cuboctahedral | ~30 | 32–45 | 32–45 | [161,162,163] |
| Magnetospirillum kuznetsovii LBB-42 | Fe3O4 | cuboctahedral | ~25 | 40–50 | 40–50 | [164] |
| Magnetospirillum magneticum AMB-1 | Fe3O4 | cuboctahedral | ~20 | ~45 | ~40 | [165,166,167] |
| Magnetospirillum magnetotacticum MS-1 | Fe3O4 | cuboctahedral | ~25 | 40–50 | 40–50 | [167,168,169] |
| Magnetospirillum marisnigri SP-1 | Fe3O4 | cuboctahedral | ~25 | 40–50 | 40–50 | [157,170] |
| Magnetospirillum moscoviense BB-1 | Fe3O4 | cuboctahedral | ~25 | 40–50 | 40–50 | [157,171] |
| Ca.Magneticavibrio boulderlitore LM-1 | Fe3O4 | prismatic | ~15 | ~50 | ~40 | [172,173] |
| Magnetovibrio blakemorei MV-1 | Fe3O4 | prismatic | ~10 | ~55 | ~35 | [174,175,176] |
| Ca.Terasakiella magnetica PR-1 | Fe3O4 | prismatic | ~15 | ~45 | ~35 | [177] |
| Magnetospira sp. QH-2 | Fe3O4 | prismatic | ~15 | ~80 | ~60 | [178] |
| Gammaproteobacteria | ||||||
| BW-2 | Fe3O4 | octahedral | ~30 | ~65 | ~60 | [179,180] |
| GRS-1 | Fe3O4 | octahedral | ~300 | ~65 | ~55 | [181] |
| FZSR-1 | Fe3O4 | prismatic | ~20 | ~80 | ~55 | [182] |
| FZSR-2 | Fe3O4 | prismatic | ~20 | ~80 | ~55 | [182] |
| NS-1 | Fe3O4 | prismatic | ~10 | ~70 | ~60 | [183] |
| SHHR-1 | Fe3O4 | prismatic | ~15 | ~75 | ~55 | [184] |
| SS-5 | Fe3O4 | prismatic | ~20 | ~85 | ~65 | [180,185] |
| Magnetococcia | ||||||
| Magnetococcus marinus MC-1 | Fe3O4 | prismatic | ~15 | ~80 | ~70 | [186,187,188] |
| Ca. Magnetaquicoccus inordinatus UR-1 | Fe3O4 | prismatic | ~30 | ~75 | ~45 | [189] |
| Ca. Magnetococcus massalia MO-1 | Fe3O4 | cuboctahedral | ~20 | ~65 | ~55 | [190,191] |
| Magnetofaba australis IT-1 | Fe3O4 | cuboctahedral | ~10 | ~85 | ~75 | [192,193] |
| Thermodesulfobacteriota | ||||||
| Ca.Belliniella magnetica LBB04 | Fe3O4 | bullet | ~35 | ~100 | ~35 | [194,195] |
| Desulfamplus magnetovallimortis BW-1 | Fe3O4Fe3S4 | bulletpleomorphic | NDND | ~55~33 | ~35~32 | [155,156,196] |
| Desulfovibrio magneticus RS-1 | Fe3O4 | irregular/bullet | ~10 | ~40 | ~20 | [197,198,199] |
| Ca.Magnetananas rongchenensis RPA | Fe3O4 | bullet | ~70 | ~115 | ~40 | [200,201] |
| Ca. Magnetoglobus multicellularis | Fe3S4 | pleomorphic | 60–100 | ~90 | ~70 | [202,203,204] |
| Nitrospirota | ||||||
| Ca. Magnetobacterium bavaricum | Fe3O4 | bullet | ~1000 | ~130 | ~40 | [154,205] |
| Ca. Magnetobacterium casensis MYR-1 | Fe3O4 | bullet | ~1000 | ~105 | ~40 | [206,207] |
| Ca. Magnetobacterium cryptolimnobacter XYR | Fe3O4 | bullet | ~150 | ~130 | ~30 | [208] |
| Ca.Magnetomicrobium cryptolimnococcus XYC | Fe3O4 | bullet | ~100 | ~135 | ~45 | [208] |
| Ca.Magnetominusculus linsii LBB02 | Fe3O4 | bullet | ~40 | ~120 | ~40 | [194,195] |
| Ca. Magnetomonas plexicatena LBB01 | Fe3O4 | bullet | ~35 | ~110 | ~45 | [194,195] |
| Omnitrophota | ||||||
| Ca. Omnitrophus magneticus SKK-01 | Fe3O4 | bullet | ~175 | ~110 | ~35 | [205,209] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimina, T.M.; Sitkov, N.O.; Gareev, K.G.; Fedorov, V.; Grouzdev, D.; Koziaeva, V.; Gao, H.; Combs, S.E.; Shevtsov, M. Biosensors and Drug Delivery in Oncotheranostics Using Inorganic Synthetic and Biogenic Magnetic Nanoparticles. Biosensors 2022, 12, 789. https://doi.org/10.3390/bios12100789
Zimina TM, Sitkov NO, Gareev KG, Fedorov V, Grouzdev D, Koziaeva V, Gao H, Combs SE, Shevtsov M. Biosensors and Drug Delivery in Oncotheranostics Using Inorganic Synthetic and Biogenic Magnetic Nanoparticles. Biosensors. 2022; 12(10):789. https://doi.org/10.3390/bios12100789
Chicago/Turabian StyleZimina, Tatiana M., Nikita O. Sitkov, Kamil G. Gareev, Viacheslav Fedorov, Denis Grouzdev, Veronika Koziaeva, Huile Gao, Stephanie E. Combs, and Maxim Shevtsov. 2022. "Biosensors and Drug Delivery in Oncotheranostics Using Inorganic Synthetic and Biogenic Magnetic Nanoparticles" Biosensors 12, no. 10: 789. https://doi.org/10.3390/bios12100789
APA StyleZimina, T. M., Sitkov, N. O., Gareev, K. G., Fedorov, V., Grouzdev, D., Koziaeva, V., Gao, H., Combs, S. E., & Shevtsov, M. (2022). Biosensors and Drug Delivery in Oncotheranostics Using Inorganic Synthetic and Biogenic Magnetic Nanoparticles. Biosensors, 12(10), 789. https://doi.org/10.3390/bios12100789









